
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 23 May 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.865131
This article is part of the Research Topic Challenges in Inflammatory Bowel Disease: Current, Future and Unmet Needs View all 13 articles
 Seyed Mobin Khoramjoo1
Seyed Mobin Khoramjoo1 Nesa Kazemifard2
Nesa Kazemifard2 Shaghayegh Baradaran Ghavami1*
Shaghayegh Baradaran Ghavami1* Maryam Farmani1
Maryam Farmani1 Shabnam Shahrokh1
Shabnam Shahrokh1 Hamid Asadzadeh Aghdaei1
Hamid Asadzadeh Aghdaei1 Ghazal Sherkat3
Ghazal Sherkat3 Mohammad Reza Zali2
Mohammad Reza Zali2Inflammatory bowel disease (IBD) is a disorder, which involves the gastrointestinal (GI) tract consisting Crohn's disease (CD) and ulcerative colitis (UC). The etiology of this disease is not yet clear and, hence, there are numerous medications and treatments for patients with IBD, although a definite and permanent treatment is still missing. Therefore, finding novel therapeutic approaches are vital for curing patients with IBD. In the GI tract, there are various lineages of cells with different roles that their existence is necessary for the barrier function of intestinal epithelial cells (IECs). Therefore, signaling pathways, which manage the hemostasis of cell lineages in intestine, such as Wnt, Notch, and Hippo, could have crucial roles in regulation of barrier function in the intestine. Additionally, these signaling pathways function as a governor of cell growth, tissue homeostasis, and organ size. In patients with IBD, recent studies have revealed that these signaling pathways are dysregulated that it could result in depletion or excess of a cell lineage in the intestine. Moreover, dysregulation of these signaling pathways in different cell lineages of the immune system could lead to dysregulation of the immune system's responses in IBD. In this article, we summarized the components and signaling of Wnt, Notch, and Hippo pathways and their role in the intestine and immune system. Furthermore, we reviewed latest scientific literature on the crosstalk among these three signaling pathways in IBD. An overview of these three signaling pathways and their interactions in IBD could provide a novel insight for prospective study directions into finding efficient medications or treatments.
Inflammatory bowel disease (IBD) is a regressive inflammatory condition, which occurs in the gastrointestinal tract (1). Patients with IBD fall into two clinical types: ulcerative colitis (UC) and Crohn's disease (CD). In patients with UC, part of involvement is limited to the colon and it can spread from the rectum to the cecum. In this type of IBD, these parts show large mucosal ulceration. On the other hand, in patients with CD, the parts, which are affected the most, are the ileum and the colon, but other parts of the gastrointestinal (GI) tract could be influenced patchily (2). In spite of the vast studies to find a causative factor for etiology of IBD, it is still known as a multifactorial disorder (3). Different factors, such as internal triggers (genetic susceptibility and immunoregulatory impairments), environmental factors (diet and chemicals), and microbial exposure, are considered to cause IBD (2, 4, 5). Moreover, recent studies have shown that the dysbiosis of gut microbiota profoundly contributes to the development of IBD (2, 6–8).
Various cell lineages arise by differentiation and proliferation of intestinal stem cells (ISCs), which are controlled by multiple signaling pathways, including Hippo, Notch, and Wnt (9–13). These signaling pathways accelerate undifferentiated columnar cells, named crypt base columnar cells, to regenerate into absorptive and secretory cell types in the GI tract (14). Some studies have illustrated that in patients who suffer from IBD, especially those with ulcerative colitis (UC), overexpression of Notch and inhibition of Wnt lead to a lack of Paneth cells that exist in crypts (15). Similarly, other studies have demonstrated that an imbalance in components of Hippo signaling pathway in the intestine of patients with IBD resulted in excess of ISCs and shortage of secretory cells, such as goblet cells and Paneth cells (16). Therefore, dysregulation in pathways that play a role in proliferation and differentiation may explain the defective mucus secretion and wound healing, which could ultimately induce the failure of intestinal barrier in patients with IBD (15). Furthermore, recent studies on Wnt, Notch, and Hippo showed that these signaling pathways play a regulatory role in the function and generation of various immune cells' types that in IBD, it could be dysregulated (17–19). However, numerous studies have been conducted on understanding the function and regulation of the proliferation pathways in the gut epithelium and their specific role in IBDs is still unknown (20).
