- 1Department of Pharmacy, Peking University Third Hospital, Beijing, China
- 2Department of Hematology, Lymphoma Research Center, Peking University Third Hospital, Beijing, China
Objective: Chemotherapy regimens containing rituximab (RTX) have been extensively used to treat diffuse large B cell lymphoma (DLBCL). However, data looking at long-term safety of DLBCL patients with hepatitis B-related cirrhosis are still lacking. This study aims to report the safety and outcomes of RTX administration in DLBCL patients with hepatitis B-related cirrhosis.
Methods: A retrospective case series was designed and implemented, using data from January 1, 2011 to December 31, 2020. Consecutive patients who were diagnosed with DLBCL and hepatitis B-related cirrhosis receiving RTX treatment were included. The primary outcomes included HBV reactivation, hepatitis flares or abnormal liver function. Survival status, the secondary outcome measure, was observed until death, loss to follow-up, or the end of follow-up, whichever occurred first.
Results: A total of 8 DLBCL patients combined with hepatitis B-related cirrhosis were included in this study [4 men; median age 62.5 years (range, 44–77 years); median RTX-containing regimen course 5 (range, 2–11)]. Of them, 6 patients had current HBV infection with HBsAg-positive and anti-HBc-positive, whereas 2 patients had previously resolved HBV infection with HBsAg-negative and anti-HBc-positive. The HBV reactivation was observed in only one patient, who received 11 courses of RTX-containing immunochemotherapies within 15 months. No hepatitis flares or abnormal liver function occurred in any patients included. All patients received standardized antiviral therapy for a lifelong time. Of 8 patients included, 3 patients died, and 1 patient was lost to follow-up, and the median overall survival among patients was 39 months (range, 7–82 months).
Conclusion: The findings provide support for the concept that, on the premise of standardized and valid management strategy, RTX containing regimens may be a safe option for use as the treatment of DLBCL patients combined with hepatitis B-related cirrhosis.
Introduction
The anti-CD20 monoclonal antibody rituximab (RTX), first approved for clinical use in 1997, has changed the standard of care for patients with various non-Hodgkin lymphoma (NHL) and non-malignant immune-mediated diseases (1, 2). It has been well recognized that the addition of RTX to standard chemotherapy regimens substantially improves both response rates and survival outcomes in patients with diffuse large B cell lymphoma (DLBCL), which represents the major subtype of NHL worldwide (3, 4). Over recent years, RTX has been extensively used as a mainstay therapeutic agent for DLBCL. For example, an R-CHOP regimen comprising RTX, anthracyclines, cyclophosphamide, vincristine, and prednisone is established as the first-line treatment for DLBCL by the National Comprehensive Cancer Network (NCCN) clinical practice guideline (5).
RTX is of great clinical importance, whereas a close link has been established between RTX therapy and hepatitis B virus (HBV) reactivation (6–8). Patients with HBV reactivation may postpone scheduled chemotherapy or present with abnormal liver function, leading to adverse effects on treatment outcome for the primary disease. It is estimated that 296 million people worldwide are living with chronic HBV infection in 2019 (9). Among individuals with chronic HBV infection, up to 40% of untreated patients chronic progress to cirrhosis, which may lead to liver failure, hepatocellular carcinoma and even death (10). Globally, cirrhosis caused more than 1.32 million deaths in 2017, and hepatitis B remains one of leading causes of cirrhosis worldwide (11). Thus, cirrhosis is a major cause of morbidity and mortality burden across the world, and imposes a substantial health problem on many countries (11).
In particular, with the largest population in the world, China still faces the severe problem of HBV infection and cirrhosis. Currently, as many as 7 million (0.5%) of the total Chinese population lives with cirrhosis (12). And it is worth mentioning that, the prevalence of HBV in China (6.2%) is much higher than that in developed countries (0.71-1.17%) (12–15), so there is still plenty of room for growth in the scale of cirrhosis. Therefore, the clinical management of RTX therapy in patients with hepatitis B-related cirrhosis is still posing a major challenge.
