- 1Department of Internal Medicine, Tainan Hospital, Ministry of Health and Welfare, Tainan City, Taiwan
- 2Department of Internal Medicine, College of Medicine, National Cheng Kung University Hospital, National Cheng Kung University, Tainan City, Taiwan
- 3Department of Medicine, College of Medicine, National Cheng Kung University, Tainan City, Taiwan
- 4Clinical Medicine Research Centre, College of Medicine, National Cheng Kung University Hospital, National Cheng Kung University, Tainan City, Taiwan
Introduction: Bloodstream infections are associated with high mortality rates and contribute substantially to healthcare costs, but a consensus on the prognostic benefits of appropriate empirical antimicrobial therapy (EAT) for bacteraemia is lacking.
Methods: We performed a systematic search of the PubMed, Cochrane Library, and Embase databases through July 2021. Studies comparing the mortality rates of patients receiving appropriate and inappropriate EAT were considered eligible. The quality of the included studies was assessed using Joanna Briggs Institute checklists.
Results: We ultimately assessed 198 studies of 89,962 total patients. The pooled odds ratio (OR) for the prognostic impacts of inappropriate EAT was 2.06 (P < 0.001), and the funnel plot was symmetrically distributed. Among subgroups without between-study heterogeneity (I2 = 0%), those of patients with severe sepsis and septic shock (OR, 2.14), Pitt bacteraemia scores of ≥4 (OR, 1.88), cirrhosis (OR, 2.56), older age (OR, 1.78), and community-onset/acquired Enterobacteriaceae bacteraemia infection (OR, 2.53) indicated a significant effect of inappropriate EAT on mortality. The pooled adjusted OR of 125 studies using multivariable analyses for the effects of inappropriate EAT on mortality was 2.02 (P < 0.001), and the subgroups with low heterogeneity (I2 < 25%) exhibiting significant effects of inappropriate EAT were those of patients with vascular catheter infections (adjusted OR, 2.40), pneumonia (adjusted OR, 2.72), or Enterobacteriaceae bacteraemia (adjusted OR, 4.35). Notably, the pooled univariable and multivariable analyses were consistent in revealing the negligible impacts of inappropriate EAT on the subgroups of patients with urinary tract infections and Enterobacter bacteraemia.
Conclusion: Although the current evidence is insufficient to demonstrate the benefits of prompt EAT in specific bacteraemic populations, we indicated that inappropriate EAT is associated with unfavorable mortality outcomes overall and in numerous subgroups. Prospective studies designed to test these specific populations are needed to ensure reliable conclusions.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42021270274.
Introduction
Bacteraemia is associated with a high mortality rate and contribute substantially to healthcare costs (1). Antibiotic therapy, both empirical and definitive, is the mainstay of treatment for such systemic infections. Although studies have extensively researched the association between the appropriate administration of empirical antimicrobial therapy (EAT) and short-term mortality outcomes in sepsis (2), the potential benefits of appropriate EAT in bloodstream infections remain unknown. Numerous studies have reported EAT to have trivial effects (3–6), whereas others have reported a beneficial fatality rate reduction (7–10). We believe that these variations in results are due to differences among studies in the bacteraemia severity, host immunity status, target patient populations, causative microorganisms, and bacteraemia source. Therefore, we conducted a systematic review and meta-analysis to assess the effects of appropriate EAT on mortality in specific clinical scenarios, and such an assessment is essential for the optimal antimicrobial stewardship.
Methods
Study Selection
Study Design
This analysis followed the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11), and our protocol was registered at the International Prospective Register of Systematic Reviews under the registration number CRD42021270274. We considered case–control studies, cohort studies, and clinical trials on adult patients with bacteraemia, without restrictions regarding onset location, causative microorganisms, antimicrobial-resistant microorganisms, sources of bacteraemia, illness severity, or comorbidities. Reviews, guideline articles, case reports, duplicate studies, studies with patient overlap, and studies on patients without bacteraemia were excluded. In the full-text assessment of eligible articles, articles were excluded if they (i) included participants aged <18 years, (ii) did not assess EAT or distinguish it from definitive treatment, (iii) did not assess the EAT–mortality association, (iv) did not define EAT, or (v) were published in a language other than English.
Patients
The patients included were adults with microbiologically documented bloodstream infections.
Intervention
The intervention was inappropriate (vs. appropriate) administration of empirical antimicrobials. An antimicrobial in vitro active against the causative microorganism was regarded as “appropriate” treatment. “Empirical” therapy was defined as that administered at initial sampling of blood cultures, within 24, 48, and 72 h, or 5 days after culture sampling, or prior to culture result.
Outcome
The primary outcomes in this meta-analysis included short-term (i.e., 7-day, 14-day, 21-day, 28- or 30-day), in-hospital, and long-term mortality.
Literature Search
The PubMed, Cochrane Library, and Embase databases were searched from their inception to July 2021 for articles on appropriate or inappropriate antimicrobial administration in adults with bacteraemia. The following terms for searches were applied: (antibiotic OR antimicrobial) AND (inappropriate OR appropriate) AND (empirical or initial) AND (bacteremia OR bacteraemia OR bloodstream) AND (mortality OR fatality OR death OR dead OR alive OR survival) NOT (children OR neonate OR adolescent OR infant OR pediatric). Details regarding the search strategy are presented in Supplementary Table 1. Additional studies were identified by perusing reference lists of systematic reviews.
Study Selection, Data Extraction, and Quality Assessment
Firstly, the results of the literature searches were screened based on study eligibility criteria and discrepancies were periodically resolved by consensus in the team conference. Focusing on the included studies, the extracted data included the study design, types and severity of patient comorbidities, sources of bacteraemia, causative microorganism, the type and proportion of antibiotic-resistant isolates, inclusion and exclusion criteria, EAT definition, the percentage of patients receiving appropriate EAT, mortality, results of unadjusted and adjusted analyses, and covariates adjusted for in multivariable analyses.
Because none of randomized clinical trials or studies was recognized in our systematic review, the quality of the included studies was evaluated using the Newcastle-Ottawa Quality (NOQ) assessment for all the included cohort or case-control studies (12). This assessment includes the appropriateness of the cohort selection and comparison between case and control groups, outcome evaluation, and patient follow-up. The maximum score of the NOQ assessment is 9 (the highest quality), and the scores of 7–9, 4–6, and 0–3 were regarded as the high, moderate, and low quality of studies, respectively (12).
Two investigators (C.-C.L. and Y.-P. H.) independently screened the search results according to exclusion criteria, recorded the clinical information, and assessed study quality from each study; and a third investigator (W.-C.K.) was consulted to resolve any disagreements during periodic meetings.
Data Synthesis and Analysis
Unadjusted Analysis
We computed the numbers of assessed and fatal patients for individual studies and pooled them for the meta-analysis. We investigated heterogeneity through subgroup analyses based on the following: infection acquisition (community-onset/acquired, hospital-onset/acquired, or healthcare-associated), comorbidity types (liver cirrhosis or haemato-oncological), age (≥65 years), neutropenia status, bacteraemia sources (vascular catheter, pneumonia, or biliary tract, or urinary tract), bacteraemia severity (intensive care unit [ICU] admission, severe sepsis and septic shock, Pitt bacteraemia score of ≥4 at onset, or non-ICU admission), microbial groups (Enterobacteriaceae or glucose non-fermentative rods), specific microorganisms (Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., Pseudomonas spp., or Acinetobacter spp.), and antibiotic-resistant microorganisms (methicillin-resistant S. aureus, extended-spectrum beta-lactamase [ESBL]-producing Enterobacteriaceae, multidrug-resistant [MDR] Enterobacteriaceae, or carbapenem-resistant Enterobacteriaceae). Moreover, because of the different EAT cut-offs and mortality assessments of each study, the subgroup analyses included subgroups of various EAT delays (0, 24, 48, and 72 h, 5 days, or prior to culture results) and different (7-, 14-, 21-, 28-, or 30-day; in-hospital; or long-term) mortality measures after the initial culture sampling.
Adjusted Analysis
Of the 198 studies initially included, 73 did not report an adjusted analysis and were therefore excluded because we could not input adjusted odds ratios (ORs) for our analyses. Among the 125 studies that used multivariate analyses, 16 reported nonsignificant results but no numerical data; another reported a significant multivariable result but no numerical data; and the remaining 108 studies provided their multivariate results in terms of adjusted ORs and 96% confidence intervals (CIs). For these 108 studies, the adjusted ORs for mortality of inappropriate EAT were inputted for 84 studies that directly reported the effects of inappropriate EAT. For the remaining 24 studies that only demonstrated the effect of appropriate EAT on mortality, the adjusted ORs and 95% CIs were inversely inputted. Moreover, for the 16 studies that reported nonsignificant effects of inappropriate EAT without providing any numerical data, an adjusted OR of 1 and the 95% CI from the univariable analysis were inputted as a dispersion measure. For one study reporting a significant effect of inappropriate EAT but no numerical data from multivariable analysis, the OR and 95% CI were inputted from univariable analyses. Accordingly, our main adjusted analyses included all studies that assessed the effects of inappropriate EAT on mortality through multivariable regression. As in the univariable analysis, further subgroup analyses were performed to minimize the effects of between-study heterogeneity.
Statistical Methods
Consistent with the previously established methods (13, 14), irrespective of unadjusted and adjusted ORs, the meta-analysis was conducted to recognize the pooled effects of inappropriate ETA on patient outcomes, and adopted a random-effects model under the assumption that the considerably heterogeneity in study results is due to the diverse study populations and multivariate regression models used for adjusting for confounding factors. Between-study heterogeneity and consistency were assessed using Cochran's Q test and I2; we aimed to eliminate this heterogeneity through the subgroup analyses. To assess the effect of small-population studies, a funnel plot of standard errors against ORs was constructed for the univariate and multivariate results of all included studies. All analyses were performed using Review Manager (version 5.3).