In IBD, the process of wound healing and mucus secretion is dysregulated that could lead to impaired barrier function of the GI tract and ultimately leaky gut. Herein, we briefly explain the barrier functionality of intestinal epithelial cells (IECs) and the process of wound healing in IBD. In addition, it is illustrated that some proliferation pathways, including Wnt, Notch, and Hippo, could have critical impacts on these processes and the immune system. We also summarize these three signaling pathways and their role in the intestine and immune system. Finally, we concisely discuss the interactions of these signaling pathways in IBD.
The intestinal epithelial cells (IECs) establish a barrier, which is selectively permeable and sets apart luminal content from beneath tissues (21, 22). Basically, IECs function as a barrier, which prevents unacceptable solutes, microorganisms, viruses, and luminal antigens from passing the epithelium and entering the lamina propria (22, 23). Multiple components that participate in the intestinal barrier consist of the epithelial cells with tight junctions, adherens junctions, and luminal secretions, such as mucus or unstirred layers, on the apical side of the epithelium (22).
The process of wound healing starts when a part of the intestinal epithelium gets injured. Intestinal wound healing depends on the accurate balance between migration, proliferation, and differentiation of the epithelial cells, which are nearby the wounded area (24). First, epithelial cells surround the wounded area, which loses their columnar polarity. Then, they proliferate to surge the pool of cells for resurfacing the wound. Finally, to maintain the mucosal barrier function, maturation and differentiation of epithelial cells are vital (25). Dysfunction of these three steps during the wound healing's process in patients with IBD results in the broken differentiation and proliferation of different cell lineages in gut, such as goblet cells or Paneth cells, that lead to flawed mucosal secretion and leaky gut (26).
Many cell lineages are vital for maintenance of intestinal epithelial barrier integrity, which arise by differentiation and proliferation of intestinal stem cells (ISCs) (27). Crypt-based stem cells, which are near the base of the crypts, need to actively proliferate to maintain continuous renewal of different cell lineages (28). As these cells move up from crypt to villus, proliferation ends gradually and differentiation into one of the four primary cell types occurs (i.e., enterocytes, goblet, Paneth, and enteroendocrine cells) (29, 30). Multiple signaling pathways, such as Hippo, Notch, and Wnt, are responsible for regulating the proliferation and the differentiation in intestinal epithelial cells (9–13, 31). Finally, an imbalance among these types of pathways in epithelium could lead to colorectal cancer and IBD (27).
The Wnt signaling pathways are a group of signaling pathways, which commence with proteins that transmit signals into a cell by cell surface receptors (32, 33). Therefore, this pathway is activated by the cell–cell communications and it has been conserved throughout the biological evolution (27). Wnt pathway is divided into β-catenin dependent (canonical) and β-catenin independent (noncanonical) types (34). This signaling pathway gets activated when Wnt proteins contact with Frizzled (Frz) receptor on the cells' surface (18, 33). Thereafter, Wnt proteins run a complex signaling cascade that plays an important role in regulating cell proliferation and differentiation by regulating the β-catenin, which is an important mediator (34–36). When the Wnt pathway is silenced, β-catenin can be phosphorylated by the ubiquitin-proteasome system [including glycogen synthase kinase 3 (GSK3), casein kinase Iα (CKIα), axin, and adenomatosis polyposis coli (APC)] and then transcription complexes prohibit gene transcriptional activity (37, 38). In opposition, when the Wnt signaling pathway has been activated, β-catenin degradation is banned, which is leading to its aggregation (38, 39). As a result, transcription complexes are changed by accumulated β-catenin, which activates targeted expression of genes related to cell proliferation and migration, EphB2/B3, Cylind-1, and c-Myc (40, 41).
