
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 19 April 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.867293
This article is part of the Research Topic Challenges in Inflammatory Bowel Disease: Current, Future and Unmet Needs View all 13 articles
 Mohamed Elbadry1
Mohamed Elbadry1 Mohamed O. Nour2,3
Mohamed O. Nour2,3 Mohamed Hussien4
Mohamed Hussien4 Elsayed Awad Ghoneem5
Elsayed Awad Ghoneem5 Mohammed A. Medhat6
Mohammed A. Medhat6 Hany Shehab7
Hany Shehab7 Sherif Galal8
Sherif Galal8 Mohamed Eltabbakh9
Mohamed Eltabbakh9 Fathiya El-Raey10
Fathiya El-Raey10 Mohamed Negm7
Mohamed Negm7 Shimaa Afify11
Shimaa Afify11 Walaa Abdelhamed12
Walaa Abdelhamed12 Ahmed Sherief9
Ahmed Sherief9 Ahmed Abdelaziz10
Ahmed Abdelaziz10 Mohamed Abo Elkasem11
Mohamed Abo Elkasem11 Aya Mahrous4
Aya Mahrous4 Ghada Kamal6
Ghada Kamal6 Maha Maher5
Maha Maher5 Omar Abdel-Hameed5
Omar Abdel-Hameed5 Abubakr Elbasuny13
Abubakr Elbasuny13 Islam El-Zayyadi5
Islam El-Zayyadi5 Ahmed Bassiony9
Ahmed Bassiony9 Abdelmajeed Moussa14
Abdelmajeed Moussa14 Essam Bedewy15
Essam Bedewy15 Asem Elfert16
Asem Elfert16 Mohamed El Kassas1*
Mohamed El Kassas1*Background and Aims: Ulcerative colitis (UC) and Crohn's disease (CD) are the most common types of Inflammatory bowel disease (IBD), with variable responses to traditional therapies and unpredicted prognosis. In Egypt and most developing countries, the lack of recent epidemiological and prognostic data adversely affects management strategies. We collected and analyzed data of patients with IBD from multiple centers across Egypt to evaluate patients' clinical and epidemiological characteristics.
Methods: This retrospective multicenter study included patients diagnosed with IBD between May 2018 and August 2021, at 14 tertiary gastroenterology units across Egypt. Record analysis addressed a combination of clinico-epidemiological characteristics, biochemical tests, stool markers, endoscopic features, histological information, and different lines for IBD treatment.
Results: We identified 1104 patients with an established diagnosis of IBD; 81% of them had UC, and 19% showed CD. The mean age of onset was 35.1 ± 12.5 years ranging from 5 to 88 years, the mean duration of illness at inclusion was 13.6 ± 16.7 years, gender distribution was almost equal with a significant male dominance (60.4%, p = 0.003) among patients with CD, 57% were living in rural areas, and 70.5% were from Delta and Coastal areas. Two hundred nineteen patients (19.8%) displayed comorbid conditions, primarily associated with CD. The most frequent complaints were diarrhea (73.2%), rectal bleeding (54.6%) that was significantly higher among patients with UC (64%, p < 0.001), and 46.8% with abdominal pain (more often with CD: 71%, p < 0.001). Conventional therapy was effective in treating 94.7% of patients. The main lesion in patients with CD was ileal (47.8%); patients with UC mainly exhibited proctosigmoiditis (28.4%). Dysplasia was detected in 7.2% of patients, mainly subjects with UC.
Conclusions: To our knowledge, our effort is the first and largest cohort of Egyptian patients with IBD to describe clinical and epidemiological characteristics, and diagnostic and management approaches. More extensive prospective studies are still needed to fully characterize disease distribution, environmental factors, and pathological features of the disease.
In 2017, 6.8 million Inflammatory bowel disease (IBD) cases were reported globally. The prevalence of IBD has increased substantially in many regions of the world, creating a substantial social and economic burden on health systems (1). Ulcerative colitis (UC) and Crohn's disease (CD) are major chronic IBD conditions in addition to indeterminate colitis that cause varying degrees of gastrointestinal (GI) tract inflammation. This classification was addressed by Montreal working party and commonly used in clinical practice and also for future serological and genetic studies in IBD (2). Data from several studies report a relationship between smoking and CD. However, quitting smoking may be accompanied by an increased risk of UC (3). Dietary fiber (particularly vegetables and fruits), saturated fats, sleep disorders, depression, and low vitamin D levels have been associated with increased IBD incidence. Also, stress, microbiota, some medications, such as NSAIDs, and early antibiotic exposure during infancy are factors that might increase the risk for IBD incidence, especially in genetically susceptible individuals (4, 5). Therefore, some studies concluded that, modification of one or more of these environmental factors has a bidirectional effect on the disease activity (6). Disease presentation includes signs and symptoms such as diarrhea, rectal bleeding, and abdominal pain. Fever, weight loss, extraintestinal symptoms, and fatigue may be observed (7). A serious complication of IBD is the malignant transformation of colonic mucosa that increases the incidence of colorectal carcinoma. Further, psychiatric health is negatively affected by IBD, especially in young patients (8). Also, extraintestinal manifestations (EIMs) can occur in some patients and affect joints, the hepatobiliary system, skin, and eyes (9).