Severe and even fatal HBV reactivation has been described in patients with previous HBV infection undergoing RTX-containing chemotherapy. Anti-CD20 monoclonal antibody therapy (e.g., RTX) has been widely recognized as a very high risk for HBV reactivation in patients with chronic infection, which means that the chance of HBV reactivation is >20% (16, 17). Additionally, previous studies have confirmed a high risk of HBV reactivation in Asian lymphoma patients with previously resolved HBV infection (18–20). Nevertheless, none of the published studies have evaluated the safety of RTX therapy and the risk of HBV reactivation in patients with hepatitis B-related cirrhosis. Currently, in real clinical practice, there still exist knowledge gaps concerning the safety of RTX therapy in lymphoma patients with hepatitis B-related cirrhosis.
Herein, the objective of this retrospective case series was to report the safety and outcomes of RTX administration in DLBCL patients with hepatitis B-related cirrhosis. We aimed to fill the gaps between knowledge and clinical practice, and provide evidence for further RTX therapy in patients with hepatitis B-related cirrhosis.
Patients And Methods
Study Design and Patients
A retrospective case series was designed and implemented. During a 10-year period from January 1, 2011 to December 31, 2020, the study population included consecutive patients who were diagnosed with DLBCL and hepatitis B-related cirrhosis and underwent RTX treatment at the Lymphoma Research Center of the Peking University Third Hospital. This retrospective study was approved by the hospital institutional review board (No. IRB00006761-M2022059). The requirement for informed patient consent was waived, because it was determined that this study did not directly involve human participants and was a secondary analysis of existing data of deidentified patients.
The diagnosis and classification of DLBCL were mainly based on a constellation of clinical, morphologic, immunophenotypic, and molecular genetic features. The histological classification was based on the 2016 World Health Organization classification standard of hematopoiesis and lymphoid tissue tumor diseases (21). The clinical staging was derived from the Ann Arbor staging system (22, 23). The diagnosis of hepatitis B-related cirrhosis was based on the etiology, history, clinical manifestations, complications, treatment process, laboratory tests, imaging, and histology. The Child-Pugh scoring system was used to classify cirrhosis patients into grade A, B or C. A total score of 5 to 6 was considered grade A, 7 to 9 was considered grade B, and 10 to 15 was considered grade C (24). In grade A, the cirrhosis was compensated, while in grade B or C, it was decompensated.
Data Collection and Follow-Up
Demographic, clinical, and follow-up data were collected with database templates from electronic medical records. The demographic and basic characteristics collected included medical record number, patient name, age, sex, classification and staging of DLBCL, Child–Pugh score, and HBV serum markers (HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc, and HBV DNA burden) at baseline at admission to the hospital for DLBCL diagnosis. The patients' treatment process was recorded, including the regimen and course of chemotherapy (immunochemotherapy), the course of radiotherapy, the dosage and course of RTX therapy, and the dosage and course of antiviral drugs.
HBV serum markers (HBV DNA burden and HBsAg) were collected before and 1 to 3 months after administrating the first dose of RTX, as well as after prolonged follow-up. In addition, liver function tests were recorded before and 1–3 months after administrating the first dose, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T-BIL), albumin (ALB), prothrombin time (PT) and prothrombin activity (PTA). Survival status was ascertained from follow-up medical clinic records of the hospital information system or by contacting the patients or their families by telephone. All patients were followed up from the diagnosis of DLBCL until death, loss to follow-up, or the end of follow-up on 10 October, 2021, whichever occurred first.
Outcome Measures
The primary outcome measures were HBV reactivation, and hepatitis flares or abnormal liver function. (1) HBV reactivation, whose definition is in line with the American Association for the Study of Liver Diseases (AASLD) 2018 hepatitis B guidance and Chinese consensus 2021 (25, 26). For HBV infection patients (HBsAg-positive, anti-HBc-positive), HBV reactivation is reasonably defined as 1 of the following: (i) a ≥100-fold increase in HBV DNA compared to the baseline level, (ii) reverse HBV DNA positivity in a patient with baseline HBV DNA negativity, or (iii) HBV DNA ≥ 10,000 IU/ml if the baseline level is not available. For previously resolved HBV infection patients (HBsAg-negative, anti-HBc-positive), the following criteria are reasonable for HBV reactivation: (i) HBV DNA is detectable, or (ii) reverse HBsAg seroconversion occurs (reappearance of HBsAg). (2) Hepatitis flares or abnormal liver function. According to the AASLD guidance (25), a hepatitis flare is reasonably defined as an ALT increase to ≥3 times the baseline level and >100 U/L. Based on the Common Terminology Criteria for Adverse Events established by the American National Cancer Institute (NCI-CTC) (27), abnormal liver function was defined as an ALT or AST increase to ≥3 times the baseline level, a T-BIL increase to ≥1.5 times the baseline level, or hypoalbuminemia with ALB <30 g/L.
