- 1Cardiovascular Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran
- 2Department of Clinical Toxicology, Loghman-Hakim Hospital, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4School of Medicine, Shahid Beheshti University of Medical Science, Tehran, Iran
- 5Department of Internal Medicine, University of Central Florida and Florida State University, Orlando, FL, United States
Background: We aimed to investigate the clinical, laboratory, and electrocardiographic (ECG) findings of colchicine poisoning and to evaluate if there is a correlation between them and the two major outcomes of this toxicity which are respiratory/cardiovascular failure and death.
Materials and Methods: Medical records of 34 colchicine-intoxicated patients that were treated in our center during the past 10 years were retrospectively evaluated. The patient's clinical presentation, vital signs, laboratory tests, ECGs, and outcomes were reviewed.
Results: Abdominal pain, and hypotension at presentation had significant correlation with mortality (p = 0.003, OR: 2.2 [4.1, 7.9], p = 0.029, OR: 13.0 [1.5, 111.8]). Mortality significantly occurred in those with sinus tachycardia, hypokalemia, metabolic acidosis, and impaired liver and kidney function tests (p-values = 0.025, 0.007, 0.04, and 0.008, respectively). All the patients had some ECG abnormalities. Most frequent ECG abnormalities were pathologic ST segment elevation and depression (70%), left atrial enlargement (48%), and sinus tachycardia (37%), PR elevation in aVR lead (37%), and T wave inversion (37%).
Conclusions: Colchicine toxicity is a dangerous entity regarding the cardiovascular events and requires close general and cardiac monitoring.
Introduction
Colchicine is a natural alkaloid compound primarily used to treat gouty arthritis, familial Mediterranean fever, amyloidosis, and severe constipation refractory to standard medical therapy (1, 2). It has also been suggested as a potential treatment for Bechet's disease, pericarditis, and atrial fibrillation after cardiac surgery. The mechanism of action is to forestall microtubule aggregation and subsequently down-regulate microtubule-based inflammatory cycles including chemotaxis (3). Colchicine is available as intravenous injection, solution, tablet, and capsule (4).
In addition to the therapeutic effects, colchicine can also be toxic due to its narrow therapeutic range. Acute overdose of colchicine is rare but constitutes one of the most serious clinical toxicology emergencies with high morbidity and mortality rates (5). Supportive care is the cornerstone of treatment and Fab fragment antibodies that are used as experimental antidote are not commercially available, making it a potentially dangerous entity (6, 7).
The toxicity of colchicine seems to be dose-dependent (6, 8). It is generally accepted that the maximum safe dose of colchicine is about 1.2 mg/day (9). Colchicine is therapeutic at 0.015 mg/kg, toxic at 0.1 mg/kg and fatal at 0.8 mg/kg (6, 7). However, death and severe sequelae are well documented at lower doses (7, 8). Fatal overdose of colchicine has been delineated in several articles where blood or plasma levels varied from 10 to 250 ng/ml (1, 2, 10).
Abdominal pain, nausea, vomiting, and diarrhea are the principal clinical manifestations of colchicine toxicity. Multi-Organ involvement is the major cause of death. In the first 10-24 h after ingestion, patients present with symptoms mimicking gastroenteritis, which are absent for intravenous users. 24 h to 7 days after ingestion is the time for multi-organ presentation and recovery follows after 7 days (7).
Cardiogenic shock secondary to direct colchicine-induced cardiac toxicity is another major cause of death. Colchicine causes direct cellular toxicity that ends up in decreased myocardial contractility (11). It is also considered to interfere with cardiac pulse generation and conduction. However, the mechanism behind the abnormal rhythms is not well understood (12, 13).
We designed this study to evaluate the different aspects of colchicine toxicity as related to the clinical, laboratory, and ECG manifestations and to see which one correlates more with respiratory/cardiovascular failure and death. Also, we aimed to find more about ECG changes that can happen from cardiac toxicity.
Materials and Methods
Study Design
Retrospective single center evaluation of colchicine toxic cases who were referred to the emergency department of Loghman-Hakim Hospital—the main referral toxicology center in Tehran, Iran—between January 2011 and January 2021. The study was reviewed and approved by Institutional Review Board of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1398.116).
Inclusion and Exclusion Criteria
Colchicine-poisoned patients either as the sole agent of toxicity or as the main cause of toxicity were included. Colchicine poisoning was confirmed based on the patient history as well as signs and symptoms of the toxicity on presentation. Symptoms such as abdominal pain, nausea, vomiting were noted and subsequent manifestations such as bone marrow suppression and alopecia were included. Evaluation of serum colchicine level was not available in our center.
Patients with history of ischemic heart disease, heart failure, severe valvular heart disease, and severe renal failure [Glomerular Filtration Rate (GFR) < 30 ml/min] (14) or hepatic failure [cirrhosis or Aspartate Aminotransferase/alanine aminotransferase (AST/ALT) > 1,000 U/L] (15) were excluded. Patients who had concomitantly overdosed on a cardiotoxic medication or substances such as amphetamine or methadone were also excluded.
Variables
Patients' demographic characteristics and point of care signs and symptoms, dosage of colchicine causing the toxicity, ECG, and lab tests were reviewed. Respiratory status and the need for mechanical ventilation was noted. Mortality was noted.