Wnt signaling pathway plays a vital role in the intestinal epithelium, specifically in regulating the stem cells' behavior, proliferation, differentiation, and migration (42). This pathway is one of the many signaling pathways for the maintenance of stem cells (43). Recent studies have shown that deletion of β-catenin's encoding gene (CTNNB1) results in disruption of secretory cells' differentiation (44). Wnt pathway is able to direct the beginning development of secretory cells lineage and the endpoint of differentiation of Paneth cells for sustaining homeostasis (45). Reduced Wnt pathway, particularly diminished expression in its transcription factor 4 (TCF-4), could mediate Paneth cells differentiation flaws that it induces specific deficiency of Paneth cell defensins, which is a principal factor in IBD pathogenesis (15, 46). In addition, evidence found in TCF-4 knockout mice illustrated that the reduced level of defensins in gut permits bacteria to invade the epithelium and resulting in colitis (42, 47). On the other hand, excessive Wnt pathway accumulates β-catenin in the cytoplasm, then they are translocated to the nuclear, and finally induces overexpression of Wnt target genes, which lead to colon cancer (48). Furthermore, recent studies on IBD-associated colorectal cancer (CRC) revealed that negative regulators of Wnt, such as AXIN2 and RFN43, are downregulated in 31 tissue sample of patients with IBD-CRC (49).
One of the important roles of Wnt pathway is in multiple layers of immune regulation. Presence or absence of Wnt proteins could have impact on different immune cells, such as dendritic cells, macrophages, CD8+ T cells, and CD4+ T cells (18, 50). Based on recent studies on Wnt proteins and dendritic cells (DCs), it is demonstrated that Wnt proteins may be involved in promoting DCs into a tolerogenic state (51). Manicassamy et al. showed that reduced expression of β-catenin in DCs enhances inflammatory responses in the mice model of inflammatory bowel disease. Therefore, in DCs, β-catenin signaling causes a tolerogenic state and prevents them from inflammatory responses (52). Recent studies on Wnt pathway and macrophages showed that in macrophages, Wnt ligands have crucial roles in repairing injured tissues, since macrophages' role in tissue repairing and wound healing is well known (53, 54); however, in some studies, it is demonstrated that macrophage-derived Wnt5a maintains immune functions and stimulates the secretion of proinflammatory cytokines (55). Some previous studies showed that proteins of Wnt signaling play a regulatory role in the function of CD8+ T-cell effector and generating memory T-cell pool (18, 56). Functional regulation of β-catenin-mediated CD8+ T-cell immune responses remains unclear (57–60). The role of Wnt pathway in CD4+ T-cells is not yet clear and it needs more accurate investigations for various Wnt ligands; however, some studies suggested that the overexpression of β-catenin in regulatory T (Treg) cells enhanced Treg function in IBD (61, 62). The canonical Wnt signaling proteins are able to induce their roles in T-cell differentiation and effector function in various inflammatory diseases, such as IBD, cancer, as well as in autoimmunity and viral infections (18). Wnt signaling is important in inflammatory and fibrotic diseases and it is in harmony with the roles of Wnt proteins in repairing injured tissue. Recent studies' outcomes demonstrated that Wnt signaling plays vital roles in lymphomyelopoiesis and immune responses (56). Moreover, Wnt pathway and inflammatory signaling pathways, such as nuclear factor-kappa B (NF-κB), Janus kinase-signal transducer and activator of transcription 3 (JAK-STAT3), and mitogen-activated protein kinase (MAPK), affect each other, which regulate inflammatory factors' secretion during the pathogenesis of colitis (18, 63).