The secondary outcome measure was overall survival. Overall survival was calculated based on the date of diagnosis and the date of death, the loss to follow-up, or the last follow-up for any cause (months).
Statistical Analysis
The data collected were recorded using Microsoft Excel 2020 software. Categorical variables were commonly represented as counts or frequencies. Continuous variables were expressed as the median (range). Then, descriptive analysis was performed using Microsoft Excel 2020 software. Statistical analysis of overall survival was performed using IBM SPSS, version 26 software (IBM Corp., Armonk, N.Y., USA).
Results
Patient Characteristics
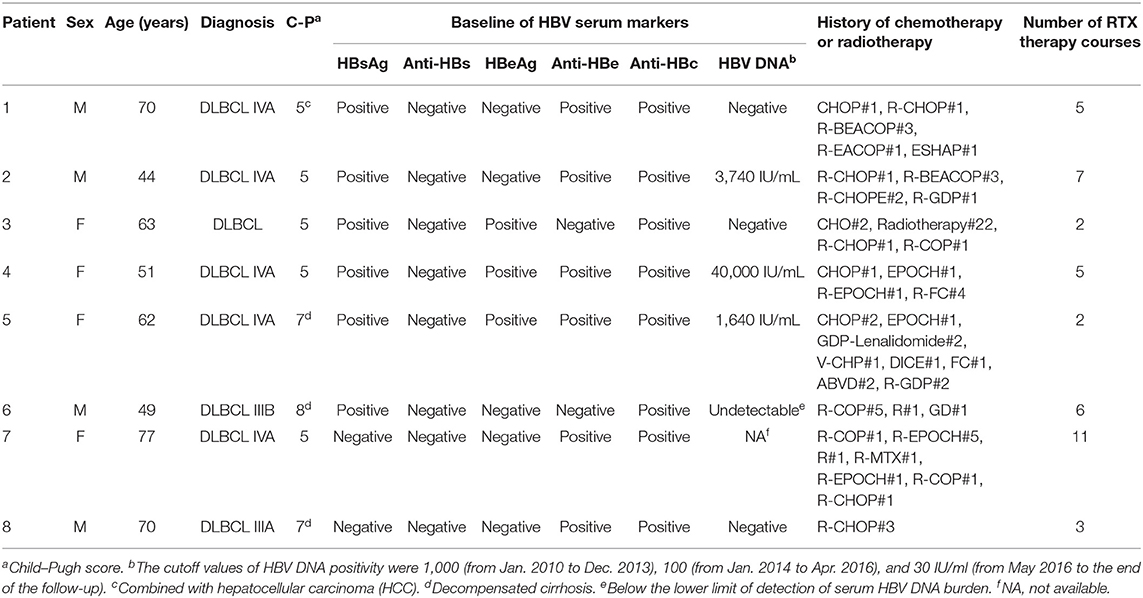
A total of 8 patients receiving RTX-containing chemotherapy were included in this study, of whom all were diagnosed with DLBCL combined with hepatitis B-related cirrhosis. In addition, one patient had hepatocellular carcinoma (HCC). The median age of the included patients was 62.5 years (range, 44-77 years). Among the 8 patients, 5 patients had compensatory cirrhosis with Child–Pugh grade A, whereas 3 patients had decompensated cirrhosis with Child–Pugh grade B. At admission, 6 patients had current HBV infection with HBsAg-positive and anti-HBc-positive, of whom 3 patients had serum HBV DNA levels > 1,000 IU/mL. Two patients had previously resolved HBV infection with HBsAg-negative and anti-HBc-positive. The median chemotherapy course was 7 (range, 3-12), the median RTX-containing regimen course was 5 (range, 2-11), and the single dose of RTX administration was consistently 375 mg/m2. The characteristics and the course of RTX treatment of the included patients are shown in Table 1.
