
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Med. , 29 April 2022
Sec. Family Medicine and Primary Care
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.872362
This article is part of the Research Topic Acupuncture for Pain Management View all 20 articles
Background: Chronic neck pain is a prevalent condition adversely impacting patients' wellbeing in both life and work experience. Electro-thumbtack needle (ETN) therapy, combining acupuncture with transcutaneous stimulation, might be one of the effective complementary and alternative medicine (CAM) therapies in treating chronic neck pain, although the evidence is scarce. This study aims to estimate the efficacy and safety of ETN therapy for chronic neck pain.
Methods and Analysis: This is a sham-controlled, randomized clinical trial. A total of 180 subjects will be randomly allocated to either the ETN group or the sham ETN group. Treatment will be administrated three times a week for four consecutive weeks, with a 6-month follow-up. The primary outcome measure will be the Numerical Rating Scale for neck pain (NRS-NP) over a period of the 4 weeks. Secondary outcome measures include the Northwick Park Neck Pain Questionnaire (NPQ), Neck Disability Index (NDI), Patient Global Impression of Change (PGIC), patient expectation, and preference assessment. The chi-square test or Fisher's exact test will be used for proportions of participants having clinically meaningful improvement. Analysis of covariance or repeated-measures analysis of variance will be applied to examine changes in the outcome measures from baseline.
Discussions: This prospective trial will contribute to evaluating the efficacy and safety of ETN in the treatment of chronic neck pain, with an intermediate-term follow-up. This study will provide further evidence for clinical neck pain management.
Ethics and Dissemination: This trial has been approved by the Research Ethical Committee of Guang'anmen Hospital (ethical approval number: 2021-039-KY-01). Recruitment began in March 2022 and will continue until December 2023. Dissemination plans include posters, WeChat, websites, and bulletin boards in hospital and communities.
Clinical Trial Registration: This trial is registered at ClinicalTrials.gov (identifier: NCT04981171).
Chronic neck pain is a common physical complaint across the world and the fourth leading impairment in terms of years lived with disability (1, 2). Its lifetime prevalence is around 48.5% (3). Neck pain is mainly due to poor posture or anxiety, especially in women and the middle-aged population (4). Previous clinical trials have demonstrated that therapies including exercise, acupuncture, and transcutaneous electrical nerve stimulation (TENS) have moderate effects in pain alleviation (5–8). However, management of chronic neck pain remains complex and challenging.
Acupuncture has been widely used for pain alleviating around the world. In previous studies, acupuncture or electroacupuncture is more effective than sham controls (6, 8–11). Clarified evidence supported that acupuncture could produce opioids and other bioactive antalgic chemicals (12). It is thus recommended for the treatment of chronic neck pain by consensus (6, 8, 13). Nevertheless, the application of acupuncture is limited to a large extent due to the patients' fright of and intolerance to needling pain. TENS, a non-invasive physical therapy, is more popular and comfortable. Evidence from clinical trials supported the effectiveness of TENS for chronic neck pain (14–16). The pain could be alleviated via stimulation either on acupoints or trigger points, both immediately after treatment and during follow-up periods (15, 17, 18). However, the difference between TENS and sham control is not significant, especially at the long-term follow-ups (14, 18).
A combination of acupuncture with conservational treatment, such as TENS, is considered to benefit the patients more in comparison with single therapy (8, 13). The emergence of electro-thumbtack needle (ETN) therapy is in response to this recommendation, aiming to achieve better effectiveness while maintaining comfortable. The device of ETN is composed of a thumbtack needle with 2 mm long body and a small rechargeable and portable electric stimulator, which outputs current. The surface of thumbtack needle is modified with conductive material to reinforce electric stimulation on acupoints. It is a convenient therapy that can be easily operated by patients on their own after training. In light of the good effectiveness of acupuncture and TENS published in previous studies, as well as the convenience of ETN, we plan to conduct this study to estimate the efficacy and safety of ETN therapy for chronic neck pain in comparison with sham ETN therapy.
This study is a single-center, participant-blinded, randomized controlled trial, which will be conducted from March 2022 to December 2023 in Guang'anmen Hospital, China Academy of Chinese Medical Sciences. The study flowchart is shown in Figure 1. We design this protocol conforming to the guidelines of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (19) (Table 1) and the Consolidated Standards of Reporting Trials (CONSORT). This trial has been approved by the Institutional Review Board of Guang'anmen Hospital, China Academy of Chinese Medical Sciences (ethical approval number: 2021-039-KY-01), and it has been registered at ClinicalTrials.gov on July 18, 2021 (Identifier: NCT04981171). Each participant will receive 4-week treatment and will be followed up for 6 months.