Assessment of patients with IBD includes physical examination with a focus on extraintestinal complications, laboratory evaluation [C-reactive protein (CRP), erythrocytes sedimentation rate (ESR), and fecal calprotectin (FC)], endoscopy, and different imaging modalities (10). All these clinical examinations are used to confirm the diagnosis, gauge disease extent, and evaluate severity. Treatment depends on disease extent and severity (7).
The disease is a worldwide concern, but the incidence is highest in the United States, Sweden, and United Kingdom (11–13). IBD has a growing incidence in the Middle East and North Africa; however, a lack of accurate registry and epidemiological cohort studies are still obstacles to evaluating the current situation (14–16). UC is more common than CD in various parts of the world. In Egypt, few data regarding the epidemiology of IBD are available; however, some studies suggest the relative incidence ratio of UC and CD is 6:1 (17).
We conducted this retrospective cross-sectional multicenter study to represent all of Egypt. We collected clinical information to evaluate epidemiological characteristics, laboratory and imaging findings, colonoscopy results, and different treatment strategies. We aimed to assess updated data to draw a real map of IBD in Egypt in order to help us introduce scientific recommendations to optimize IBD diagnosis and management strategies.
This retrospective multicenter study was conducted on patients diagnosed with IBD at 14 tertiary GI units. These centers are affiliated with universities, distributed across Egypt, and represent the main geographical areas within the country where most of the Egyptian population is concentrated along the banks of the Nile River and on the river's delta including (1) Greater Cairo (Helwan, Cairo, Ain Shams, Al-Azhar, and the National Hepatology and Tropical Medicine Research Institute), (2) Delta region (Mansoura, Tanta, Zegazeg, and Kafr Elsheikh), (3) Coastal region (Alexandria and Damietta), (4) Upper Egypt (Sohag, Assiut, and Aswan). Patients from remote areas in Egypt, Sinai Peninsula, and Oases are largely referred to these tertiary centers. Data were collected both manually and electronically from available medical records between May 2018 and August 2021. Medical records of patients of all ages and both sexes with a confirmed diagnosis of IBD during the study period were enrolled.
Demographic features recorded were age of onset, gender, residence, geographic area, duration of illness, history of smoking, associated comorbidities, and other autoimmune diseases. Disease characteristics, including the severity of symptoms, presence of EIMs, type of medical treatment, and history of surgical intervention, were tabulated. Remission was defined as complete resolution of signs and symptoms, and endoscopic and histological healing of colon mucosa.
The diagnosis of CD or UC was based on a combination of clinical manifestations, biochemical tests, stool markers, endoscopic features, and histological evaluation.
The recorded biochemical information included complete blood count (CBC), CRP, ESR, liver, and kidney function tests. Also, FC as a stool marker of intestinal inflammation was noted. Reports of abdominal ultrasonography and upper endoscopy were retrieved for all enrolled patients.
Endoscopic features consistent with UC were continuous and confluent colon inflammation with clear demarcation and rectal involvement. Endoscopic features consistent with CD were discontinuous lesions, mucosal nodularity, ulceration (both aphthous and linear), and strictures. Disease distribution and activity of UC were evaluated using the Montreal classification and Mayo score, respectively. While patients with CD were evaluated according to the severity of the onset of the disease using Harvey-Bradshaw score and Phenotypic distribution according to Montreal classification (2, 18).
All recorded data about treatment approach either step up or step down, type of treatment either topical or systemic or biological in addition to antibiotic and steroid therapy were extracted from the patients‘ medical records and statistically analyzed.
All procedures involving human participants were carried out according to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments, or comparable ethical standards. The study was approved by the Research Ethics Committee for human subject research at the Faculty of Medicine, Helwan University (Serial: 76-2021). The study data set was fully anonymized.
Analysis used SPSS version 25.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp., USA). Mean ± SD was used for quantitative variables, and frequency and percentage were used for qualitative variables. Mann-Whitney and Wilcoxon tests were used to assess the differences in means of quantitative non-parametric variables. Chi-square and Fisher's Exact tests were used to assess differences in the frequency of qualitative variables. The statistical methods assumed a significance level of p < 0.05 and a highly significant level of p < 0.001.
The study included 1,104 patients with an established diagnosis of IBD. Ulcerative colitis was diagnosed in 81.3% of the study population, while −18.7% exhibited CD. The mean age of onset was 35.1 ± 12.5 years ranging from 5 to 88 years with mean duration of illness at inclusion of 13.6 ± 16.7 years for the cohort. The gender distribution was almost equal; however, a significant male dominance (60.4%, p = 0.003) was observed among patients with CD. The residence of the recruited patients was found to be in rural areas in 57% of cases. These patients displayed a higher prevalence of UC (59.2%, p = 0.002). Further, 70.5% were from North of Egypt. About of patients suffered from comorbid conditions, mainly hypertension (HTN) (9.1%) and diabetes (DM) (8.4%). Most comorbidities were more frequent among patients with CD, except for DM and other autoimmune diseases (Table 1).
