- 1Department of Hepatobiliary Surgery, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 2Department of Pathology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Extramammary Paget’s disease (EMPD) is a rare cutaneous neoplasm with distant metastases and a poor prognosis. We report the case of a 63-year-old male patient exhibiting stage IV primary EMPD with neuroendocrine differentiation, and harboring a somatic mutation in AMER1. After four cycles of Anlotinib combined with Tislelizumab, the patient achieved partial response for the metastatic lesions according to mRECIST1.1 criteria. Total positron emission tomography and computed tomography (PET-CT) scans revealed a significant reduction in SUV from 18.9 to 5.3, and the serum CEA decreased to normal levels after the treatment regimen. However, the patient developed fractures of the fourth and fifth thoracic vertebrae during the treatment. Therefore, percutaneous vertebroplasty was performed, and the patient experienced severe postoperative pneumonia and died from pulmonary encephalopathy and respiratory failure in June 2021. The overall and progression-free survival of the patient after diagnosis were 9 and 8 months, respectively. During the systemic treatment, the patient suffered grade 1 rash in the back and thigh and grade 1 hypertension. Nevertheless, the combination treatment of anlotinib and tislelizumab had a favorable clinical outcome and provided a survival advantage, and should be considered a therapeutic option for patients with AMER1-mutant metastatic EMPD.
Introduction
Extramammary Paget’s disease (EMPD) is a rare cutaneous neoplasm that accounts for 6.5% of all Paget’s disease cases (1). Most EMPD cases are diagnosed as carcinoma in situ, which usually shows indolent disease progression. However, once Paget cells invade deeply into the dermis, they frequently metastasize to the regional lymph nodes (LN) and distant organs (2). Distant metastasis is associated with poor prognosis and <10% 5-year survival rate due to the limited efficacy of conventional chemotherapies (1, 3).
Recent studies have elucidated the pathways underlying EMPD development and progression, which can lead to the development of novel treatment approaches for metastatic EMPD. For instance, genomic analyses of EMPD lesions have identified somatic mutations in various genes, including TP53, ERBB, NRAS, BRAF, PIK3CA, and AKT1 (4, 5). This strongly suggests that the pathways downstream of ERBB2/HER2, such as the RAS/RAF-MEK-ERK and PI3K-AKT-mTOR pathways, may drive EMPD progression (5), and should be explored further as potential therapeutic targets for metastatic EMPD. Patients with metastatic EMPD may be considered for chemotherapy, targeted therapy, or immune checkpoint inhibitors. The tyrosine kinase inhibitor Anlotinib (6) has multiple targets and exerts its anti-tumor effect by inhibiting PI3K/AKT phosphorylation (7). Combined targeted therapy and immune checkpoint inhibitors may be appropriate for patients without good performance status.
Herein, we report a case of metastatic EMPD that responded to the combination therapy of Anlotinib and the checkpoint inhibitor Tislelizumab.
Case Presentation
A 63-year-old male who was diagnosed with eczema of the scrotum in 2012 and did not pursue treatment visited our hospital on 12th October, 2020 on account of back pain. Positron emission tomography computed tomography (PET-CT) showed erosion and metastatic nodules in the occipital bone, entire vertebra, bilateral ribs, appendicular skeleton and the pelvic bone (Figures 1A,B). The patient was also positive for hepatitis B, and the serum levels of CA19.9, AFP, NSE, and CEA were 43.24 U/ml, 2.90 ng/ml, 15.62 ng/L, and 1,488 ng/ml, respectively. Fine-needle aspiration cytology (FNAC) was performed on the hepatic metastatic node and right groin lymph node, and the biopsies indicated neuroendocrine differentiation. However, based on the clinical presentation and case history, we suspected that the primary tumor was eczema of scrotum (Figure 2). Perineal skin mass resection surgery was subsequently performed, and histological examination of the epidermis and dermis revealed lightly stained tumor cells with dense cytoplasm that were arranged in the form of sheets, small nests or scattered masses. These histological features were consistent with Paget’s disease with infiltrating adenocarcinoma. The tumor grew 0.4 cm deep into the dermis and involved the skin appendages. Immunohistochemical staining indicated that the EMPD tumor cells were positive for GATA3 (3+), CK7 (3+), GCDFP15 (1+), androgen receptor (2+), HER2 (2+), ER (1+), CEA (3+), and synaptophysin (focal+), and negative for CK20, P16, S-100, HMB-45, P40, PR, CD56, and ChrA. However, the FISH test for HER2 was negative (Figure 3). Based on the histopathological and immunohistochemical findings, we diagnosed the case as cT1N2M1 primary EMPD with neuroendocrine differentiation on 20th October, 2020.