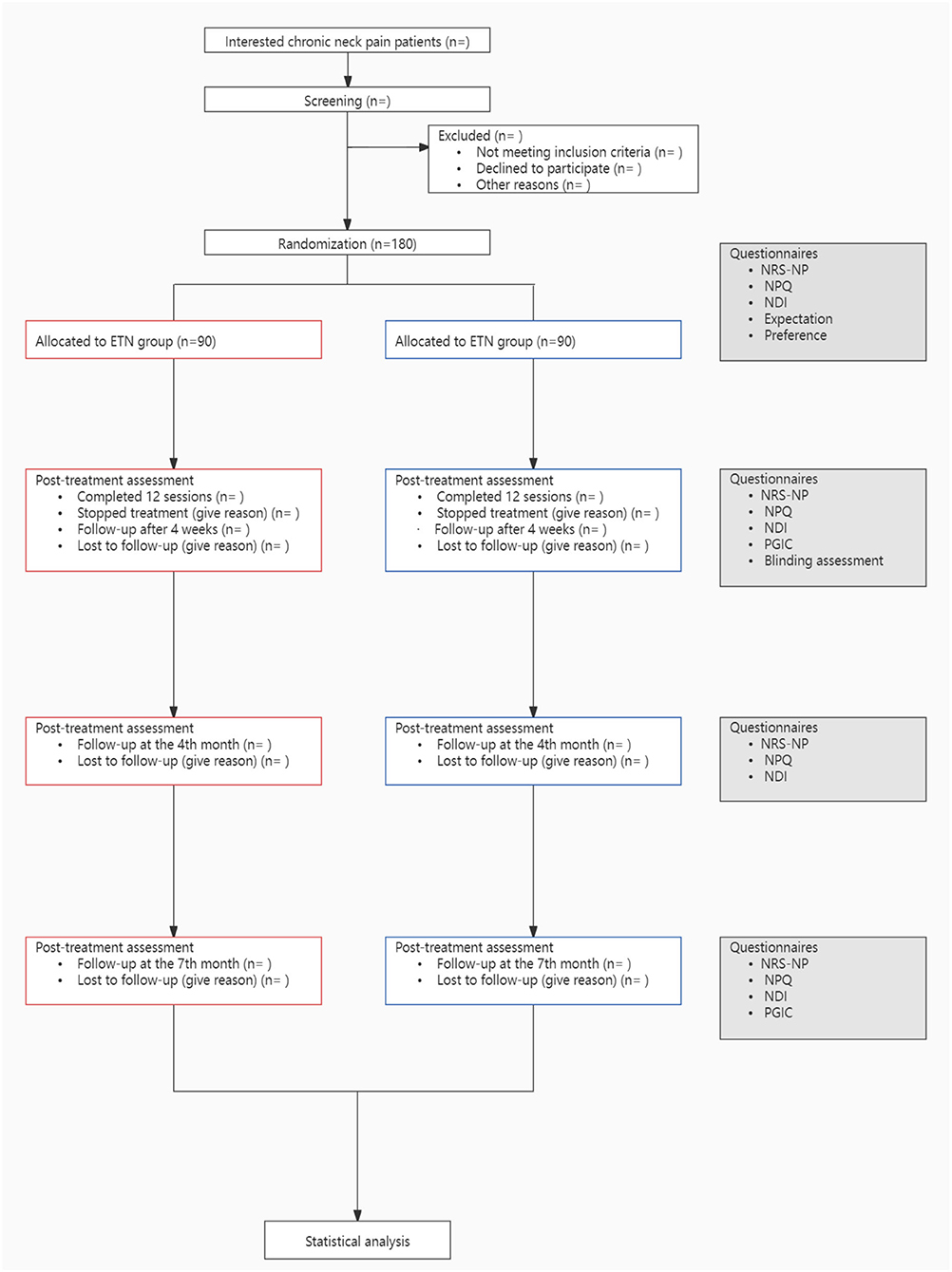
Figure 1. Study flowchart. ETN, electro-thumbtack needle; NRS-NP, numerical rating scale for neck pain; NPQ, the northwick park neck pain questionnaire; NDI, neck disability index; PGIC, patient global impression of change.

Table 1. SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents*.
Participants conforming to the following criteria are included:
(1) Neck pain accompanied with headaches or movement coordination impairments according to the Orthopedic Section of the American Physical Therapy Association (APTA) (7);
(2) Aged 18–65 years;
(3) History of neck pain for at least 3 months;
(4) Scoring at least 4 on Numerical Rating Scale for Neck Pain (NRS-NP) assessing the average neck pain intensity over the last 7 days (12, 20–23).
Participants who meet any of the following criteria are excluded:
(1) Neck pain with mobility deficits or radiating neck pain according to the Orthopedic Section, APTA (7);
(2) Neck pain secondary to specific diseases, such as tumor, immune disease, endocrine and metabolic disorders, neurological abnormalities, cervical vertebra fracture, and cervical dislocation;
(3) Acute or radiating neck pain or pain accompanied with upper limb symptoms;
(4) Neck pain with sensory or motor disturbance;
(5) Prior cervical spine surgery or congenital abnormalities;
(6) Experiencing medical dispute litigation;
(7) With the experience of acupuncture in the past 30 days;
(8) In the administration of analgesic, muscle relaxant, hormones, or having greater pain in other regions of the body;
(9) Allergic to metal or the adhesive tape, implantation of cardiac pacemaker, or ulceration of skin at selected acupoints;
(10) Disable to communicate or critically ill;
(11) Drug or alcohol dependent;
(12) Currently or planning to be pregnant.