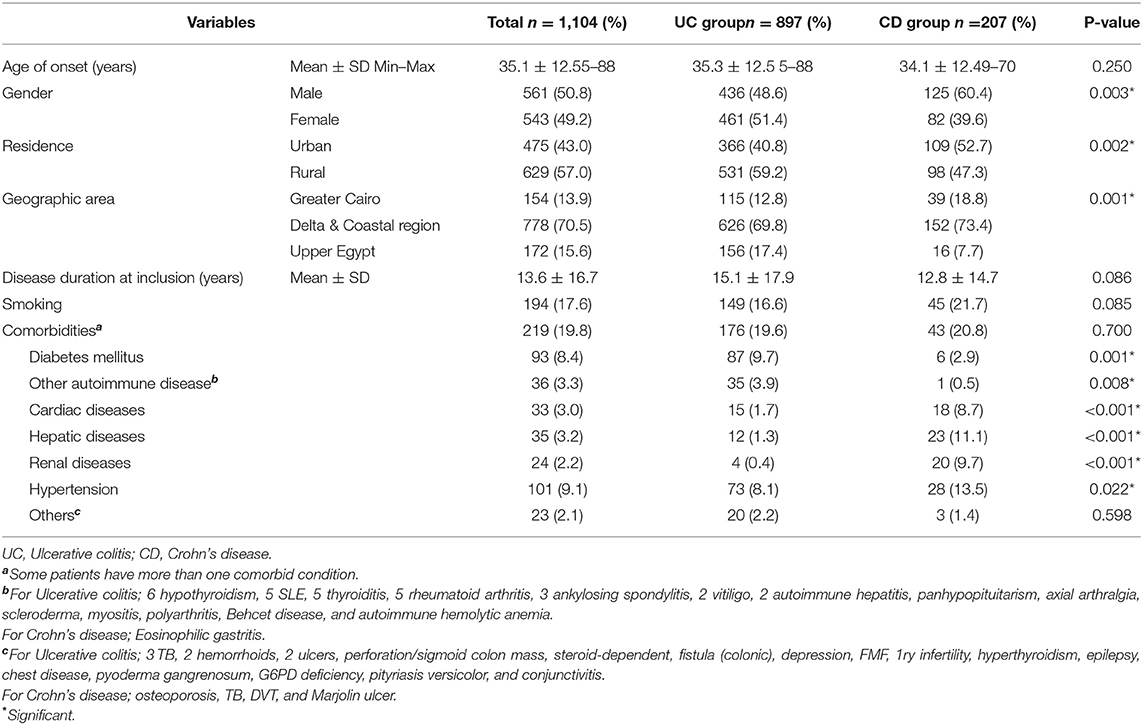
Table 1. General characteristics of patients with inflammatory bowel disease in Egypt (May 2018 to August 2021).
The most frequent manifestations, at the time of diagnosis, among all patients was diarrhea (73.2%) which was similar for both UC and CD. Bowel movements/day was 4.7 ± 2.2 ranging from 0 to 20. -Rectal bleeding was reported by 54.6% of patients, and it was significantly higher among UC subjects, while 48.6% -of patients complained from abdominal pain. Abdominal pain was higher among patients with CD, but the difference was insignificant. Weight loss was seen in 15.3% of patients, and 11.7% displayed EIM, mainly arthropathy. Fever was the least frequent symptom (8.5%) and was significantly higher among subjects with CD (Table 2).
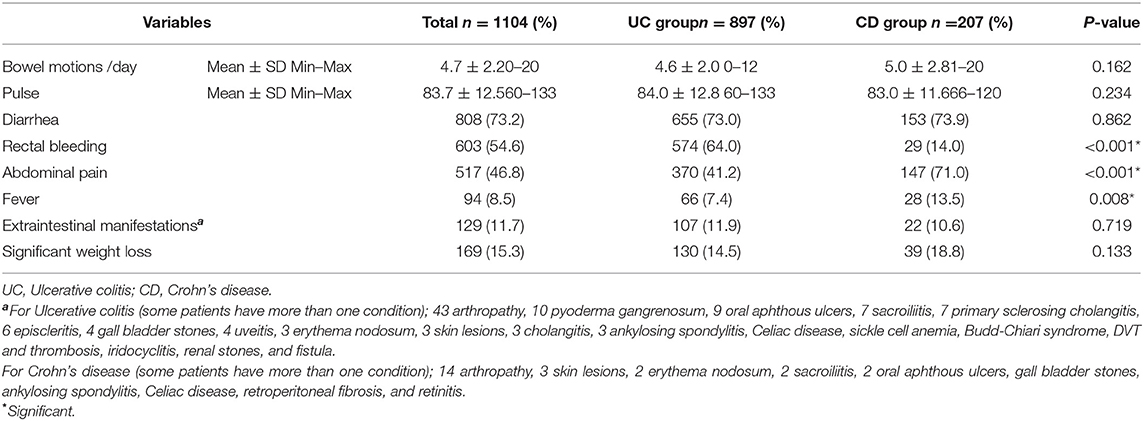
Table 2. Clinical characteristics of patients with inflammatory bowel disease in Egypt (May 2018 to August 2021).
Laboratory characteristics at the time of diagnosis showed significantly improved HB, CRP, ESR, WBC, and FC levels after treatment in both UC and CD patients (Table 3). PLT and AST were decreased in CD and UC patients, respectively. No notable change was observed in serum levels of total protein, albumin, and ALT.
