
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med., 26 April 2022
Sec. Regulatory Science
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.878942
This article is part of the Research TopicInsights in Regulatory Science: 2021View all 25 articles
Biomarkers are important tools in medicines development and clinical practice. Besides their use in clinical trials, such as for enrichment of patients, monitoring safety or response to treatment, biomarkers are a cornerstone of precision medicine. The European Medicines Agency (EMA) emphasised the importance of the discovery, qualification, and use of biomarkers in their Regulatory Science Strategy to 2025, which included the recommendation to enhance early engagement with biomarker developers to facilitate regulatory qualification. This study explores the journey of biomarkers through the EU regulatory system and beyond, based on a review of interactions between developers and the EMA from 2008 to 2020, as well as the use of qualified biomarkers in clinical trials. Of applicants that used early interaction platforms such as the Innovation Task Force, less than half engaged in fee-related follow-up procedures. Results showed that, as compared to companies, consortia were more likely to opt for the Qualification of Novel Methodologies procedure and engage in follow-up procedures. Our results highlight the importance of early engagement with regulators for achieving biomarker qualification, including pre-submission discussions in the context of the qualification procedure. A review of clinical trials showed that all qualified biomarkers are used in practice, although not always according to the endorsed context of use. Overall, this study highlights important aspects of biomarker qualification, including opportunities to improve the seamless support for developers by EMA. The use of qualified biomarkers in clinical trials underlines the importance of regulatory qualification, which will further enable precision medicine for the benefit of patients.
Biomarkers are powerful tools that can serve many purposes in medicines development and clinical practice (1). Examples include the selection of patients and enrichment of study populations for clinical trials, monitoring safety or response to treatment during trials, but also supporting decision-making in the context of precision medicine. In 2001, the Biomarker Definitions Working Group proposed that a biomarker could be defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (2). This definition was further elaborated in the Biomarkers, EndpointS, and other Tools (BEST) resource, in which subcategories were defined that reflect different biomarker functions (3, 4). The crucial role of biomarkers in drug development has long been recognised and supported by regulators, who have established frameworks for review of biomarker validation plans and/or data, which may result in regulatory qualification (5). In 2007, a joint pilot procedure by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) concerning a panel of nephrotoxicity biomarkers marked the beginning of regulatory qualification of biomarkers in the EU (6, 7). Recently, the EMA underlined the importance of the discovery, qualification and use of biomarkers in their “Regulatory Science Strategy to 2025” (8, 9). One of the primary strategic goals related to regulatory science for human medicines focuses on the integration of science and technology in medicines development, including support of developments in the fields of precision medicine and biomarkers. “Enhancing early engagement with novel biomarker developers to facilitate regulatory qualification” is mentioned as a key step to achieve this goal, accompanied by the recommendation to “critically review the EMA’s biomarker validation process, including duration and opportunities to discuss validation strategies in advance, in order to encourage greater uptake and use.” Encouraged by these objectives, we aimed to assess the past and current situation, by reviewing the interactions between developers and regulators at various stages of the process leading up to regulatory biomarker qualification. Moreover, our aim was to assess the impact of this regulatory “stamp of approval,” in terms of uptake by the scientific community as well as use in clinical trials.
At the EMA, the Innovation Task Force (ITF) acts as a first point of contact for developers in early-stage projects with innovative aspects for drug development (10). Interactions take place in the form of informal briefing meetings between applicants and experts from the EU network, and address mainly strategic aspects of regulatory, scientific, and legal nature. A primary goal of the ITF is to fill the gap between early-stage research, performed by academic groups or small to medium-sized companies, but also large companies, and formal regulatory procedures that involve fees, such as Scientific Advice (SA) and the Qualification of Novel Methodologies (QoNM) (Figure 1) (11). The former is an interaction platform at the EMA, where medicine developers can discuss strategies to generate robust evidence for the benefit-risk assessment during the marketing authorisation application (MAA) (12). Biomarkers may constitute an essential part of this strategy and are therefore a common topic of discussion in SA procedures. The QoNM procedure is a voluntary pathway towards regulatory qualification of methodologies in drug development, which also includes biomarkers, and can result in a Qualification Advice (QA) or Qualification Opinion (QO) (Figure 1) (13). A QA typically concerns projects in earlier stages, potentially including review of preliminary data, and is the way to agree on evidence generation plans and protocols for studies intended to support a QO. When the submitted evidence supports a QO, the draft opinion document is published for consultation by the scientific community before final adoption by EMA’s Committee for Medicinal Products for Human Use (CHMP). Upon final adoption of the QO, it is considered that the proposed method (e.g., biomarker) is an acceptable regulatory standard for the defined context of use in drug development. By making the QO publicly available, others may use the qualified method or biomarker in their drug development efforts. In this study, we analysed the journey of biomarkers through the EMA’s pre-submission interaction platforms and beyond, by reviewing interactions between developers and the Agency as well as the use of qualified biomarkers in clinical trials. In doing so, we aimed to identify potential points for improvement, with the goal to enhance the seamless support by the EMA for biomarker validation, qualification, and subsequent use in drug development.