Results
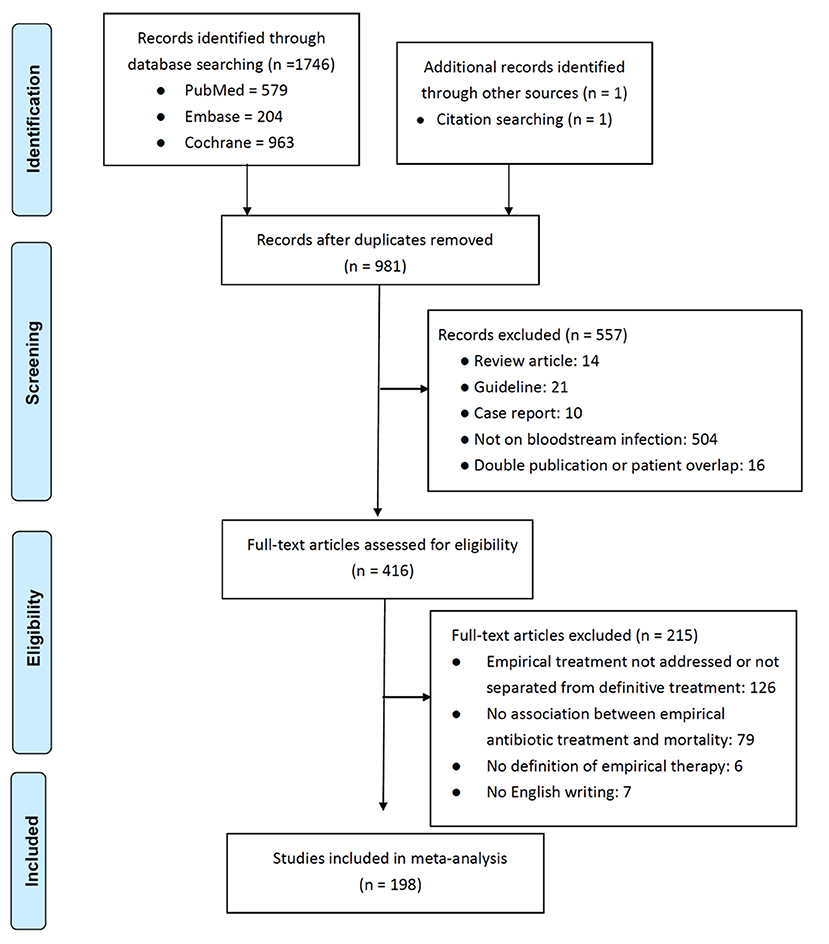
In total, 198 studies (3–10, 15–204), were selected from 981 potentially relevant studies on the basis of the inclusion and exclusion criteria (Figure 1). Total 89,962 patients were assessed; the majority (n = 64,008, 71.2%) received appropriate EAT, and the overall mortality rate was 21.3% (n = 19,181). The publication year, geographic location, design, acquisition sources, bacteraemia sources, target patient population, causative microorganisms, mortality rate, numbers of patients receiving appropriate EAT, ORs and adjusted ORs of inappropriate EAT, and study quality of each study are presented in Supplementary Table 2. The majority (190 studies) of the included studies were cohort studies and the remaining eight studies were case–control studies, which had a median (interquartile range [IQR]) NOQ score of 7 (6–7).
Univariate (Unadjusted) Analysis for Mortality
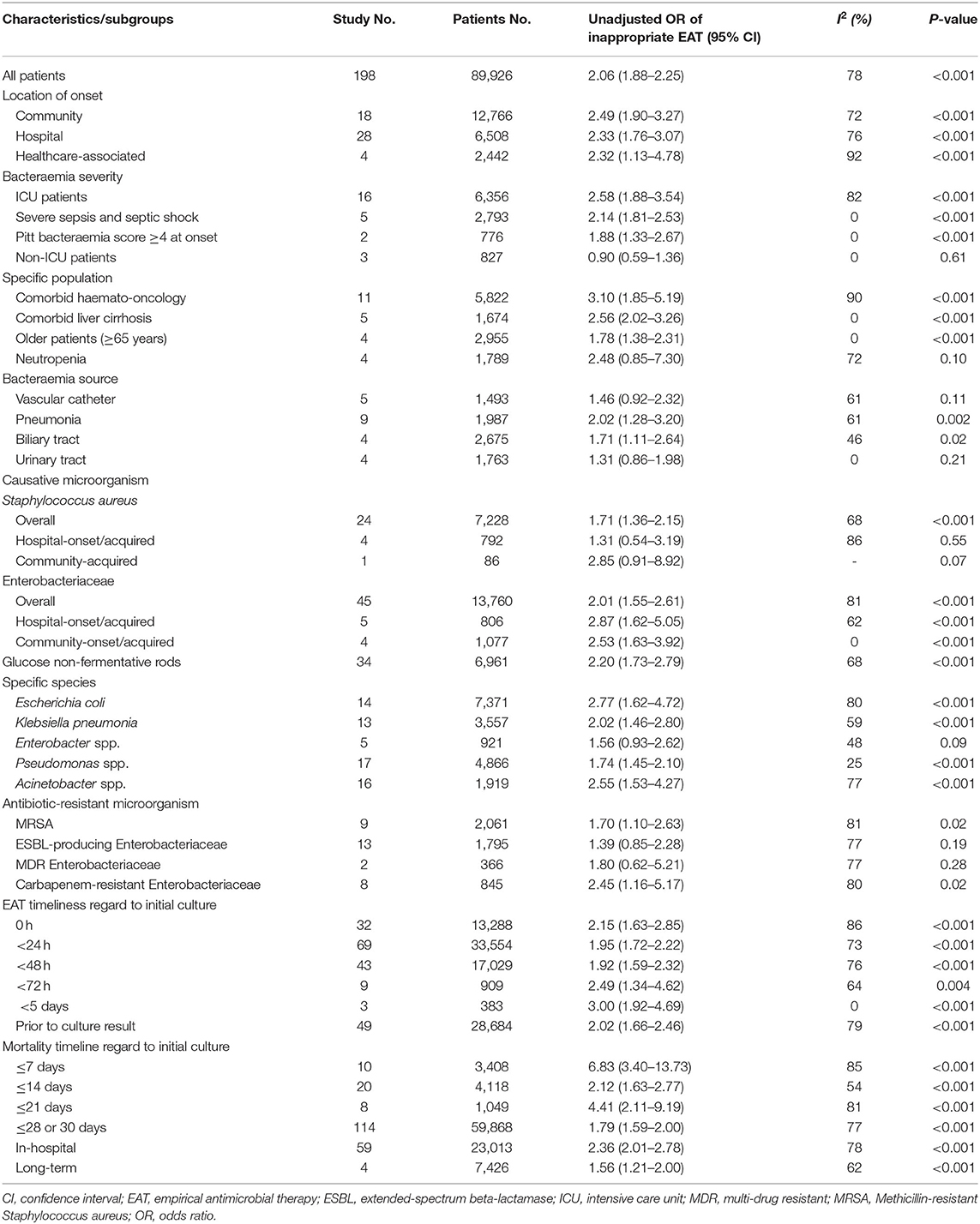
For the 89,962 patients assessed in 198 studies, the pooled OR for prognostic impacts of inappropriate EAT was 2.06 (95% CI, 1.88–2.25; P < 0.001), as shown in Supplementary Figure 1A; and these studies were symmetrically distributed around the pooled OR in the funnel plot (Supplementary Figure 1B). Because considerable between-study heterogeneity was observed (P < 0.001, I2 = 72%), subgroup meta-analyses based on the acquisition sources, bacteraemia sources, target patient populations, causative microorganisms, differential timeline cutoffs (after initial sampling of blood cultures) for EAT definition, and varied timeline cutoffs assessed for mortality outcomes were conducted (Table 1). The detailed forest plots for these subgroup analyses are presented in Supplementary Figure 2. The effects of inappropriate EAT remained significant in nearly all subgroups (Table 1); however, the between-study heterogeneity remained significant in most of the subgroups. Among the eight subgroups without between-study heterogeneity (I2 = 0%), inappropriate EAT was significantly associated with mortality in the following: patients experiencing severe sepsis and septic shock (OR, 2.14; 95% CI, 1.81–2.53), patients with Pitt bacteraemia scores of ≥4 (OR, 1.88; 95% CI, 1.33–2.67), patients with cirrhosis (OR, 2.56; 95% CI, 2.02–3.26), patients aged >65 years (OR, 1.78; 95% CI, 1.38–2.31), patients with community-onset/acquired Enterobacteriaceae bacteraemia (OR, 2.53; 95% CI, 1.63–3.92), and studies with an EAT assessment period of <5 days (OR, 3.00; 95% CI, 1.92–4.69). Conversely, the effect of inappropriate EAT on mortality was negative among non–ICU patients (OR, 0.90; 95% CI, 0.59–1.36) and patients with urinary tract infection–induced bacteraemia (OR, 1.31; 95% CI, 0.86–1.98). Notably, between-study heterogeneity remained significant in each subgroup detailing the varied assessment periods for EAT or mortality, but the association of inappropriate EAT with mortality was consistently significant.
Multivariate (Adjusted) Analysis for Mortality
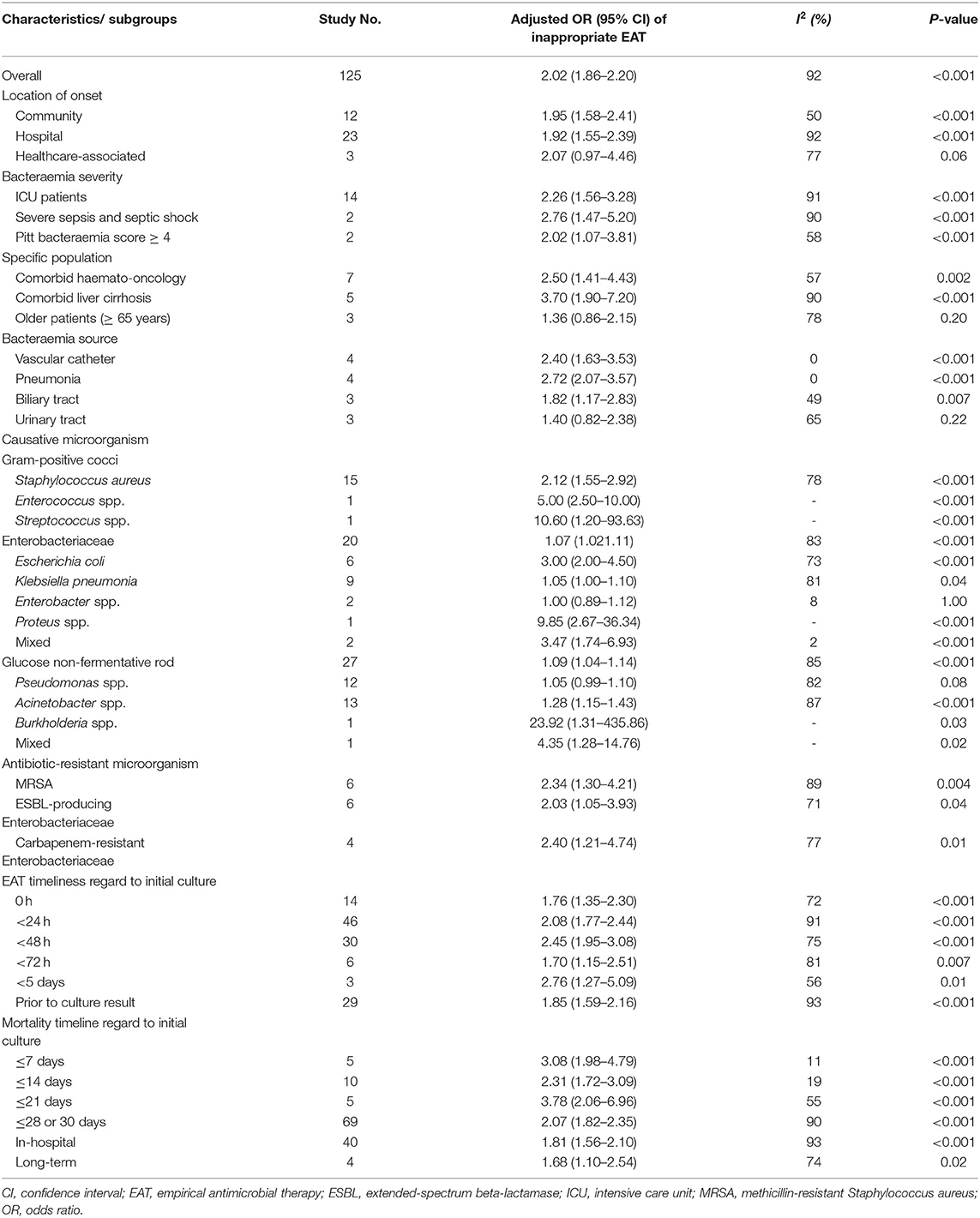
Of the 125 studies reporting adjusted multivariable results for mortality risk, the pooled adjusted OR for prognostic impacts of inappropriate EAT was 2.02 (95% CI, 1.86–2.49), with an asymmetrical distribution of studies around the pooled OR in the funnel plot (Supplementary Figure 3). Because considerable heterogeneity was observed (I2 = 92%, P < 0.001), we conducted further subgroup analyses (Table 2). The forest plots of these subgroup analyses are presented in Supplementary Figure 4. The effects of inappropriate EAT remained significant in nearly all subgroups, and the between-study heterogeneity remained considerable in most subgroups. Among the subgroups without heterogeneity, inappropriate EAT remained significant impacts in the subgroups of patients acquiring bacteraemia from a vascular catheter (adjusted OR, 2.40; 95% CI, 1.63–3.53) or pneumonia (adjusted OR, 2.72; 95% CI, 2.07–3.57), those with mixed Enterobacteriaceae bacteraemia (adjusted OR, 4.35; 95% CI, 1.28–14.76), and those in studies measuring mortality within 7 (adjusted OR, 3.08; 95% CI, 1.98–4.79) or 14 (adjusted OR, 2.31; 95% CI, 1.72–3.09) days after the initial culture sampling. However, the prognostic impact of inappropriate EAT was nonsignificant in patients with Enterobacter bacteraemia (OR, 1.00; 95% CI, 0.89–1.12). As in the univariable analyses, considerable heterogeneity remined in all subgroups with different assessment periods of EAT and mortality outcomes, and the predictive ability of inappropriate EAT was significant in all these subgroups.