A total of 180 participants with chronic neck pain will be recruited in Guang'anmen Hospital via posters, WeChat, websites, and bulletin boards in hospital and communities. Diagnosis will be made by an orthopedist. Eligible participants will be officially enrolled only after signing a written informed consent, and all participants have the right to withdraw their consent later at any time.
Eligible participants will be randomly assigned in a 1:1 ratio to either the ETN group or the sham ETN group. The randomization sequence will be generated using a fixed block size by an independent investigator through the SAS 9.4 (SAS Institute, Cary, NC, USA). The randomization number will be sealed in opaque and sequentially labeled envelopes, with the name of the investigator and the date signed over the seals. The envelop will be opened sequentially after the enrollment of participant, and the group allocation will be only informed to the acupuncturist before treatment.
In this trial, the participants, outcome assessors, and the statisticians will all be blinded. The sham ETN was designed to mimic true ETN: the sham thumbtack needle is 0.2 mm long with a blunt tip (instead of a sharp tip), and the electric stimulation is minimal and transient. They will produce a similar sensation of tingling as true ETN, which makes it hard for participants to distinguish. To avoid conversation between groups, participants will be treated separately on alternate days by appointments. After either session in the last week of treatment, blinding assessment will be completed by all participants. They will be told before group allocation: you may be randomly allocated to either the ETN group or the sham ETN group; the electric stimulation is weak in both groups, and you may even be incapable to sense it during the process out of body adaption. Participants will be asked “Which therapy do you think was provided to you?” The available answers include “ETN therapy,” “sham ETN therapy,” or “unclear.”
Thumbtack needle is in the shape of a thumbtack. It has a short body and a circular handle buried in a piece of round medical adhesive tape (Figure 2), which was produced by conductive material. In this trial, thumbtack needles of 0.25 × 2 mm size (Zhenxing, Hangzhou, China) will be used. Acupoints of Dazhui (GV14), bilateral Wangu (GB1 2), bilateral Jiaji at C4 and C6 levels, two Ashi points, and bilateral Houxi (SI3) will be selected in accordance with the theory of traditional Chinese medicine and the consensus of experts.
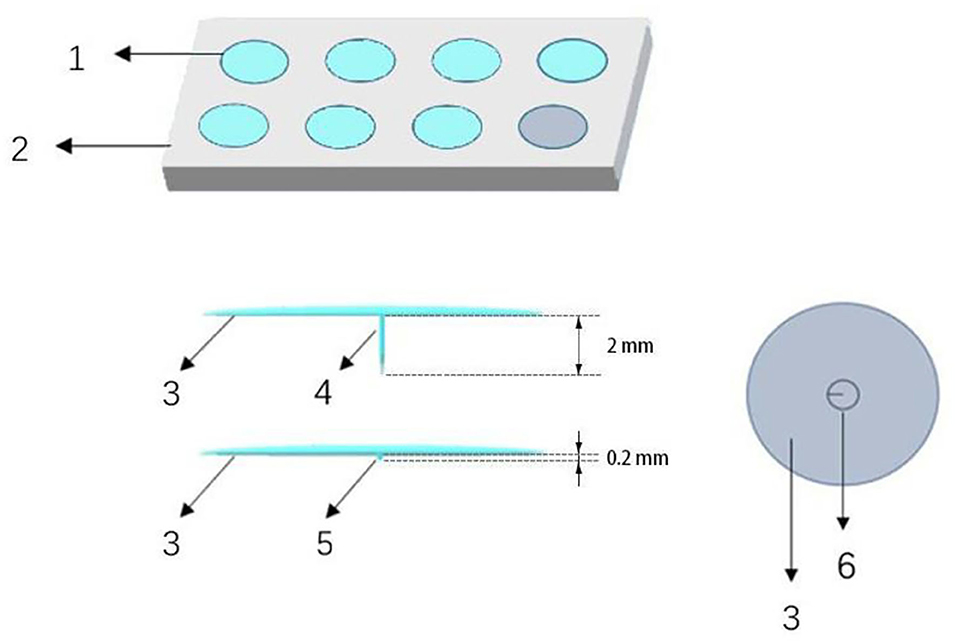
Figure 2. Electro-thumbtack needle and sham thumbtack needle. (1) Thumbtack needle or sham needle, (2) plastic plate, (3) adhesive tape, (4) needle, (5) blunt tip, (6) circular handle.