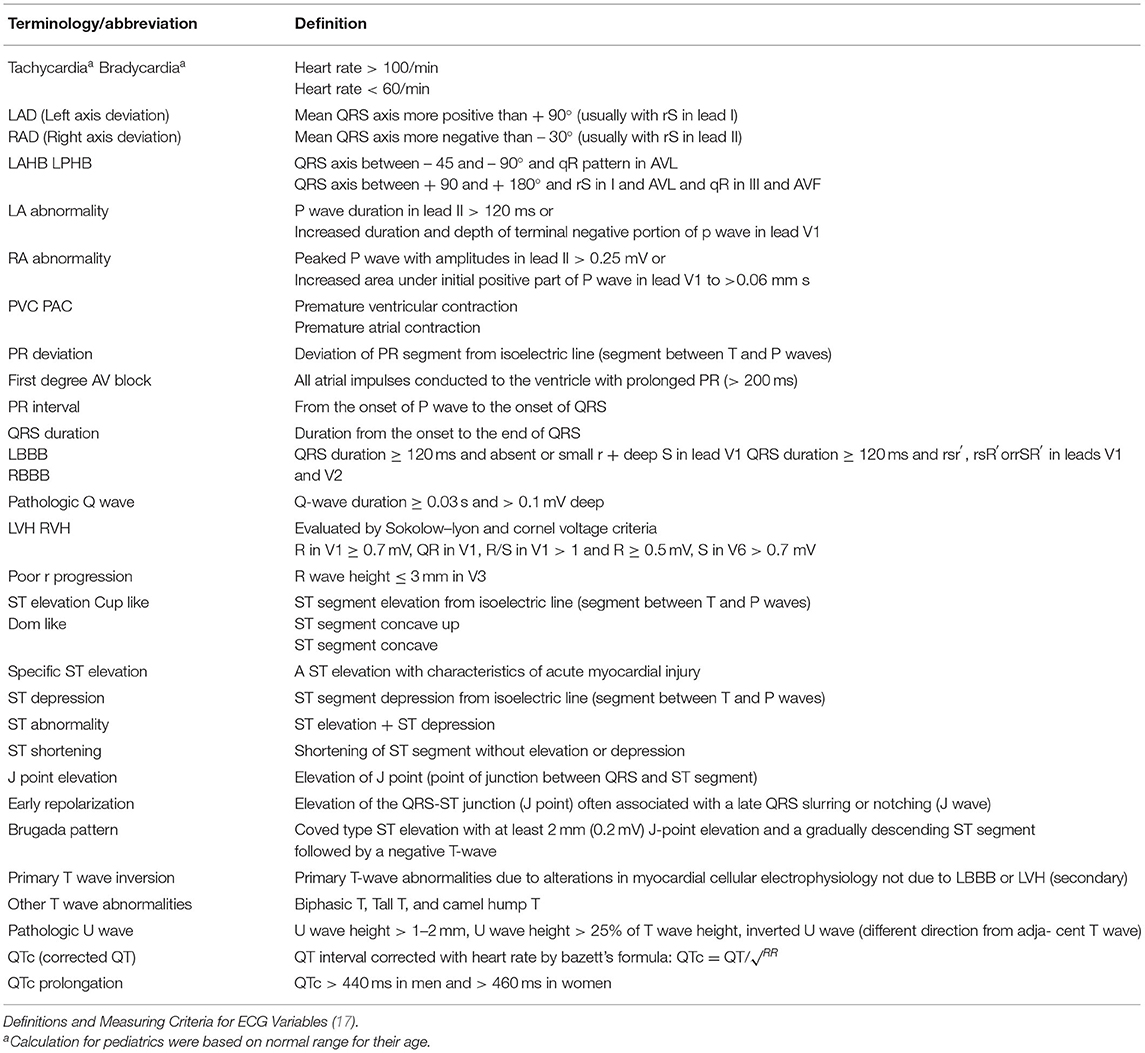
Point of care ECGs were evaluated by a cardiologist using the standard normal ranges for each (16) (Table 1). Heart rate, rhythm, axis, atrial abnormalities, PR interval, PR deviation, pathologic Q wave, QRS duration, ventricular enlargement, ST-T abnormalities, J-point elevation, early repolarization, Brugada pattern, abnormal U wave, and corrected QT intervals (QTc) were all evaluated.
Statistics
Quantitative variables were given as mean (±SD) and median [interquartile range; IQR] for variables with normal and non-normal distribution, respectively. Data was analyzed using Pearson Chi-square, Fisher's exact test, t-test, and Mann-Whitney U-test. Measure of association between binomial exposure and outcome was expressed using odds ratio (OR) and 95% confidence interval. Roc curve was used to define the best cut-off for instantaneous sensitivity and specificity in continuous variable with significant association with mortality. Data was analyzed using statistical package for social sciences (SPSS) software version 27. P-values < 0.05 were considered to be statistically significant.
Results
A total of 34 colchicine-intoxicated patients were evaluated. There were no patients with known cardiovascular or other significant comorbidities. Sixteen patients had intentionally overdosed to attempt suicide; two had accidentally overdosed on colchicine, and in other 16, the intention was not clear.
History and Primary Symptoms
Almost all patients were conscious upon arrival and were able to communicate with the physician and report symptoms. Subsequently, some gradually lost consciousness.
