
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 14 April 2022
Sec. Hepatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.869190
This article is part of the Research TopicNAFLD in the World StageView all 7 articles
 Jae Seung Lee1,2,3
Jae Seung Lee1,2,3 Hye Won Lee1,2,3
Hye Won Lee1,2,3 Beom Kyung Kim1,2,3
Beom Kyung Kim1,2,3 Jun Yong Park1,2,3
Jun Yong Park1,2,3 Do Young Kim1,2,3
Do Young Kim1,2,3 Sang Hoon Ahn1,2,3
Sang Hoon Ahn1,2,3 Jae Young Jang4
Jae Young Jang4 Soo Young Park5
Soo Young Park5 Hyun Woong Lee1,2,6
Hyun Woong Lee1,2,6 Chun Kyon Lee7
Chun Kyon Lee7 Seung Up Kim1,2,3*
Seung Up Kim1,2,3*Identification of non-alcoholic steatohepatitis (NASH) with high activity and fibrosis is a major priority in patients with non-alcoholic fatty liver disease. We validated the predictive value of the FibroScan-aspartate aminotransferase (FAST) score and other non-invasive fibrosis surrogates in predicting high-risk NASH criteria. This multicenter retrospective study recruited 251 biopsy-proven non-alcoholic fatty liver disease (NAFLD) patients (132 [52.6%] men) between 2011 and 2014. The FAST score was calculated using transient elastography data and aspartate aminotransferase (AST) levels. The NAFLD fibrosis score (NFS), fibrosis-4 index (FIB-4), and AST to platelet ratio index (APRI) were calculated using biochemical data. The area under the receiver operating characteristic curves (AUCs) of the FAST score, liver stiffness, NFS, FIB-4, and APRI were 0.752, 0.718, 0.609, 0.650, and 0.722 for NAFLD activity score (NAS) ≥5 (n = 117, 46.6%); 0.788, 0.754, 0.649, 0.701, and 0.747 for fatty liver inhibition of progression-NASH with histologic activity ≥3 (n = 202, 80.5%); 0.807, 0.806, 0.691, 0.732, and 0.760 for severe disease with activity ≥3 and/or fibrosis ≥3 (n = 132, 52.6%); and 0.714, 0.812, 0.748, 0.738, and 0.669 for NASH with NAS ≥4 and fibrosis ≥2 (n = 70, 27.9%), respectively. The FAST score had the highest AUC for the most high-risk NASH criteria, except for in predicting NAS ≥4 and fibrosis ≥2. The liver stiffness value showed consistently acceptable performance in predicting all high-risk NASH criteria. The FAST score has acceptable performance in identifying high-risk NASH. However, liver stiffness alone was not inferior to the FAST score.
The global prevalence of non-alcoholic fatty liver disease (NAFLD) is estimated to be 24%, which is now an emerging cause of advanced liver diseases such as cirrhosis and hepatocellular carcinoma (HCC) (1–4). The prevalence of NAFLD in the Republic of Korea also increased rapidly from 18.6% in 1998–2001 to 21.5% in 2016–2017, with an increasing prevalence of obesity and diabetes (5). Approximately 25–40% of patients with NAFLD progress to non-alcoholic steatohepatitis (NASH), which exhibits more aggressive histologic findings with necroinflammatory activity and has an increased risk of liver fibrosis (6). The priority of identifying patients with NASH and significant fibrosis arises from their prognosis, with an increased risk of cirrhosis and HCC (2, 7). Moreover, the lack of effective and evidence-based pharmacotherapy necessitated various clinical trials for NAFLD that require the identification of patients with advanced inflammation and fibrosis. Although liver biopsy is the gold standard for evaluation, histological assessment is impractical because of its invasiveness, cost, sampling error, and interobserver variability. Therefore, efforts have been made to estimate histologic inflammation and fibrosis through non-invasive methods using biomarkers or imaging techniques (8–11).