After sterilizing the skin around the acupoints, the acupuncturist will hold the handle of the needle between the thumb and forefinger, vertically insert the needle into the skin instantly, and adhere medical adhesive tape to skin. After that, the acupuncturist will setup the gel electrodes and the portable stimulation devices (Zhenxing, Hangzhou, China) (Figure 3) to the adhesive tape of all thumbtack needles, switch the mode to low-frequency and discontinuous wave, and adjust the current intensity from level 1 to an appropriate level, which the participant can tolerate. The acupuncturist will gently remove the medical adhesive tape and needles in 30 min.
Participants will receive three 30-min treatment sessions per week (ideally every other day) for 4 consecutive weeks after baseline assessment.
We will use sham thumbtack needle specially designed to mimic the true needling. The sham thumbtack needle is 0.2 mm in length and has a blunt tip (rather than sharp) at the bottom. Other than these differences, the conductive surface and other structure of sham thumbtack needle are totally the same as true needle (Figure 2). In the sham ETN group, the blunt tip of needle will stay on the surface and not penetrate the skin, which avoids treatment effect to the largest extent and confuses the participants meanwhile.
Acupoints used in the sham ETN group are the same as those in the ETN group. Acupuncturist will first sterilize the areas of acupoints and then apply the sham needles on the surface of the skin. After assembling the same gel electrodes and portable stimulation devices as in the ETN group (Figures 3, 4), the acupuncturist will adjust the current intensity to level 1 (i.e., the minimum level). The minimal stimulus will last for only 30 s, after which the device will be turned down to cutoff the electric current. The duration of each session will also be 30 min. The frequency of treatment will be all the same as that in the ETN group.
Standardized neck exercise of stretching and strengthening can help participants to relax the neck and acknowledge the appropriate posture. The exercise program consists of head retraction, neck extension, neck flexion, and rotation (Figure 5) (24). Each stretch must reach the maximum angle as possible and be hold for 5–10 s with a 10-s interval (15). When a participant is undergoing severe neck pain, he/she could conduct the exercise in clinostatism (24).
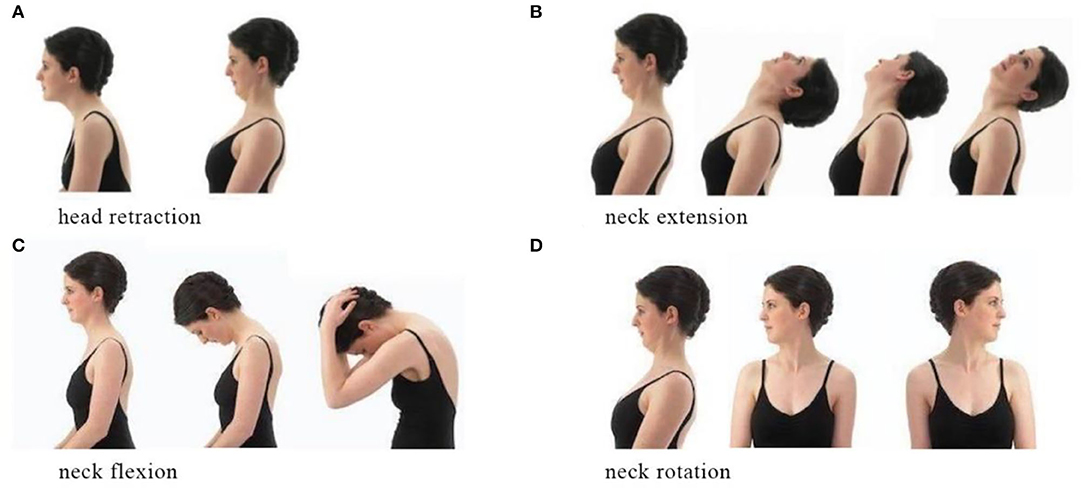
Figure 5. Neck exercise. (A) Head retarction. (B) Neck extension. (C) Neck flexion. (D) Neck rotation.
All participants will receive standardized neck exercise instruction at their first treatment session. The neck exercise will be performed at home later. A handbook with detailed illustrations to the exercise will be distributed to participants for guidance. Participants will be asked to perform the standardized exercise one time every day (20) (preferably in the morning) throughout the 4-week treatment. An exercise diary will also be distributed for supervision.
Any other therapy will be discouraged during this trial. Acetaminophen (sustained release type, 500 mg/T) will be provided as a rescue medication with the largest dose of 4 tablets per day for no more than 3 days in total in the condition of unbearable pain. Rescue medication or any other unexpected intervention will be recorded in detail. Proportion of participants and the total days taking rescue medication will be calculated and properly analyzed.