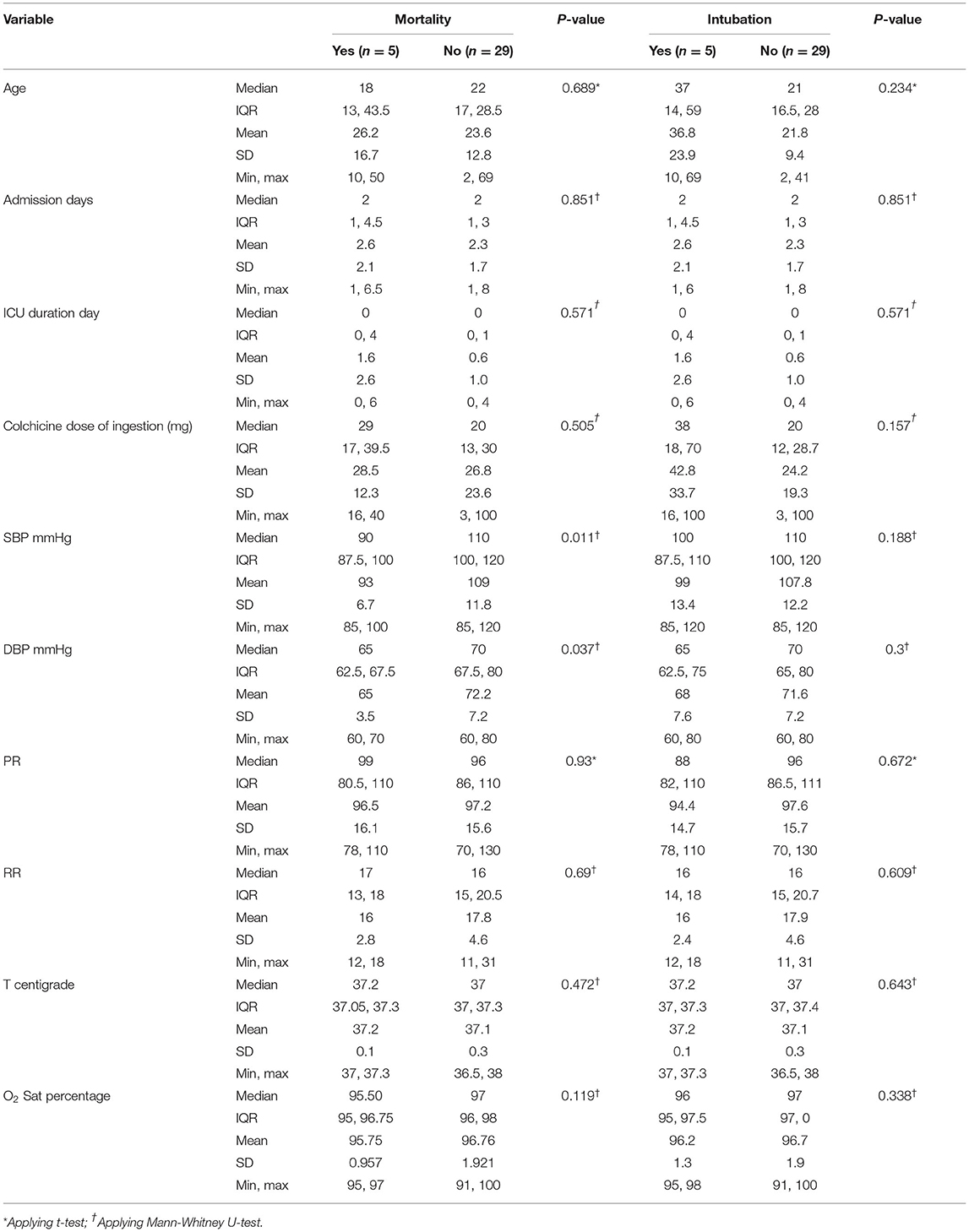
The most common presentations were nausea in 28 cases (82%) and vomiting in 24 (70%). Twelve patients (35%) had abdominal pain and 11 (32%) had diarrhea. Eleven (35%) reported dizziness as the main presentation. Other less frequent clinical presentations included dyspnea in 4 (12%), limb numbness in 3 (9%), facial paresthesia/numbness in 3 (9%), headache in 3 (9%), tremor in 2 (6%), and ataxia in one patient. The patients' characteristics are summarized in Tables 2, 3.
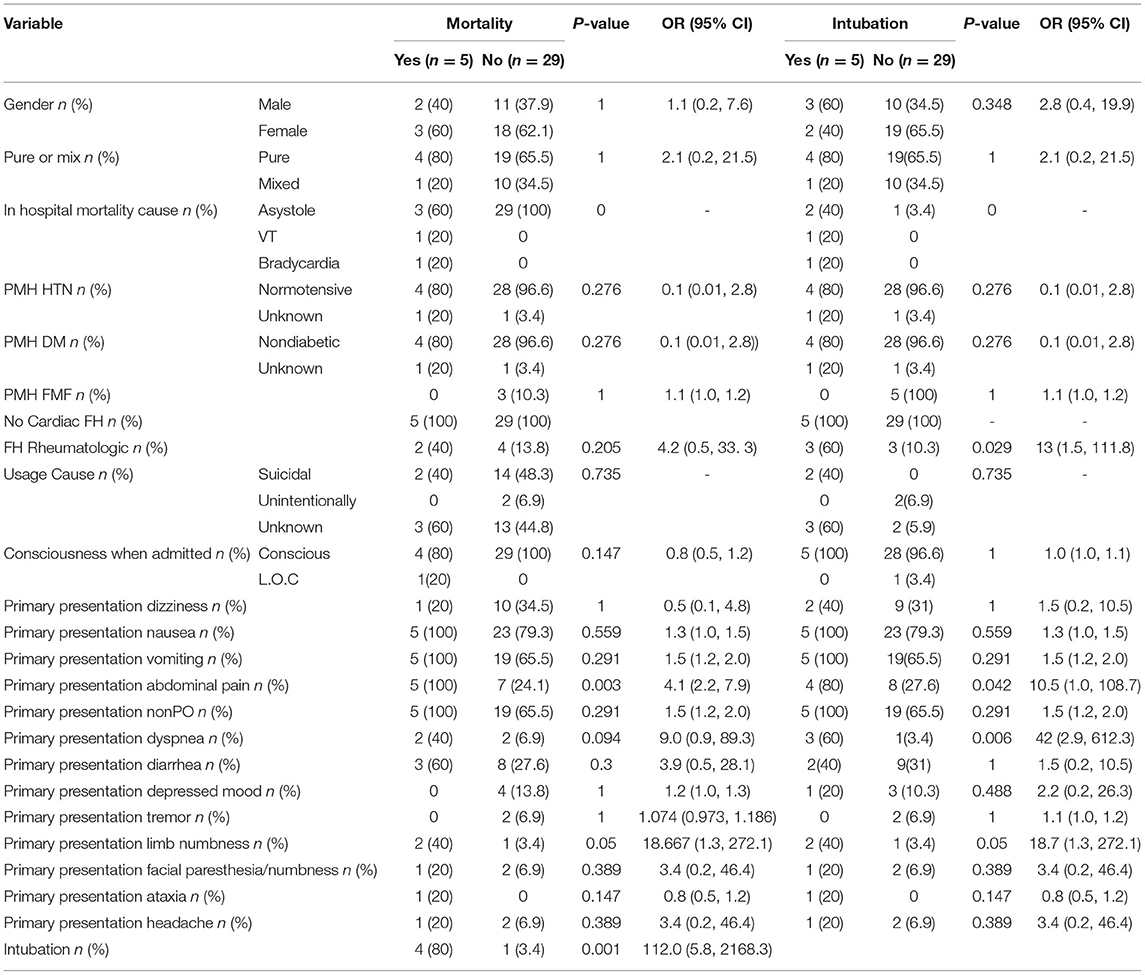
Table 2. Patient history and primary symptoms in mortality and mechanical ventilation groups (qualitative variables).
Significant correlation was present between abdominal pain and limb numbness with both mechanical ventilation and mortality. All patients who did not survive as well as 80% of the mechanical ventilation patients reported abdominal pain on arrival (p = 0.003, OR [95% CI] 2.2 [4.1, 7.9], and p = 0.04, OR [95% CI] 10.5 [1.1, 108.7], respectively). In the mortality and mechanical ventilation group, 60% of patients reported