Recently, Newsome et al. suggested the FibroScan-aspartate aminotransferase (FAST) score for the non-invasive identification of patients with NASH with NAFLD activity score (NAS) ≥4 and fibrosis stage ≥2, which represent high-risk NASH (12). The score, combining liver stiffness (LS) and controlled attenuation parameter (CAP) values using transient elastography (TE) with AST levels, showed high performance (area under the receiver operating characteristic curve [AUC]: 0.74–0.95) in the derivation and validation cohorts. Moreover, recent studies have supported that the FAST score is reliable in stratifying high-risk NASH in Japanese (AUC: 0.76) and US veterans (AUC: 0.75), regardless of the TE probe type (13, 14). However, the method of determining high-risk NASH can be different, and studies directly comparing the FAST score with other non-invasive surrogates such as LS value by TE, NAFLD fibrosis score (NFS), fibrosis index based on four factors (FIB-4), and AST to platelet ratio index (APRI) in predicting high-risk NASH according to various criteria are lacking (15).
Therefore, this study aimed to validate the performance of the FAST score in predicting high-risk NASH defined by various criteria and compare its performance to that of other non-invasive surrogates, using the biopsy-proven NAFLD cohort.
Patients with biopsy-proven NAFLD were recruited between January 2011 and December 2020 through a retrospective review using data from five tertiary medical centers in the Republic of Korea (Yonsei University Severance Hospital, Yonsei University Gangnam Severance Hospital, Soonchunhyang University Seoul Hospital, Kyungpook National University Hospital, and National Health Insurance Cooperation Ilsan Hospital). The inclusion criteria were as follows: (1) age ≥19 years, (2) histological findings of hepatic steatosis, (3) no significant alcohol intake (>30 g/day for men and 20 g/day for women), and (4) TE performed with an M probe on the day of liver biopsy. The exclusion criteria were as follows: (1) other causes of chronic hepatitis, such as viral hepatitis B and C; (2) any histological findings suggesting other chronic liver disease or secondary to other etiologies (e.g., autoimmune hepatitis, congestive hepatopathy, primary biliary cholangitis, primary sclerosing cholangitis, and Wilson's disease); (3) previous hepatic or other malignancies; (4) any signs of acute hepatitis or liver failure, defined as AST level >300 IU/L, alanine aminotransferase level >300 IU/L, total bilirubin level >3.0 mg/dL, or serum albumin level <2.5 g/dL; (5) drug exposure that can induce secondary hepatic steatosis (for example, corticosteroids, tamoxifen, and amiodarone); (6) TE assessment failure or unreliable TE results; and (7) insufficient clinical data.
The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The need for written informed consent was waived because of the retrospective nature of the study. The study procedure was approved by the institutional review board of each institute (IRB No. 4-2021-0239).
All patients underwent ultrasonography-guided liver biopsy using a 19-gauge biopsy needle. To acquire adequate samples, at least two tissues, approximately 2 cm in length, were obtained from each patient. Liver biopsies were routinely formalin-fixed, paraffin-embedded, and assessed by individual pathologists at each institute.
Steatosis (0–3), ballooning (0–2), and lobular inflammation (0–3) were scored to calculate the NAS using the NASH Clinical Research Network (NASH CRN) scoring system (16). The NAS was the sum of steatosis, ballooning, and lobular inflammation grades and ranged from 0 to 8 (16). NASH was defined in two ways: one using NAS ≥5, which is known as a criterion for definite NASH (16), and the other using the fatty liver inhibition of progression (FLIP) definition as the presence of steatosis, hepatocyte ballooning, and lobular inflammation with at least 1 point for each category (FLIP-NASH) (17). Steatosis, activity, and fibrosis (SAF) scores were calculated through the separate assessment of the grade of steatosis (S0–S3), activity (A0–A4 through the addition of ballooning and lobular inflammation), and the stage of fibrosis (F0–F4 with a single modification of pooling the three substages [1a, 1b, and 1c]) according to the NASH CRN (17).
At each hospital, TE (EchoSens, Paris, France) was performed by experienced operators who had conducted at least 500 examinations. Patients were examined after overnight fasting using M probes due to the limited availability of the XL probe in only one institute. LS (kPa) and CAP (dB/m) measurements were recorded until 10 valid measurements were obtained for each patient. The median value was considered representative of the elastic modulus of the liver. Only procedures with at least 10 valid measurements, a success rate of at least 60%, and an interquartile range (IQR) to median value ratio of 30% were considered reliable (18–20).