The Notch signaling pathway is conserved during the evolution that is presented in most animals (27). It regulates the differentiation and development of cells, tissues, and organs by interactions among nearby cells (27). The pathway consists of receptors, ligands, transformation complexes, and several regulatory molecules (64). The Notch transmembrane receptor plays a critical role in the signaling pathway that regulates the fate and development of a wide range of metazoan cells through local cell interactions (65–67). There are at least four different Notch receptors (Notch 1–4) in mammals that the Notch-1 is dominant in the intestine (66, 68). As a result of binding ligands, slight structural conformations in the membrane around the binding site activate matrix metalloproteinases (MMPs) and γ-secretase (69). With the assistance of activated γ-secretase and a disintegrin and metalloproteinase (ADAM)-family MMPs, Notch intracellular domain (NICD), which is the activated form of Notch receptors, is generated (64, 66, 70). Thereafter, NICD enters the nuclear and by the help of activator transcription complexes, it regulates the HES genes to determine the fate of the cell (64, 71–74).
In the intestine, Notch is necessary for the survival of ISCs. Notch is also responsible for determining ISCs differentiation into secretory or absorptive lineages (63). High Notch signaling leads to absorptive differentiation, whereas low Notch signaling induces differentiation of secretory cells (75). Abnormal activity of the Notch pathway in IBD induces increased expression of the HES1 transcription factor in human colon cell lines, which thereafter inhibits differentiation of secretory cell lineages and weakens the mucus barrier, which is linked to chronic colitis (76). Accordingly, it is demonstrated that the Notch signaling pathway has a crucial role in maintaining goblet cells in the lesion of patients with UC that is so important in mucosal and wound healing in IBD. Zheng et al. illustrated that abnormality in expression of Notch intracellular domain (NICD) in ulcers induces reduction in the quantity of goblet cells in patients with UC (77). NICDs imposed expression leads to a decrease in phenotypic genes for goblet cells located in human IECs (77). In addition, several signaling pathways and cytokines cascade with Notch pathway mediate epithelial regeneration, such as interleukin-22 (IL-22) and tumor necrosis factor-α (TNF-α) (27, 78). Accordingly, Kuno et al. found that messenger RNA (mRNA) expression of OFLM4, intestinal stem cell marker, is upregulated by TNF-α and Notch pathway in patients with IBD (63). Moreover, it is reported that Notch signaling pathway contributes to the maintenance of tight junctions and adherens junction proteins in mice. Ahmed et al. showed that during the infection with Citrobacter rodentium and absence of Notch pathway, the function of tight junctions and adherens junctions impaired, which could result in increased permeability of epithelial cells and more exposure of luminal contents with immune system and inflammation (79). Notch dysregulation has also been demonstrated in colon cancer (80).
Notch signaling is important pervasively all over the immune system, since it has lineage and context-dependent impacts on a broad range of cells. In the immune system, Notch1 ligands, especially Jagged1, are present in regulatory T-cells (Tregs) (17). The activation of Notch1 in dendritic cells (Notch1 intracellular domain) induces the interaction of signaling elements and components that result in overexpression and the transport of pSmad3, which is known to facilitate the effector function of Tregs (17, 81). Notch signaling is also involved in the improvement of inflammatory conditions. Some studies revealed that Notch signaling pathway improves an inflammatory cascade in macrophages in inflammation and blocking Notch reduces the production of proinflammatory cytokines, such as interleukin-1β (IL-1β) (82, 83).