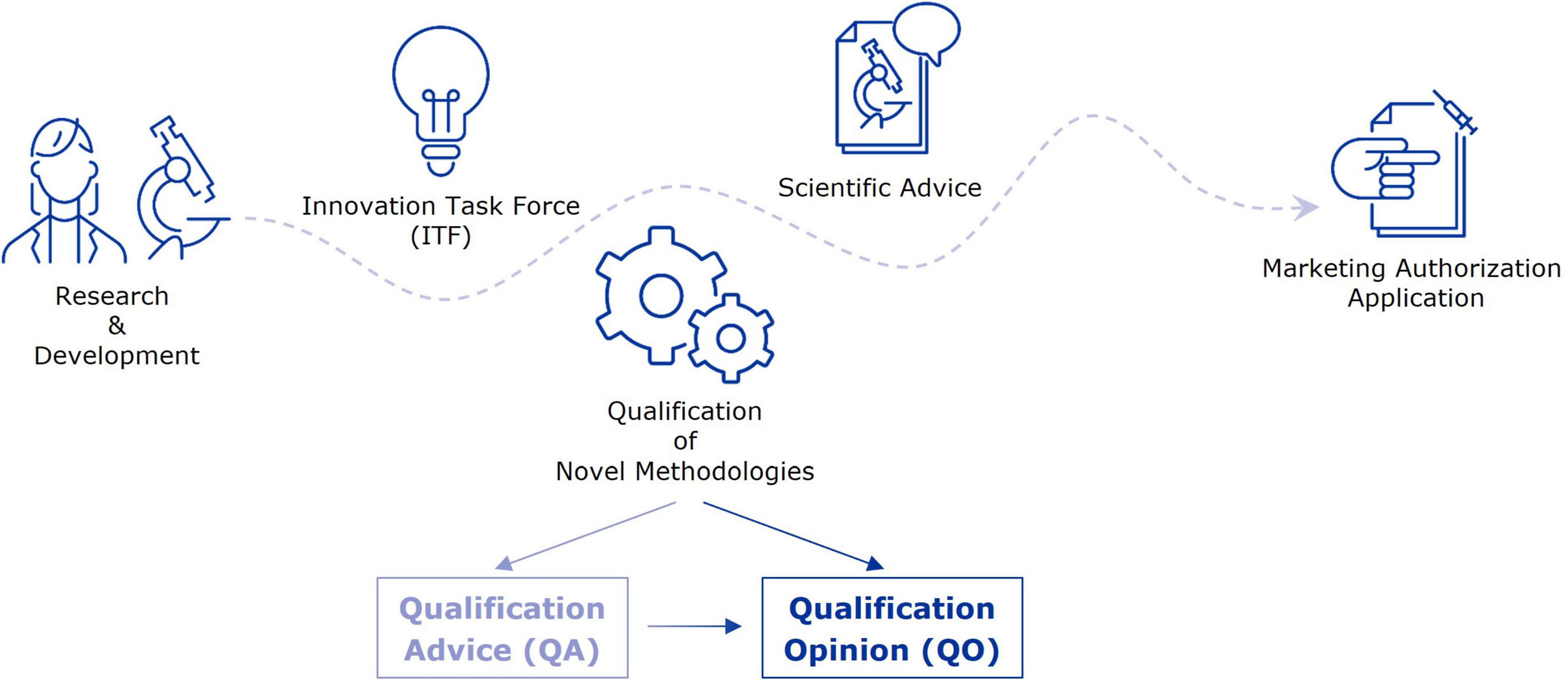
Figure 1. From early-stage research to marketing authorisation–EMA support mechanisms for evidence generation strategies. This figure highlights a selection of interaction platforms at EMA through which applicants can receive guidance and feedback on their evidence generation strategy towards a marketing authorisation application.
Minutes from ITF briefing meetings that took place between January 1st, 2008 and December 31st, 2020 were collected from EMA’s internal database. The most recent version of the minutes file was used and, wherever possible, the final version reviewed and approved by ITF and participants. Minutes of preliminary meetings or informal teleconferences were excluded from the analysis. This collection was searched using keywords biomarker, in vitro, companion, diagnostic and qualification. Initially, all documents were selected that (1) included the word biomarker, (2) contained the word companion if it occurred together with the term diagnostic or test, or (3) contained the word qualification in combination with procedure, advice, or novel methodologies. Minutes that referred to both biomarkers and QoNM (1), minutes that referred to the use of in vitro diagnostics in combination with biomarkers (2), and minutes in which biomarkers were the main topic of discussion (3) were marked as relevant. Related fee-associated procedures were identified as follows: for QoNM, applicant names and relevant keywords from the ITF minutes were used to search a collection of biomarker-related QoNM procedures that took place between 2008 and 2020 (described in the next section) as well as QoNM applications that had been withdrawn or rejected. Similarly, the applicant’s name and relevant keywords (including biomarker) were used to search all finished SA procedures that had been started in the year of the ITF meeting or later. Hits from these searches were inspected manually and those that discussed the biomarker from the ITF meeting were marked as relevant.