The patients' body mass index (BMI; kg/m2) and biochemical data were obtained at the time of liver biopsy. The FAST score was calculated according to a previously reported formula using recently measured LS, CAP, and AST levels. Using the patients' biochemical data, individual NFS, FIB-4, and APRI were also calculated according to a previously reported formula (21–23).
The main outcome was the diagnosis of high-risk NASH. The outcomes were assessed in various ways, as suggested in the literature: (1) definite NASH (NAS ≥5) according to the NASH CRN (16), (2) severe FLIP-NASH (with activity ≥A3) (17, 24), (3) severe disease according to the SAF scoring system (severe SAF, activity ≥A3, and/or fibrosis ≥F3) (17, 24), and (4) FLIP-NASH with NAS ≥4 and fibrosis ≥2 (NASH + NAS ≥4 + F ≥2) according to a previous study that suggested the FAST score (12).
Data were expressed as the median and IQR for quantitative data and as numbers with percentages in parentheses for qualitative data, as appropriate. The significance of differences between variables was evaluated using Student's t-test or the Mann–Whitney U test (continuous variables) and the chi-square or Fisher's exact test (categorical variables). Ordinal logistic regression analyses were performed to evaluate the relationship between each non-invasive fibrosis surrogate and each histologic finding (e.g., steatosis, ballooning, lobular inflammation, and fibrosis). The surrogates with higher McFadden's pseudo R-squared values (R2), higher χ2 values through the likelihood ratio, and lower values for Akaike information criteria (AIC) were considered to have a better fit to the scores of the histologic stages. The predictive performance of the FAST score and other non-invasive surrogates for histologic findings and each outcome were assessed using the AUC. AUCs were compared using the calculated 95% confidence intervals (CIs) using the DeLong test. Optimal cutoff values were chosen to maximize the sum of the sensitivity and specificity of the Youden index. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of each surrogate for each outcome were calculated using the cutoff value.
All statistical analyses were conducted using SPSS version 26.0 for Windows (IBM Corp., Armonk, NY, USA) and R package (version 4.1.1, http://cran.r-project.org/). Two-sided P < 0.05 were considered statistically significant.
A flowchart of patient selection is summarized in Figure 1. A total of 496 patients with biopsy-proven NAFLD were considered eligible. After excluding 245 patients who met the exclusion criteria, 251 patients with NAFLD were finally included.
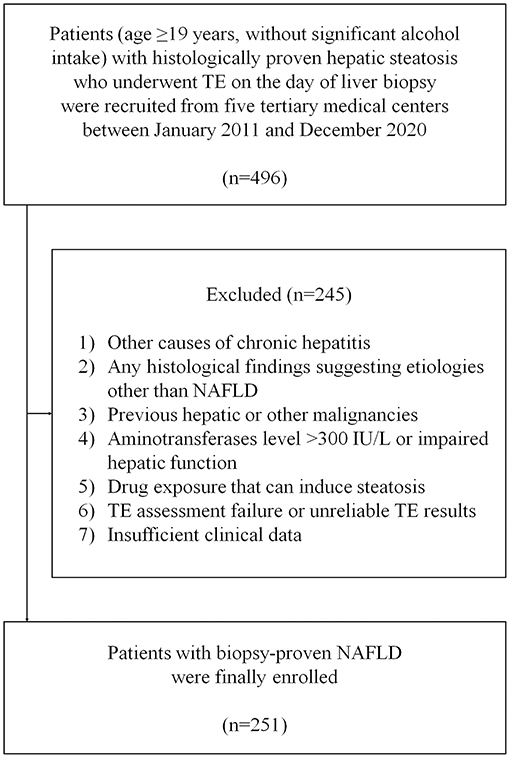
Figure 1. Flowchart of the study. NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; TE, transient elastography.