HBV Reactivation During Follow-Up
For three patients with baseline positive HBV DNA at admission, antiviral therapy, including entecavir (ETV) in combination with or without adefovir dipivoxil (ADV), was administered until HBV DNA turned negative before the initiation of chemotherapy. For all patients, the serum levels of HBsAg and/or HBV DNA were confirmed negative before administrating RTX. For six infection patients with HBsAg-positive and anti-HBc-positive, HBV DNA remained negative or undetectable, and HBV reactivation was not observed. For two previously resolved infection patients with HBsAg-negative and anti-HBc-positive, one patient remained HBsAg-negative and HBV DNA-negative, whereas HBV reactivation was observed in the other patient. For the latter, the patient received 11 courses of RTX-containing immunochemotherapies within 15 months, whose courses of RTX therapy were the most. In addition, the patient's reappearance of HBsAg and reverse HBV DNA positivity might be associated with the progression of the primary disease, since the reversal of HBV DNA and HBsAg occurred after lymphoma progression [with a lactate dehydrogenase (LDH) level of 770 U/L]. The serum HBV DNA and HBsAg of the included patients before and after RTX administration are shown in Table 2.
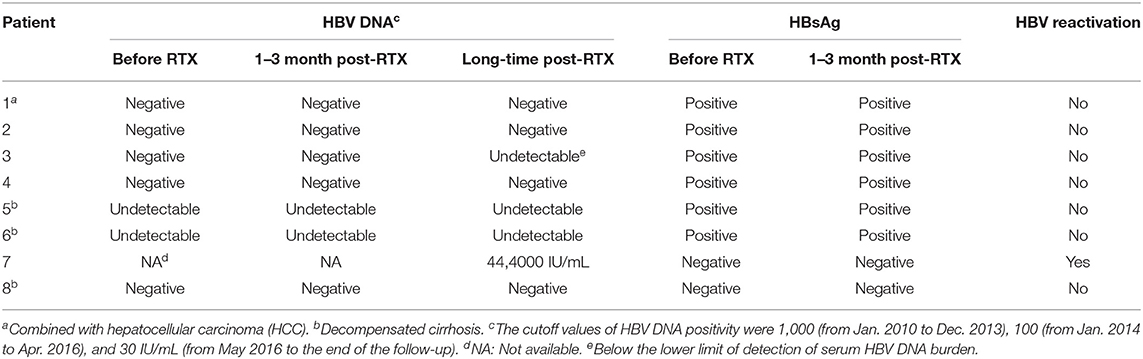
Table 2. The serum levels of hbv dna and hbsag in the included patients before and after rtx administration.
Hepatitis Flares or Abnormal Liver Function During Follow-Up
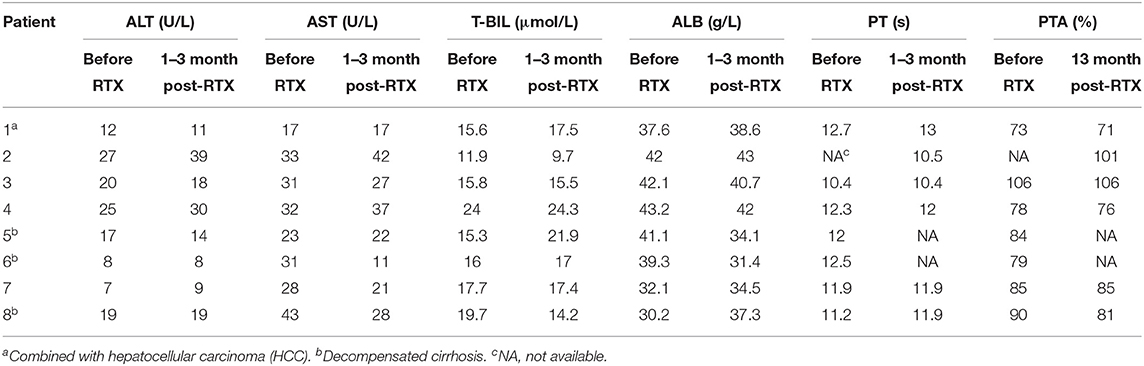
No hepatitis flares or abnormal liver function occurred in any patients. Regarding the serum levels of ALT, AST and T-BIL, no patient developed elevated ALT, AST, and T-BIL. Regarding the serum level of ALB, two patients' ALB levels were reduced from 41.1 g/L and 39.3 g/L to 34.1 g/L and 31.4 g/L, respectively, but none of them developed hypoalbuminemia. Regarding PT and PTA, compared with the baseline level before administrating RTX, no obvious change was observed. The liver function tests of the included patients before and after RTX administration are shown in Table 3 and Figure 1.