A document containing a list of all QoNM procedure applications was downloaded from EMA’s internal database on May 11th, 2021. Procedures that never started, or that were started after December 31st, 2020, were excluded from the analysis. All remaining procedures were assessed individually and procedures that contained modelling or simulation techniques, patient-reported outcomes, ratings or scales, methods or protocols, clinical outcome assessments, or databases or registries were excluded from the analysis.
Clinical trial searches were performed in the ClinicalTrials.gov database using the general search function or the expert search function (Supplementary Table 1). The respective disease areas of the qualified biomarkers were searched for interventional trials in which the biomarker in question was used according to the context of use endorsed by the CHMP in the qualification opinion (Table 1). For each search, all hits were downloaded, and data were extracted, including NTC number, title, status, condition, outcome measures, sponsors, total number, and age range of enrolled subjects, start and completion date, and locations of the trial. As most qualified biomarkers serve the purpose of enrichment of study populations, the “inclusion criteria” and “exclusion criteria” sections were manually extracted for each trial. The relevant sections were screened individually to determine whether the search terms occurred in the desired context, e.g., whether the biomarker was in fact used for enrichment and if so, what cut-off values were used. Based on this exercise, several initial hits were deemed irrelevant and were excluded from further analysis.
Out of the 311 ITF briefing meetings that took place from 2008 to 2020, 41 contained discussions or questions related to biomarkers and, in most cases, applicants were referred to SA or QoNM. Most biomarker-related ITF meetings were held in 2010 and 2012, with six and eight meetings, respectively, but no increasing trend was observed (Figure 2A). At least 12 of the 41 meetings could be linked to relevant SA or QoNM procedures (Supplementary Table 2). Six ITF meetings were linked to a SA procedure that referred to the same biomarker. For example, a lung clearance index that was discussed in an ITF meeting as a potential surrogate endpoint in cystic fibrosis trials was endorsed by the CHMP as a primary endpoint in the SA procedure. In another ITF meeting, a predictive biomarker for patient selection in non-small cell lung cancer trials was discussed. Two related SA procedures were identified that contained discussions on the cut-off values for that biomarker. In yet another ITF meeting, predictive biomarkers for clinical trials in multiple sclerosis were discussed, which could also be linked to a SA procedure. Interestingly, no reference was made to QoNM in any of the final SA letters. Seven ITF meetings resulted in follow-up QoNM procedures, one of which was also linked to a SA procedure. Four of the seven ITF meetings were with consortia funded by the Innovative Medicines Initiative (IMI): an EU public-private partnership funding health research and innovation (14, 15).
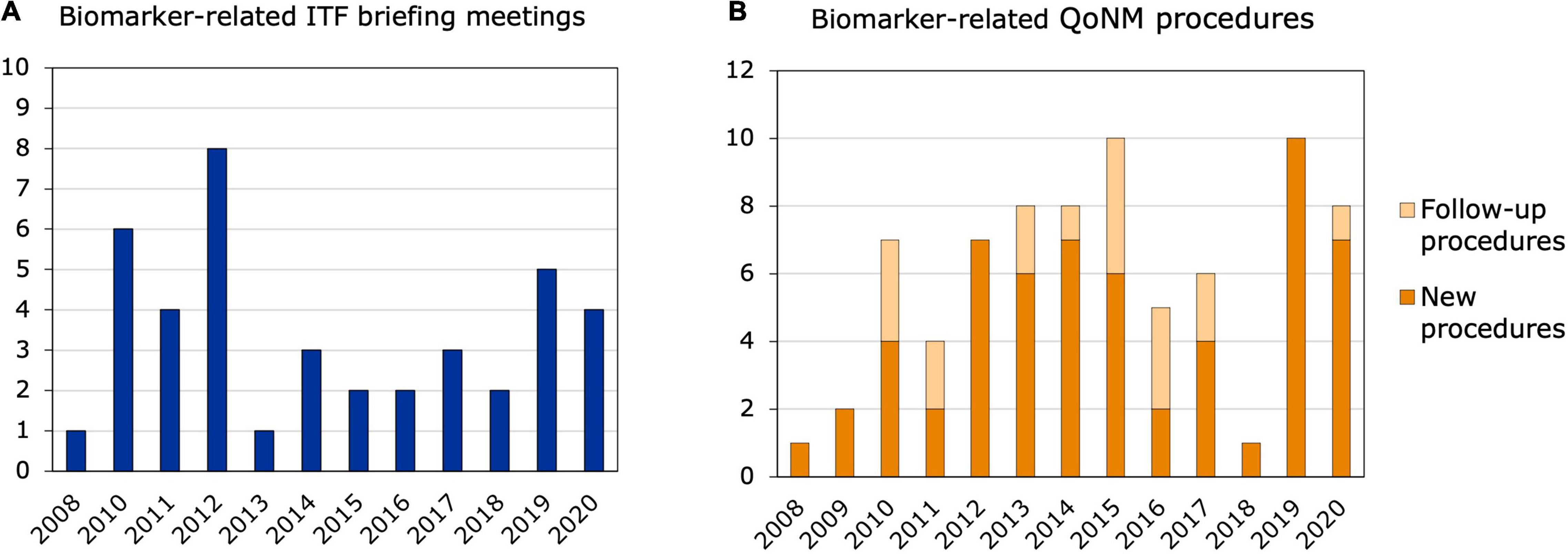
Figure 2. Biomarker-related interactions between 2008 and 2020. (A) ITF briefing meetings. The bars represent ITF briefing meetings related to biomarkers and their qualification that took place from 2008 to 2020. (B) New and follow-up qualification procedures. Qualification of Novel Methodologies procedures related to biomarkers that were started between 2008 and 2020.