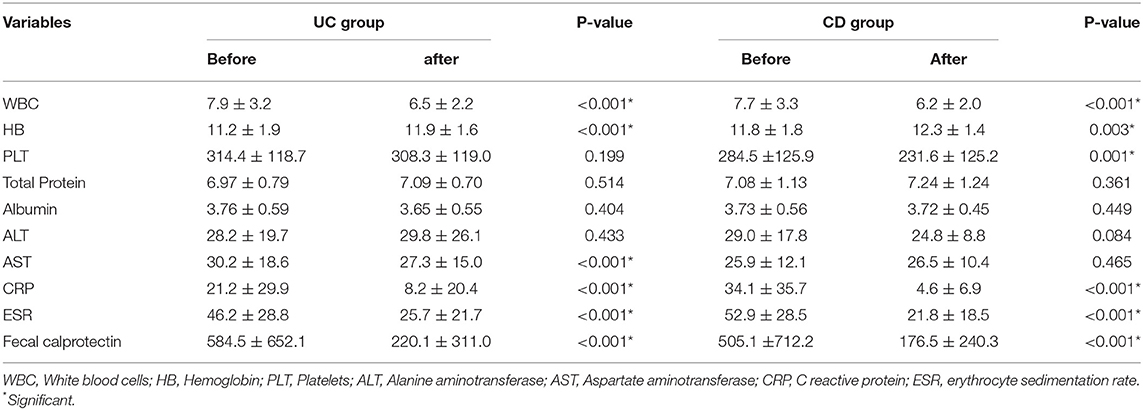
Table 3. Laboratory characteristics of patients with inflammatory bowel disease in Egypt (May 2018 to August 2021) before and after treatment.
patients with patients with The lesions in CD subjects at diagnosis were mainly ileal (47.8%) and ileocolonic (38.6%). Lesions were mainly non-stricturing and non-penetrating (61.4%) and less commonly stricturing (20.8%) (Figure 1).
At diagnosis, CT/MRI revealed fistulization in about 11.1% of patients, and no abnormality was detected in about 18.4%. Colonoscopy showed proctosigmoiditis in 28.4% of patients with UC, proctitis in 25.1%, and pancolitis in 22.9%. Mild, moderate, and severe lesions were detected in 44.7, 35.8, and 19.5%, respectively (Figure 2).
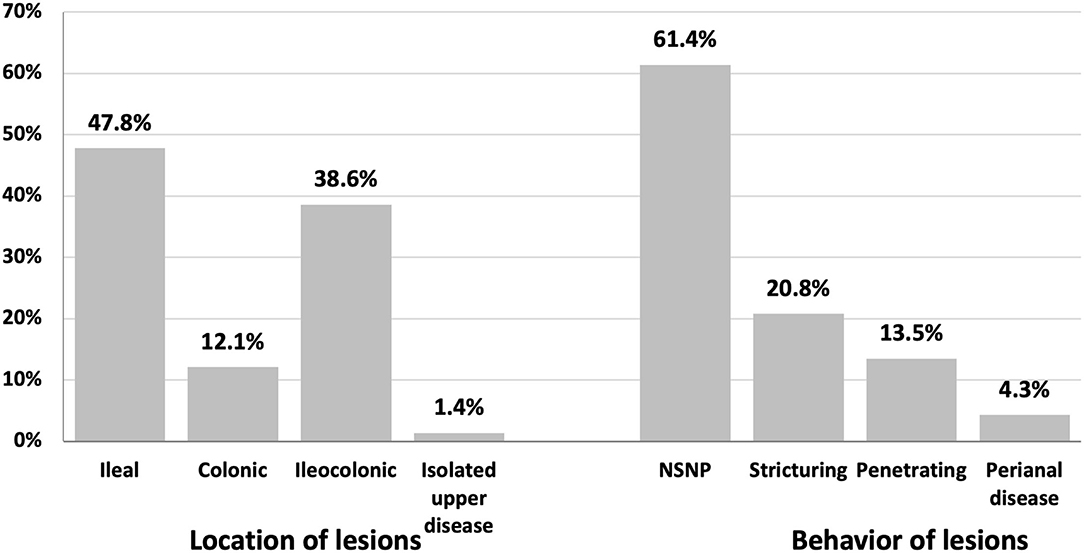
Figure 2. Location and behavior of lesions by colonoscopy in patients with Crohn's disease in Egypt (May 2018 to August 2021) at the time of diagnosis.
Dysplasia was detected histologically in 80 patients (7.2%), mainly associated with UC (75 patients). Low-grade dysplasia was predominant, seen in 68 patients (6.2%). Sixty-four patients were diagnosed with UC (p = 0.012).
Most patients (94.7%) were managed using a step-up approach. The main antibiotics used were quinolone and metronidazole (40–50%). Topical therapies available in Egypt are (5-ASA suppositories and budesonide enema, and they) were used in 21–31% of cases. Unfortunately, other forms of topical therapies (5-ASA enemas, 5-ASA foams, 5-ASA gels, Corticosteroids foams, tacrolimus suppositories, and cyclosporin enemas) are not present in Egypt. Systemic treatment was prescribed for 50–86%, mainly with 5-ASA. About one-fourth received biological treatment, and only 2.6% were referred for coloniccolectomy or intestinal resection and anastomosis (Table 4).