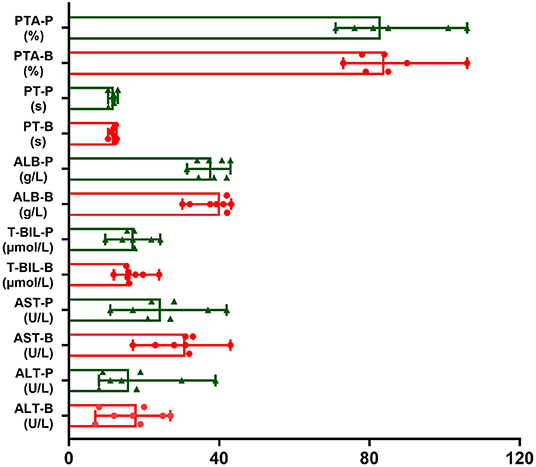
Figure 1. The trends of liver function tests of included patients before and after RTX administration. The red one is value of liver function before RTX treatment (B). The green one is value of liver function post RTX treatment for 1–3 months (P). Data were expressed as median and minimum and maximum values.
Antiviral Prophylaxis and Clinical Outcomes
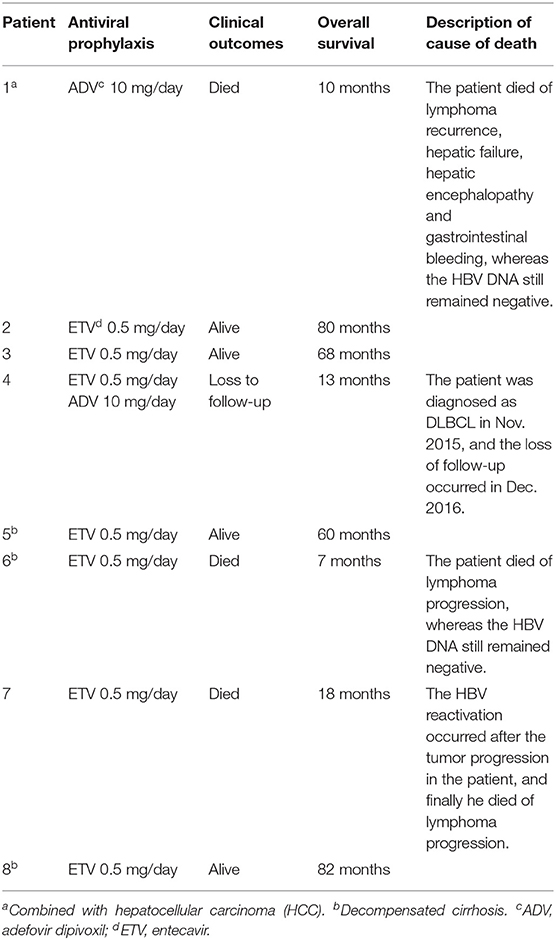
In line with clinical practice guidelines from the American Society of Clinical Oncology (ASCO) (28) and AASLD (25), antiviral prophylaxis is recommended for chronic infection patients with positive HBsAg or resolved infection patients receiving anti-CD20 antibody therapy. All included patients were at high risk of HBV reactivation and received standardized antiviral therapy for a lifelong time. Among them, ETV prophylaxis was used for 6 patients, ADV prophylaxis was used for one patient, and ETV and ADV were combined for one patient. During follow-up, 4 (50%) patients survived, 1 (12.5%) patient was lost to follow-up, and 3 (37.5%) patients died. HBV reactivation occurred after tumor progression in one of the deceased patients. The median overall survival (mOS) of the included patients was 39 months (range, 7–82 months). The antiviral prophylaxis and clinical outcomes of the included patients are shown in Table 4.