Out of the 77 biomarker-related qualification procedures that took place between 2008 and 2020, 18 were follow-ups to previous procedures (Figure 2B). Nine of the 77 procedures resulted in a QO, of which four were follow-up procedures (Table 1). Two QOs from 2011 (16, 17) were follow-ups to the same QO procedure in 2010 (18) and qualified biomarkers for Alzheimer’s Disease (AD). These three QOs are the only QO procedures that were brought forward by a company and aimed at qualifying a biomarker for a specific clinical development program. All three qualified biomarkers are enrichment biomarkers for clinical trials with BMS-708163, or avagacestat: an amyloid precursor protein secretase (γ-secretase) inhibitor that was developed by Bristol-Myers Squibb for the treatment of predementia and mild-to-moderate AD. Another QO, which was a follow-up to previous QA procedures, qualified low hippocampal volume as an enrichment biomarker for clinical trials in predementia AD (19). The fourth follow-up QO qualified neuroimaging of the dopamine transporter as an enrichment biomarker for Parkinson’s Disease (PD) (20) and was preceded by two previous QA procedures—an initial QA procedure and a follow-up QA. Of all biomarker-related qualification procedures, 36 were started by companies, of which 20 related to a specific clinical development program. On the other hand, 41 procedures were initiated by consortia or foundations and the vast majority of those by IMI consortia and the Critical Path Institute (C-Path). The C-Path initiative is a non-profit public-private partnership with the FDA, which aims to accelerate the pace and reduce the costs of medical product development through the creation of new standards, including biomarkers, that aid in the scientific evaluation of the efficacy and safety of new therapies (21, 22). Interestingly, only three out of the 18 follow-up procedures were initiated by companies, and the remaining 15 by consortia or foundations. Among the follow-up procedures, a large number related to safety biomarkers for drug-induced injury in different organ systems: five of them in the kidney, but also in the liver and cardiovascular system. These procedures were also mainly driven by the C-Path Preclinical Safety Testing Consortium and the IMI SAFE-T consortium. The IMI EU-AIMS consortium also initiated three follow-up QAs in autism spectrum disorder (23).
Since 2008, eight qualification opinions on biomarkers for clinical use have been adopted by the EMA (Table 1). The first four of these concern prognostic/predictive biomarkers for enrichment in clinical trials in AD and were published between April 2011 and February 2012. This includes the three procedures initiated by Bristol-Myers Squibb for the clinical development of avagacestat, which was discontinued in November 2012 as the Phase II clinical trial programme did not establish the desired efficacy profile. The three QOs qualify cerebrospinal fluid (CSF) biomarkers “low Aβ1-42 and high tau concentrations” as well as “a positive amyloid PET signal” for enrichment in clinical trials for predementia AD and mild to moderate AD (16–18, 24). The company performed two Phase II studies with avagacestat (NCT00890890 and NCT00810147), only one of which used inclusion criteria related to the QOs. This study was started in 2009, before the first qualification procedure, and “CSF aβ42 levels < 200 pg/mL or Total Tau/aβ42 ratio of ≥ 0.39” were used as inclusion criteria. A search of the clinicaltrials.gov database for trials using amyloid beta and/or tau in their inclusion criteria yielded 28 relevant hits, two of which were started before publication of the opinions (Table 2). Interestingly, the qualified biomarker “low Aβ1-42 and high tau” is used in only six out of 28 trials. Although the QO does not specify what would be considered “low” and “high” values, two trials mention similar cut-offs: “A-beta 42 concentration of less than 638 ng/L AND total tau >375 ng/L” (NCT02389413) and “low Aβ1-42 concentrations (<640 pg/mL) and increased total tau concentrations (>375 pg/ml)” (NCT02240693). In several cases, “low Aβ1-42” or “high tau” are used, but not in the right combination. Another observation is that the ratio between Aβ1-42 and either total tau, tau, or phosphorylated tau is mentioned as a biomarker in 13 trials, with varying thresholds. A total of 12 out of 28 trials also mention the qualified biomarker “positive amyloid PET scan” as part of their inclusion criteria, however, always as an alternative to CSF biomarkers and never a combination of the two. A search of the clinicaltrials.gov database for trials using “amyloid AND PET” in their inclusion criteria yielded 120 relevant hits, 12 of which are included in Table 2. The fourth procedure related to AD qualified low hippocampal volume, as measured by MRI, and considered as a dichotomised variable (low volume or not), as a prognostic marker of progression to dementia in subjects with cognitive deficit compatible with predementia stage of AD (19, 25). Like for the CSF biomarkers, no cut-off value for “low volume” was mentioned in the QO. A search of the clinicaltrials.gov database for trials using “hippocampal volume” in their inclusion criteria yielded 4 hits in total, all of which were started after publication of the QO. Besides the trial included in Table 2, which mentions “hippocampal volume loss,” the other three contain the following inclusion criteria:
“Magnetic resonance imaging (MRI) scans of the brain within the past six months reveal evidence and findings consistent with Alzheimer’s disease, including hippocampal volume loss and/or overall cerebral atrophy (cerebral volume loss).”