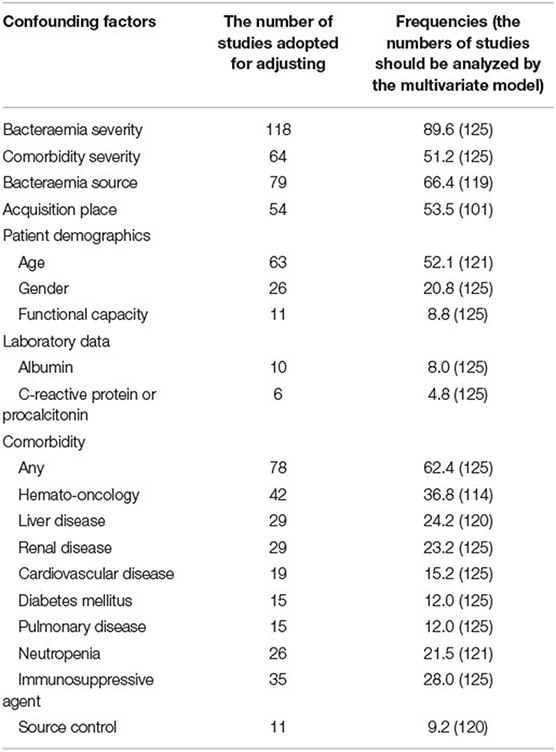
Confounding factors for mortality outcomes for potential enrolment in multivariable analyses were calculated in each individual study (Table 3). Nearly all studies (89.6%) assessed bacteraemia severity, using scores such as the Acute Physiology Age and Chronic Health Evaluation; Acute Physiology Score; Sepsis-related (Sequential) Organ Failure Assessment score; and Pitt bacteraemia score. Formal scores for comorbidity severity (i.e., Charlson comorbidity index or McCabe classification) were used in approximately 50% of the studies. Other confounding factors assessed in more than half of the studies were the bacteraemia source, acquisition source, patient age, and the presence of any comorbidity. The median (IQR) number of confounding factors included in the multivariable models was 5 (4–7), and the median (IQR) of the ratio of confounding factors to deaths was 13.0 (7.6–28.8).
Non-specific Comorbid Patients With Overall Bacteraemia
We evaluated the prognostic effect of inappropriate EAT in 14 bacteraemia studies of patients with nonspecific comorbidities and bacteraemia without the specific causative microorganism and specific acquisition source. Both the univariable (Figure 2A) and multivariable (Figure 2B) analyses revealed significant adverse effects of inappropriate EAT, with an OR of 2.31 and adjusted OR of 1.78 for mortality; the between-study heterogeneity remained considerable (I2 = 91% and 71%, respectively).
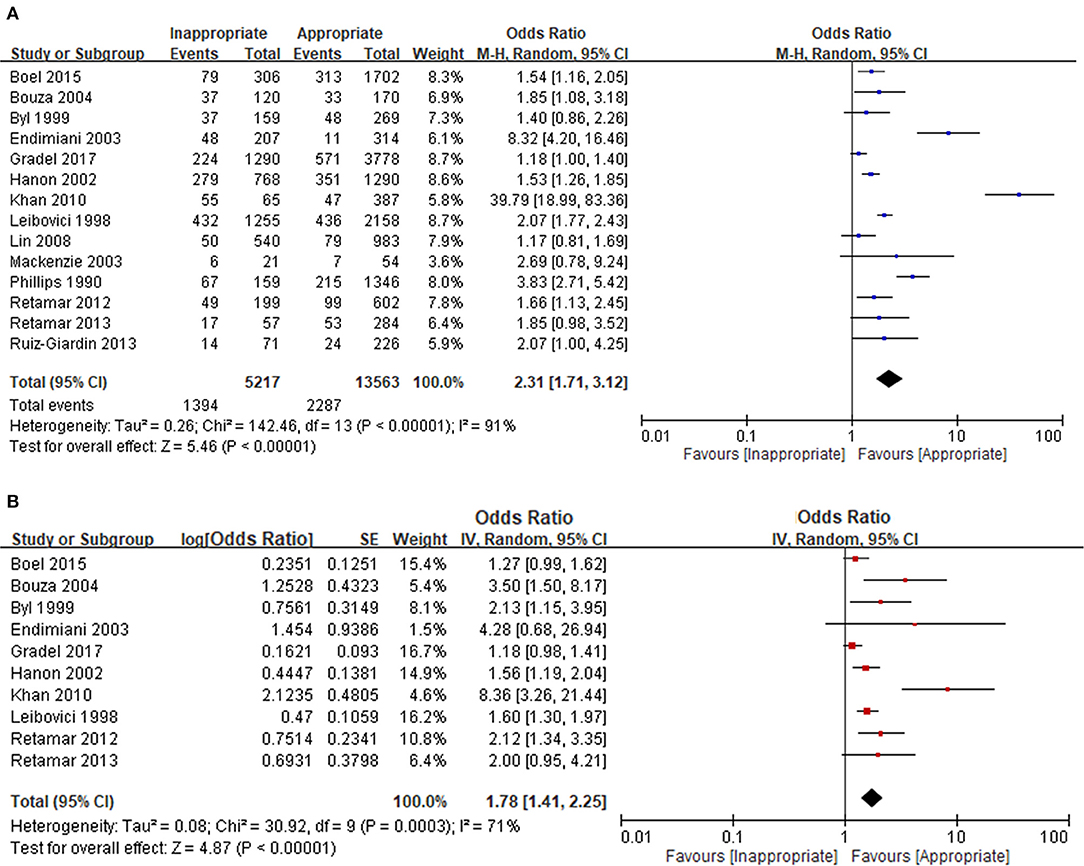
Figure 2. Unadjusted (A) and adjusted (B) analyses in studies dealing with overall bacteraemia. *Only included non-specific comorbid patients with bacteraemia without the unique focus, causative microorganisms, and acquisition place.
Discussion
Bacteraemia ia an common and complex disease with mortality rates widely ranged from 1.2% (122) to 90% (63), depending on host's immune status or comorbidities, severity of illness at onset, and bacteraemia sources. Of numerous studies previously reported the prognostic effect of inappropriate EAT, the definition of “empirical” administration and mortality assessed for study outcomes were not consistent. To diminish the publication bias, the study adopted by any reasonable definition of “empirical” administration and “short- or long-term outcomes” was comprehensively enrolled in our analyses. Despite the existence of considerable between-study heterogeneity herein, the prognostic impacts of inappropriate EAT remained significant in all the subgroups categorized by different cutoff timelines of EAT or mortality. Furthermore, irrespectively of whether through univariable or multivariable analyses, the pooled effect of inappropriate EAT was significant in all included patients and its impact was nearly all evidenced in patients sub-grouped by different acquisition places, bacteraemia severity, bacteraemia sources, aimed patient populations, and causative microorganisms. Accordingly, the prognostic disadvantage of delayed administration of appropriate antimicrobials was emphasized in our analyses.
A pooled analysis of the univariable results revealed the negligible impact of inappropriate EAT on four subgroups, namely the bacteraemia source of urinary tract infections (four studies), neutropenia individuals (four), MDR-Enterobacteriaceae bacteraemia (two), and non–ICU patients (three). However, other than for studies dealing with urinary tract infections, multivariable analyses were not performed within these studies, because the majority (8/9, 88.9%) revealed similar mortality rates for patients who did not receive appropriate EAT and those who received through the univariate analyses. Currently in literature search, few studies have evaluated the prognostic effect of inappropriate EAT on these subgroups; we believe this is because the nonsignificant effects limit their publications. Although the current evidence is insufficient to highlight the prognostic disadvantage of inappropriate EAT, further studies focusing on these specific populations are warranted.
The pooled results of the univariable and multivariable analyses consistently indicated that inappropriate EAT significantly impacted the prognoses of nearly all subgroup patients. Moreover, the pooled univariable and multivariable analyses consistently revealed negligible impacts of inappropriate EAT on two subgroups: the bacteraemia source of urinary tract infections and Enterobacter bacteraemia. However, the pooled results of the univariable and multivariable analyses differed for the prognostic effects in several subgroups herein, including those of healthcare-associated acquisition, older patients, and bacteraemia caused by vascular catheter infections or ESBL-producing Enterobacteriaceae. In such the situation, we believe that the multivariable analysis is necessary to clarify the independent effectiveness of antimicrobial therapy. Taking the ESBL-producer as an example, its crucial association with vascular catheter infections (205), severe comorbid patients (205), older patients (41), or healthcare-associated bacteraemia (205) has been established. Moreover, the association of ESBL-producers and delayed EAT or unfavorable prognoses had been evidenced (19, 111, 144, 145). Consequently, to diminish the ESBL-producer, a crucial confounding factor, affecting the prognostic effects of inappropriate EAT, adjustments for the above parameters were essential. Accordingly, based on the pooled result of multivariable analyses herein, the prognostic effect of delayed EAT was trivial in patients with healthcare-associated bacteraemia or the older patients experiencing bacteraemia and significant in those with bacteraemia caused by vascular catheter infections or ESBL-producing Enterobacteriaceae.