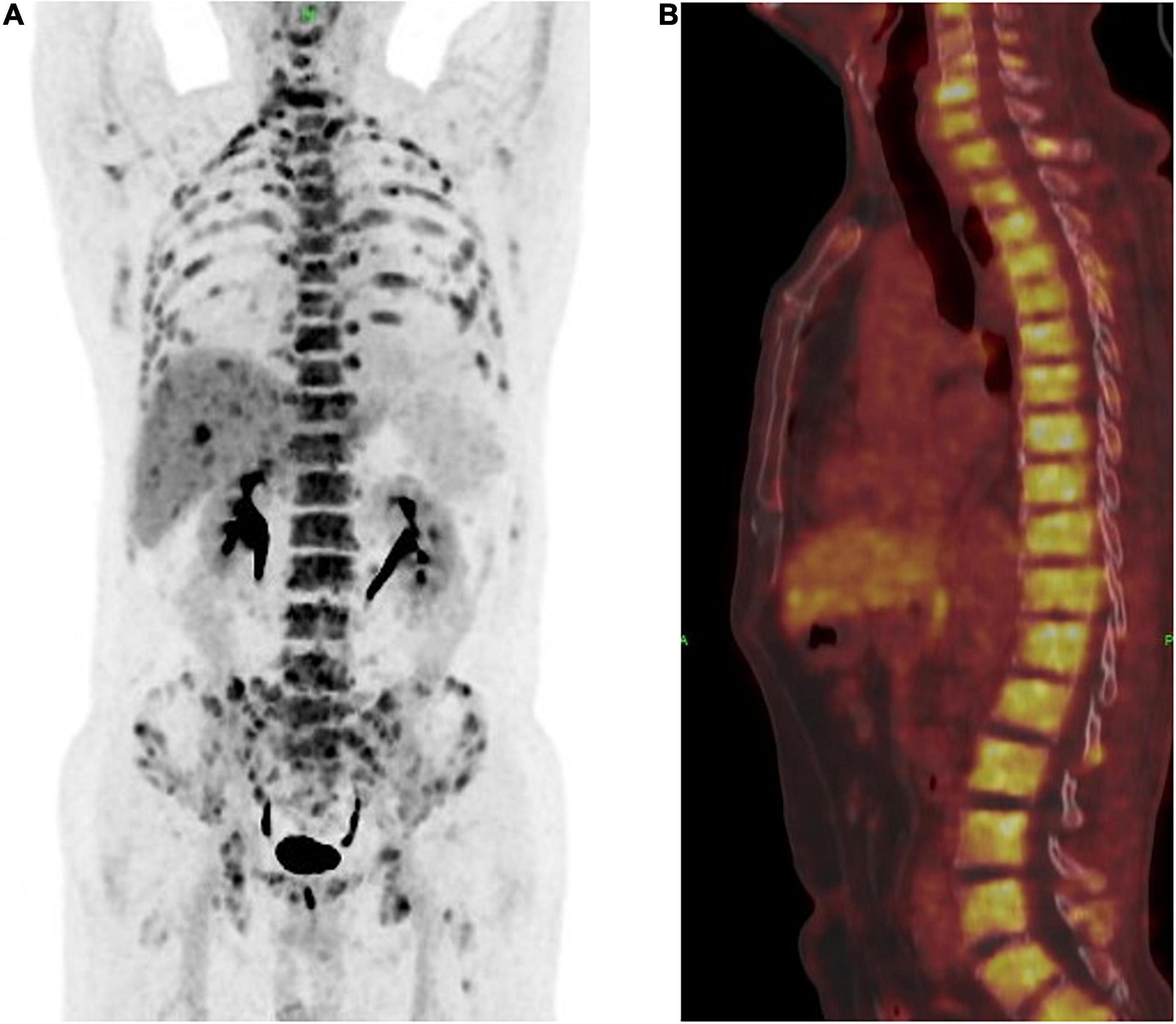
Figure 1. (A) Positron emission tomography and computed tomography (PET-CT) showed a multiple bone destruction with bone metastases including whole vertebra, occipital bone, ribs of double side, appendicular skeleton, and pelvis. (B) PET-CT showed a whole vertebra metastatic Primary EMPD, showing that a most obvious SUV of 18.9.