“Magnetic resonance imaging (MRI) confirmation of atrophy of the hippocampus or the medial temporal lobe volume, MRI manifestation of high possibility of Alzheimer’s Disease.”
“Magnetic Resonance Imaging (MRI) confirmation of atrophy of the hippocampus or the medial temporal lobe volume.”
The remaining four QOs regarding biomarkers for clinical use were published between 2015 and 2020. In October 2015, baseline total kidney volume (TKV) was qualified as a prognostic biomarker for renal decline in autosomal dominant polycystic kidney disease (26, 27). The QO mentions that baseline TKV should be used in combination with other markers, e.g., patient age and eGFR, to identify patients that are likely to experience a progressive decline in renal function. A search of clinicaltrials.gov for interventional trials in kidney disease yielded a total of eight trials, four of which started after the publication of the QO (Supplementary Table 3). Six of the eight trials refer to the “total” or “combined” kidney volume, of which five refer to a specific value that ranges from >500 to > 1,200 mL. Kidney volume progression, specifically with a yearly increase of more than 6%, is also mentioned as an inclusion criterium in three trials. None of the trials use TKV in combination with other factors.
In April 2018, changes in plasma fibrinogen levels were qualified as a prognostic biomarker in chronic obstructive pulmonary disease, with 350 mg/dl as the threshold considered to be most useful (28, 29). A search of clinicaltrials.gov yielded three hits, only one of which was started after publication of the QO (Supplementary Table 4). All three trials referred to plasma fibrinogen concentration at baseline, rather than changes in levels: two trials referred to a threshold of 350 mg/dL and the third to 300 mg/dL.
In April 2018, reduced dopamine transporter (DAT) levels, as measured by Single-Photon Emission Computed Tomography (SPECT) neuroimaging were qualified as an enrichment biomarker for clinical trials targeting patients with early Parkinsonian symptoms (20, 30). The qualification procedure was initiated by the Critical Path for Parkinson’s consortium with the aim to demonstrate the predictive accuracy of visual assessment of DAT neuroimaging scans at baseline for identifying those subjects with high likelihood of progressing in clinical motor disability. A search of clinicaltrials.gov yielded 14 relevant hits, half of which were started after publication of the QO (Supplementary Table 5). Two out of the trials used DAT SPECT as means of exclusion.
In April 2019, stride velocity 95th centile (SV95C) measured at the ankle was qualified as a secondary endpoint in clinical trials for ambulant Duchenne Muscular Dystrophy patients of 5 years or older (31, 32). From the adopted QO document: “Stride velocity 95th centile measured at the ankle (SV95C) is an acceptable secondary endpoint in pivotal or exploratory drug therapeutic studies for regulatory purposes when measured by a valid and suitable wearable device to quantify a patient’s ambulation ability directly and reliably in a continuous manner in a home environment and as an indicator of maximal performance.” A search of clinicaltrials.gov yielded two hits by different sponsors, one of which started after publication of the QO (Supplementary Table 6).
Support for biomarker discovery, validation, and qualification is an important objective at the European Medicines Agency (8, 9). Biomarker developers can make use of various interaction platforms offered by the Agency, ranging from briefing meetings with the Innovation Task Force as a first point of contact, to the Qualification of Novel Methodologies procedure, where a biomarker can be qualified for a specific context of use (13). Since the start of this voluntary procedure in 2008, nine biomarkers have obtained this regulatory “stamp of approval” by the EMA, which has sparked the question how the support from the regulatory system for biomarker qualification can be improved. The aim is to enable precision medicine for the benefit of patients, by facilitating a seamless interaction with biomarker and medicine developers. Encouraged by this objective, we reviewed biomarker-related pre-authorisation interactions that took place between 2008 and 2020 at the EMA and explored the impact of qualified biomarkers, by assessing their uptake in clinical trials.