The median age of the patients (men: 132 [52.6%]) was 44 (IQR: 34–56) years. The proportion of patients with BMI >25 kg/m2 and 30 kg/m2 was 81.7% (n = 205) and 37.8% (n = 95), respectively. Diabetes mellitus or impaired fasting glucose and hypertension were observed in 117 (46.6%) and 78 (31.1%) patients, respectively. The median LS and CAP using TE assessment were 7.8 (IQR: 6.2–11.9) kPa and 316 (IQR: 281–342) dB/m, respectively. The median NFS, FIB-4, and APRI value using biochemical data were −1.976 (IQR: −3.367 to −0.645), 1.09 (IQR: 0.67–1.99), and 0.62 (0.37–0.94), respectively. The median calculated FAST score was 0.54 (IQR: 0.33–0.69) (Table 1). All patients underwent TE on the day of liver biopsy.
The histological information is summarized in Table 2. Grade 3 steatosis, grade 3 lobular inflammation, and grade 2 ballooning were identified in 43 (17.1%), 9 (3.6%), and 88 (35.1%) patients, respectively. Fibrosis stages 0, 1, 2, 3, and 4 were identified in 33 (13.1%), 137 (54.6%), 34 (13.5%), 33 (13.1%), and 14 (5.6%) patients, respectively.
Regarding endpoints, definite NASH, severe FLIP-NASH, severe SAF, and NASH + NAS ≥4 + F ≥2 were reported in 117 (46.6%), 202 (80.5%), 132 (52.6%), and 70 (27.9%) patients, respectively (Table 2).
Ordinal logistic regression analyses of the FAST score, LS, CAP, NFS, FIB-4, and APRI for histologic steatosis, activity, and fibrosis stages are summarized in Supplementary Table 1. A significant correlation was observed between the CAP value (dB/m) and FAST score and the histologic steatosis stages (all P < 0.001) with low R2 values (0.043 and 0.047, respectively). For histologic activity, a significant correlation was found between all noninvasive fibrosis surrogates (all P < 0.05): FAST score showed the highest R2 (0.120) and the lowest AIC values (673.003) compared to those of the other surrogates (all R2 <0.1 and AIC >700, respectively). The LS value (kPa) best explained the degree of histological fibrosis stage (P < 0.001, R2 = 0.196, and AIC = 533.289) compared with other surrogates (all P < 0.001, R2 <0.2, and AIC > 600).
The diagnostic accuracy of each non-invasive surrogate for the prediction of each fibrosis stage (≥F2, ≥F3, and ≥F4) is presented in Table 3. LS values showed higher AUCs in predicting ≥F2 (0.822; 95% CI, 0.767–0.876), ≥F3 (0.925; 95% CI, 0.893–0.957), and ≥F4 (0.963; 95% CI, 0.935–0.991) than FAST, NFS, FIB-4, and APRI values (especially for ≥F3, all P < 0.001). The cutoff values of LS with maximal Youden index were 8.7 kPa for ≥F2, 9.8 kPa for ≥F3, and 15.7 for ≥F4, respectively.
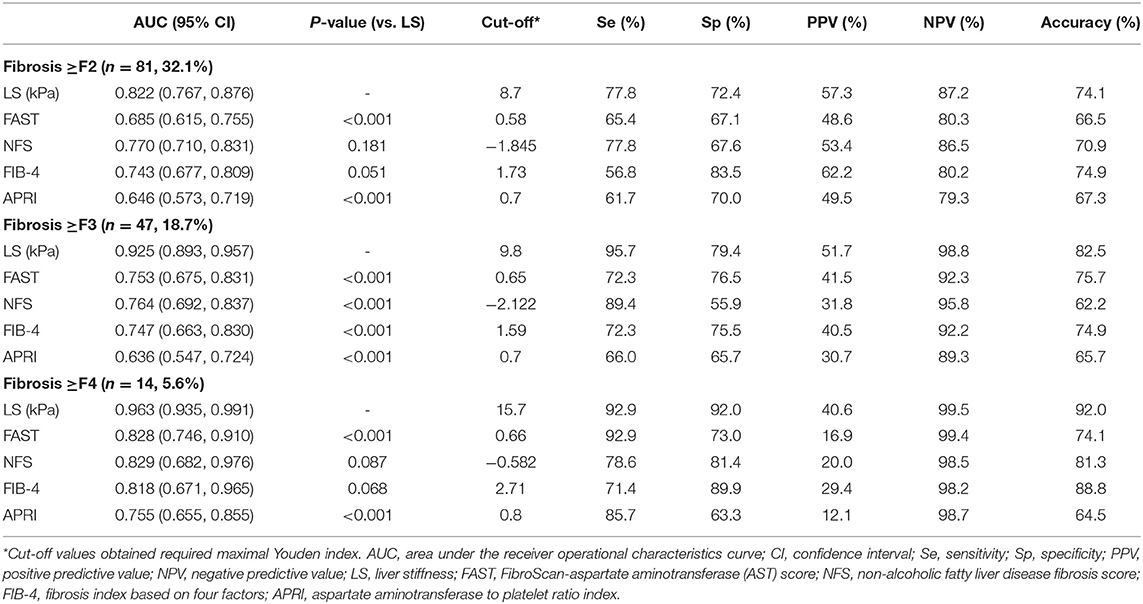
Table 3. Comparisons between non-invasive fibrosis surrogates for predicting histologic fibrosis stage.