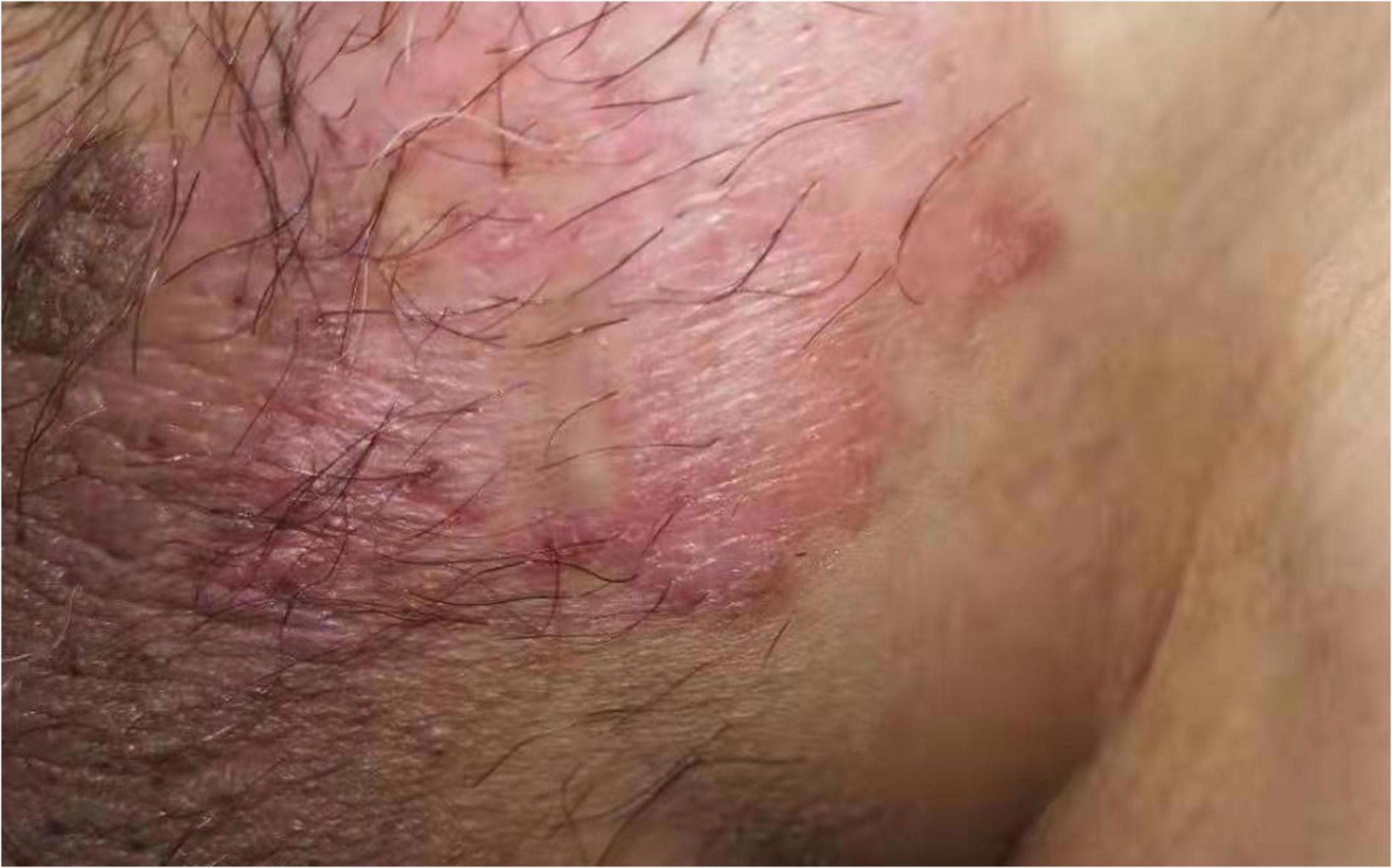
Figure 2. The perineal lesions were about 4 cm × 3 cm in size, with obvious rash, erythema and erosion, accompanied by pruritus, no pain and exudation.
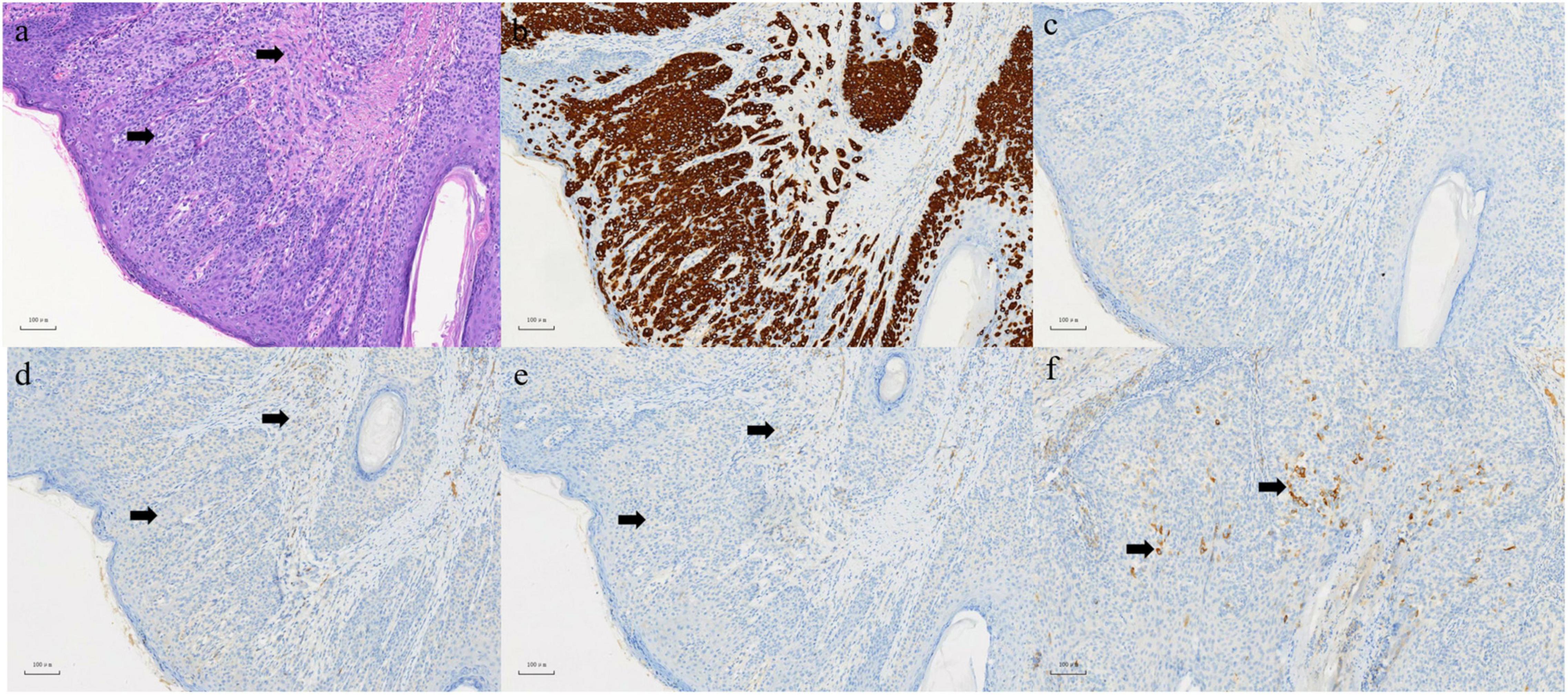
Figure 3. (a) In epidermis and dermis, there are abundant pale-staining cytoplasmic, which are flaky, small nests or scattered cells in HE staining, involving both the dermis and skin appendages. Left arrow: Paget cell. Right arrow: infiltrating adenocarcinoma. (b) Positive expression of CK (7) in EMPD section 100×. (c) Negative expression of CK (20) in EMPD section 100×. (d) Negative expression of CD (56) in EMPD section 100×. (e) Negative expression of ChrA in EMPD section 100×. (f) Focal positive expression of synaptophysin in EMPD section 100×.
Sequencing analysis of the biopsied specimens revealed a mutation in AMER1 exon 2 (c.1724G > A, p.R575Q) (Supplementary Figure 1), which has been identified as the causative mutation of aberrant PI3K/AKT/mTOR signaling in gastric cancer cells, and promotes the progression and metastasis of gastric cancer (8). In addition, the patient had a tumor mutational burden (TMB) of 2.38 mut/Mb and a stable microsatellite status (MSS), along with low expression of programmed death-ligand 1 (PD-L1). Given the extensive bone metastases, especially in the entire vertebra, we did not consider conventional chemotherapy due to its myelosuppressive effects. Based on the poor physical condition of the patient and genetic results, we initiated a regimen of Anlotinib (12 mg) in combination with the IgG4 anti-PD-1 humanized monoclonal antibody Tislelizumab (9, 10) after a multi-disciplinary panel discussion.
The patient achieved partial response (PR) for the metastatic lesions according to the mRECIST1.1 criteria after four cycles of treatment. The total PET-CT scan (Figures 4A,B), showed an obvious decrease in SUV from 18.9 to 5.3, and the serum level of CEA also dropped within the normal range. Computed tomography (CT) and magnetic resonance imaging (MRI) showed that both hepatic and lymph node (regional and distant) metastases achieved stable disease (SD) status as per RECIST1.1. However, the patient developed fractures in the fourth and fifth thoracic vertebrae during the treatment period due to an accident. Therefore, the treatment regimen was suspended for 4 weeks and percutaneous vertebroplasty was performed in that interval. External thoraco-lumbosacral orthosis was used postoperatively.