The primary outcome of this trial will be the proportion of participants with at least 50% decrease from baseline in the score of NRS-NP at the 4th week (25). The NRS-NP is a valid and reliable 11-point rating scale to assess the average intensity of neck pain during the last 3 days (26, 27). It is sensitive to small changes with a lower failure rate and can be delivered either graphically or verbally (26–28). The score of NRS-NP ranges from 0 (i.e., no pain at all) to 10 (i.e., the worst pain imaginable). Participants will be asked to circle out 1 of the 11 numbers to describe their pain. The scores of NRS-NP can be categorized into four degrees: 0 (i.e., no pain), 1–3 (i.e., mild), 4–6 (i.e., moderate), and 7–10 (i.e., severe). The reduction of 50% from baseline is regarded as golden standard of the NRS-NP to interpret significant clinically improvement (15, 28, 29).
Secondary outcomes include the proportion of participants with an at least 50% decrease from baseline in NRS-NP score at the 16th and 28th weeks; the changes from baseline in NRS-NP score at the 4th, 16th, and 28th weeks; and the proportion of participants with an at least 30% decrease from baseline in NRS-NP at the 4th, 16th, and 28th weeks. Other secondary outcomes include:
(1) Changes in the Northwick Park Neck Pain Questionnaire (NPQ) percentage score from the baseline at the 4th, 16th, and 28th weeks.
(2) The proportion of participants with an at least 25% decrease in NPQ percentage score together with a “better” or “much better” at the 4th, 16th, and 28th weeks.
The NPQ is a validated questionnaire to assess the overall symptoms of neck pain (30). It contains 9 questions, the score of each ranging from 0 to 4, on the aspects of intensity, duration, numbness, sleep disturbance, and disability in carrying, watching television, working, social life, and driving. The total score of the 9 questions will be converted to NPQ percentage score (divided by 36 for people who drive and by 32 for those who do not), which ranges from 0 to 100%, and with higher scores indicating worse condition. A 25% decrease in NPQ percentage score and a “better” or “much better” for the 5-scale global effectiveness rating are both needed (31) to reach Minimal Clinically Important Difference (MCID).
(3) Changes from the baseline in Neck Disability Index (NDI) score at the 4th, 16th, and 28th weeks.
(4) The proportion of participants with an at least 10-point decrease from the baseline in NDI score at the 4th, 16th, and 28th weeks.
The NDI is a frequently used instrument to evaluate the self-reporting functional status of patients with neck pain. It consists of 10 items concerning pain, headache, concentration, and daily obligatory activities. Each section is scored with an ordinal scale from 0 to 5, with a maximum score of 50 points (32, 33). Higher scores indicate greater functional limitation. The NDI has been translated into the simplified-Chinese version, which has been shown to have reproducibility, reliability, and validity (34). A 10-point decrease is required to achieve clinically important meaning (27, 35–37).
(5) The proportion of participants reported “much improved” or “very much improved” on Patient Global Impression of Change (PGIC) at the 4th week.
PGIC is a seven-point categorical scale to assess the participant's subjective satisfaction of the treatment (38). It is considered the most reliable instrument for trials concerning chronic pain (38–40). Participants are supposed to report their overall conditions in comparison with before the treatment using one of the following options: “very much worse,” “much worse,” “minimally worse,” “no change,” “minimally improved,” “much improved,” and “very much improved.” Those reported “much improved” or “very much improved” are considered responders of the intervention. It reflects not only the benefits of treatment but also the importance of the benefits for participants themselves (41).
(6) Expectation and belief.
Two questions will be elicited to assess the expectation of and belief in ETN, respectively. For expectation, participants will be asked “How do you think your neck pain will be in 4 weeks?” with the optional of “worse,” “unchanged,” “no idea,” “better,” and “much better.” For belief in ETN therapy, they will be asked “Do you think ETN therapy will be effective in treating chronic neck pain?” with the option of “not effective,” “little effective,” “not sure,” “effective,” and “very effective.” These questions will be elicited at the baseline.
Acupuncture-associated side effects such as bruising, dizziness, nausea, hematomas, infection, palpitations, or severe pricking pain [visual analog score (VAS) ≥ 4], and all other adverse events (AEs) irrelevant to acupuncture, will be carefully observed and recorded. Serious AEs (SAEs) will be reported to the Medical Ethics Committee of Guang'anmen Hospital within 24 h. After each session, the acupuncturist and outcome assessors will document adverse events and side effects if happens.
The outcome assessor will fill the initial data in case report form and then, input the data into an excel template for data collecting. Two inspectors will check and make sure that all initial data have been correctly input. Any deviation from protocol will be reported to ZL, who will make the final decision if we have to change the protocol or terminate the trial.
Both ETN therapy and sham ETN therapy are safe for participants recruited in our trial; therefore, no intervention modification is permitted, and no unblinding during the trial is permissible. Group allocation will be revealed after the completion of statistical analysis. Participants in the sham ETN group will receive 12 supplementary sessions of ETN therapy if they want. The follow-ups can be conveniently finished by filling in a questionnaire in the clinic, over telephone or through WeChat. This approach could probably improve adherence and make it possible for those who have to discontinue the treatment to complete follow-ups.