limb numbness, drowsiness, or tingling (p = 0.05, OR [95% CI] 18.7 [1.3, 272.1]). Dyspnea at presentation had significant association with mechanical ventilation. Almost 60% of intubated patients had shortness of breath on presentation (p = 0.006, OR [95% CI] 42.0 [2.9, 612.3]). 80% of those who died required mechanical ventilation. Low systolic and diastolic blood pressure (<90 and 60 mmHg, respectively) were significantly related to mortality (p = 0.029, OR [95% CI] 13.0 [1.5, 111.8]). 60% of patients who needed mechanical ventilation had a positive family history of rheumatoid arthritis (p = 0.029, OR: 13.0 [1.5, 111.8]). Dose of ingested colchicine has no significant correlation with mortality in this study.
Laboratory Findings
Patients with poor outcomes had higher blood urea nitrogen (BUN) and creatinine, creatine phosphokinase (CPK) and lactate dehydrogenase (LDH), liver function tests, and international normalized ratio (INR) (Table 4).
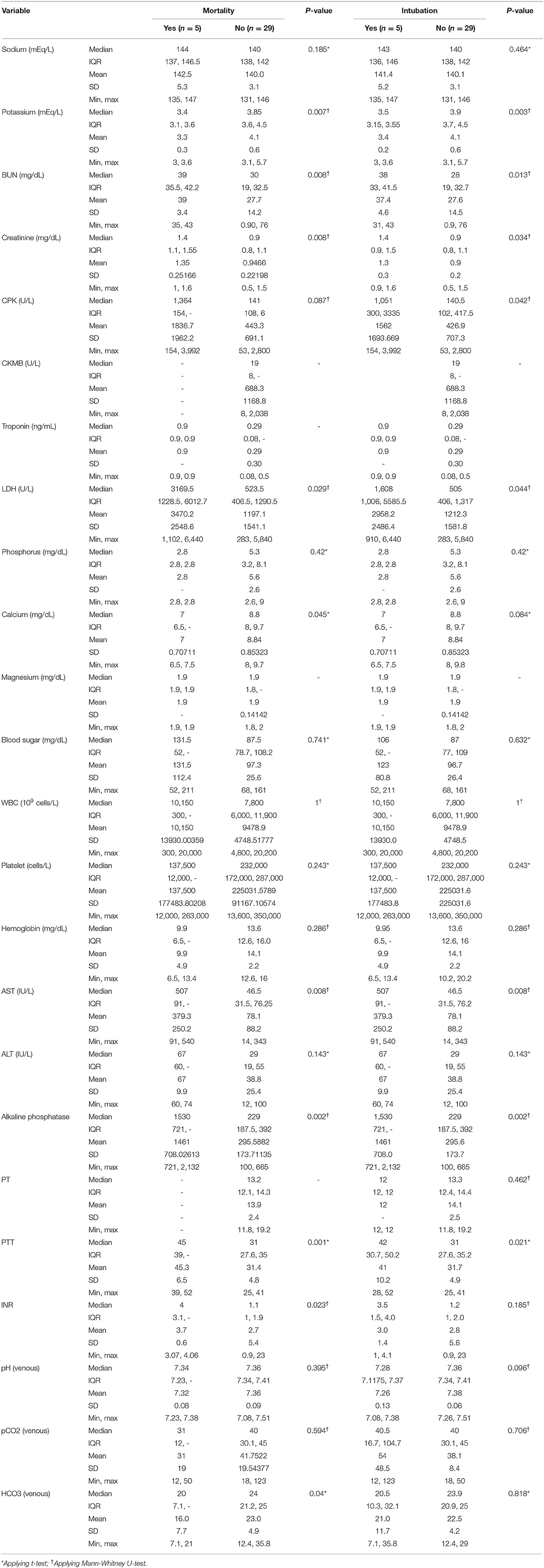
Table 4. Lab test results in patients who eventually underwent intubation and those who were expired.