Of the 41 biomarker-related ITF briefing meetings that were identified between 2008 and 2020, 12 could be linked to interactions between the applicant and the EMA through Scientific Advice or QoNM. The fact that 70% of meetings did not result in any other interaction might be due to the early stage of some projects, or the fees associated with the abovementioned procedures. A decreasing trend in the use of QoNM by pharmaceutical companies had previously been identified (33), suggesting that companies are more likely to include biomarker-related questions in SA procedures. This hypothesis is supported by the data on interactions following ITF briefing meetings, which show that half of the applicants that initiated a follow-up opted for SA, all of which were companies, when they had been referred to the qualification procedure by ITF experts. On the other hand, most ITF meetings that resulted in QoNM were with consortia funded by the Innovative Medicines Initiative, which was launched in 2008 to address challenges in drug development and regulation (14, 15). Many IMI projects generate data that is relevant for stakeholders in medicines development and, therefore, involvement of regulators is a cornerstone of the IMI programme. Yet, despite various IMI projects aiming at biomarker qualification, none of them have resulted in qualified biomarkers thus far. This could be due to the limited timespan of the IMI-funded projects, typically 5–6 years, which may be too short for complex biomarker validation exercises. This also became evident from analysis of QA final advice letters and is in line with what has been reported by Laverty and Meulien, who state that the interaction with regulatory bodies is often initiated too late in the project (15). However, consortia such as IMI EU-AIMS are mentioned as a success story due to their early interaction with regulators, which has resulted in multiple follow up QA procedures in the field of autism spectrum disorder (23). These findings highlight that the benefit of transparency and availability of biomarker qualification data should be communicated clearly, in order to engage a wide range of stakeholders in this procedure.
The difference between the number of ITF meetings in which QoNM was recommended and the number of applicants that follow this recommendation suggests that QoNM application may be perceived as challenging. Given that applicants are encouraged to make use of this procedure early on in their biomarker qualification effort, and that the interactions should ideally follow an iterative approach (34), lowering potential hurdles is key to achieving these aims. As part of the QoNM procedure, applicants can participate in a preparatory meeting with the Scientific Advice Office, in which members of the qualification team may join as appropriate (34). Such meetings, which take place before any fee is due, allow for an informal scientific discussion and may offer preliminary feedback on the maturity of the data. However, preparatory meetings take place only after submission of a complete draft dossier for assessment, which may discourage applicants, particularly in early stages of a project. In such cases, questions around the qualification procedure and the level of evidence required for biomarker qualification are often addressed in ITF briefing meetings. To facilitate access to the QoNM procedure, beyond the information currently available (35, 36), additional guidance may aid applicants in preparation of the draft dossier, especially in early-stage projects. Further support can be obtained through informal interactions with the EMA Scientific Advice Office (c2NpZW50aWZpY2FkdmljZUBlbWEuZXVyb3BhLmV1).
To investigate some aspects of the potential impact of a QO on medicines development, the use of qualified biomarkers in clinical trials was assessed. A search of the clinicaltrials.gov database revealed that the first qualified biomarkers, CSF proteins (sometimes combined with amyloid PET) as enrichment biomarkers for AD, were used as inclusion criteria in 26 trials after publication of the opinion. However, an investigation of the inclusion criteria showed that only six trials used the biomarkers according to the qualified context of use (“high tau and low Aβ1-42”). It is unclear what exactly is meant by “high” and “low” tau, which has also been discussed in the comments from the public consultation (37). The same applies to the QOs on Low Hippocampal Volume, Total Kidney Volume, and Plasma Fibrinogen levels—a threshold that was considered “most useful” was only given for TKV. These findings highlight the importance of a clearly defined context of use, in order to ensure optimal use of the qualified biomarker. Overall, qualified biomarkers are used in clinical trials, albeit not always according to their qualified context of use. This observation is also supported by findings from SA procedures, where sponsors often refer to specific qualified biomarkers but want to use the biomarker in a different context of use (data not shown). It should be noted that a biomarker may be scientifically valid in different contexts of use, which may not all be covered in a QO procedure. In general, as clinical trials are the foundation of evidence generation for MAAs, the uptake of qualified biomarkers highlights that regulatory qualification is relevant for medicine developers.
In summary, regulatory qualification of biomarkers is a cornerstone of the EMA’s strategy to enable precision medicine for the benefit of patients. This study presents a review of biomarker-related pre-authorisation interactions at the EMA since 2008, which highlights opportunities to enhance the seamless support for biomarker developers. More detailed guidance may facilitate QoNM application for applicants that are referred to the qualification procedure during ITF briefing meetings, enabling sponsors to engage in preparatory meetings with members of the Qualification Team. Moreover, early initiation of dialogue with regulators is key to successful biomarker qualification by consortia, such as those initiated by the IMI initiative or CriticalPath Institute. In general, the use of qualified biomarkers in clinical trials illustrates the positive impact of regulatory qualification on evidence generation for MAAs. However, a review of inclusion criteria and outcome measures of the trials showed that, although the biomarker may be scientifically valid for the intended purpose, the context of use endorsed by the CHMP is not always applied. An assessment of the impact on MAA evaluation may contribute to understanding the value of a QO and may encourage potential applicants to engage in the procedure, which in turn would contribute to the development of precision medicine.