This meta-analysis has several limitations. First, because of ethical concerns with testing the negative effects of inappropriate EAT on mortality, randomized clinical trials comparing the outcomes of appropriate and inappropriate EAT are limited in the literature. Therefore, consistent with that in previous meta-analyses of appropriate EAT administration in patients with sepsis (206–208), between-study heterogeneity might arise from nonrandomised studies herein. Second, in accordance with previous methods (206), we used reasonable assumptions to comprehensively capture the multivariate result of each study to minimize publication bias, such as the inverse input of adjusted ORs and 95% CIs for included studies reporting only the prognostic effects of appropriate EAT. However, the publication bias in the adjusted analyses remained greater than that in the unadjusted analyses. We believe that this bias was partially caused by the lack of included studies that found no significant impact of inappropriate EAT and those that reported their multivariate results only qualitatively, resulting in their true values of adjusted ORs being unavailable for our collection. Moreover, because of the publication bias, another leading limitation of the present study is the prognostic benefits of appropriate EAT only 293 disclosed in specific bacteraemic populations. Third, to avoid confusing the reader with a “massive” meta-analysis, only studies with mortality as the assessed outcome were included in our analyses. Therefore, information detailing the impacts of inappropriate EAT on the economic outcome, microbiological clearance rate, and hospitalization length was not presented herein.
Although current evidence cannot sufficiently support the disadvantage of inappropriate EAT in specific populations with bacteraemia, such as elder patients, non–ICU patients, causative Enterobacter species, and the source of urinary tract infections, this review and meta-analysis of contemporaneous literature demonstrates that inappropriate EAT is associated with unfavorable mortality outcomes overall and in most patient subgroups. Our findings underscore the necessity of precision medicine for the rapid diagnosis and for “treating the right patient with the right drug at the right time.”
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
C-CL executed the main database searches and helped to extract data from individual studies by using prespecified methods determined by all study authors and drafted this manuscript. C-CL and Y-PH independently reviewed 416 studies and helped to capture data from individual studies by using prespecified methods determined by all authors. W-CK revised it carefully from a professional point of view. All authors contributed to the inception of the research question, study design, read, and approved the final manuscript.
Funding
This study was partially supported by research grants from the Ministry of Science and Technology (MOST 110-2314-B-006-068), the Ministry of Health and Welfare (MOHW109-TDU-B-211-114003), and National Cheng Kung University Hospital (NCKUH-11003036 and NCKUH-11104005), Tainan, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.869822/full#supplementary-material
References
1. Bates DW, Pruess KE, Lee TH. How bad are bacteremia and sepsis?: Outcomes in a cohort with suspected bacteremia. Arch Intern Med. (1995) 1556:593–598. doi: 10.1001/archinte.155.6.593
2. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. (2018) 44:925–8. doi: 10.1007/s00134-018-5085-0
3. Endimiani A, Tamborini A, Luzzaro F, Lombardi G, Toniolo A. A two-year analysis of risk factors and outcome in patients with bloodstream infection. Jpn J Infect Dis. (2003) 56:1–7.
4. Kim SH, Park WB, Lee CS, Kang CI, Bang JW, Kim HB, et al. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin Microbiol Infect. (2006) 12:13–21. doi: 10.1111/j.1469-0691.2005.01294.x
5. Retamar P, Lopez-Prieto MD, Nátera C, de Cueto M, Nuño E, Herrero M, et al. Reappraisal of the outcome of healthcare-associated and community-acquired bacteramia: a prospective cohort study. BMC Infect Dis. (2013) 13:1–10. doi: 10.1186/1471-2334-13-344
6. Boel J, Søgaard M, Andreasen V, Jarløv JO, Arpi M. Evaluating antibiotic stewardship programs in patients with bacteremia using administrative data: a cohort study. Eur J Clin Microbiol Infect Dis. (2015) 34:1475–84. doi: 10.1007/s10096-015-2378-x
7. Lee CC, Lee CH, Yang CY, Hsieh CC, Tang HJ, Ko WC. Beneficial effects of early empirical administration of appropriate antimicrobials on survival and defervescence in adults with community-onset bacteremia. Crit Care. (2019) 23:1–12. doi: 10.1186/s13054-019-2632-1
8. Falcone M, Bassetti M, Tiseo G, Giordano C, Nencini E, Russo A, et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care. (2020) 24:1–12. doi: 10.1186/s13054-020-2742-9
9. Chen HC, Lin WL, Lin CC, Hsieh WH, Hsieh CH, Wu MH, et al. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J Antimicrob Chemother. (2013) 68:947–53. doi: 10.1093/jac/dks475
10. Seo H, Lee SC, Chung H, Ra SH, Sung H, Kim MN, et al. Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant enterobacteriaceae bacteremia. Int J Antimicrob Agents. (2020) 56:106126. doi: 10.1016/j.ijantimicag.2020.106126
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12. doi: 10.1016/j.jclinepi.2021.02.003
12. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online at: www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed April 18, 2022).
13. Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. (2005) 143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006
14. Petrelli F, Ghidini M, Ghidini A, Perego G, Cabiddu M, Khakoo S, et al. Use of antibiotics and risk of cancer: a systematic review and meta-analysis of observational studies. Cancers. (2019) 11:1174. doi: 10.3390/cancers11081174
15. Babar ZU, Dodani SK, Nasim A. Treatment outcome and adverse effects of colistin in adult patients with carbapenem-resistant gram-negative bacteremia from Pakistan. Int J Infect Dis. (2021) 106:171–5. doi: 10.1016/j.ijid.2021.03.004
16. Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, Cardozo C, Albasanz-Puig A, Marco F, et al. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis. (2020) 70:1068–74. doi: 10.1093/cid/ciz319
17. Álvarez-Marín R, Navarro-Amuedo D, Gasch-Blasi O, Rodríguez-Martínez JM, Calvo-Montes J, Lara-Contreras R, et al. A prospective, multicenter case control study of risk factors for acquisition and mortality in Enterobacter species bacteremia. J Infect. (2020) 80:174–81. doi: 10.1016/j.jinf.2019.09.017
18. Shargian-Alon L, Gafter-Gvili A, Ben-Zvi H, Wolach O, Yeshurun M, Raanani P, et al. Risk factors for mortality due to Acinetobacter baumannii bacteremia in patients with hematological malignancies–a retrospective study. Leuk Lymphoma. (2019) 60:2787–92. doi: 10.1080/10428194.2019.1599113
19. Lim CL, Spelman D. Mortality impact of empirical antimicrobial therapy in ESBL-and AmpC-producing Enterobacteriaceae bacteremia in an Australian tertiary hospital. Infect Dis Health. (2019) 24:124–33. doi: 10.1016/j.idh.2019.02.001
20. Chusri S, Chongsuvivatwong V, Silpapojakul K, Singkhamanan K, Hortiwakul T, Charernmak B, et al. Clinical characteristics and outcomes of community and hospital-acquired Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. (2019) 52:796–806. doi: 10.1016/j.jmii.2019.03.004
21. Yamaga S, Shime N. Association between appropriate empiric antimicrobial therapy and mortality from bloodstream infections in the intensive care unit. J Infect Chemother. (2018) 24:267–71. doi: 10.1016/j.jiac.2017.11.011
22. Saliba P, Hornero A, Cuervo G, Grau I, Jimenez E, García D, et al. Mortality risk factors among non-ICU patients with nosocomial vascular catheter-related bloodstream infections: a prospective cohort study. J Hosp Infect. (2018) 99:48–54. doi: 10.1016/j.jhin.2017.11.002
23. Park SY, Lee EJ, Kim T, Yu SN, Park K-H, Lee MS, et al. Early administration of appropriate antimicrobial agents to improve the outcome of carbapenem-resistant Acinetobacter baumannii complex bacteraemic pneumonia. Int J Antimicrob Agents. (2018) 51:407–12. doi: 10.1016/j.ijantimicag.2017.10.018
24. Liu LH, Wang NY, Wu AYJ, Lin CC, Lee CM, Liu CP. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J Microbiol Immunol Infect. (2018) 51:565–72. doi: 10.1016/j.jmii.2016.08.016
25. Kuo SH, Lin WR, Lin JY, Huang CH, Jao YT, Yang PW, et al. The epidemiology, antibiograms and predictors of mortality among critically-ill patients with central line-associated bloodstream infections. J Microbiol Immunol Infect. (2018) 51:401–10. doi: 10.1016/j.jmii.2017.08.016
26. Kleinhendler E, Cohen MJ, Moses AE, Paltiel O, Strahilevitz J, Cahan A. Empiric antibiotic protocols for cancer patients with neutropenia: a single–center study of treatment efficacy and mortality in patients with bacteremia. Int J Antimicrob Agents. (2018) 51:71–6. doi: 10.1016/j.ijantimicag.2017.06.016