Discussion
Strength and General Findings of This Study
To our knowledge, this is the first study to report the long-term safety of RTX in patients with hepatitis B-related cirrhosis. HBV infection has been shown to increase the risk of developing DLBCL and other NHL, and the incidence of HBV infection is higher in DLBCL patients than in other malignancy patients (29–31). Meanwhile, the size of the population with hepatitis B-related cirrhosis is not to be underestimated. But there is extremely limited information about long-term outcomes of rituximab administration in patients with hepatitis B-related cirrhosis. Therefore, we paid more attention to DLBCL patients with hepatitis B-related cirrhosis in this present study. This study is unique in its included hepatitis B-cirrhosis population with both current HBV infection and previously resolved HBV infection, whereas most other publications have focused on lymphoma patients with previously resolved HBV infection and without cirrhosis (18–20).
Our findings pinpointed to a few salient points among the case series. First, the close link has not been established between DLBCL patients with hepatitis B cirrhosis and HBV reactivation or hepatitis flares, which is in agreement with a former study in patients with hepatitis C-related cirrhosis (32). Besides, the only case of HBV reactivation suggests that long-term and frequent RTX therapy may contribute to increasing the risk of HBV reactivation, and consequently, more effective prevention and close monitoring are warranted. Third, this study provides support for the concept that clinical management strategies, including screening prior to treatment and early and lifelong antiviral treatment, contribute to avoiding HBV-related liver complications and thereby improving patients' long-term outcomes.
Recommendation for Clinical Practice
From a clinician or clinical pharmacist's point of view, concerns about the risk of HBV reactivation always limit RTX use in hepatitis B-related cirrhosis, leading to adverse effects on patients' outcome. Therefore, the clinical treatment of DLBCL patients with hepatitis B-related cirrhosis still remains a challenge we have to face. Herein, we tried to discuss recommendations regarding the clinical management of HBV reactivation in DLBCL patients with hepatitis B-related cirrhosis, who usually need to receive first-line RTX-containing chemotherapy.
First, we strongly recommend that the prevention of HBV reactivation begin with patient screening before the initiation of therapy (33). The baseline HBV serum markers (e.g., HBV DNA burden, HBsAg) should be tested and recorded. Second, after screening, we think it is necessary to initiate antiviral prophylaxis before RTX-containing therapies are given (33). Especially for chronic infection patients who are HBsAg-positive and HBV DNA-positive, antiviral prophylaxis should be initiated until HBV DNA is undetectable or negative. Third, it is well recognized that nucleoside analogs (NAs) have been shown to decrease the risk of HBV reactivation. In line with relevant clinical guidelines or research evidence (5, 25, 34), we would like to recommend ETV or tenofovir (especially ETV) as the standard agent for antiviral prophylaxis.
Furthermore, although most guidelines recommend that the antiviral duration is at least 12 months after the end of immunochemotherapy (25, 28, 35), we would like to recommend life-long antiviral prophylaxis for DLBCL patients with hepatitis B-related cirrhosis. The reason for extending the duration of antiviral prophylaxis is long-term immunosuppression of hematological malignancy patients, and HBV reactivation has been reported at 55 months after the completion of chemotherapy (36).
Last but not least, regarding the monitoring and follow-up, the monitoring of liver function tests and HBV serum markers is recommended every 1-3 months after the first year of chemotherapy completion, followed by every 3-6 months. It is worth mentioning that ALT elevations may occur earlier than the increase in HBV DNA replication (37), and monitoring of ALT levels may contribute to suggestive HBV reactivation. Thus, we recommend monitoring liver enzymes and HBV serum markers at the same time.
Limitations
Our findings must be interpreted with caution considering several limitations. First, this study was limited by a small sample size, although we included consecutive patients during the 10-year duration period. Second, although our single-center experience suggested that liver injury usually occurred within 1-3 months after RTX-containing chemotherapy (38), the possibility of delayed liver injury could not be completely excepted. In addition, we only included Chinese patients with hepatitis B-related cirrhosis, while the genotypes of HBV may vary from one geographic area to another, and the pathogenic differences between HBV genotypes should be taken into consideration (39). Future larger prospective validation studies, including different patient populations, are still warranted to draw definitive conclusions.
Conclusions
To conclude, the findings from this retrospective case series provide support for the concept that RTX containing regimens may be a safe option for use as the treatment of DLBCL patients combined with hepatitis B-related cirrhosis. Clinical management strategies, including screening for HBV serum markers prior to commencing treatment, life-long antiviral prophylaxis and careful and regular monitoring, are of the utmost importance to avoid HBV reactivation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Peking University Third Hospital Institutional Review Board.