The original contributions presented in the study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding authors.
NH, FE, JL, and AH designed the study. NH performed the research and analysed the data. NH, FE, and TV wrote the manuscript. All authors contributed to the article and approved the submitted version.
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
The research presented was conducted in the context of employment at the European Medicines Agency.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The passing of JL during this research project came as a great shock to all of us. We acknowledge his input and contribution to this study and appreciate his insights and expertise in regulatory science and innovation, which he used to guide this project forward. We thank Ralf Herold for his advice on the search of clinical trials. We also acknowledge and thank Iordanis Gravanis, Michael Berntgen, and Spiros Vamvakas for their input in the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.878942/full#supplementary-material
1. Aronson JK, Ferner RE. Biomarkers—a general review. Curr Protoc Pharmacol. (2017) 76:9.23.1–9.23.17. doi: 10.1002/cpph.19
2. Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69:89–95. doi: 10.1067/mcp.2001.113989
3. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. Silver Spring, MD: Food and Drug Administration (US) (2016).
5. Papaluca M, Goodsaid F. Evolution of biomarker qualification at the health authorities. Nat Biotechnol. (2010) 28:441–3. doi: 10.1038/nbt0510-441
6. European Medicines Agency. Final Conclusions on the Pilot Joint EMEA/FDA VXDS Experience on Qualification of Nephrotoxicity Biomarkers. London: European Medicines Agency (2009).
7. Flamion B, Jayadev S, Webb CP, Beushausen SA, Abadie E, Vidal JM, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat Biotechnol. (2010) 28:455–62. doi: 10.1038/nbt.1625
8. European Medicines Agency. EMA Regulatory Science to 2025. Amsterdam: European Medicines Agency (2020).
9. Hines PA, Guy RH, Humphreys AJ, Papaluca-Amati M. The European medicines agency’s goals for regulatory science to 2025. Nat Rev Drug Discov. (2019) 18:403–4. doi: 10.1038/d41573-019-00071-2
11. Ehmann F, Papaluca Amati M, Salmonson T, Posch M, Vamvakas S, Hemmings R, et al. Gatekeepers and enablers: how drug regulators respond to a challenging and changing environment by moving toward a proactive attitude. Clin Pharmacol Ther. (2013) 93:425–32. doi: 10.1038/clpt.2013.14
12. Hofer MP, Jakobsson C, Zafiropoulos N, Vamvakas S, Vetter T, Regnstrom J, et al. Regulatory watch: impact of scientific advice from the European medicines agency. Nat Rev Drug Discov. (2015) 14:302–3. doi: 10.1038/nrd4621
13. Manolis E, Vamvakas S, Isaac M. New pathway for qualification of novel methodologies in the European medicines agency. Proteomics Clin Appl. (2011) 5:248–55. doi: 10.1002/prca.201000130
14. Goldman M, Seigneuret N, Eichler H-G. The innovative medicines initiative: an engine for regulatory science. Nat Rev Drug Discov. (2014) 14:1–2. doi: 10.1038/nrd4520
15. Laverty H, Meulien P. The innovative medicines initiative -10 years of public-private collaboration. Front Med. (2019) 6:275. doi: 10.3389/fmed.2019.00275
16. European Medicines Agency. Qualification Opinion of Alzheimer’s Disease Novel Methodologies/Biomarkers for Pet Amyloid Imaging (Positive/Negative) as a Biomarker for Enrichment, for Use in Regulatory Clinical Trials in Predementia Alzheimer’s Disease. Amsterdam: European Medicines Agency (2012).
17. European Medicines Agency. Qualification Opinion of Alzheimer’s Disease Novel Methodologies/Biomarkers for the use of CSF AB 1-42 and T-Tau and/or PET-Amyloid Imaging as Biomarkers for Enrichment for Clinical Trials in Mild and Moderate Alzheimer’s Disease. London: European Medicines Agency (2012).
18. European Medicines Agency. Qualification Opinion of Alzheimer’s Disease Novel Methodologies/Biomarkers for BMS-708163. London: European Medicines Agency (2011).
19. European Medicines Agency. Qualification Opinion of Low Hippocampal Volume by MRI for Use in Clinical Trials in Pre-Dementia Stage of Alzheimer’s Disease. London: European Medicines Agency (2011).
20. European Medicines Agency. Qualification Opinion on Dopamine Transporter Imaging as an Enrichment Biomarker for Parkinson’s Disease Clinical Trials in Patients with Early Parkinsonian Symptoms. London: European Medicines Agency (2017).