27. Haruki Y, Hagiya H, Haruki M, Sugiyama T. Clinical characteristics and outcome of critically ill patients with bacteremia caused by extended-spectrum β-lactamase-producing and non-producing Escherichia coli. J Infect Chemother. (2018) 24:944–7. doi: 10.1016/j.jiac.2018.04.016
28. Bassetti M, Righi E, Del Giacomo P, Sartor A, Ansaldi F, Trucchi C, et al. Predictors of mortality with Staphylococcus aureus bacteremia in elderly adults. J Am Geriatr Soc. (2018) 66:1284–9. doi: 10.1111/jgs.15391
29. Bartoletti M, Giannella M, Lewis R, Caraceni P, Tedeschi S, Paul M, et al. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin Microbiol Infect. (2018) 24:546. e541–546. e548. doi: 10.1016/j.cmi.2018.01.013
30. Zhang Y, Du M, Chang Y, Chen L-a, Zhang Q. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp bloodstream infections in a tertiary-care hospital in Beijing, China: a four-year retrospective study. Antimicrob Resist Infect Control. (2017) 6:1–11. doi: 10.1186/s13756-017-0231-y
31. Yu WL, Lee MF, Chen CC, Tang HJ, Ho CH, Chuang YC. Impacts of hypervirulence determinants on clinical features and outcomes of bacteremia caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Microb Drug Resist. (2017) 23:376–83. doi: 10.1089/mdr.2016.0018
32. Xie Y, Tu B, Xu Z, Zhang X, Bi J, Zhao M, et al. Bacterial distributions and prognosis of bloodstream infections in patients with liver cirrhosis. Sci Rep. (2017) 7:1–9. doi: 10.1038/s41598-017-11587-1
33. Wang X, Zhang L, Sun A, Yang X, Sang W, Jiang Y, et al. Acinetobacter baumannii bacteraemia in patients with haematological malignancy: a multicentre retrospective study from the Infection Working Party of Jiangsu Society of Hematology. Eur J Clin Microbiol Infect Dis. (2017) 36:1073–81. doi: 10.1007/s10096-016-2895-2
34. Wang W, Jiang T, Zhang W, Li C, Chen J, Xiang D, et al. Predictors of mortality in bloodstream infections caused by multidrug-resistant gram-negative bacteria: 4 years of collection. Am J Infect Control. (2017) 45:59–64. doi: 10.1016/j.ajic.2016.08.008
35. Tagashira Y, Sakamoto N, Isogai T, Hikone M, Kosaka A, Chino R, et al. Impact of inadequate initial antimicrobial therapy on mortality in patients with bacteraemic cholangitis: a retrospective cohort study. Clin Microbiol Infect. (2017) 23:740–7. doi: 10.1016/j.cmi.2017.02.027
36. Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, et al. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrobial Agents Chemother. (2017) 61:e02349–02316. doi: 10.1128/AAC.02349-16
37. Royo-Cebrecos C, Gudiol C, García J, Tubau F, Laporte J, Ardanuy C, et al. Characteristics, aetiology, antimicrobial resistance and outcomes of bacteraemic cholangitis in patients with solid tumours: a prospective cohort study. J Infect. (2017) 74:172–8. doi: 10.1016/j.jinf.2016.10.008
38. Man MY, Shum HP, Chan YH, Chan K, Yan WW, Lee R, et al. Clinical predictors and outcomes of Klebsiella pneumoniae bacteraemia in a regional hospital in Hong Kong. J Hosp Infect. (2017) 97:35–41. doi: 10.1016/j.jhin.2017.06.007
39. Ma J, Li N, Liu Y, Wang C, Liu X, Chen S, et al. Antimicrobial resistance patterns, clinical features, and risk factors for septic shock and death of nosocomial E coli bacteremia in adult patients with hematological disease: a monocenter retrospective study in China. Medicine. (2017) 96:e6959. doi: 10.1097/MD.0000000000006959
40. Li L, Huang H. Risk factors of mortality in bloodstream infections caused by Klebsiella pneumonia: a single-center retrospective study in China. Medicine. (2017) 96:e7924. doi: 10.1097/MD.0000000000007924
41. Lee CC, Wang JL, Lee CH, Hung YP, Hong MY, Chang CM, et al. Age-related trends in adults with community-onset bacteremia. Antimicrob Agents Chemother. (2017) 61:e01050–01017. doi: 10.1128/AAC.01050-17
42. Gradel KO, Jensen US, Schønheyder HC, Østergaard C, Knudsen JD, Wehberg S, et al. Impact of appropriate empirical antibiotic treatment on recurrence and mortality in patients with bacteraemia: a population-based cohort study. BMC Infect Dis. (2017) 17:1–9. doi: 10.1186/s12879-017-2233-z
43. Adrie C, Garrouste-Orgeas M, Essaied WI, Schwebel C, Darmon M, Mourvillier B, et al. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. (2017) 74:131–41. doi: 10.1016/j.jinf.2016.11.001
44. Yoon YK, Park DW, Sohn JW, Kim HY, Kim YS, Lee CS, et al. Effects of inappropriate empirical antibiotic therapy on mortality in patients with healthcare-associated methicillin-resistant Staphylococcus aureus bacteremia: a propensity-matched analysis. BMC Infect Dis. (2016) 16:1–12. doi: 10.1186/s12879-016-1650-8
45. Yilmaz M, Elaldi N, Balkan II, Arslan F, Batirel AA, Bakici MZ, et al. Mortality predictors of Staphylococcus aureus bacteremia: a prospective multicenter study. Ann Clin Microbiol Antimicrob. (2016) 15:1–10. doi: 10.1186/s12941-016-0122-8
46. Trecarichi EM, Pagano L, Martino B, Candoni A, Di Blasi R, Nadali G, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. (2016) 91:1076–81. doi: 10.1002/ajh.24489
47. Savage RD, Fowler RA, Rishu AH, Bagshaw SM, Cook D, Dodek P, et al. The effect of inadequate initial empiric antimicrobial treatment on mortality in critically ill patients with bloodstream infections: a multi-centre retrospective cohort study. PLoS ONE. (2016) 11:e0154944. doi: 10.1371/journal.pone.0154944
48. Migiyama Y, Yanagihara K, Kaku N, Harada Y, Yamada K, Nagaoka K, et al. Pseudomonas aeruginosa bacteremia among immunocompetent and immunocompromised patients: relation to initial antibiotic therapy and survival. Jpn J Infect Dis. (2016) 69:91–6. doi: 10.7883/yoken.JJID.2014.573
49. Guillamet CV, Vazquez R, Noe J, Micek ST, Kollef MH. A cohort study of bacteremic pneumonia: The importance of antibiotic resistance and appropriate initial therapy? Medicine. (2016) 95:e4708. doi: 10.1097/MD.0000000000004708
50. Gudiol C, Royo-Cebrecos C, Laporte J, Ardanuy C, Garcia-Vidal C, Antonio M, et al. Clinical features, aetiology and outcome of bacteraemic pneumonia in neutropenic cancer patients. Respirology. (2016) 21:1411–8. doi: 10.1111/resp.12848
51. De la Calle C, Morata L, Cobos-Trigueros N, Martinez J, Cardozo C, Mensa J, et al. Staphylococcus aureus bacteremic pneumonia. Eur J Clin Microbiol Infect Dis. (2016) 35:497–502. doi: 10.1007/s10096-015-2566-8
52. Cheng WL, Hsueh PR, Lee CC, Li CW, Li MJ, Chang CM, et al. Bacteremic pneumonia caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: appropriateness of empirical treatment matters. J Microbiol Immunol Infect. (2016) 49:208–15. doi: 10.1016/j.jmii.2014.05.003
53. Abraham K, Dolman HS, Zimmerman LH, Faris J, Edelman DA, Baylor A, et al. Impact of inappropriate initial antibiotics in critically ill surgical patients with bacteremia. Am J Surg. (2016) 211:593–8. doi: 10.1016/j.amjsurg.2015.10.025
54. Wu JN, Gan TE, Zhu YX, Cao JM, Ji CH, Wu YH, et al. Epidemiology and microbiology of nosocomial bloodstream infections: analysis of 482 cases from a retrospective surveillance study. J Zhejiang Univ Sci B. (2015) 16:70–7. doi: 10.1631/jzus.B1400108
55. Picot-Guéraud R, Batailler P, Caspar Y, Hennebique A, Mallaret M-R. Bacteremia caused by multidrug-resistant bacteria in a French university hospital center: 3 years of collection. Am J Infect Control. (2015) 43:960–4. doi: 10.1016/j.ajic.2015.05.004
56. Park S, Kwon K, Chung JW, Huh H, Chae S. Coagulase-negative staphylococcal bacteremia: risk factors for mortality and impact of initial appropriate antimicrobial therapy on outcome. Eur J Clin Microbiol Infect Dis. (2015) 34:1395–401. doi: 10.1007/s10096-015-2364-3
57. Park H, Jang KJ, Jang W, Park SH, Park JY, Jeon TJ, et al. Appropriate empirical antibiotic use and 30-d mortality in cirrhotic patients with bacteremia. World J Gastroenterol. (2015) 21:3587. doi: 10.3748/wjg.v21.i12.3587
58. Lee HY, Chen CL, Liu SY, Yan YS, Chang CJ, Chiu CH. Impact of molecular epidemiology and reduced susceptibility to glycopeptides and daptomycin on outcomes of patients with methicillin-resistant Staphylococcus aureus bacteremia. PLoS ONE. (2015) 10:e0136171. doi: 10.1371/journal.pone.0136171
59. Hsieh CC, Lee CC, Chan TY, Hong MY, Chi CH, Ko WC. Clinical features and impact of empirical therapy in cirrhotic adults with community-onset bacteremia. Am J Emerg Med. (2015) 33:222–8. doi: 10.1016/j.ajem.2014.11.024
60. Hernández C, Fehér C, Soriano A, Marco F, Almela M, Cobos-Trigueros N, et al. Clinical characteristics and outcome of elderly patients with community-onset bacteremia. J Infect. (2015) 70:135–43. doi: 10.1016/j.jinf.2014.09.002
61. Dimopoulos G, Koulenti D, Tabah A, Poulakou G, Vesin A, Arvaniti K, et al. Bloodstream infections in ICU with increased resistance: epidemiology and outcomes. Minerva Anestesiol. (2015) 81:405–18.