Author Contributions
Conceptualization: ZS and FD. Data curation: ZS, YM, and FD. Formal analysis, investigation, and methodology: ZS and YM. Funding, acquisition, project administration, and validation: FD. Visualization: YM and DJ. Writing—original draft: ZS. Supervision and writing—review and editing: RZ and FD. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Beijing Medical Award Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our gratitude to Yuanbo Su from Department of Infectious Diseases, Peking University Third Hospital for consultation. We thank the peer reviewers for constructive criticism and suggested additions, which have all been addressed and have significantly improved this article.
References
1. Hennessy BT, Hanrahan EO, Daly PA. Non-hodgkin lymphoma: an update. Lancet Oncol. (2004) 5:341–53. doi: 10.1016/S1470-2045(04)01490-1
2. Smolen JS, Aletaha D, McInnes LB. Rheumatoid arthritis. Lancet. (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
3. Tilly H, Gomes Da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26:v116–25. doi: 10.1093/annonc/mdv304
4. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. (2018) 50:74–87. doi: 10.1016/j.pathol.2017.09.006
5. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: B-cell Lymphomas (Version 5, 2021). (2021). Available online at: http://nccn.org/ (accessed January 12, 2022).
6. Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood. (2019) 133:137–46. doi: 10.1182/blood-2018-04-848044
7. Wu JQ, Song YP, Su LP, Zhang MZ, Li W, Hu Y, et al. Three-year Follow-up on the safety and effectiveness of rituximab plus chemotherapy as first -line treatment of diffuse large b-cell lymphoma and follicular lymphoma in real-world clinical settings in China: a prospective, multicenter, noninterventional study. Chin Med J (Eng). (2018) 131:1767–75. doi: 10.4103/0366-6999.237401
8. Food Drug Administration (FDA) U.S. RITUXAN Drug Instructions. (2021). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103705s5467lbl.pdf (accessed January 12, 2022).
9. World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact. (2021). Available online at: https://www.who.int/publications/i/item/9789240027077 (accessed January 12, 2022).
10. Tang LSY, Covert E, Wilson E, Shyam K. Chronic hepatitis B infection: a review. JAMA. (2018) 319:1802–13. doi: 10.1001/jama.2018.3795
11. Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. (2020) 5:245–66. doi: 10.1016/S2468-1253(19)30349-8
12. Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. (2019) 71:212–21. doi: 10.1016/j.jhep.2019.03.004
13. Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. (2012) 56:422–33. doi: 10.1002/hep.24804
14. Ohishi W, Chayama K. Current treatment for chronic hepatitis B in Japan. Clin J Gastroenterol. (2009) 2:325–30. doi: 10.1007/s12328-009-0100-1
15. European Centre for Disease Prevention and Control (ECDC) (2017). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/HepatitisBC-testing-in-EU-May2017.pdf (accessed January 12, 2022).
16. Ziogas DC, Kostantinou F, Cholongitas E, Anastasopoulou A, Diamantopoulos P, Haanen J, et al. Reconsidering the management of patients with cancer with viral hepatitis in the era of immunotherapy. J Immunother Cancer. (2020) 8: e000943. doi: 10.1136/jitc-2020-000943
17. Ozoya OO, Chavez J, Sokol L, Dalia S. Optimizing antiviral agents for hepatitis B management in malignant lymphomas. Ann Transl Med. (2017) 5:39. doi: 10.21037/atm.2016.12.25
18. Kim SJ, Hsu C, Song Y, Tay K, Hong X, Cao J, et al. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer. (2013) 49:3486–96. doi: 10.1016/j.ejca.2013.07.006
19. Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. (2014) 59:2092–100. doi: 10.1002/hep.26718
20. Seto W, Chan TSY, Hwang Y, Wong DK, Fung J, Liu KS, et al. Hepatitis B Reactivation in patients with previous hepatitis B virus exposure undergoing Rituximab—containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. (2014) 32:3736–43. doi: 10.1200/JCO.2014.56.7081
21. Steven H Swerdlow ECSA, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375-90. doi: 10.1182/blood-2016-01-643569
22. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. (1971) 31:1860–1.
23. Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: cotswolds meeting. J Clin Oncol. (1989) 7:1630–6. doi: 10.1200/JCO.1989.7.11.1630
24. Durand F, Valla D. Assessment of the prognosis of cirrhosis: child–pugh versus MELD. J Hepatol. (2005) 42:S100–7. doi: 10.1016/j.jhep.2004.11.015
25. Terrault NA, Lok ASF, McMahon BJ, Chang K, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
26. Zhonghua X, Ye X, Za Z. Chinese society of lymphoma, Chinese anti-cancer association, Chinese society of hematology, Chinese Medical association. the consensus on the prophylaxis and treatment of HBV reactivation in B or plasma cell-directed CAR-T cell therapy. Chin J Hematol. (2021) 42:441-6. doi: 10.3760/cma.j.issn.0253-2727.2021.06.001
27. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) (2017). Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed January 12, 2022).
28. Hwang JP, Feld JJ, Hammond SP, Wang SH, Alston-Johnson DE, Cryer DR, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: asco provisional clinical opinion update. J Clin Oncol. (2020) 38:3698–715. doi: 10.1200/JCO.20.01757
29. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. (2010) 11:827–34. doi: 10.1016/S1470-2045(10)70167-4
30. Qi Z, Wang H, Gao G. Association of risk of non-Hodgkin's lymphoma with hepatitis B virus infection: a meta-analysis. Int J Clin Exp Med. (2015) 8:22167–74.
31. Su T, Liu C, Tseng T, Chou S, Liu C, Yang H, et al. Chronic hepatitis B is associated with an increased risk of B-cell non-Hodgkin's lymphoma and multiple myeloma. Aliment Pharm Ther. (2019) 49:589–98. doi: 10.1111/apt.15132
32. Parker SM, Hyder MA, Fesler MJ. Bendamustine and rituximab for indolent B-Cell non-hodgkin lymphoma in patients with compensated hepatitis c cirrhosis: a case series. Clin Lymphoma Myeloma Leuk. (2013) 13:e15–7. doi: 10.1016/j.clml.2013.07.002
33. Cao X, Wang Y, Li P, Huang W, Lu X, Lu H, et al. Reactivation during the treatment of non-hodgkin lymphoma and management strategies. Front Oncol. (2021) 11:685706. doi: 10.3389/fonc.2021.685706
34. Zheng JN, Zou TT, Zou H, Zhu GQ, Ruan LY, Cheng Z, et al. Comparative efficacy of oral nucleotide analogues for the prophylaxis of hepatitis B virus recurrence after liver transplantation: a network meta-analysis. Expert Rev Anti Infect Ther. (2016) 14:979–87. doi: 10.1080/14787210.2016.1220831
35. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. (2016) 10:1–98. doi: 10.1007/s12072-015-9675-4
36. Hara T, Oka K, Iwai N, Inada Y, Tsuji T, Okuda T, et al. Hepatitis B virus reactivation 55 months following chemotherapy including rituximab and autologous peripheral blood stem cell transplantation for malignant lymphoma. Internal Med. (2021) 60:417–21. doi: 10.2169/internalmedicine.5678-20
37. Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. (2006) 43:209–20. doi: 10.1002/hep.21051
38. Jing HM, Tian L, Li DJ, Ke XY. Hepatic dysfunction in asymptomatic carrier of HBV after rituximab combined with chemotherapy in non-Hodgkin lymphoma patients (report of 5 cases). Beijing Med J. (2007) 29:534–6. doi: 10.15932/j.0253-9713.2007.09.025
Keywords: diffuse large B cell lymphoma, cirrhosis, rituximab, hepatitis B virus, reactivation
Citation: Song Z, Ma Y, Jiang D, Zhao R and Dong F (2022) Long-Term Safety of Rituximab in DLBCL Patients With Hepatitis B-Related Cirrhosis: A Retrospective Case Series. Front. Med. 9:890339. doi: 10.3389/fmed.2022.890339
Received: 17 March 2022; Accepted: 11 May 2022;
Published: 30 May 2022.
Edited by:
Mutlu Arat, Istanbul Florence Nightingale Hospital, TurkeyReviewed by:
Onur Kirkizlar, Trakya University, TurkeyApurva Patel, Gujarat Cancer and Research Institute, India
Copyright © 2022 Song, Ma, Jiang, Zhao and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Dong, a25vd2ZseWluZzc5NzkmI3gwMDA0MDsxNjMuY29t
 Zaiwei Song
Zaiwei Song Yi Ma
Yi Ma Dan Jiang1
Dan Jiang1