The sample size calculation was based on the primary outcome of the proportion of participants with an at least 50% decrease from the baseline in average neck pain measured by NRS-NP at the 4th week. According to the previous literature, the response rate of TENS plus neck exercise for 4 weeks was reported to be 50% (15); therefore, we presume a 60% response rate of ETN plus neck exercise. For sham ETN plus neck exercise, we presume the response rate to be 38%. Accordingly, a two-tailed testing with an alpha of 0.05, a desired power set at 0.8, and an allowing dropout rate of 20% resulted in 90 participants needed in each group.
Data calculation will be performed using the SPSS software Ver.20.0 under the ITT principle. Baseline data before intervention will be analyzed to see if the two groups are comparable. The continuous data will be represented as mean ± SD deviation or median [interquartile range (IQR)]. Proportions of participants having clinically meaningful improvement will be analyzed using chi-square test or Fisher's exact test. Changes in the outcome measures from the baseline will be examined by using analysis of covariance for normally distributed data to exclude the possible impact on the effect by covariates of gender and work habits. Repeated-measures analysis of variance will be used if the data are not normally distributed. The Cochran-Mantel-Haenszel test will be used to analyze categorical data, which will be represented by percentages and case. We will use linear regression to discuss the association of participants' expectations and the treatment. Missing research data will be imputed by multiple imputations. For all statistical calculation, the significance level will be two-sided and set at 0.05. A p < 0.05 will be considered to indicate statistical significance.
The objective of this study is to evaluate the efficacy and safety of ETN therapy for non-specific chronic neck pain. Demographic characteristics will be collected including age, duration, job, psychosocial or physical risk factors, level of exercise, history of injury, and history of autoimmune disease. The outcomes are designed in accordance with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations for core outcomes of chronic pain (41). Both the reduction of 30 and 50% on NRS are applied as outcome measures in this trial. Although the reduction of 30% from baseline in NRS is consistently acknowledged as MCID in current studies (15, 23, 25, 27, 28), a 50% reduction is widely used in clinical trials as a rigorous outcome measurement interpreting more significant improvement in symptoms (15, 28, 29). Additionally, multiple outcomes will be evaluated, as various as pain intensity, disability, quality of life, and the degree of subjective satisfaction.
The effectiveness of both manual acupuncture and TENS has been identified by previous studies (15, 42) in reducing chronic neck pain. Since ETN treatment has been an integration of thumbtack needling and electric stimulation, the treatment effect will probably be stronger and comprehensive. Notably, it is easy to administrate thumbtack needles on acupoints, even by patients themselves at home.
In this trial, blunt-tip thumbtack needle with no penetration and transient minimal electric stimulation is designed as sham control. It can produce acupuncture-like sensation of tingling or pain by pressing the blunt tip on acupoints without skin penetrating, similar to the validated placebo needle (43, 44). To maintain the success of blindness, participants from different groups will be avoided to contact with each other. The outcome assessor and the statistician will also be concealed to group allocation. These approaches are constructed to reduce possible biases attached to the results.
Limitations of this study are as follows. Group allocation will be not blinded to the acupuncturist, whose attitude for or against an intervention might be inevitably transferred to participants. Besides, this study does not implement a waitlist control in consideration of ethics principles, in which condition we cannot exclude the influence of spontaneous remission of disease.
This prospective trial will evaluate the efficacy and safety of ETN for chronic neck pain and provide evidence for clinical health recommendations.
The studies involving human participants were reviewed and approved by the Research Ethical Committee of Guang'anmen Hospital, China Academy of Chinese Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
ZL, HS, and XW designed this trial and drafted the manuscript over discussion. The study protocol was revised by YY, LZ, YC, and SG. HS and XW contributed to the ethical application and the implementation of the trial. All authors approved the publication of this final manuscript.
This study is funded by Guang'anmen Hospital, China Academy of Chinese Medical Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank everyone who participated in this study, including the participants, statisticians, orthopedists, acupuncturists, assessors, and the Research Ethical Committee of Guang'anmen Hospital, China Academy of Chinese Medical Sciences. The design of this study was supported by Dr. Yuanjie Sun, who provided the guidance and advice on the design and registration of this trial.