The AUCs of CAP for ≥S2 (n = 147, 58.3%) and ≥S3 (n = 43, 17.1%) were 0.685 (95% CI, 0.618–0.752; cutoff, 280 dB/m) and 0.570 (95% CI, 0.482–0.658; cutoff, 281 dB/m), respectively.
The diagnostic accuracies of all surrogates for high-risk NASH are summarized in Table 4. The FAST score showed the highest AUC in predicting definite NASH, severe FLIP-NASH, and severe SAF. For definite NASH (NAS ≥5), the AUC of the FAST score (cutoff: 0.48) was 0.752 (95% CI: 0.692–0.812), which was statistically similar to those of LS (0.718, P = 0.202) and APRI (0.722, P = 0.209) and significantly higher than those of the NFS (0.609, P = 0.002) and FIB-4 (0.650, P = 0.009). For severe FLIP-NASH, the AUC of the FAST score (cutoff: 0.58) was 0.788 (95% CI: 0.732–0.843), which was statistically similar to those of LS (0.754, P = 0.198) and APRI (0.747, P = 0.087) and significantly higher than those of the NFS (0.649, P = 0.002) and FIB-4 (0.701, P = 0.023). For severe SAF, the AUC of the FAST score (cutoff: 0.64) was 0.807 (95% CI: 0.753–0.860), which was similar to that of LS (0.806, P = 0.971) and significantly higher than those of the NFS (0.691, P = 0.009), FIB-4 (0.732, P = 0.048), and APRI (0.760, P = 0.046).
In predicting NASH + NAS ≥4 + F ≥2, the FAST score did not show a superior AUC (0.714; 95% CI, 0.646–0.782), considering the significantly higher AUCs of LS (0.812, P < 0.001), NFS (0.748, P < 0.001), and FIB-4 (0.738, P = 0.021). The cutoff values of the FAST score were obtained as 0.37 for 90% sensitivity and 0.79 for 90% specificity, respectively. Using the Youden index-based cutoff values of the FAST score (0.57), sensitivity, specificity, PPV, NPV, and accuracy were 69.4, 67.0, 45.9, 84.5, and 67.7%, respectively. Using the previously reported rule-out zone (≤ 0.35) and rule-in zone (≥0.67) of the FAST score (12), the sensitivity, specificity, PPV, NPV, and accuracy were 93.1, 35.2, 36.6, 92.6, and 51.8%, respectively, and 56.9, 77.1, 50.0, 81.7, and 71.3%, respectively.
Remarkably, LS itself showed consistently acceptable performances for all outcomes with a fixed cutoff value of 7.7 kPa using the Youden index (Table 4).