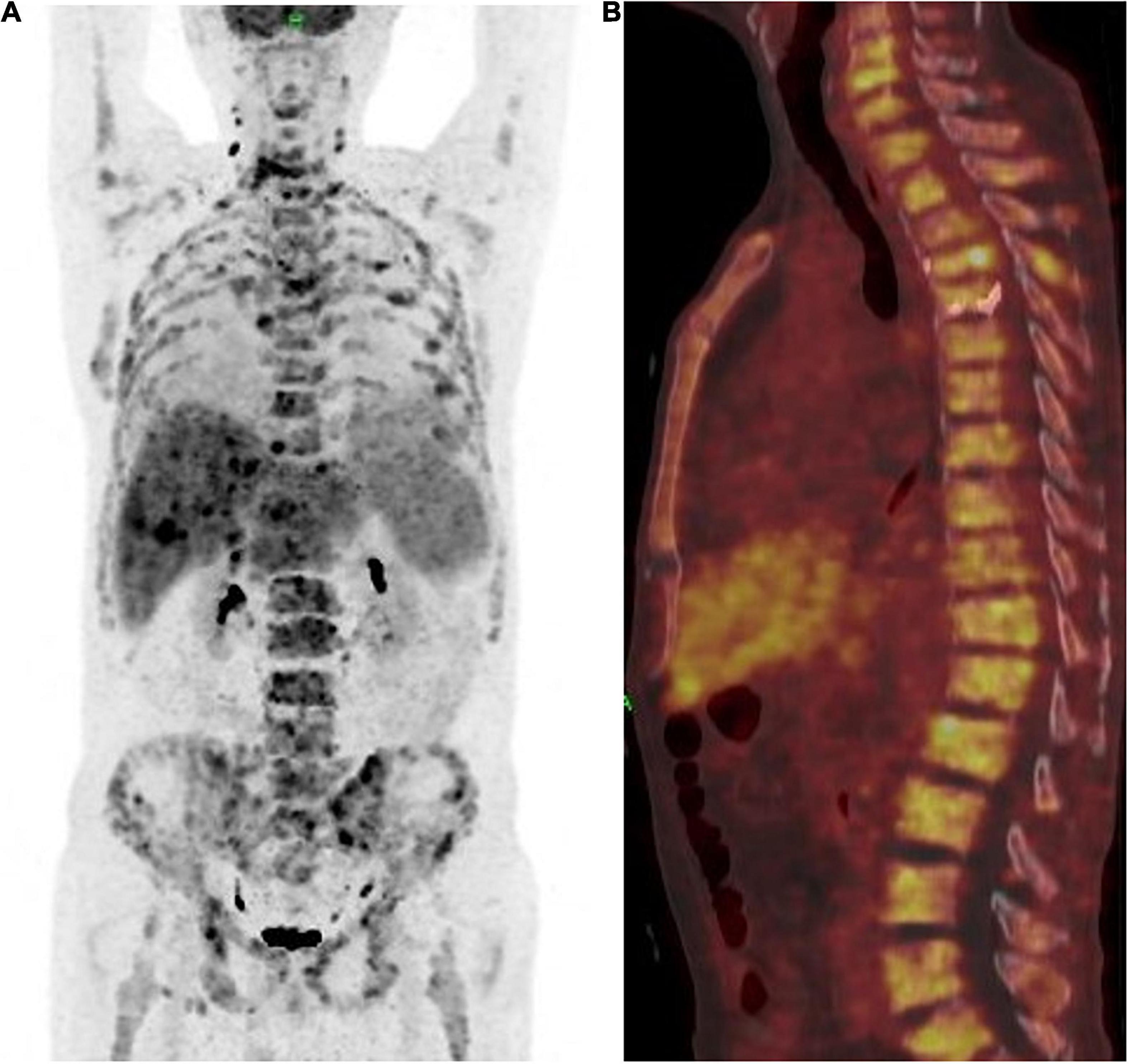
Figure 4. (A) Positron emission tomography and computed tomography showed the patient achieved partial response (PR) according to the mRECIST1.1 on metastatic lesions. (B) PET-CT showed a whole vertebra metastatic Primary EMPD, showing that a most obvious decrease of SUV from 18.9 to 5.3.
The patient experienced severe postoperative pneumonia. Chest CT showed that the metastatic lymph nodes of thoracic vertebrae and mediastinum were stable. He died from pulmonary encephalopathy and respiratory failure in June, 2021 with overall survival (OS) of 9 months and progression-free survival (PFS) of 8 months. During the treatment regimen, the patient experienced grade 1 rashes on the back and thigh, as well as grade 1 hypertension.