The odd [95% CI] of 84 times [4.1, 1715.6] for death was achieved using the cut-off of 1.35 mg/dl for creatinine level (p = 0.003). It was 17 [1.3, 223.1] for LDH ≥ 1,551 (p = 0.035), 5 [2.1, 12.0] for AST ≥ 85 mg/dl (p = 0.02), 8 [2.2, 29.2] for INR ≥ 2.64 (p = 0.01), 7.2 [2.9, 18.0] for BUN ≥ 34.5 (p = 0.002), and 7 [1.9, 25.2] for PTT ≥ 37.5 s (p = 0.015).
Bicarbonate level (but not pH) was significantly lower in the mortality group and so were serum potassium and calcium levels (Table 4).
Electrocardiographic Findings
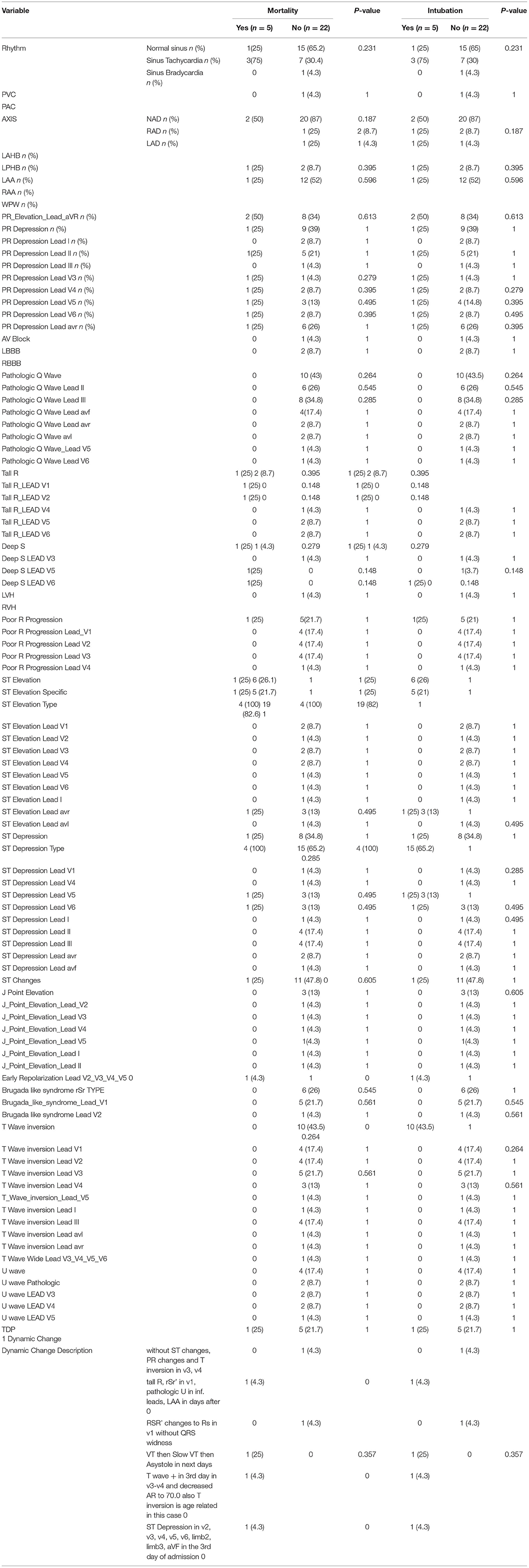
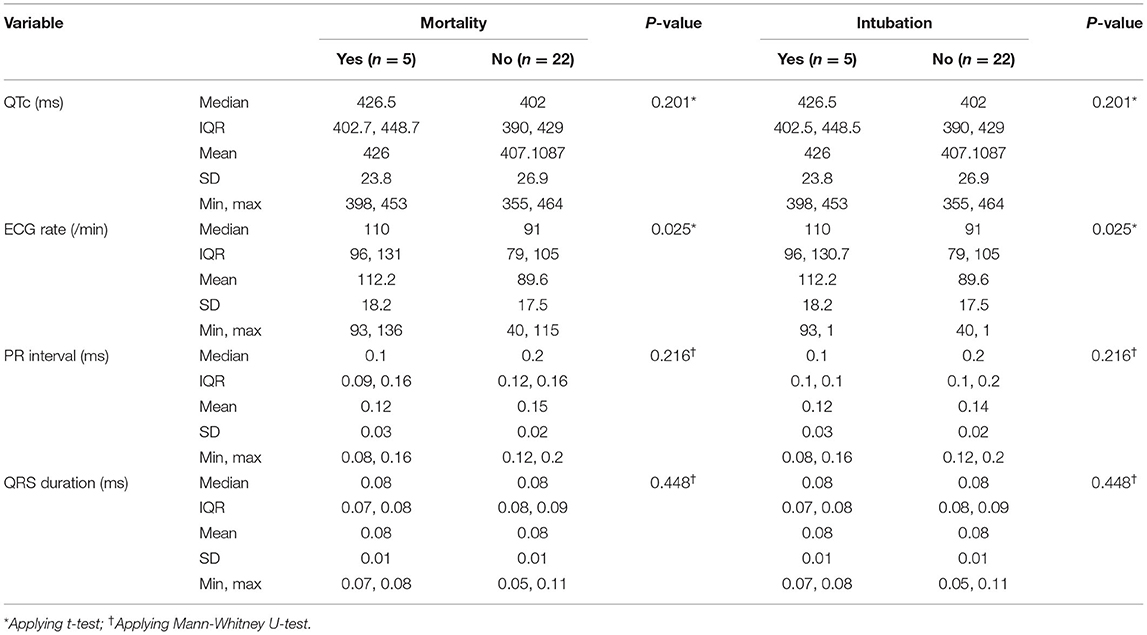
Point of care ECGs for 27 patients were available. Thirteen (38%) were male and median [IQR] age was 22 years. Five patients were intubated and five passed away during the hospital stay. All patients had some ECG abnormalities. Sinus tachycardia and bradycardia were detected in 10 (37%) and one patient, respectively. Mean heart rate was significantly higher in patients who were eventually intubated or died (p = 0.02). One patient was reported to have premature ventricular complexes (PVC). Other abnormalities seen were left atrial enlargement in 13 patients (48%), PR elevation in aVR lead in 10 (37%), pathologic Q wave in 10 (37%), ST segment elevation in 7 (37%), ST segment depression in 9 (33%), Brugada pattern in 6 (22%), T wave inversion in 10 (37%), and pathologic U wave in 2 (7%). None of these changes were related to mechanical ventilation and death in a statistically significant manner. ECG abnormalities reported are summarized in Tables 5, 6.
Three of the cases (11%) had prolonged QTc interval. Mean QTc [IQR] was 426 ms [402.75 to 448.75 ms] in the mechanical ventilation and mortality groups (p = 0.20). QTc, PR interval, and QRS duration were not significantly different between mortality and survival groups.
There were 13 cases of left atrial enlargement and no cases of right atrial enlargement. PR elevation in lead aVR and PR depression in the limb leads, anterolateral leads, and aVR leads were found in 10 patients. Inverted T waves and pathological Q wave in II and III were seen in 10 cases each. ST-elevation was detected in 7 patients in precordial leads, I, aVR, and aVL. Brugada-like pattern was recorded in six cases in leads V1 and V2. Poor R progression was detected in 6 ECGs in the anteroseptal precordial leads. Three cases of left posterior hemiblock were detected. Pathological U waves and left bundle branch block were detected in two cases. J point elevation, first degree atrioventricular block, and early repolarization were present in one case each.