This multicenter retrospective cohort study validated the performance of the FAST score and other non-invasive surrogates in detecting high-risk NASH among patients with histologically confirmed NAFLD. The LS value by TE showed the best ordinal correlation (R2 = 0.196 and AIC = 533.289) and predictive performance (AUCs: 0.822–0.963) with the histologic fibrosis stages, whereas the FAST score (R2 = 0.120 and AIC = 673.033) had the best ordinal correlation with the histologic activity compared to that of the LS value only (R2 = 0.047 and AIC = 728.749). The FAST score showed acceptable predictive performance (AUCs: 0.714–0.807) for the various criteria for high-risk NASH: higher AUCs than that of the LS, NFS, FIB-4, and APRI for definite NASH, severe FLIP-NASH, and severe SAF; however, they were significantly inferior in predicting NASH + NAS ≥4 + F ≥2 compared to those of the LS, NFS, and FIB-4 (AUCs: 0.812, 0.748, and 0.738, respectively, all P < 0.05). Comparably, LS alone also showed consistent acceptable predictive performance (AUCs: 0.718–0.812) for all criteria and was significantly higher for NASH + NAS ≥4 + F ≥2 among the surrogates.
Our study had several clinical implications. First, our study is the first to apply the various criteria for “high-risk NASH” for the validation of non-invasive surrogates among patients with histologically confirmed NAFLD. Recent evidence suggests that the association between liver fibrosis and liver- and non-liver-related mortality in patients with NAFLD is stronger than that of NAS or its individual components (25, 26). However, considering that the high histological activity is related to the higher prevalence of metabolic risk factors and can accelerate fibrosis progression (24, 27) and the suggested data that patients with elevated NAS commonly acquire histological responses to trial medication (28), both fibrosis and activity should be included to define “high-risk NASH.” For this reason, Newsome et al. suggested the FLIP-NASH + NAS ≥4 + F ≥2 criteria that reflected the combination of high activity and significant fibrosis (12). However, it is uncertain whether these criteria affect the long-term prognosis of patients with NAFLD. Therefore, this study utilized additional known criteria for histologic NASH diagnosis, NASH with high activity, and NAFLD with high activity and/or fibrosis that were applied in the literature (16, 17, 24), and evaluated that the accuracy of each non-invasive surrogate is consistent among the various criteria.
Second, the predictive performance of various non-invasive surrogates, including the FAST score, LS, NFS, FIB-4, and APRI, was compared for the various criteria with comparable sample sizes (n = 251). The components of the FAST score include the LS, CAP, and AST, which may represent histologic fibrosis, steatosis, and activity, respectively (12), and its dominant performance in the separate validation cohorts compared to that of the FIB-4 and NFS was validated only for the NASH + NAS ≥4 + F ≥2 (12). Ordinal logistic regression analyses showed that the FAST score sufficiently explained the stages of histological activity. For this reason, although not significant, the FAST score showed the highest AUCs in predicting NAS ≥4, severe FLIP-NASH, and severe SAF among the surrogates. These findings suggest that the FAST score may be advantageous in predicting the histologic activity and its related NASH criteria.
However, the predictive performance of the FAST score for the NASH + NAS ≥4 + F ≥2 (AUC: 0.714) was inferior to those of LS, NFS, and FIB-4 and of previously reported AUCs [0.85 in external cohorts (12), 0.76 in the Japanese cohort (14), and 0.75 in the US veterans cohort (13)]. The low number (27.9%) of patients who met the criteria might have caused this result, although numbers were similar in the previous studies (12, 14). Moreover, the insufficient accuracy of the CAP value for histologic steatosis grade might have affected the prediction of the steatosis component of NAS ≥4. In this study, the rule-out cutoff (90% sensitivity) was 0.37, similar to 0.35 (sensitivity, 93.1%; NPV, 92.6%); however, the rule-in cutoff (90% specificity) was 0.79, far higher than 0.65 (specificity, 77.1%; PPV, 50.0%). Therefore, the FAST score may also be advantageous in accurately excluding high-risk NASH considering the similar rule-out cutoff; however, the performance to rule-in high-risk NASH and its reproducibility should be further evaluated.