Discussion
Chemotherapy remains the cornerstone of EMPD management. However, there is currently no standardized treatment for metastasis (11, 12). Kato et al. retrospectively analyzed 17 EMPD patients with multiple metastases, of which eight were treated with 5-fluoruuracil (FU)/cisplatin and nine patients chose best supportive care (13). The median PFS (mPFS) and median OS (mOS) of the 5-FU/cisplatin group were 6.2 and 19.4 months, respectively, and were not significantly longer than that of the supportive care group (13). Tokuda et al. retrospectively examined 22 patients with advanced EMPD who received 5-FU/cisplatin therapy, and found that mPFS and mOS were 5.2 and 12 months, respectively (14). Yoshino et al. conducted a multicenter, retrospective study to evaluate the efficacy of docetaxel as a first line chemotherapy for 13 metastatic EMPD patients, and found that the mPFS and mOS were 7.1 and 16.6 months, respectively (15). Docetaxel and low-dose 5-FU/cisplatin (FP) have been used to treat locally advanced EMPD, although data is limited. While docetaxel monotherapy can result in grade 3 or 4 myelosuppressive events, FP therapy usually requires repeated hospitalization (15). Although conventional chemotherapies have been used to treat distant metastases for a long time, some studies indicated possible efficacy of chemotherapies, however, no prospective study has shown that conventional chemotherapy improves overall survival. Guercio et al. reported a case of metastatic EMPD with treatment response to checkpoint inhibitor immunotherapy (16). The patient had diffuse metastasis and had previously progressed after receiving chemotherapy and alpelisib, an oral α-specific PIK3CA inhibitor. The treatment was discontinued due to the toxic effects of alpelisib such as nausea, grade 3 rash and hyperglycemia, as well as disease progression. The new treatment regimen consisting of four cycles of 1 mg/kg ipilimumab plus 3 mg/kg nivolumab resulted in a durable partial response lasting 7 months (16).
Evidence-Based Clinical Practice Guidelines recommends chemotherapy, targeted therapy or immune checkpoint inhibitors for patients with metastatic EMPD (11). Around 15–58% of EMPD patients overexpress HER2, which likely plays a crucial role in the development and progression of EMPD (17). Multiple cases of HER2-positive advanced EMPD have been reported that respond to the anti-HER2 antibody trastuzumab, either as a monotherapy or combined with other chemotherapies (18). HER2 lies upstream of the RAS–RAF–MEK–ERK and PI3K–AKT–mTOR pathways, which accelerates cell growth and promotes survival (19). Furthermore, DNA sequencing of EMPD tumors has shown that upregulation of HER2 and its downstream effectors is associated with the progression of EMPD (3). However, our patient was HER negative and harbored a mutation in the AMER1 exon 2 (c.1724G > A, p.R575Q). AMER1 inhibits the PI3K/AKT/mTOR pathway by blocking PI3K phosphorylation (8). Unfortunately, we did not have access to the PI3K specific inhibitor alpelisib. It had been demonstrated that treatment with alpelisib prolonged DFS among patients with PIK3CA-mutated, HER2-negative advanced breast cancer who had received endocrine therapy previously (20). The tyrosine kinase inhibitor Anlotinib (6) has multiple targets and exerts its anti-tumor effect by inhibiting PI3K/Akt phosphorylation (7). In a recent study, Anlotinib mainly inhibited angiogenesis and tissue tumor cell migration by inhibiting c-Kit, VEGFR2, VEGFR3, FGFR1-4, PDGFR α and β, and other targets, thus inhibiting tumor by inhibited PI3K/AKT/Bad phosphorylation and promoted apoptosis of tumor cells by activating RAS protein expression (21, 22). In the present study, the combination of Anlotinib and Tislelizumab significantly prolonged the DFS of the patient to 8 months.
Tislelizumab was an investigational humanized IgG4 monoclonal antibody and engineered to minimize binding to FcγR on macrophages in order to limit antibody-dependent phagocytosis, a potential mechanism of resistance to anti-PD-1 therapy. Dose-escalation and dose-expansion study had assessed the safety, tolerability, pharmacology and clinical activity of tislelizumab in patients with advanced solid tumors (10). Previous studies have reported low rates (10%) of PD-L1 expression in EMPD tumor cells (23–25), and checkpoint inhibitor blockade is recommended for patients with mismatch repair or microsatellite instability, or high mutational burden (11), that does not mean that there would be poor anti-tumor effect from checkpoint inhibitors in all EMPD cases. A prior study identified germline mismatch repair gene missense mutations in 8 of 20 EMPD tumors, with a proportion of cases exhibiting microsatellite instability (MSI), lending rationale for consideration of checkpoint inhibitor immunotherapy in such cases (26). Our patient responded to immune checkpoint blockade despite microsatellite stability. Although PD-L1 expression correlates with the response to PD-1/PD-L1 targeted therapies in some settings, it often fails to predict treatment response. Furthermore, there are currently no established criteria for high versus low TMB in EMPD. One study reported successful outcome of immune checkpoint inhibition for metastatic EMPD with low PD-L1 expression, microsatellite stability and TMB of 4.4 mut/MB (16). Likewise, our study also provides preliminary evidence for extending the indication for immunotherapy to primary metastatic EMPD.