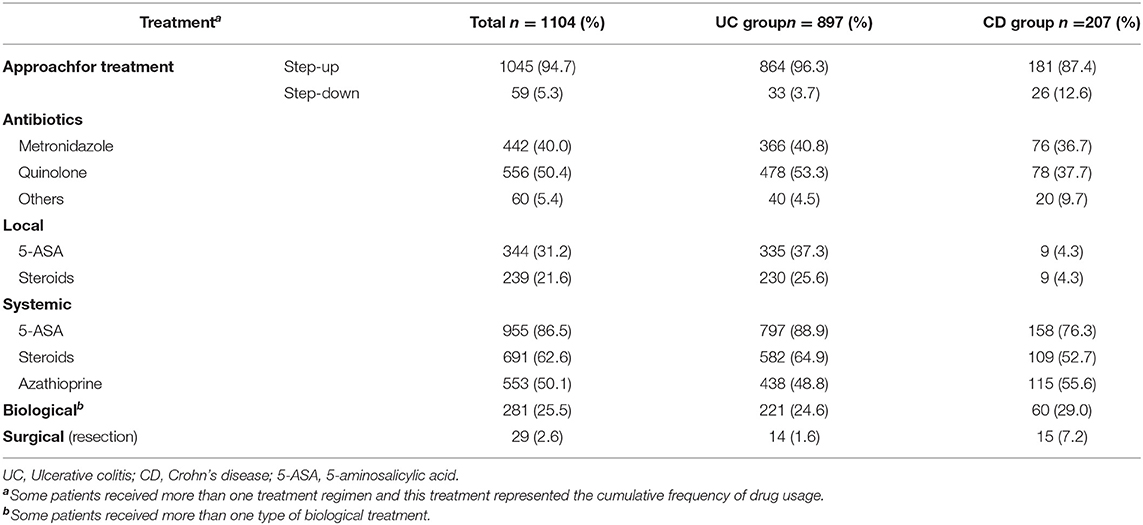
Table 4. Treatment characteristics of patients with inflammatory bowel disease in Egypt (May 2018 to August 2021).
This study is the largest cohort evaluated for IBD in Egypt to the best of our knowledge. We found that the prevalent cases of UC are about four-fold greater than CD. This ratio is higher than reported elsewhere. In 2009, census data estimated that 1,171,000 Americans exhibited IBD (565,000 CD and 593,000 UC) (19). In 2018, the overall prevalence of IBD, CD, and UC in the UK were 725, 276, and 397 per 100 000 people, respectively (20). The occurrence of bleeding, which is more common in UC, is a potent stimulant for seeking medical advice, and so UC cases can be identified more frequently than CD cases. Furthermore, the diagnosis of CD may require exhaustive workup including detailed history taking, laboratory investigations, imaging modalities, and colonoscopy with ileal intubation or even enterostomy. These diagnostic facilities are expensive (not fully covered by insurance) and not available in all centers, particularly that more than half of our study population are from rural areas. However, such differences may be due to many factors that should be studied in community-based research. Key issues may be socioeconomic status, environmental conditions, and access to diagnostic and treatment facilities.
Our study's mean age of onset was 35 and 34 years for UC and CD subjects, respectively. The onset of IBD in adults was reported as 31–34 years in North America, Western Europe, and Oceania (21–25). In Asia, the median age at diagnosis of CD was 34 years, and for UC was 42 years. UC was also found to start at more advanced ages, up to 79 years (26, 27).
The development of UC in our cohort was independent of gender, and however, CD was more common in males. These findings are consistent with epidemiological data from Europe, North America, and Oceania (21–25). However, UC was more common in males in data from Asia (28).
More than half of the study population was from rural areas, inconsistent with the available literature on IBD demographics. The urban population in Egypt has been almost stable since 2010 and represents 42.8% of the Egyptian population (29). However, the lifestyle in rural areas has been urbanized, and this change should be studied selectively. Zuo et al. (30) indicated that rapid urbanization in the developing world is associated with an increasing incidence of several autoimmune diseases, including IBD. Urbanization impacts gut microbiota through westernization of diet, raised pollution levels, increased usage of antibiotics, and better hygiene status. A westernized diet is low in carbohydrates and high in animal proteins and fats. This diet will alter gut microbiota.
The clinical presentation of IBD in our cohort is consistent with globally published data. The predominant manifestations of UC were diarrhea, rectal bleeding, and mucous discharge from the rectum (31). Conversely, CD was characterized by prolonged intermittent diarrhea with abdominal pain (32–35).
Ileal (47.8%) and ileocolonic (38.6%) regions were predominant sites of CD lesions, Colonic lesions were seen in (12.1%), while isolated upper GI CD was detected in only 1.4% of our study population. Slightly less than two-thirds of our patients (61.4%) showed non-complicated disease. Twenty-point eight percent exhibit stricturing CD, and 13.5% show penetrating CD. These data are consistent with data from Europe and North America, where 27–42% of patients with CD have ileal lesions at the time of diagnosis, 23–33% exhibit ileocolonic disease, 28–35% show colonic lesions, and only 1–6% of patients present with upper GI CD (24, 36–39). Data from stricturing CD is estimated to occur in 29% and 19% of patients with CD in Europe (38) and North America (36), respectively. Conversely, Asian cohorts reported that more than half of patients with CD have an ileocolonic disease (40–42).
Multiple serum biomarkers were evaluated for their ability to confirm the IBD disease type and to predict the disease course. The atypical antineutrophil cytoplasmic antibody (atypical pANCA) and anti-Saccharomyces cerevisiae antibody (ASCA) are the most studied antibodies in IBD patients. is most often expressed by patients with ulcerative colitis. Atypical pANCA is more common patients in patients with UC while ASCA in more frequent in CD patients (43). However, these tests are expensive and covered by health insurance, so they are commonly tested in Egypt.