Third, this study first presented the dominant predictive performance of the LS value compared to that of the FAST score. The results suggest that the LS value alone can sufficiently predict the various criteria of the “high-risk NASH” with consistently high AUCs, even for NASH + NAS ≥4 + F ≥2. In this study, the LS value showed the highest accuracy in predicting the actual histologic fibrosis stages compared to those of the NFS, FIB-4, and APRI. Moreover, the LS value showed the best accuracy for the fibrosis-added criteria (severe SAF and NASH + NAS ≥4 + F ≥2) and acceptable performance even for the activity-dominant criteria (NAS ≥5 and severe FLIP-NASH), with the consistently calculated best cutoff value (7.2 kPa) using the Youden index. Considering that this study was based on a retrospective review of real clinical data, the decision to perform further invasive procedures such as liver biopsy using only the LS value can be more convenient and reliable in clinical practice. However, we should consider that the discordance between CAP and the degree of steatosis might have caused a suboptimal AUC of FAST score for NASH + NAS ≥4 + F ≥2 in this study. Further studies are warranted to determine whether LS or FAST score is more predictable for patients with high-risk NASH.
Our study has several limitations. First, due to its retrospective nature and cross-sectional design, this study could have been subject to selection bias and may only present snapshots of NASH that could be resolved and/or progressed over time (29). Second, the availability of the XL probe in only one institute has limited further analysis regarding the probe type. In this study, 8 of 245 patients (3.3%) were excluded due to TE assessment failure. Even if the effect may be negligible considering that the previous study by Oeda et al. (14) showed a statistically similar accuracy of the FAST score between the M and XL probes in Asian patients, the exclusion of patients due to TE failure associated with high BMI or obesity may have influenced the study results. Third, the absence of a central review of histologic findings by independent pathologists blinded to the clinical data might critically affect the diagnosis of NASH by various criteria, which could disturb the predictive performance of the FAST score in this study. Fourth, further comparison using a magnetic resonance elastography (MRE)-based model, which reported higher accuracy in detecting significant fibrosis (30), was impossible due to the lack of paired MRE data.
In conclusion, the FAST score has acceptable performance in identifying high-risk NASH in Korean patients with histologically confirmed NAFLD and may help avoid unnecessary invasive procedures considering the high NPV. However, LS alone would still be effective, even slightly better than the FAST score, in identifying patients with high-risk NASH.
The datasets presented in this article are not publicly available due to patients' privacy. Requests to access the datasets should be directed to a3N1a29yZWFAeXVocy5hYw==.
The studies involving human participants were reviewed and approved by Yonsei University Health System, Severance Hospital, Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceptualization, data curation, project administration, and writing—original draft by JL and SK. Formal analysis and visualization by JL. Funding acquisition and supervision by SK. Investigation, resources, and writing—review and editing by JL, HL, BK, JP, DK, SA, JJ, SP, HL, CL, and SK. All authors contributed to the article and approved the submitted version.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (2019R1A2C4070136). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.869190/full#supplementary-material
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
2. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Non-alcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. (2019) 17:748–55.e3. doi: 10.1016/j.cgh.2018.05.057
3. Wong RJ, Cheung R. Ahmed A. Non-alcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the US. Hepatology. (2014) 59:2188–95. doi: 10.1002/hep.26986
4. Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, et al. Relative etiological role of prior hepatitis B virus infection and non-alcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion. (2011) 84(Suppl 1):17–22. doi: 10.1159/000333210
5. Park SH, Plank LD, Suk KT, Park YE, Lee J, Choi JH, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998–2017. Clin Mol Hepatol. (2020) 26:209–15. doi: 10.3350/cmh.2019.0065
6. Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. (2016) 17:774. doi: 10.3390/ijms17050774
7. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