Dynamic ECG changes were noted in six patients during their hospital stay. Except for the heart rate, none of the ECG variables were found to be related to mortality and intubation. Mean heart rate was 112/min (range: 93-136/min) and 90/min (range: 40-115/min) in the mortality and mechanical ventilation groups, respectively. Mode of death was bradycardia/asystole in 4, and ventricular tachycardia in 1 patient.
Discussion
Previous studies on colchicine poisoning are generally case reports and small case series (17–19). The clinical effects of colchicine on different organs are shown in Table 7. In this study, we evaluated clinical presentation, detailed ECG abnormalities, laboratory data on arrival, and outcome of 34 colchicine-intoxicated patients who were referred to our tertiary toxicology center during a 10-year period.
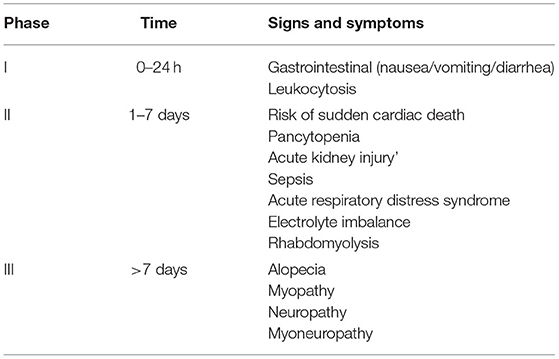
Table 7. General involvements of different organ systems in colchicine poisoning (phases of colchicine poisoning).
Although most of the ECG abnormalities we found in our study were not significantly related to the patient's outcome, our detailed findings seem to be singular. Previously reported cases had shown ST elevation in patients with colchicine poisoning (20). Bradycardia and arrhythmia had been reported in severe toxicities after intravenous administration of colchicine (21). However, to the best of our knowledge, no studies have clearly detailed the ECG effects of colchicine in intoxicated patients. Colchicine effect on skeletal muscles has been discussed (22).
Clinical results are also of importance. Based on our findings, tachycardia is significantly related with intubation and mortality in colchicine-intoxicated patients; a finding that had never been reported before. Troponin level does not significantly change after colchicine poisoning although cardiac changes are common showing the fact that the nature of the damage to the cardiac cells is not ischemic or necrosis-inducing in this poisoning.
Also, previous studies have reported laboratory abnormalities in colchicine poisoning, but none had evaluated the relation of these abnormalities with mortality (23). High BUN and Cr and lower serum potassium and calcium significantly increased the risk of mortality. Renal failure, hypokalemia, and hypocalcemia are due to diarrhea and GI loss. Metabolic acidosis was another important laboratory finding associated with mortality in these patients.