Proctosigmoiditis was the most common disease site in UC cases in our cohort. Proctitis, left-sided colitis, and pancolitis were also seen in order of descending incidence. Most cases were mild to moderate at diagnosis. Disease extent and severity by colonoscopy in patients with ulcerative colitis are shown in Figure 3. Other cohorts showed that left-sided colitis was the most common disease site in Europe (39, 44).
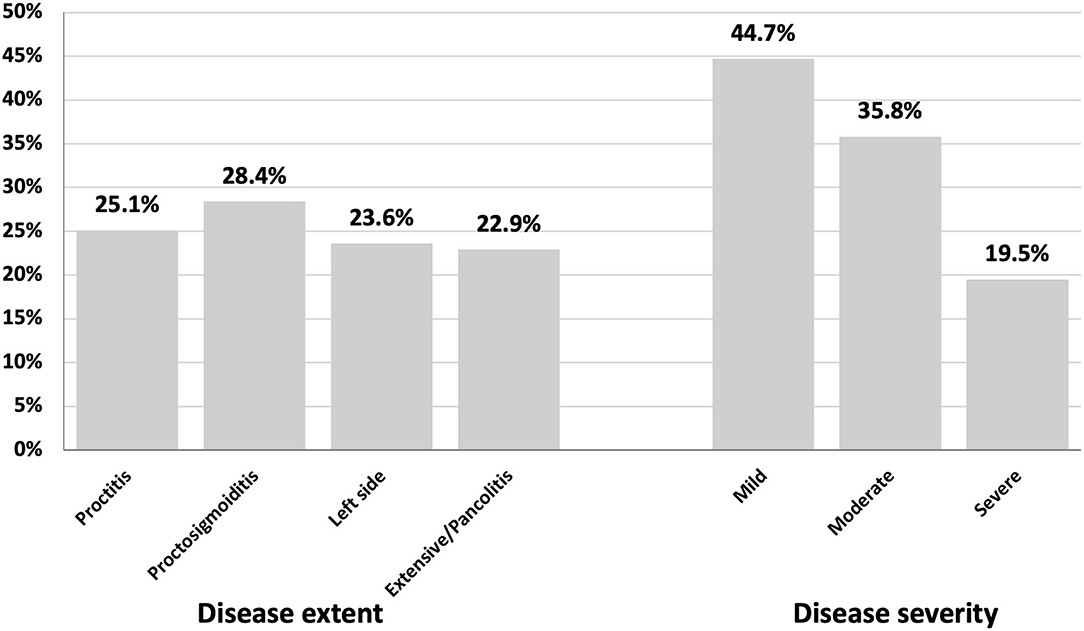
Figure 3. Disease extent and severity by colonoscopy in patients with ulcerative colitis in Egypt (May 2018 to August 2021).
Most of our patients (96.3% of patients with UC and 87.4% of patients with CD) received conventional medical therapy as a first-line treatment even if they were indicated for biological therapy. The high costs of biological therapy likely limit its use, which may not be sustainable. However, health insurance and government-sponsored treatment have started to cover the costs of biological therapy at many centers in Egypt. Infliximab and adalimumab began to be used in 2013, and new biological therapies, such as ustekinumab have been used more recently (45).
Fortunately, conventional step-up therapy is a practical approach for treating Egyptian patients with IBD. Significant improvement in inflammatory markers, including CRP, ESR, and FC, were observed after treatment of both UC and CD.
Colorectal cancer is the most serious complication for patients with IBD, and dysplasia usually precedes the development of colorectal cancer (46). In our study, cases with confirmed dysplastic lesions constituted 7.2% of the total population. Most were low-grade and discovered on the first diagnosis.
Available evidence suggests that IBD incidence in Africa is increasing. Data are still lacking to understand the disease pattern across this continent. A few reports from North and South Africa are available (47).
The major strengths of our study are its large sample size and geographic diversity of patients, and collected data provide a comprehensive, updated picture of IBD in Egypt. Data describe differences in some clinical, epidemiological aspects of the disease. However, the study has some limitations. First, the distinction between UC and CD was not always clear. We depended on the most likely diagnosis from the treating physician. Lacking the distinction between IBD subtypes is a global problem. In a recently published population-based cohort study in European countries including 1,289 patients with IBD, the confirmation of IBD subtype was impossible in 7% of the study population, even after 5 years of follow-up (48). Second, the incomplete data for the follow-up of some patients did not allow us to track the changes in the disease patterns or to do a statistical analysis to see the histological features at time diagnosis will affect the outcome of our patients. Third, we may have missed early mild cases of IBD, especially in the private sector. Fourth, risk factors and environmental determinants of IBD were not discussed. Finally, certain socioeconomic factors, such as income and education, in addition to the yearly incidence of new cases could not be assessed.