8. Soon G, Wee A. Updates in the quantitative assessment of liver fibrosis for non-alcoholic fatty liver disease: histological perspective. Clin Mol Hepatol. (2021) 27:44–57. doi: 10.3350/cmh.2020.0181
9. Castera L, Friedrich-Rust M, Loomba R. Non-invasive assessment of liver disease in patients with non-alcoholic fatty liver disease. Gastroenterology. (2019) 156:1264–81. doi: 10.1053/j.gastro.2018.12.036
10. Muthiah MD, Cheng Han N, Sanyal AJ. A clinical overview of non-alcoholic fatty liver disease: a guide to diagnosis, the clinical features, and complications-what the non-specialist needs to know. Diabetes Obes Metab. (2022) 24(Suppl 2):3–14. doi: 10.1111/dom.14521
11. Sanyal AJ, Shankar SS, Calle RA, Samir AE, Sirlin CB, Sherlock SP, et al. Non-invasive biomarkers of non-alcoholic steatohepatitis: the FNIH NIMBLE project. Nat Med. (2022). doi: 10.1038/s41591-021-01652-8
12. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. (2020) 5:362–73. doi: 10.1016/s2468-1253(19)30383-8
13. Puri P, Jain S, Fuchs M. Use of FibroScan-AST Score to Stratify High-Risk Nonalcoholic steatohepatitis in US veterans. Clin Gastroenterol Hepatol. (2020) 18:3060–1. doi: 10.1016/j.cgh.2020.07.063
14. Oeda S, Takahashi H, Imajo K, Seko Y, Kobayashi T, Ogawa Y, et al. Diagnostic accuracy of FibroScan-AST score to identify non-alcoholic steatohepatitis with significant activity and fibrosis in Japanese patients with non-alcoholic fatty liver disease: comparison between M and XL probes. Hepatol Res. (2020) 50:831–9. doi: 10.1111/hepr.13508
15. Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. (2021) 41:261–70. doi: 10.1111/liv.14669
16. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology. (2005) 41:1313–21. doi: 10.1002/hep.20701
17. Bedossa P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of non-alcoholic fatty liver disease. Hepatology. (2014) 60:565–75. doi: 10.1002/hep.27173
18. Lee HW, Chon YE, Kim SU, Kim BK, Park JY, Kim DY, et al. Predicting liver-related events using transient elastography in chronic hepatitis c patients with sustained virological response. Gut Liver. (2016) 10:429–36. doi: 10.5009/gnl15021
19. Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol. (2012) 18:163–73. doi: 10.3350/cmh.2012.18.2.163
20. Zhang X, Wong GL, Wong VW. Application of transient elastography in non-alcoholic fatty liver disease. Clin Mol Hepatol. (2020) 26:128–41. doi: 10.3350/cmh.2019.0001n
21. Treeprasertsuk S, Björnsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol. (2013) 19:1219–29. doi: 10.3748/wjg.v19.i8.1219
22. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple non-invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. (2006) 43:1317–25. doi: 10.1002/hep.21178
23. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38:518–26. doi: 10.1053/jhep.2003.50346
24. Nascimbeni F, Bedossa P, Fedchuk L, Pais R, Charlotte F, Lebray P, et al. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J Hepatol. (2020) 72:828–38. doi: 10.1016/j.jhep.2019.12.008
25. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with non-alcoholic fatty liver disease. Gastroenterology. (2015) 149:389–97. doi: 10.1053/j.gastro.2015.04.043
26. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. (2015) 61:1547–54. doi: 10.1002/hep.27368
27. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in non-alcoholic fatty liver vs. non-alcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. (2015) 13:643–54. doi: 10.1016/j.cgh.2014.04.014
28. Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an Agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of non-alcoholic steatohepatitis without fibrosis worsening. Gastroenterology. (2016) 150:1147–59. doi: 10.1053/j.gastro.2016.01.038
29. Ng CH, Xiao J, Lim WH, Chin YH, Yong JN, Tan DJH, et al. Placebo effect on progression and regression in NASH: evidence from a meta-analysis. Hepatology. (2022). doi: 10.1002/hep.32315 [Epub ahead of print].
Keywords: fatty liver, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, liver function tests, fibrosis, elasticity imaging techniques
Citation: Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Jang JY, Park SY, Lee HW, Lee CK and Kim SU (2022) Comparison of FibroScan-Aspartate Aminotransferase (FAST) Score and Other Non-invasive Surrogates in Predicting High-Risk Non-alcoholic Steatohepatitis Criteria. Front. Med. 9:869190. doi: 10.3389/fmed.2022.869190
Received: 04 February 2022; Accepted: 11 March 2022;
Published: 14 April 2022.
Edited by:
Daniel Q. Huang, National University of Singapore, SingaporeReviewed by:
Ng Cheng Han, National University of Singapore, SingaporeCopyright © 2022 Lee, Lee, Kim, Park, Kim, Ahn, Jang, Park, Lee, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung Up Kim, a3N1a29yZWFAeXVocy5hYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.