1. Blanpied PR, Gross AR, Elliott JM, Devaney LL, Clewley D, Walton DM, et al. Neck pain guidelines: revision 2017: using the evidence to guide physical therapist practice. J Orthop Sports Phys Ther. (2017) 47:511–2. doi: 10.2519/jospt.2017.0507
2. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2163–96. doi: 10.1016/S0140-6736(12)61729-2
3. Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. (2006) 15:834–48. doi: 10.1007/s00586-004-0864-4
4. Hoy DG, Protani M, De R, Buchbinder R. The epidemiology of neck pain. Best Pract Res Clin Rheumatol. (2010) 24:783–92. doi: 10.1016/j.berh.2011.01.019
5. Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. BMJ. (2017) 358:j3221. doi: 10.1136/bmj.j3221
6. Côté P, Wong JJ, Sutton D, Shearer HM, Mior S, Randhawa K, et al. Management of neck pain and associated disorders: a clinical practice guideline from the Ontario protocol for traffic injury management (OPTIMa) collaboration. Eur Spine J. (2016) 25:2000–22. doi: 10.1007/s00586-016-4467-7
7. Childs JD, Cleland JA, Elliott JM, Teyhen DS, Wainner RS, Whitman JM, et al. Neck pain: Clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopedic section of the American physical therapy association. J Orthop Sports Phys Ther. (2008) 38:A1–A34. doi: 10.2519/jospt.2008.0303
8. Hurwitz EL, Carragee EJ, van der Velde G, Carroll LJ, Nordin M, Guzman J, et al. Treatment of neck pain: noninvasive interventions: results of the bone and joint decade 2000-2010 task force on neck pain and its associated disorders. Spine. (2008) 33(4 Suppl):S123–52. doi: 10.1097/BRS.0b013e3181644b1d
9. Yuan Q-L, Wang P, Liu L, Sun F, Cai Y-S, Wu W-T, et al. Acupuncture for musculoskeletal pain: a meta-analysis and meta-regression of sham-controlled randomized clinical trials. Sci Rep. (2016) 6:30675. doi: 10.1038/srep30675
10. He D, Bo Veiersted K, Høstmark AT, Ingulf Medbø J. Effect of acupuncture treatment on chronic neck and shoulder pain in sedentary female workers: a 6-month and 3-year follow-up study. Pain. (2004) 109:299–307. doi: 10.1016/j.pain.2004.01.018
11. Vas J, Perea-Milla E, Méndez C, Sánchez Navarro C, León Rubio JM, Brioso M, et al. Efficacy and safety of acupuncture for chronic uncomplicated neck pain: a randomised controlled study. Pain. (2006) 126:245–55. doi: 10.1016/j.pain.2006.07.002
12. Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. (2014) 120:482–503. doi: 10.1097/ALN.0000000000000101
13. Seo SY, Lee K-B, Shin J-S, Lee J, Kim M-R, Ha I-H, et al. Effectiveness of acupuncture and electroacupuncture for chronic neck pain: a systematic review and meta-analysis. Am J Chin Med. (2017) 45:1573–95. doi: 10.1142/S0192415X17500859
14. Martimbianco ALC, Porfírio GJ, Pacheco RL, Torloni MR, Riera R. Transcutaneous electrical nerve stimulation (TENS) for chronic neck pain. Cochrane Database Syst Rev. (2019) 12:CD011927. doi: 10.1002/14651858.CD011927.pub2
15. Chan DKC, Johnson MI, Sun KO, Doble SJ, Jenkins S. Electrical acustimulation of the wrist for chronic neck pain: a randomized, sham-controlled trial using a wrist-ankle acustimulation device. Clin J Pain. (2009) 25:320–6. doi: 10.1097/AJP.0b013e318192ce39
16. Ardiç F, Sarhus M, Topuz O. Comparison of two different techniques of electrotherapy on myofascial pain. J Back Musculoskelet Rehabil. (2002) 16:11–6. doi: 10.3233/BMR-2002-16103
17. Maayah M, Al-Jarrah M. Evaluation of transcutaneous electrical nerve stimulation as a treatment of neck pain due to musculoskeletal disorders. J Clin Med Res. (2010) 2:127–36. doi: 10.4021/jocmr2010.06.370e
18. Chiu TTW, Hui-Chan CWY, Chein G. A randomized clinical trial of TENS and exercise for patients with chronic neck pain. Clin Rehabil. (2005) 19:850–60. doi: 10.1191/0269215505cr920oa
19. Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, KrleŽa-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
20. Bronfort G, Evans R, Anderson AV, Svendsen KH, Bracha Y, Grimm RH. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. (2012) 156 (1 Pt. 1):1–10. doi: 10.7326/0003-4819-156-1-201201030-00002
21. White P, Lewith G, Prescott P, Conway J. Acupuncture versus placebo for the treatment of chronic mechanical neck pain: a randomized, controlled trial. Ann Intern Med. (2004) 141:911–9. doi: 10.7326/0003-4819-141-12-200412210-00007
22. Lee S, Nam D, Leem J, Han G, Lee S, Lee J. Efficacy and safety of myofascial-meridian release acupuncture (MMRA) for chronic neck pain: a study protocol for randomized, patient- and assessor-blinded, sham controlled trial. BMC Complement Altern Med. (2016) 16:45. doi: 10.1186/s12906-016-1027-y
23. Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. (2008) 30:974–85. doi: 10.1016/j.clinthera.2008.05.011