If rhabdomyolysis occurs, hemoglobinuria may also cause azotemia (24). This can be considered a possible cause of azotemia in four of our patients who had CPK of more than 1,000 mg/dl and higher mortality. Arslan et al. reported a case of colchicine poisoning with evidence of myocytolysis in autopsy (25) confirming the possible effect of the drug on myocytes. Colchicine has been known to induce myopathy in several other studies, as well (26, 27).
Similar to previous studies, the most common presentation of our patients were GI manifestations including nausea, vomiting, abdominal pain and diarrhea (8, 10) which can occur due to mucosal damage and cholera-like syndrome (28). Elevated liver enzymes and higher INR in our cases were significantly related to mortality. We think hypovolemia and hypotension might have played a role through ischemic hepatitis. Dyspnea was an important presentation of our patients and was significantly related to intubation. Case reports of acute respiratory distress syndrome due to colchicine poisoning exist in the literature but none of our patients had ARDS (29). Direct toxic effect of colchicine on respiratory muscles have already been suggested as the mechanism of respiratory involvement (30, 31) which can be argued through our results, as well.
Neuropathy was not prominent in our patients. Limb and facial numbness and paresthesia, headache, tremor, and ataxia were the most common presentations. Patients who complained of numbness, tingling, and facial paresthesia on admission had a higher risk of mortality and need for intubation. Different forms of neuropathy including myo-neuropathy, distal sensory abnormalities, nerve conduction impairment, and axonal neuropathy have been reported in previous studies and changes in microtubular network has been suggested as the probable mechanism of myopathy in these patients (7, 32).
Considering the results of the current study, BUN, creatinine, potassium, and calcium disturbances on presentation accompany with a poorer prognosis. Admission of patients with these problems to the intensive care unit and obsessive correction of the electrolyte and kidney disturbances may yield better prognosis in these patients.
Limitations and Recommendations
Our main limitation was inability to check serum colchicine level and to correlate it with symptoms. Another limitation was retrospective nature of the study that led to some missing data.
The low prevalence of colchicine poisoning makes it difficult to conduct a prospective study with attention to levels and cardiac poisoning. Therefore, we recommend more close attention to ECG changes, hemodynamics, and laboratory abnormalities in colchicine toxic patients to detect high risk patients who are at risk of intubation and death.
Conclusion
Colchicine toxicity is a potentially dangerous situation that needs close monitoring and management. Abdominal pain, sinus tachycardia, hypotension, elevated renal function tests and low potassium are signs of danger and might require intensive care to avoid mechanical ventilation and mortality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was reviewed and approved by Institutional Review Board of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1398.116). The need for written informed consent was waived due to the retrospective nature of the study.
Author Contributions
MS developed the concept, made the questionnaire, wrote the manuscript, and made the tables. NZ developed the concept, edited the manuscript, and did critical thinking on the subject. AG collected the data and drafted the manuscript. HA edited the final manuscript. HH-M edited the manuscript, submitted it to the journal, and is responsible for the overall content as guarantor. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This manuscript is written based on thesis of Amir Hushang Gerami for School of Medicine.
References
1. Kintz P, Jamey C, Tracqui A, Mangin P. Colchicine poisoning: report of a fatal case and presentation of an HPLC procedure for body fluid and tissue analyses. J Anal Toxicol. (1997) 21:70-2. doi: 10.1093/jat/21.1.70
2. Jones GR, Singer PP, Bannach B. Application of LC-MS analysis to a colchicine fatality. J Anal Toxicol. (2002) 26:365-9. doi: 10.1093/jat/26.6.365
3. Leung YY, Yao Hui LL, Kraus VB. Colchicine–update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. (2015) 45:341-50. doi: 10.1016/j.semarthrit.2015.06.013
4. Bonnel RA, Villalba ML, Karwoski CB, Beitz J. Deaths associated with inappropriate intravenous colchicine administration. J Emerg Med. (2002) 22:385-7. doi: 10.1016/S0736-4679(02)00430-4
5. Bismuth C, Gaultier M, Conso F. Aplasie médullaire après intoxication aiguë à la colchicine. 20 cas [Medullary aplasia after acute colchicine poisoning. 20 cases]. Nouv Presse Med. (1977) 6:1625-9. French.
6. Roddy E, Mallen CD. Colchicine in overdose. Br J Gen Pract. (2017) 67:61. doi: 10.3399/bjgp17X688993
7. Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. (2010) 48:407-14. doi: 10.3109/15563650.2010.495348
8. Aghabiklooei A, Zamani N, Hassanian-Moghaddam H, Nasouhi S, Mashayekhian M. Acute colchicine overdose: report of three cases. Reumatismo. (2014) 65:307-11. doi: 10.4081/reumatismo.2013.720
9. Colchicine. Colchicine (Oral Route) Proper Use. Mayo Clinic. Available online at: https://www.mayoclinic.org/drugs-supplements/colchicine-oral-route/description/drg-20067653
10. Rahimi M, Alizadeh R, Hassanian-Moghaddam H, Zamani N, Kargar A, Shadnia S. Clinical manifestations and outcomes of colchicine poisoning cases; a cross sectional study. Arch Acad Emerg Med. (2020) 8:e53.