There is no report at present of primary metastatic EMPD with neuroendocrine differentiation. Furthermore, only one case of canal adenocarcinoma with neuroendocrine features accompanying secondary EMPD has been reported in an elderly male patient, who responded to 5-FU, leucovorin and oxaliplatin (FOLFOX6) (27). The present case was that of a rare primary EMPD with neuroendocrine features, which indicated poor prognosis on account of metastases. For advanced malignancies lacking standard treatment, therapy based on mutated genes and pathways may improve prognosis. While our findings support further investigation into the safety and efficacy of targeted therapy combined with immune checkpoint inhibitors for metastatic EMPD, achieving more than 8 months of DFS and 9 months of OS in the poor performance status patient. Tislelizumab concomitant with Anlotinib has tolerable toxicity and favorable antitumor activity in patients with previously treated advanced tumors (28), no additional toxicity was observed. These findings contribute new, important information on Anlotinib plus Tislelizumab in metastatic EMPD, particularly demonstrated the Anlotinib inhibit the PI3K/AKT signaling pathway in the EMPD with AMER1 mutations, provided evidences and a basic principle for using Anlotinib plus Tislelizumab to treat patients with advanced malignancy who lack effective treatment choices.
In conclusion, an AMER1-mutant patient with rare primary EMPD presenting neuroendocrine features responded to the combination treatment of Anlotinib and Tislelizumab, which suggest an effective and tolerable therapeutic option for this rare disease.
Author Contributions
LX, XL, ML, and QS contributed to conception and design of the study. YL, XC, SM, QM, ZH, ZZ, and XC contributed to the acquisition, analysis, or interpretation of data for the work. XY and XH wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Sanming Project of Medicine in Shenzhen (No. SZSM202011010) and Shenzhen High-level Hospital Construction Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge Hong Zhao and Xinyu Bi work in National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, for the writing assistance and critical revision of the manuscript for important intellectual content.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.891958/full#supplementary-material
Supplementary Figure 1 | The IGV picture of AMER1 exon 2 (c.1724G > A, p.R575Q).
References
1. Alo PL, Galati GM, Sebastiani V, Ricci F, Visca P, Mariani L, et al. Fatty acid synthase expression in Paget’s disease of the vulva. Int J Gynecol Pathol. (2005) 24:404–8. doi: 10.1097/01.pgp.0000170065.53813.81
2. Fujisawa Y, Yoshino K, Kiyohara Y, Kadono T, Murata Y, Uhara H, et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget’s disease: multi-center, retrospective study of 151 patients. J Dermatol Sci. (2015) 79:38–42. doi: 10.1016/j.jdermsci.2015.03.014
3. Fukuda K, Funakoshi T. Metastatic extramammary Paget’s disease: pathogenesis and novel therapeutic approach. Front Oncol. (2018) 8:38. doi: 10.3389/fonc.2018.00038
4. Kiniwa Y, Yasuda J, Saito S, Saito R, Motoike IN, Danjoh I, et al. Identification of genetic alterations in extramammary Paget disease using whole exome analysis. J Dermatol Sci. (2019) 94:229–35. doi: 10.1016/j.jdermsci.2019.03.006
5. Ishida Y, Kakiuchi N, Yoshida K, Inoue Y, Irie H, Kataoka TR, et al. Unbiased detection of driver mutations in extramammary Paget disease. Clin Cancer Res. (2021) 27:1756–65. doi: 10.1158/1078-0432.ccr-20-3205
6. Chi Y, Ji G, Zhang J, Tang H, Yang Y, Liu W, et al. Efficacy and safety of anlotinib in patients with advanced malignancy: a single-center, single-arm, phase 2 trial. Int J Clin Oncol. (2021) 26:1611–8. doi: 10.1007/s10147-021-01959-z
7. Lu Y, Lin J, Duan M, Rui Y, Zheng H, Zhu L, et al. Anlotinib suppresses oral squamous cell carcinoma growth and metastasis by targeting the RAS protein to inhibit the PI3K/Akt signalling pathway. Anal Cell Pathol (Amst). (2021) 2021:5228713. doi: 10.1155/2021/5228713
8. Li J, Ye D, Shen P, Liu X, Zhou P, Zhu G, et al. Mir-20a-5p induced WTX deficiency promotes gastric cancer progressions through regulating PI3K/AKT signaling pathway. J Exp Clin Cancer Res. (2020) 39:212. doi: 10.1186/s13046-020-01718-4
9. Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. (2020) 8:e000437. doi: 10.1136/jitc-2019-000437
10. Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. (2020) 8:e000453. doi: 10.1136/jitc-2019-000453