To our knowledge, our study is the first Egyptian cohort study from multiple highly specialized GI centers from all over the country. It examines the largest cohort of Egyptian patients with IBD and describes the clinical, epidemiological presentation, diagnostic procedures, disease behavior, and prognostic implications along with available therapeutic options. The number of IBD cases was higher in rural than urban areas despite limited resources and relatively poor facilities in rural areas. More effort should be directed toward screening patients with IBD in rural areas for early detection and proper management of the disease. Such effort may relieve the burden of unexpected serious maladies. Step-up conventional therapy for patients with IBD is still recommended and effective, especially in countries with limited resources. More extensive prospective epidemiological studies in Egypt, other countries of the Middle East, and Africa are still needed to fully characterize disease distribution, environmental factors, and pathological features of IBD. Such data can be compared with other parts of the world to complete the global map of IBD and produce worldwide guidelines for managing this severe expanding disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Research Ethics Committee for human subject research at the Faculty of Medicine, Helwan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MElb and MEK: study design. MON: data analysis. MElb, MON, MH, EG, MAM, HS, SG, MElt, FE-R,MN, SA,WA, AS, AA, MA, AMa, GK, MM, OA-H, AE, IE-Z, AB, AMo, EB, and AE: patient recruitment, data collection, and writing up of the first draft of the paper. All authors revised and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4
2. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. (2006) 55:749–53. doi: 10.1136/gut.2005.082909
3. Karban A, Eliakim R. Effect of smoking on inflammatory bowel disease: Is it disease or organ specific? World J Gastroenterol. (2007) 13:2150–2. doi: 10.3748/wjg.v13.i15.2150
4. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. (2020) 145:16–27. doi: 10.1016/j.jaci.2019.11.003
5. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. (2015) 12:205–17. doi: 10.1038/nrgastro.2015.34
6. Vedamurthy A, Ananthakrishnan AN. Influence of environmental factors in the development and outcomes of inflammatory bowel disease. Gastroenterol Hepatol (N Y). (2019) 15:72–82.
7. Veauthier B, Hornecker JR. Crohn's disease: diagnosis and management. Am Fam Physician. (2018) 98:661–9.
8. Bernstein CN, Benchimol EI, Bitton A, Murthy SK, Nguyen GC, Lee K, et al. The impact of inflammatory bowel disease in Canada 2018: extraintestinal diseases in IBD. J Can Assoc Gastroenterol. (2019) 2:S73–80. doi: 10.1093/jcag/gwy053
9. Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. (2019) 12:113–22.
10. Chen P, Zhou G, Lin J, Li L, Zeng Z, Chen M, et al. Serum biomarkers for inflammatory bowel disease. Front Med. (2020) 7:123. doi: 10.3389/fmed.2020.00123
11. Trallori G, Palli D, Saieva C, Bardazzi G, Bonanomi AG, d'Albasio G, et al. A population-based study of inflammatory bowel disease in Florence over 15 years (1978-92). Scand J Gastroenterol. (1996) 31:892–9. doi: 10.3109/00365529609051998
12. Logan RF. Inflammatory bowel disease incidence: up, down or unchanged? Gut. (1998) 42:309–11. doi: 10.1136/gut.42.3.309
13. Ehlin AG, Montgomery SM, Ekbom A, Pounder RE, Wakefield AJ. Prevalence of gastrointestinal diseases in two British national birth cohorts. Gut. (2003) 52:1117–21. doi: 10.1136/gut.52.8.1117
14. Mosli M, Alawadhi S, Hasan F, Abou Rached A, Sanai F, Danese S. Incidence, prevalence, and clinical epidemiology of inflammatory bowel disease in the Arab World: a systematic review and meta-analysis. Inflamm Intest Dis. (2021) 6:123–31. doi: 10.1159/000518003
15. Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol. (2013) 5:237–47. doi: 10.2147/CLEP.S33961
16. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. (2012) 142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001
17. Esmat S, El Nady M, Elfekki M, Elsherif Y, Naga M. Epidemiological and clinical characteristics of inflammatory bowel diseases in Cairo, Egypt. World J Gastroenterol. (2014) 20:814–21. doi: 10.3748/wjg.v20.i3.814
18. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. (2008) 14:1660–6. doi: 10.1002/ibd.20520
19. Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. (2013) 58:519–25. doi: 10.1007/s10620-012-2371-5
20. Pasvol TJ, Horsfall L, Bloom S, Segal AW, Sabin C, Field N. etal. Incidence and prevalence of inflammatory bowel disease in UK primary care: a population-based cohort study. BMJ Open. (2020) 10:e036584. doi: 10.1136/bmjopen-2019-036584
21. Lakatos L, Kiss LS, David G, Pandur T, Erdelyi Z, Mester G, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. (2011) 17:2558–65. doi: 10.1002/ibd.21607
22. Vegh Z, Burisch J, Pedersen N, Kaimakliotis I, Duricova D, Bortlik M, et al. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: results of the 2011 ECCO-EpiCom inception cohort. J Crohns Colitis. (2014) 8:1506–15. doi: 10.1016/j.crohns.2014.06.004
23. Rönnblom A, Holmström T, Tanghöj H, Karlbom U, Thörn M, Sjöberg D. Low colectomy rate five years after diagnosis of ulcerative colitis. Results from a prospective population-based cohort in Sweden (ICURE) diagnosed during 2005-2009. Scand J Gastroenterol. (2016) 51:1339–44. doi: 10.1080/00365521.2016.1200141
24. Su HY, Gupta V, Day AS, Gearry RB. Rising incidence of inflammatory bowel disease in Canterbury, New Zealand. Inflamm Bowel Dis. (2016) 22:2238–44. doi: 10.1097/MIB.0000000000000829
25. Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV. Incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted county, minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. (2017) 15:857–63. doi: 10.1016/j.cgh.2016.10.039
26. Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. (2008) 14:542–9. doi: 10.1002/ibd.20310
27. Ng SC, Leung WK, Shi HY Li MK, Leung CM, Ng CK, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis. (2016) 22:1954–60. doi: 10.1097/MIB.0000000000000846
28. Zeng Z, Zhu Z, Yang Y, Ruan W, Peng X, Su Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. (2013) 28:1148–53. doi: 10.1111/jgh.12164
29. The World Bank. United Nations Population Division. World Urbanization Prospects: 2018 Revision. Urban population (% of total population)—Egypt, Arab Rep. (2018). Available online at: https://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS?locations=EG (accessed 09 October, 2021).
30. Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2018) 15:440–52. doi: 10.1038/s41575-018-0003-z
31. Kornbluth A Sachar DB Practice Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. (2010) 105:501–23. doi: 10.1038/ajg.2009.727
32. Adams DH. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. Gut. (2007) 56:1175. doi: 10.1136/gut.2007.121533
33. Thoreson R, Cullen JJ. Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am. (2007) 87:575–85. doi: 10.1016/j.suc.2007.03.001
34. Panés J, Gomollón F, Taxonera C, Hinojosa J, Clofent J, Nos P. Crohn's disease: a review of current treatment with a focus on biologics. Drugs. (2007) 67:2511–37. doi: 10.2165/00003495-200767170-00005
35. Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. (2014) 20:1082–96. doi: 10.2174/13816128113199990416
36. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. (2012) 107:1693–701. doi: 10.1038/ajg.2012.298
37. Vester-Andersen MK, Prosberg MV, Jess T, Andersson M, Bengtsson BG, Blixt T, et al. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. (2014) 109:705–14. doi: 10.1038/ajg.2014.45
38. Jeuring SF, van den Heuvel TR, Liu LY, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, et al. Improvements in the long-term outcome of crohn's disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort. Am J Gastroenterol. (2017) 112:325–36. doi: 10.1038/ajg.2016.524
39. Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, et al. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. (2019) 68:423–33. doi: 10.1136/gutjnl-2017-315568
40. Burisch J, Katsanos KH, Christodoulou DK, Barros L, Magro F, Pedersen N, et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort-an epi-IBD study. J Crohns Colitis. (2019) 13:198–208. doi: 10.1093/ecco-jcc/jjy154
41. Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. (2013) 145:158–65.e2. doi: 10.1053/j.gastro.2013.04.007
42. Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV Jr, Tysk C, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. (2013) 62:630–49. doi: 10.1136/gutjnl-2012-303661
43. Zhao J, Ng SC, Lei Y, Yi F, Li J, Yu L, et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis. (2013) 19:1839–45. doi: 10.1097/MIB.0b013e31828a6551
44. Roda G, Narula N, Pinotti R, Skamnelos A, Katsanos KH, Ungaro R, et al. Systematic review with meta-analysis: proximal disease extension in limited ulcerative colitis. Aliment Pharmacol Ther. (2017) 45:1481–92. doi: 10.1111/apt.14063
45. Eltabbakh M, Abd Alaty W, Sakr MA, Sherief AF. Commenting on the Letter (Completing the Picture in Egypt: Response to “Inflammatory Bowel Diseases in Egypt During the COVID-19 Pandemic”). Inflamm Bowel Dis. (2021) 27:e66. doi: 10.1093/ibd/izab009
46. Driessen A, Macken E, Moreels T, Jouret-Mourin A. Dysplasia in inflammatory bowel disease. Acta Gastroenterol Belg. (2017) 80:299–308.
47. Hodges P, Kelly P. Inflammatory bowel disease in Africa: what is the current state of knowledge? Int Health. (2020) 12:222–30. doi: 10.1093/inthealth/ihaa005
Keywords: ulcerative colitis, Crohn's disease, multicenter study, Egypt, inflammatory bowel disease
Citation: Elbadry M, Nour MO, Hussien M, Ghoneem EA, Medhat MA, Shehab H, Galal S, Eltabbakh M, El-Raey F, Negm M, Afify S, Abdelhamed W, Sherief A, Abdelaziz A, Abo Elkasem M, Mahrous A, Kamal G, Maher M, Abdel-Hameed O, Elbasuny A, El-Zayyadi I, Bassiony A, Moussa A, Bedewy E, Elfert A and El Kassas M (2022) Clinico-Epidemiological Characteristics of Patients With Inflammatory Bowel Disease in Egypt: A Nationwide Multicenter Study. Front. Med. 9:867293. doi: 10.3389/fmed.2022.867293
Received: 31 January 2022; Accepted: 21 March 2022;
Published: 19 April 2022.
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Ivana Trivic, Children's Hospital Zagreb, CroatiaCopyright © 2022 Elbadry, Nour, Hussien, Ghoneem, Medhat, Shehab, Galal, Eltabbakh, El-Raey, Negm, Afify, Abdelhamed, Sherief, Abdelaziz, Abo Elkasem, Mahrous, Kamal, Maher, Abdel-Hameed, Elbasuny, El-Zayyadi, Bassiony, Moussa, Bedewy, Elfert and El Kassas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed El Kassas, bV9lbGthc3Nhc0BocS5oZWx3YW4uZWR1LmVn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.