11. Biçer S, Soysal DD, Ctak A, Uçsel R, Karaböcüoglu M, Uzel N. Acute colchicine intoxication in a child: a case report. Pediatr Emerg Care. (2007) 23:314-7. doi: 10.1097/01.pec.0000270163.84076.9f
12. van Heyningen C, Watson ID. Troponin for prediction of cardiovascular collapse in acute colchicine overdose. Emerg Med J. (2005) 22:599-600. doi: 10.1136/emj.2002.004036
13. D'Amario D, Cappetta D, Cappannoli L, Princi G, Migliaro S, Diana G, et al. Colchicine in ischemic heart disease: the good, the bad and the ugly. Clin Res Cardiol. (2021) 110:1531-42. doi: 10.1007/s00392-021-01828-9
14. Dimopoulos MA, Terpos E. Renal insufficiency and failure. Hematology Am Soc Hematol Educ Program. (2010) 2010:431-6. doi: 10.1182/asheducation-2010.1.431
15. Lee LK, Gan VC, Lee VJ, Tan AS, Leo YS, Lye DC. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. PLoS Negl Trop Dis. (2012) 6:e1676. doi: 10.1371/journal.pntd.0001676
16. ECGpedia. Available online at: http://en.ecgpedia.org/index.php?title=Main_Page (accessed December 25, 2017).
17. Kilic SC, Alaygut D, Unal E, Koç E, Patiroglu T. Acute colchicine intoxication complicated with extramedullary hematopoiesis due to filgrastim in a child. J Pediatr Hematol Oncol. (2014) 36:e460-2. doi: 10.1097/MPH.0000000000000071
18. Folpini A, Furfori P. Colchicine toxicity–clinical features and treatment. Massive overdose case report. J Toxicol Clin Toxicol. (1995) 33:71-7. doi: 10.3109/15563659509020219
19. Maxwell MJ, Muthu P, Pritty PE. Accidental colchicine overdose. A case report and literature review. Emerg Med J. (2002) 19:265-7. doi: 10.1136/emj.19.3.265
21. Mubayed L, Muller BA, Jacobson JL, Hast HA, Nguyen HH. Acute pediatric colchicine toxicity is associated with marked bradydysrhythmias. J Emerg Med. (2018) 55:e65-9. doi: 10.1016/j.jemermed.2018.03.004
22. Sadoshima J, Takahashi T, Jahn L, Izumo S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA. (1992) 89:9905-9. doi: 10.1073/pnas.89.20.9905
23. Alinejad S, Zamani N, Abdollahi M, Mehrpour O. A narrative review of acute adult poisoning in Iran. Iran J Med Sci. (2017) 42:327-46.
24. Kim MG, Jung J, Hong SB, Lee SO, Choi SH, Kim YS, et al. Severe fever with thrombocytopenia syndrome presenting with rhabdomyolysis. Infect Chemother. (2017) 49:68-71. doi: 10.3947/ic.2017.49.1.68
25. Arslan MN, Özgün A, Daş T, Kumru D, Sam B, Koç S. Colchicine-induced rhabdomyolysis: an autopsy case. Am J Forensic Med Pathol. (2016) 37:57-9. doi: 10.1097/PAF.0000000000000225
26. Al Megalli M, Bashir S, Qadah H, Ameen O, Al-Harbi TM. Colchicine-induced acute myopathy: case study from Saudi Arabia. Cureus. (2021) 13:e20290. doi: 10.7759/cureus.20290
27. Du YJ, Liu WC, Chen X, Cheng YJ. [A case report of colchicine-induced myopathy in a patient with chronic kidney disease]. Beijing Da Xue Xue Bao Yi Xue Ban. (2021) 53:1188-1190. Chinese. doi: 10.19723/j.issn.1671-167X.2021.06.030
28. Rigante D, La Torraca I, Avallone L, Pugliese AL, Gaspari S, Stabile A. The pharmacologic basis of treatment with colchicine in children with familial Mediterranean fever. Eur Rev Med Pharmacol Sci. (2006) 10:173-8.
29. Domínguez de Villota E, Galdos P, Mosquera JM, Tomaś MI. Colchicine overdose: an unusual origin of multiorgan failure. Crit Care Med. (1979) 7:278-9. doi: 10.1097/00003246-197906000-00005
30. Maurizi M, Delorme N, Laprévote-Heully MC, Lambert H, Larcan A. Syndrome de détresse respiratoire aiguë de l'adulte au cours des intoxications par la colchicine [Acute respiratory distress syndrome in adults in colchicine poisoning]. Ann Fr Anesth Reanim. (1986) 5:530-2. French. doi: 10.1016/S0750-7658(86)80041-7
31. Heaney D, Derghazarian CB, Pineo GF, Ali MA. Massive colchicine overdose: a report on the toxicity. Am J Med Sci. (1976) 271:233-8. doi: 10.1097/00000441-197603000-00014
Keywords: colchicine toxicity, colchicine poisoning, intoxication, ECG manifestations, mortality
Citation: Sheibani M, Zamani N, Gerami AH, Akhondi H and Hassanian-Moghaddam H (2022) Clinical, Laboratory, and Electrocardiographic Findings in Colchicine Toxicity: 10 Years of Experience. Front. Med. 9:872528. doi: 10.3389/fmed.2022.872528
Received: 09 February 2022; Accepted: 30 March 2022;
Published: 19 May 2022.
Edited by:
Muneeb A. Faiq, New York University, United StatesReviewed by:
Ashutosh Kumar, All India Institute of Medical Sciences, IndiaMaheswari Kulandhasamy, University of Delhi, India
Copyright © 2022 Sheibani, Zamani, Gerami, Akhondi and Hassanian-Moghaddam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hossein Hassanian-Moghaddam, aGFzc2FuaWFuQHNibXUuYWMuaXI=
 Mehdi Sheibani1
Mehdi Sheibani1 Nasim Zamani
Nasim Zamani Hossein Hassanian-Moghaddam
Hossein Hassanian-Moghaddam