62. Cain SE, Kohn J, Bookstaver PB, Albrecht H, Al-Hasan MN. Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob Agents Chemother. (2015) 59:245–50. doi: 10.1128/AAC.03935-14
63. Al-Dorzi HM, Asiri AM, Shimemri A, Tamim HM, Al Johani SM, Al Dabbagh T, et al. Impact of empirical antimicrobial therapy on the outcome of critically ill patients with Acinetobacter bacteremia. Ann Thorac Med. (2015) 10:256. doi: 10.4103/1817-1737.164302
64. Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit care. (2014) 18:1–13. doi: 10.1186/s13054-014-0596-8
65. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Predictors of hospital mortality among septic ICU patients with Acinetobacter spp. bacteremia: a cohort study. BMC Infect Dis. (2014) 14:1–7. doi: 10.1186/s12879-014-0572-6
66. Rodríguez-Pardo D, Almirante B, Fernández-Hidalgo N, Pigrau C, Ferrer C, Planes A, et al. Impact of prompt catheter withdrawal and adequate antimicrobial therapy on the prognosis of hospital-acquired parenteral nutrition catheter-related bacteraemia. Clin Microbiol Infect. (2014) 20:1205–10. doi: 10.1111/1469-0691.12703
67. Marín M, Gudiol C, Garcia-Vidal C, Ardanuy C, Carratalà J. Bloodstream infections in patients with solid tumors: epidemiology, antibiotic therapy, and outcomes in 528 episodes in a single cancer center. Medicine. (2014) 93:143–9. doi: 10.1097/MD.0000000000000026
68. Lee HY, Chen CL, Wu SR, Huang CW, Chiu CH. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. (2014) 42:1081–8. doi: 10.1097/CCM.0000000000000125
69. Kim YJ, Jun YH, Kim YR, Park KG, Park YJ, Kang JY, et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis. (2014) 14:1–7. doi: 10.1186/1471-2334-14-161
70. Hernandez C, Cobos-Trigueros N, Feher C, Morata L, De La Calle C, Marco F, et al. Community-onset bacteraemia of unknown origin: clinical characteristics, epidemiology and outcome. Eur J Clin Microbiol Infect Dis. (2014) 33:1973–80. doi: 10.1007/s10096-014-2146-3
71. Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, et al. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine. (2014) 93:298–309. doi: 10.1097/MD.0000000000000111
72. Falcone M, Vena A, Mezzatesta M, Gona F, Caio C, Goldoni P, et al. Role of empirical and targeted therapy in hospitalized patients with bloodstream infections caused by ESBL. Ann Ig. (2014) 26:293–304. doi: 10.7416/ai.2014.1989
73. Davis JS, McMillan M, Swaminathan A, Kelly JA, Piera KE, Baird RW, et al. A 16-year prospective study of community-onset bacteremic Acinetobacter pneumonia: low mortality with appropriate initial empirical antibiotic protocols. Chest. (2014) 146:1038–45. doi: 10.1378/chest.13-3065
74. Bodro M, Gudiol C, Garcia-Vidal C, Tubau F, Contra A, Boix L, et al. Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support Care Cancer. (2014) 22:603–10. doi: 10.1007/s00520-013-2012-3
75. Bartoletti M, Giannella M, Caraceni P, Domenicali M, Ambretti S, Tedeschi S, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. (2014) 61:51–8. doi: 10.1016/j.jhep.2014.03.021
76. Yang CJ, Chung YC, Chen TC, Chang HL, Tsai YM, Huang MS, et al. The impact of inappropriate antibiotics on bacteremia patients in a community hospital in Taiwan: an emphasis on the impact of referral information for cases from a hospital affiliated nursing home. BMC Infect Dis. (2013) 13:1–8. doi: 10.1186/1471-2334-13-500
77. Ruiz-Giardin JM, Jimenez BC, Martin RM, Ortiz J, Arenas MHC, SanMartin JV, et al. Clinical diagnostic accuracy of suspected sources of bacteremia and its effect on mortality. Eur J Intern Med. (2013) 24:541–5. doi: 10.1016/j.ejim.2013.05.010
78. Peña C, Suarez C, Ocampo-Sosa A, Murillas J, Almirante B, Pomar V, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. (2013) 57:208–16. doi: 10.1093/cid/cit223
79. Park JH, Choi SH, Chung JW. The impact of early adequate antimicrobial therapy on 14-day mortality in patients with monomicrobial Pseudomonas aeruginosa and Acinetobacter baumannii bacteremia. J Infect Chemother. (2013) 19:843–9. doi: 10.1007/s10156-013-0571-3
80. Ortega M, Marco F, Soriano A, Almela M, Martinez J, Pitart C, et al. Epidemiology and prognostic determinants of bacteraemic catheter-acquired urinary tract infection in a single institution from 1991 to 2010. Journal of Infection. (2013) 67:282–7. doi: 10.1016/j.jinf.2013.06.003
81. Molina J, Penuela I, Lepe J, Gutierrez-Pizarraya A, Gomez M, Garcia-Cabrera E, et al. Mortality and hospital stay related to coagulase-negative Staphylococci bacteremia in non-critical patients. J Infect. (2013) 66:155–62. doi: 10.1016/j.jinf.2012.10.021
82. Lee NY, Lee JC, Li MC, Li CW, Ko WC. Empirical antimicrobial therapy for critically ill patients with Acinetobacter baumannii bacteremia: combination is better. J Microbiol Immunol Infect. (2013) 46:397–8. doi: 10.1016/j.jmii.2013.03.004
83. Kuo SC, Lee YT, Yang SP, Chiang MC, Lin YT, Tseng FC, et al. Evaluation of the effect of appropriate antimicrobial therapy on mortality associated with Acinetobacter nosocomialis bacteraemia. Clin Microbiol Infect. (2013) 19:634–9. doi: 10.1111/j.1469-0691.2012.03967.x
84. Kang CI, Wi YM, Ko KS, Chung DR, Peck KR, Lee NY, et al. Outcomes and risk factors for mortality in community-onset bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli, with a special emphasis on antimicrobial therapy. Scand J Infect Dis. (2013) 45:519–25. doi: 10.3109/00365548.2013.775479
85. Kang CI, Sung YK, Lee KH, Lee KT, Lee JK. Clinical impact of inappropriate initial antimicrobial therapy on outcome in bacteremic biliary tract infections. Scand J Infect Dis. (2013) 45:227–34. doi: 10.3109/00365548.2012.730151
86. Horcajada J, Shaw E, Padilla B, Pintado V, Calbo E, Benito N, et al. Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect. (2013) 19:962–8. doi: 10.1111/1469-0691.12089
87. Gudiol C, Ayats J, Camoez M, Domínguez MÁ, García-Vidal C, Bodro M, et al. Increase in bloodstream infection due to vancomycin-susceptible Enterococcus faecium in cancer patients: risk factors, molecular epidemiology and outcomes. PLoS ONE. (2013) 8:e74734. doi: 10.1371/journal.pone.0074734
88. Gasch O, Camoez M, Dominguez M, Padilla B, Pintado V, Almirante B, et al. Predictive factors for early mortality among patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. (2013) 68:1423–30. doi: 10.1093/jac/dkt016
89. Garnacho-Montero J, Díaz-Martín A, García-Cabrera E, Ruiz Pérez de. Pipaón M, Hernández-Caballero C, Lepe-Jiménez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp bloodstream infections. J Antimicrob Chemother. (2013) 68:206–13. doi: 10.1093/jac/dks347
90. Frakking FN, Rottier WC, Dorigo-Zetsma JW, van Hattem JM, Van Hees BC, Kluytmans JA, et al. Appropriateness of empirical treatment and outcome in bacteremia caused by extended-spectrum-β-lactamase-producing bacteria. Antimicrob Agents Chemother. (2013) 57:3092–9. doi: 10.1128/AAC.01523-12
91. Bang JH, Jung Y, Cheon S, Kim CJ, Song KH, Choe PG, et al. Pseudomonas aeruginosa bacteremia in patients with liver cirrhosis: a comparison with bacteremia caused by Enterobacteriaceae. BMC Infect Dis. (2013) 13:1–6. doi: 10.1186/1471-2334-13-332
92. Wu UI, Chen WC, Yang CS, Wang L, Hu FC, Chang SC, et al. Ertapenem in the treatment of bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli: a propensity score analysis. Int J Infect Dis. (2012) 16:e47–52. doi: 10.1016/j.ijid.2011.09.019
93. Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. (2012) 55:943–50. doi: 10.1093/cid/cis588
94. Tumbarello M, Trecarichi EM, Fiori B, Losito AR, D'Inzeo T, Campana L, et al. Multidrug-resistant Proteus mirabilis bloodstream infections: risk factors and outcomes. Antimicrob Agents Chemother. (2012) 56:3224–31. doi: 10.1128/AAC.05966-11
95. Sancho S, Artero A, Zaragoza R, Camarena J, González R, Nogueira J. Impact of nosocomial polymicrobial bloodstream infections on the outcome in critically ill patients. Eur J Clin Microbiol Infect Dis. (2012) 31:1791–6. doi: 10.1007/s10096-011-1503-8
96. Rong C, Yan ZQ, Dan F, Luo YP, Wang LL, Shen DX. Nosocomial bloodstream infection in patients caused byStaphylococcus aureus: drug susceptibility, outcome, and risk factors for hospital mortality. Chin Med J. (2012) 125:226–9. doi: 10.3760/cma.j.issn.0366-6999.2012.02.012
97. Retamar P, Portillo MM, López-Prieto MD, Rodríguez-López F, De Cueto M, García MV, et al. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother. (2012) 56:472–8. doi: 10.1128/AAC.00462-11
98. Peralta G, Lamelo M, Álvarez-García P, Velasco M, Delgado A, Horcajada JP, et al. Impact of empirical treatment in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. bacteremia A multicentric cohort study. BMC Infect Dis. (2012) 12:1–7. doi: 10.1186/1471-2334-12-245
99. Park SY, Park HJ, Moon SM, Park KH, Chong YP, Kim MN, et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis. (2012) 12:1–6. doi: 10.1186/1471-2334-12-308
100. Ortega M, Marco F, Soriano A, Almela M, Martinez J, Lopez J, et al. Epidemiology and prognostic determinants of bacteraemic biliary tract infection. J Antimicrob Chemother. (2012) 67:1508–13. doi: 10.1093/jac/dks062
101. Morata L, Cobos-Trigueros N, Martínez JA, Soriano Á, Almela M, Marco F, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. (2012) 56:4833–7. doi: 10.1128/AAC.00750-12
102. Lye D, Earnest A, Ling M, Lee TE, Yong HC, Fisher D, et al. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect. (2012) 18:502–8. doi: 10.1111/j.1469-0691.2011.03606.x
103. Lee YT, Kuo SC, Yang SP, Lin YT, Tseng FC, Chen TL, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. (2012) 55:209–15. doi: 10.1093/cid/cis385
104. Lee CC, Lee CH, Chuang MC, Hong MY, Hsu HC, Ko WC. Impact of inappropriate empirical antibiotic therapy on outcome of bacteremic adults visiting the ED. Am J Emerg Med. (2012) 30:1447–56. doi: 10.1016/j.ajem.2011.11.010
105. Labelle A, Juang P, Reichley R, Micek S, Hoffmann J, Hoban A, et al. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit Care Med. (2012) 40:2016–21. doi: 10.1097/CCM.0b013e318250aa72
106. Jung Y, Lee MJ, Sin HY, Kim NH, Hwang JH, Park J, et al. Differences in characteristics between healthcare-associated and community-acquired infection in community-onset Klebsiella pneumoniae bloodstream infection in Korea. BMC Infect Dis. (2012) 12:1–9. doi: 10.1186/1471-2334-12-239