11. Kibbi N, Owen JL, Worley B, Wang JX, Harikumar V, Downing MB, et al. Evidence-based clinical practice guidelines for extramammary Paget disease. JAMA Oncol. (2022) 8:618–28. doi: 10.1001/jamaoncol.2021.7148
12. Ishizuki S, Nakamura Y. Extramammary Paget’s disease: diagnosis, pathogenesis, and treatment with focus on recent developments. Curr Oncol. (2021) 28:2969–86. doi: 10.3390/curroncol28040260
13. Kato H, Watanabe S, Kariya K, Nakamura M, Morita A. Efficacy of low-dose 5-fluorouracil/cisplatin therapy for invasive extramammary Paget’s disease. J Dermatol. (2018) 45:560–3. doi: 10.1111/1346-8138.14247
14. Tokuda Y, Arakura F, Uhara H. Combination chemotherapy of low-dose 5-fluorouracil and cisplatin for advanced extramammary Paget’s disease. Int J Clin Oncol. (2015) 20:194–7. doi: 10.1007/s10147-014-0686-2
15. Yoshino K, Fujisawa Y, Kiyohara Y, Kadono T, Murata Y, Uhara H, et al. Usefulness of docetaxel as first-line chemotherapy for metastatic extramammary Paget’s disease. J Dermatol. (2016) 43:633–7. doi: 10.1111/1346-8138.13200
16. Guercio BJ, Iyer G, Kidwai WZ, Lacouture ME, Ghafoor S, Rossi AM, et al. Treatment of metastatic extramammary paget disease with combination ipilimumab and nivolumab: a case report. Case Rep Oncol. (2021) 14:430–8. doi: 10.1159/000514345
17. Tanaka R, Sasajima Y, Tsuda H, Namikawa K, Tsutsumida A, Otsuka F, et al. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br J Dermatol. (2013) 168:1259–66. doi: 10.1111/bjd.12249
18. Ichiyama T, Gomi D, Fukushima T, Kobayashi T, Sekiguchi N, Sakamoto A, et al. Successful and long-term response to trastuzumab plus paclitaxel combination therapy in human epidermal growth factor receptor 2-positive extramammary Paget’s disease: a case report and review of the literature. Mol Clin Oncol. (2017) 7:763–6. doi: 10.3892/mco.2017.1422
19. Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. (2014) 13:1021–31. doi: 10.1158/1535-7163.mct-13-0639
20. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
21. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. (2018) 11:120. doi: 10.1186/s13045-018-0664-7
22. Syed YY. Anlotinib: first global approval. Drugs. (2018) 78:1057–62. doi: 10.1007/s40265-018-0939-x
23. Urata K, Kajihara I, Miyauchi H, Mijiddorj T, Otsuka-Maeda S, Sakamoto R, et al. The Warburg effect and tumour immune microenvironment in extramammary Paget’s disease: overexpression of lactate dehydrogenase A correlates with immune resistance. J Eur Acad Dermatol Venereol. (2020) 34:1715–21. doi: 10.1111/jdv.16145
24. Karpathiou G, Chauleur C, Hathroubi S, Habougit C, Peoc’h M. Expression of CD3, PD-L1 and CTLA-4 in mammary and extra-mammary Paget disease. Cancer Immunol Immunother. (2018) 67:1297–303. doi: 10.1007/s00262-018-2189-x
25. Pourmaleki M, Young JH, Socci ND, Chiang S, Edelweiss M, Li Y, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. (2019) 10:6152–67. doi: 10.18632/oncotarget.27247
26. Kang Z, Xu F, Zhu Y, Fu P, Zhang QA, Hu T, et al. Genetic analysis of mismatch repair genes alterations in extramammary Paget disease. Am J Surg Pathol. (2016) 40:1517–25. doi: 10.1097/pas.0000000000000709
27. Yamaura M, Yamada T, Watanabe R, Kawai H, Hirose S, Tajima H, et al. Anal canal adenocarcinoma with neuroendocrine features accompanying secondary extramammary Paget disease, successfully treated with modified FOLFOX6: a case report. BMC Cancer. (2018) 18:1142. doi: 10.1186/s12885-018-5084-0
Keywords: extramammary Paget disease, anlotinib, tislelizumab, neuroendocrine differentiation, survival
Citation: Yin X, Li X, Li M, She Q, Liu Y, Chen X, Ma S, Ma Q, Huang Z, Xu L, Huang X, Zhan Z and Che X (2022) Treatment of Metastatic Primary Extramammary Paget Disease With Combination Anlotinib and Tislelizumab: A Case Report and Review of the Literature. Front. Med. 9:891958. doi: 10.3389/fmed.2022.891958
Received: 08 March 2022; Accepted: 27 April 2022;
Published: 24 May 2022.
Edited by:
Richard Beatson, University College London, United KingdomReviewed by:
Apurva Patel, Gujarat Cancer and Research Institute, IndiaManuel Valdebran Canales, Medical University of South Carolina, United States
Copyright © 2022 Yin, Li, Li, She, Liu, Chen, Ma, Ma, Huang, Xu, Huang, Zhan and Che. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhun Huang, ZHJoeGp1bmVAMTYzLmNvbQ==; Lin Xu, ZHJ4dWxpbkAxMjYuY29t
†These authors have contributed equally to this work
 Xin Yin1†
Xin Yin1† Lin Xu
Lin Xu Xiaozhun Huang
Xiaozhun Huang Xu Che
Xu Che