24. McKenzie R. Treat Your Own Neck. 3rd ed. Raumati Beach: Spinal Publications New Zealand Limited (2010). p. 50–64.
25. Malfliet A, Kregel J, Coppieters I, de Pauw R, Meeus M, Roussel N, et al. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain: a randomized clinical trial. JAMA Neurol. (2018) 75:808–17. doi: 10.1001/jamaneurol.2018.0492
26. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. (2005) 14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x
27. Pool JJM, Ostelo RWJG, Hoving JL, Bouter LM, Vet HCW. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine. (2007) 32:3047–51. doi: 10.1097/BRS.0b013e31815cf75b
28. Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. (2001) 94:149–58. doi: 10.1016/S0304-3959(01)00349-9
29. Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain. (1997) 69:311–5. doi: 10.1016/S0304-3959(96)03306-4
30. Leak AM, Cooper J, Dyer S, Williams KA, Turner-Stokes L, Frank AO. The northwick park neck pain questionnaire, devised to measure neck pain and disability. Br J Rheumatol. (1994) 33:469–74. doi: 10.1093/rheumatology/33.5.469
31. Sim J, Jordan K, Lewis M, Hill J, Hay EM, Dziedzic K. Sensitivity to change and internal consistency of the northwick park neck pain questionnaire and derivation of a minimal clinically important difference. Clin J Pain. (2006) 22:820–6. doi: 10.1097/01.ajp.0000210937.58439.39
32. Vernon H. The neck disability index. J Musculoskel Pain. (1996) 4:95–104. doi: 10.1300/J094v04n04_09
33. Vernon H. The neck disability index: state-of-the-art, 1991-2008. J Manipulative Physiol Ther. (2008) 31:491–502. doi: 10.1016/j.jmpt.2008.08.006
34. Lim HHR, Tang ZY, Hashim MABM, Yang M, Koh EYL, Koh KH. Cross-cultural adaptation, reliability, validity, and responsiveness of the simplified-chinese version of neck disability index. Spine. (2020) 45:541–8. doi: 10.1097/BRS.0000000000003325
35. Cerezo-Téllez E, Torres-Lacomba M, Fuentes-Gallardo I, Perez-Muñoz M, Mayoral-Del-Moral O, Lluch-Girbés E, et al. Effectiveness of dry needling for chronic nonspecific neck pain: a randomized, single-blinded, clinical trial. Pain. (2016) 157:1905–17. doi: 10.1097/j.pain.0000000000000591
36. Cleland JA, Fritz JM, Whitman JM, Palmer JA. The reliability and construct validity of the neck disability index and patient specific functional scale in patients with cervical radiculopathy. Spine. (2006) 31:598–602. doi: 10.1097/01.brs.0000201241.90914.22
37. Young BA, Walker MJ, Strunce JB, Boyles RE, Whitman JM, Childs JD. Responsiveness of the neck disability index in patients with mechanical neck disorders. Spine J. (2009) 9:802–8. doi: 10.1016/j.spinee.2009.06.002
38. Perrot S, Lantéri-Minet M. Patients' global impression of change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain. (2019) 23:1117–28. doi: 10.1002/ejp.1378
39. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Euro J Pain. (2004) 8:283–91. doi: 10.1016/j.ejpain.2003.09.004
40. Geisser ME, Clauw DJ, Strand V, Gendreau MR, Palmer R, Williams DA. Contributions of change in clinical status parameters to patient global impression of change (PGIC) scores among persons with fibromyalgia treated with milnacipran. Pain. (2010) 149:373–8. doi: 10.1016/j.pain.2010.02.043
41. Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. (2003) 106:337–45. doi: 10.1016/j.pain.2003.08.001
42. MacPherson H, Tilbrook H, Richmond S, Woodman J, Ballard K, Atkin K, et al. Alexander technique lessons or acupuncture sessions for persons with chronic neck pain: a randomized trial. Ann Intern Med. (2015) 163:653–62. doi: 10.7326/M15-0667
43. Qin Z, Zang Z, Wu J, Zhou J, Liu Z. Efficacy of acupuncture for chronic prostatitis/chronic pelvic pain syndromes: study protocol for a randomized, sham acupuncture-controlled trial. BMC Complement Altern Med. (2016) 16:440. doi: 10.1186/s12906-016-1428-y
Keywords: acupuncture, chronic neck pain, RCT, electro-thumbtack needle, efficacy and safety
Citation: Shi H, Wang X, Yan Y, Zhu L, Chen Y, Gao S and Liu Z (2022) Efficacy and Safety of Electro-Thumbtack Needle Therapy for Patients With Chronic Neck Pain: Protocol for a Randomized, Sham-Controlled Trial. Front. Med. 9:872362. doi: 10.3389/fmed.2022.872362
Received: 09 February 2022; Accepted: 21 March 2022;
Published: 29 April 2022.
Edited by:
Zheng-jie Li, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Ling Zhao, Chengdu University of Traditional Chinese Medicine, ChinaCopyright © 2022 Shi, Wang, Yan, Zhu, Chen, Gao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhishun Liu, emhpc2h1bmpvdXJuYWxAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.