107. Horino T, Chiba A, Kawano S, Kato T, Sato F, Maruyama Y, et al. Clinical characteristics and risk factors for mortality in patients with bacteremia caused by Pseudomonas aeruginosa. Intern Med. (2012) 51:59–64. doi: 10.2169/internalmedicine.51.5698
108. Bassetti M, Trecarichi EM, Mesini A, Spanu T, Giacobbe D, Rossi M, et al. Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect. (2012) 18:862–9. doi: 10.1111/j.1469-0691.2011.03679.x
109. Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. (2011) 17:1798–803. doi: 10.1111/j.1469-0691.2011.03514.x
110. Wang SS, Lee NY, Hsueh PR, Huang WH, Tsui KC, Lee HC, et al. Clinical manifestations and prognostic factors in cancer patients with bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. J Microbiol Immunol Infect. (2011) 44:282–8. doi: 10.1016/j.jmii.2010.08.004
111. Tuon FF, Kruger M, Terreri M, Penteado-Filho SR, Gortz L. Klebsiella ESBL bacteremia-mortality and risk factors. Braz J Infect Dis. (2011) 15:594–8. doi: 10.1590/S1413-86702011000600016
112. Tumbarello M, Repetto E, Trecarichi EM, Bernardini C, De Pascale G, Parisini A, et al. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol Infect. (2011) 139:1740–9. doi: 10.1017/S0950268810003055
113. Takesue Y, Nakajima K, Takahashi Y, Ichiki K, Wada Y, Tsuchida T, et al. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 μg/ml methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J Infect Chemother. (2011) 17:52–7. doi: 10.1007/s10156-010-0086-0
114. Song JY, Cheong HJ, Choi WS, Heo JY, Noh JY, Kim WJ. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J Med Microbiol. (2011) 60:605–11. doi: 10.1099/jmm.0.029439-0
115. Shime N, Satake S, Fujita N. De-escalation of antimicrobials in the treatment of bacteraemia due to antibiotic-sensitive pathogens in immunocompetent patients. Infection. (2011) 39:319–25. doi: 10.1007/s15010-011-0116-6
116. Schechner V, Gottesman T, Schwartz O, Korem M, Maor Y, Rahav G, et al. Pseudomonas aeruginosa bacteremia upon hospital admission: risk factors for mortality and influence of inadequate empirical antimicrobial therapy. Diagn Microbiol Infect Dis. (2011) 71:38–45. doi: 10.1016/j.diagmicrobio.2011.05.010
117. Reisfeld S, Paul M, Gottesman B, Shitrit P, Leibovici L, Chowers M. The effect of empiric antibiotic therapy on mortality in debilitated patients with dementia. Eur J Clin Microbiol Infect Dis. (2011) 30:813–8. doi: 10.1007/s10096-011-1161-x
118. Rebelo M, Pereira B, Lima J, Decq-Mota J, Vieira JD, Costa JN. Predictors of in-hospital mortality in elderly patients with bacteraemia admitted to an Internal Medicine ward. Int Arch Med. (2011) 4:1–9. doi: 10.1186/1755-7682-4-33
119. Park SH, Choi SM, Lee DG, Kim J, Choi JH, Kim SH, et al. Emergence of extended-spectrum β-lactamase-producing Escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. (2011) 17:537–44. doi: 10.1089/mdr.2011.0072
120. Ortega M, Marco F, Soriano A, Almela M, Martínez J, López J, et al. Cefotaxime resistance and outcome of Klebsiella spp bloodstream infection. Eur J Clin Microbiol Infect Dis. (2011) 30:1599–605. doi: 10.1007/s10096-011-1266-2
121. Lewis T, Chaudhry R, Nightingale P, Lambert P, Das I. Methicillin-resistant Staphylococcus aureus bacteremia: epidemiology, outcome, and laboratory characteristics in a tertiary referral center in the UK. Int J Infect Dis. (2011) 15:e131–5. doi: 10.1016/j.ijid.2010.09.013
122. Lee SS, Kim Y, Chung DR. Impact of discordant empirical therapy on outcome of community-acquired bacteremic acute pyelonephritis. J Infect. (2011) 62:159–64. doi: 10.1016/j.jinf.2010.10.009
123. Ku NS, Han SH, Kim CO, Baek JH, Jeong SJ, Jin SJ, et al. Risk factors for mortality in patients with Burkholderia cepacia complex bacteraemia. Scand J Infect Dis. (2011) 43:792–7. doi: 10.3109/00365548.2011.589076
124. Johnson MT, Reichley R, Hoppe-Bauer J, Dunne WM, Micek S, Kollef M. Impact of previous antibiotic therapy on outcome of Gram-negative severe sepsis. Crit Care Med. (2011) 39:1859–65. doi: 10.1097/CCM.0b013e31821b85f4
125. Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sánchez-Ortega I, et al. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother. (2011) 66:657–63. doi: 10.1093/jac/dkq494
126. Feodoroff B, Lauhio A, Ellström P, Rautelin H. A nationwide study of Campylobacter jejuni and Campylobacter coli bacteremia in Finland over a 10-year period, 1998–2007, with special reference to clinical characteristics and antimicrobial susceptibility. Clin Infect Dis. (2011) 53:e99–e106. doi: 10.1093/cid/cir509
127. Enoch D, Phillimore N, Mlangeni D, Salihu H, Sismey A, Aliyu S, et al. Outcome for Gram-negative bacteraemia when following restrictive empirical antibiotic guidelines. QJM. (2011) 104:411–9. doi: 10.1093/qjmed/hcq228
128. De Rosa FG, Pagani N, Fossati L, Raviolo S, Cometto C, Cavallerio P, et al. The effect of inappropriate therapy on bacteremia by ESBL-producing bacteria. Infection. (2011) 39:555–61. doi: 10.1007/s15010-011-0201-x
129. Cheol-In K, Chung DR, Ko KS, Peck KR, Song JH, Diseases KNftSoI. Risk factors for mortality and impact of broad-spectrum cephalosporin resistance on outcome in bacteraemic intra-abdominal infections caused by Gram-negative bacilli. Scand J Infect Dis. (2011) 43:202–8. doi: 10.3109/00365548.2010.539257
130. Asgeirsson H, Kristjansson M, Kristinsson KG, Gudlaugsson O. Staphylococcus aureus bacteraemia–Nationwide assessment of treatment adequacy and outcome. J Infect. (2011) 62:339–46. doi: 10.1016/j.jinf.2011.03.003
131. Vitkauskiene A, Skrodeniene E, Dambrauskiene A, Macas A, Sakalauskas R. Pseudomonas aeruginosa bacteremia: resistance to antibiotics, risk factors, and patient mortality. Medicina. (2010) 46:490. doi: 10.3390/medicina46070071
132. Tumbarello M, Spanu T, Di Bidino R, Marchetti M, Ruggeri M, Trecarichi EM, et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-β-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. (2010) 54:4085–91. doi: 10.1128/AAC.00143-10
133. Tam VH, Rogers CA, Chang KT, Weston JS, Caeiro JP, Garey KW. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother. (2010) 54:3717–22. doi: 10.1128/AAC.00207-10
134. Son JS, Song JH, Ko KS, Yeom JS, Ki HK, Kim SW, et al. Bloodstream infections and clinical significance of healthcare-associated bacteremia: a multicenter surveillance study in Korean hospitals. J Korean Med Sci. (2010) 25:992–8. doi: 10.3346/jkms.2010.25.7.992
135. Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, et al. Empiric antibiotic therapy for Staphylococcus aureus bacteremia may not reduce in-hospital mortality: a retrospective cohort study. PLoS ONE. (2010) 5:e11432. doi: 10.1371/journal.pone.0011432
136. Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Ruíz M, Peña C, et al. Community-onset bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. (2010) 50:40–8. doi: 10.1086/649537
137. Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Cisneros JM, Peña C, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-β-lactamase-producing Escherichia coli. J Clin Microbiol. (2010) 48:1726–31. doi: 10.1128/JCM.02353-09
138. Paul M, Kariv G, Goldberg E, Raskin M, Shaked H, Hazzan R, et al. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. (2010) 65:2658–65. doi: 10.1093/jac/dkq373
139. Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. (2010) 54:1742–8. doi: 10.1128/AAC.01365-09
140. Martinez J, Cobos-Trigueros N, Soriano A, Almela M, Ortega M, Marco F, et al. Influence of empiric therapy with a β-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob Agents Chemother. (2010) 54:3590–6. doi: 10.1128/AAC.00115-10
141. Lin PY, Chen HL, Huang CT, Su LH, Chiu CH. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteraemia. Int J Antimicrob Agents. (2010) 36:436–40. doi: 10.1016/j.ijantimicag.2010.06.033
142. Khan FY, Elshafie SS, Almaslamani M, Abu-Khattab M, El Hiday AH, Errayes M, et al. Epidemiology of bacteraemia in Hamad general hospital, Qatar: a one year hospital-based study. Travel Med Infect Dis. (2010) 8:377–87. doi: 10.1016/j.tmaid.2010.10.004
143. Corona A, Bertolini G, Lipman J, Wilson AP, Singer M. Antibiotic use and impact on outcome from bacteraemic critical illness: the BActeraemia Study in Intensive Care (BASIC). J Antimicrob Chemother. (2010) 65:1276–85. doi: 10.1093/jac/dkq088
144. Abhilash K, Veeraraghavan B, Abraham O. Epidemiology and outcome of bacteremia caused by extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in a tertiary care teaching hospital in south India. J Assoc Physicians India. (2010) 58(Suppl):13–7.
145. Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, et al. Incidence and clinical. Impact of extended-spectrum-β-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect. (2009) 58:299–307. doi: 10.1016/j.jinf.2009.02.002
146. Su CP, Huang PY, Yang CC, Lee MH. Fusobacterium bacteremia: clinical significance and outcomes. J Microbiol Immunol. (2009) 42:336–42.
147. Ortega M, Marco F, Soriano A, Almela M, Martínez J, Munoz A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. (2009) 63:568–74. doi: 10.1093/jac/dkn514
148. Metan G, Sariguzel F, Sumerkan B. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur J Intern Med. (2009) 20:540–4. doi: 10.1016/j.ejim.2009.05.005
149. Klevay MJ, Horn DL, Neofytos D, Pfaller MA, Diekema DJ. Initial treatment and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. (2009) 64:152–7. doi: 10.1016/j.diagmicrobio.2009.03.007
150. Evans CT, Burns SP, Chin A, Weaver FM, Hershow RC. Predictors and outcomes of antibiotic adequacy for bloodstream infections in veterans with spinal cord injury. Arch Phys Med Rehabil. (2009) 90:1364–70. doi: 10.1016/j.apmr.2009.02.012
151. Erbay A, Idil A, Gözel MG, Mumcuoglu I, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. (2009) 34:575–9. doi: 10.1016/j.ijantimicag.2009.07.006
152. Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, et al. Prospective observational study of the impact of VIM-1 metallo-β-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. (2009) 53:1868–73. doi: 10.1128/AAC.00782-08
153. Chang EP, Chiang DH, Lin ML, Chen TL, Wang FD, Liu CY. Clinical characteristics and predictors of mortality in patients with Enterobacter aerogenes bacteremia. J Microbiol Immunol Infect. (2009) 42:329–35.
154. Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M, et al. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. (2009) 49:997–1005. doi: 10.1086/605555
155. Wareham D, Bean DC, Khanna P, Hennessy E, Krahe D, Ely A, et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis. (2008) 27:607–12. doi: 10.1007/s10096-008-0473-y
156. Tumbarello M, Sali M, Trecarichi EM, Leone F, Rossi M, Fiori B, et al. Bloodstream infections caused by extended-spectrum-β-lactamase-producing Escherichia coli: risk factors for inadequate initial antimicrobial therapy. Antimicrob Agents Chemother. (2008) 52:3244–52. doi: 10.1128/AAC.00063-08
157. Soriano A, Marco F, Martínez JA, Pisos E, Almela M, Dimova VP, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. (2008) 46:193–200. doi: 10.1086/524667
158. Marschall J, Agniel D, Fraser VJ, Doherty J, Warren DK. Gram-negative bacteraemia in non-ICU patients: factors associated with inadequate antibiotic therapy and impact on outcomes. J Antimicrob Chemother. (2008) 61:1376–83. doi: 10.1093/jac/dkn104
159. Marcos M, Inurrieta A, Soriano A, Martínez JA, Almela M, Marco F, et al. Effect of antimicrobial therapy on mortality in 377 episodes of Enterobacter spp. bacteraemia. J Antimicrob Chemother. (2008) 62:397–403. doi: 10.1093/jac/dkn155
160. Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother. (2008) 52:3188–94. doi: 10.1128/AAC.01553-07
161. Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. (2008) 36:2967–72. doi: 10.1097/CCM.0b013e31818b3477
162. Cheong HS, Kang CI, Wi YM, Kim ES, Lee JS, Ko KS, et al. Clinical significance and predictors of community-onset Pseudomonas aeruginosa bacteremia. Am J Med. (2008) 121:709–14. doi: 10.1016/j.amjmed.2008.03.034
163. Peralta G, Sanchez MB, Garrido JC, De Benito I, Cano ME, Martínez-Martínez L, et al. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J Antimicrob Chemother. (2007) 60:855–63. doi: 10.1093/jac/dkm279
164. Osih RB, McGregor JC, Rich SE, Moore AC, Furuno JP, Perencevich EN, et al. Impact of empiric antibiotic therapy on outcomes in patients with Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. (2007) 51:839–44. doi: 10.1128/AAC.00901-06
165. Ortega M, Almela M, Martinez J, Marco F, Soriano A, Lopez J, et al. Epidemiology and outcome of primary community-acquired bacteremia in adult patients. Eur J Clin Microbiol Infect Dis. (2007) 26:453–7. doi: 10.1007/s10096-007-0304-6
166. Lodise TP Jr, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, et al. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. (2007) 51:3510–5. doi: 10.1128/AAC.00338-07
167. Bassetti M, Trecarichi EM, Righi E, Sanguinetti M, Bisio F, Posteraro B, et al. Incidence, risk factors, and predictors of outcome of candidemia. Survey in 2 Italian university hospitals. Diagn Microbiol Infect Dis. (2007) 58:325–31. doi: 10.1016/j.diagmicrobio.2007.01.005
168. Ye Y, Li J, Ye D, Jiang Z. Enterobacter bacteremia: Clinical features, risk factors for multiresistance and mortality in a Chinese University Hospital. Infection. (2006) 34:252–7. doi: 10.1007/s15010-006-5038-3
169. Schramm GE, Johnson JA, Doherty JA, Micek ST, Kollef MH. Methicillin-resistant Staphylococcus aureus sterile-site infection: the importance of appropriate initial antimicrobial treatment. Crit Care Med. (2006) 34:2069–74. doi: 10.1097/01.CCM.0000227655.41566.3E
170. Marra AR, Edmond MB, Forbes BA, Wenzel RP, Bearman GM. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol. (2006) 44:1342–6. doi: 10.1128/JCM.44.4.1342-1346.2006
171. Lin YC, Chen TL, Ju HL, Chen HS, Wang FD, Yu KW, et al. Clinical characteristics and risk factors for attributable mortality in Enterobacter cloacae bacteremia. J Microbiol Immunol Infect. (2006) 39:67–72.
172. Khatib R, Saeed S, Sharma M, Riederer K, Fakih M, Johnson L. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dise. (2006) 25:181–5. doi: 10.1007/s10096-006-0096-0
173. Guilarde A, Turchi M, Martelli C, Primo M. Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. J Hosp Infect. (2006) 63:330–6. doi: 10.1016/j.jhin.2006.02.011
174. Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. (2006) 43:25–31. doi: 10.1086/504810
175. Fang CT, Shau WY, Hsueh PR, Chen YC, Wang JT, Hung CC, et al. Early empirical glycopeptide therapy for patients with methicillin-resistant Staphylococcus aureus bacteraemia: impact on the outcome. J Antimicrob Chemother. (2006) 57:511–9. doi: 10.1093/jac/dkl006
176. Falagas ME, Kasiakou SK, Rafailidis PI, Zouglakis G, Morfou P. Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J Antimicrob Chemother. (2006) 57:1251–4. doi: 10.1093/jac/dkl130
177. Wang FD, Lin ML, Liu CY. Bacteremia in patients with hematological malignancies. Chemotherapy. (2005) 51:147–53. doi: 10.1159/000085623
178. Shih HI, Lee HC, Lee NY, Chang CM, Wu CJ, Wang LR, et al. Serratia marcescens bacteremia at a medical center in southern Taiwan: high prevalence of cefotaxime resistance. J Microbiol Immunol Infect. (2005) 38:350–7.
179. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. (2005) 49:1306–11. doi: 10.1128/AAC.49.4.1306-1311.2005
180. Metan G, Zarakolu P, Çakir B, Hascelik G, Uzun O. Clinical outcomes and therapeutic options of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Int J Antimicrob Agents. (2005) 26:254–7. doi: 10.1016/j.ijantimicag.2005.06.012
181. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. (2005) 49:760–6. doi: 10.1128/AAC.49.2.760-766.2005
182. Hung MN, Chen SY, Wang JL, Chang SC, Hsueh PR, Liao CH, et al. Community-acquired anaerobic bacteremia in adults: one-year experience in a medical center. J Microbiol Immunol Infect. (2005) 38:436–43.
183. Bouza E, Pintado V, Rivera S, Blázquez R, Muñoz P, Cercenado E, et al. Nosocomial bloodstream infections caused by Streptococcus pneumoniae. Clin Microbiol Infect. (2005) 11:919–24. doi: 10.1111/j.1469-0691.2005.01260.x
184. Lin JC, Yeh KM, Peng MY, Chang FY. Community-acquired methicillin-resistant Staphylococcus aureus bacteremia in Taiwan: risk factors for acquisition, clinical features and outcome. J Microbiol Immunol Infect. (2004) 37:24–8.
185. Bouza E, Sousa D, Munoz P, Rodríguez-Créixems M, Fron C, Lechuz JG. Bloodstream infections: a trial of the impact of different methods of reporting positive blood culture results. Clin Infect Dis. (2004) 39:1161–9. doi: 10.1086/424520
186. Anatoliotaki M, Valatas V, Mantadakis E, Apostolakou H, Mavroudis D, Georgoulias V, et al. Bloodstream infections in patients with solid tumors: associated factors, microbial spectrum and outcome. Infection. (2004) 32:65–71. doi: 10.1007/s15010-004-3049-5
187. Zaragoza R, Artero A, Camarena J, Sancho S, Gonzalez R, Nogueira J. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin Microbiol Infect. (2003) 9:412–8. doi: 10.1046/j.1469-0691.2003.00656.x
188. Valles J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. (2003) 123:1615–24. doi: 10.1378/chest.123.5.1615
189. MacKenzie A, Robertson L, Jappy B, Laing R, Gould IM. Audit of an antibiotic policy and microbiological investigations for treating bacteraemia in a large teaching hospital. Int J Antimicrob Agents. (2003) 22:618–21. doi: 10.1016/j.ijantimicag.2003.05.002
190. Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. (2003) 36:1418–23. doi: 10.1086/375057
191. Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. (2003) 37:745–51. doi: 10.1086/377200
192. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. (2003) 47:2756–64. doi: 10.1128/AAC.47.9.2756-2764.2003
193. Hanon FoX, Sørensen TL, Mølbak K, Schønheyder H, Monnet DL, Pedersen G. Survival of patients with bacteraemia in relation to initial empirical antimicrobial treatment. Scand J Infect Dis. (2002) 34:520–8. doi: 10.1080/00365540110080827
194. Soriano A, Martinez J, Mensa J, Marco F, Almela M, Moreno-Martinez A, et al. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. (2000) 30:368–73. doi: 10.1086/313650
195. Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. (2000) 118:146–55. doi: 10.1378/chest.118.1.146
196. Byl B, Clevenbergh P, Jacobs F, Struelens MJ, Zech F, Kentos A, et al. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis. (1999) 29:60–6. doi: 10.1086/520182
197. Salonen J, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. (1998) 26:1413–7. doi: 10.1086/516355
198. Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik S. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. (1998) 244:379–86. doi: 10.1046/j.1365-2796.1998.00379.x
199. Carratalà J, Rosón B, Fernández-Sevilla A, Alcaide F, Gudiol F. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med. (1998) 158:868–72. doi: 10.1001/archinte.158.8.868
200. Elhanan G, Sarhat M, Raz R. Empiric antibiotic treatment and the misuse of culture results and antibiotic sensitivities in patients with community-acquired bacteraemia due to urinary tract infection. J Infect. (1997) 35:283–8. doi: 10.1016/S0163-4453(97)93194-7
201. Phillips I, King A, Gransden WR, Eykyn SJ. The antibiotic sensitivity of bacteria isolated from the blood of patients in St Thomas' Hospital, 1969–1988. J Antimicrob Chemother. (1990) 25(suppl_C):59–80. doi: 10.1093/jac/25.suppl_C.59
202. Feldman C, Smith C, Levy H, Ginsburg P, Miller S, Koornhof H. Klebsiella pneumoniae bacteraemia at an urban general hospital. J Infect. (1990) 20:21–31. doi: 10.1016/S0163-4453(90)92258-M
203. Meyers BR, Sherman E, Mendelson MH, Velasques G, Srulevitch-Chin E, Hubbard M, et al. Bloodstream infections in the elderly. Am J Med. (1989) 86:379–84. doi: 10.1016/0002-9343(89)90333-1
204. Bodey GP, Elting L, Kassamali H, Lim BP. Escherichia coli bacteremia in cancer patients. Am J Med. (1986) 81:85–95. doi: 10.1016/0002-9343(86)90518-8
205. Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae–a case–control study in a low prevalence country. PLoS ONE. (2013) 8:e69581. doi: 10.1371/journal.pone.0069581
206. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. (2010) 54:4851–63. doi: 10.1128/AAC.00627-10
207. Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care. (2015) 19:1–12. doi: 10.1186/s13054-015-0795-y
Keywords: empirical antibiotic, bacteraemia, mortality, systematic review, meta-analysis
Citation: Hung Y-P, Lee C-C and Ko W-C (2022) Effects of Inappropriate Administration of Empirical Antibiotics on Mortality in Adults With Bacteraemia: Systematic Review and Meta-Analysis. Front. Med. 9:869822. doi: 10.3389/fmed.2022.869822
Received: 05 February 2022; Accepted: 03 May 2022;
Published: 30 May 2022.
Edited by:
Longxiang Su, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Wentao Ni, Peking University People's Hospital, ChinaAnca Butiuc-Keul, Babeş-Bolyai University, Romania
Copyright © 2022 Hung, Lee and Ko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Chi Lee, Y2hpY2hpbmdibTg1QGdtYWlsLmNvbQ==; Wen-Chien Ko, d2luc3RvbjM0MTVAZ21haWwuY29t
 Yuan-Pin Hung
Yuan-Pin Hung Ching-Chi Lee
Ching-Chi Lee Wen-Chien Ko
Wen-Chien Ko