21. Woodcock J. FDA’s critical path initiative. Drug Discov Today Technol. (2007) 4:33. doi: 10.1016/j.ddtec.2007.11.002
22. Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Ann Rev Med. (2008) 59:1–12. doi: 10.1146/annurev.med.59.090506.155819
23. Murphy D, Spooren W. EU-AIMS: a boost to autism research. Nat Rev Drug Discov. (2012) 11:815–6. doi: 10.1038/nrd3881
24. Isaac M, Vamvakas S, Abadie E, Jonsson B, Gispen C, Pani L. Qualification opinion of novel methodologies in the predementia stage of Alzheimer’s disease: cerebro-spinal-fluid related biomarkers for drugs affecting amyloid burden — regulatory considerations by European medicines agency focusing in improving benefit/risk in regulatory trials. Eur Neuropsychopharmacol. (2011) 21:781–8. doi: 10.1016/j.euroneuro.2011.08.003
25. Hill DLG, Schwarz AJ, Isaac M, Pani L, Vamvakas S, Hemmings R, et al. Coalition against major diseases/European medicines agency biomarker qualification of hippocampal volume for enrichment of clinical trials in predementia stages of Alzheimer’s disease. Alzheimers Dement. (2014) 10: 421–429.e3. doi: 10.1016/j.jalz.2013.07.003
26. European Medicines Agency. Total Kidney Volume (TKV) as a Prognostic Biomarker for Use in Clinical trials Evaluating Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD). London: European Medicines Agency (2015).
27. Perrone RD, Mouksassi MS, Romero K, Czerwiec FS, Chapman AB, Gitomer BY, et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. (2017) 2:442–50. doi: 10.1016/j.ekir.2017.01.003
28. Miller BE, Tal-Singer R, Rennard SI, Furtwaengler A, Leidy N, Lowings M, et al. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am J Respir Crit Care Med. (2016) 193:607–13. doi: 10.1164/rccm.201509-1722PP
29. European Medicines Agency. Qualification Opinion on Plasma Fibrinogen as a Prognostic Biomarker (Drug Development Tool) for All-Cause Mortality and COPD Exacerbations in COPD Subjects. London: European Medicines Agency (2018).
30. Romero K, Conrado D, Burton J, Nicholas T, Sinha V, Macha S, et al. Molecular neuroimaging of the dopamine transporter as a patient enrichment biomarker for clinical trials for early Parkinson’s disease. Clin Transl Sci. (2019) 12:240–6. doi: 10.1111/cts.12619
31. Haberkamp M, Moseley J, Athanasiou D, de Andres-Trelles F, Elferink A, Rosa MM, et al. European regulators’ views on a wearable-derived performance measurement of ambulation for Duchenne muscular dystrophy regulatory trials. Neuromuscul Disord. (2019) 29:514–6. doi: 10.1016/j.nmd.2019.06.003
32. European Medicines Agency. Qualification Opinion on Stride Velocity 95th Centile as a Secondary Endpoint in Duchenne Muscular Dystrophy Measured by a Valid and Suitable Wearable Device. Amsterdam: European Medicines Agency (2019).
33. Bakker E, Hendrikse NM, Ehmann F, van der Meer DS, Llinares Garcia J, Vetter T, et al. Biomarker qualification at the European medicines agency: a review of biomarker qualification procedures from 2008 to 2020. Clin Pharmacol Ther. (2022). doi: 10.1002/cpt.2554
34. European Medicines Agency. Qualification of Novel Methodologies for Drug Development: Guidance to Applicants. London: European Medicines Agency (2014).
35. European Medicines Agency. Essential Considerations for Successful Qualification of Novel Methodologies. Amsterdam: European Medicines Agency (2017).
36. European Medicines Agency. Questions and Answers: Qualification of Digital Technology-Based Methodologies to Support Approval of Medicinal Products. Amsterdam: European Medicines Agency (2020).
Keywords: biomarkers, biomarker qualification, Qualification of Novel Methodologies, regulatory science, European Medicines Agency, Innovation Task Force
Citation: Hendrikse NM, Llinares Garcia J, Vetter T, Humphreys AJ and Ehmann F (2022) Biomarkers in Medicines Development—From Discovery to Regulatory Qualification and Beyond. Front. Med. 9:878942. doi: 10.3389/fmed.2022.878942
Received: 18 February 2022; Accepted: 14 March 2022;
Published: 26 April 2022.
Edited by:
Bruno Sepodes, University of Lisbon, PortugalReviewed by:
Carla Matos Torre, University of Lisbon, PortugalCopyright © 2022 Hendrikse, Llinares Garcia, Vetter, Humphreys and Ehmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalie M. Hendrikse, bmF0YWxpZW1oZW5kcmlrc2VAZ21haWwuY29t; Falk Ehmann, ZmFsay5laG1hbm5AZW1hLmV1cm9wYS5ldQ==
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.