The Hippo pathway is a pathway, which is remained conserved throughout the evolution and it controls the size and homeostasis of an organ by regulating cell proliferation, survival, apoptosis, and stemness (84, 85). Specifically, intercellular contacts and membrane adhesion complexes modulate the transduction of a signal by the fundamental constituents of this pathway, which are highly conserved in mammals (86). These components contain the mammalian sterile 20-like kinases, MST1 and MST2, with their regulatory protein WW45 (SAV1) and the large tumor suppressor 1 and 2 kinases (LATS1 and LATS2) with their regulatory protein MOBKL1A/B (MOB1) (87). When the MST1/2 kinases get activated and LATS kinases get phosphorylated, it leads to negative regulation of cell proliferation (88). Concisely, phosphorylation of LATS kinases results in a process of phosphorylation of the transcriptional coactivators Yes-associated protein (YAP) at Ser127 and PDZ-binding motif (TAZ) at Ser89, so it makes binding sites for 14-3-3 proteins that accumulate YAP/TAZ in the cytoplasm (88). Once this inhibitory phosphorylation does not work, these transcription factors will be able to enter to the nucleus and contact with other transcriptional factors that enhance cell proliferation (89, 90). In cells that are in apoptotic phase because of exposure to severe DNA damage stress, YAP activates the transcription of proapoptotic genes through binding to the p73 transcription factor that is a p53-like tumor suppressor. This process is moderated by phosphorylation of YAP at the Tyr357 position through c-Abl protein, which provides a higher affinity of YAP compared to p73 (89, 91).
It is confirmed that YAP/TAZ enhances regeneration of tissues in the mammalian intestine (92). Accordingly, Yui et al. demonstrated that YAP/TAZ is associated with the expression of Sca1, which is a cell surface protein representing a marker for the repairing epithelium (93). YAP/TAZ has two different roles in the renewal of the intestinal epithelium: one is ISCs' proliferation that happens through collaboration of YAP/TAZ with transcription factor TEADs (94) and the other role is encouraging goblet cells differentiation by cooperation with transcription factor klf4 (95, 96). The activity of MST1/2 gets higher, as cells move from the crypts toward the lumen, so MST1/2 has decreased activity in the crypts (97). Contrarily, YAP is plentiful in the nucleus of the cells, which are located in lower crypts; however, this molecule is also found in cytoplasm of upper cells in the villi (97). In general, the expression of YAP in the nucleus of cells diminishes as cells move from the crypts to the villi; in contrary, expression of YAP in cytoplasm surges (16). Deletion of MST1/2 in mouse intestinal epithelium cells induces an improved amount of nuclear YAP; as a result, it increases proliferation of undifferentiated ISCs and lack of secretory cells both in the small and large intestines (16). In another study, it is demonstrated that in IECs, conditional knockout of MST1/2 results in disorganized villus structures, increased undifferentiated cells, and dysplastic epithelia (98). It is also reported that in mouse gut, deficiency of SAV1 induces enlargement of crypt structures (92). YAP modulates the regeneration of mucus both in the patients with IBD and the DSS-induced colitis mouse model (99). Moreover, previous studies conducted by Ou et al. illustrated that YAP/TAZ expression is linked with promotion of fibrosis in patients with CD by activating intestinal fibroblasts (100). To sum up, YAP in the nucleus has a positive role in the regeneration of intestinal epithelium in IBD and may provide a novel therapeutic target for IBD.
Recent studies have shown that the Hippo pathway plays an important role in the modulation of immune system. MST1/2 has a pivotal role in mediating, migration, adherence, and survival of T cells by its downstream effectors, such as LATS1/2, NDR1/2, and YAP (101). MST1/2 promotes the function of regulatory T-cell (Treg) through modulating Foxp3 acetylation (16, 102). It is shown the deficiency of MST1/2 leads to impairment of Foxp3 expression and Treg cell development and its function in mice (16, 102). It is also illustrated that the deficiency of MST1/2 might induce the lack of naïve T cells, which could lead to autoimmune demonstrations or resulting in recurrent bacterial or viral infections (103, 104). Additionally, in a study by Geng et al., it has been found that the TAZ is able to determine the fate of a T cell to become a proinflammatory T-helper (Th) 17 cell or an immunosuppressive Treg cell (105). Particularly, lack of TAZ improves the differentiation of Treg cells; however, activation of TAZ enhances Th17 cell differentiation (105). On the other hand, YAP in macrophages was shown to deteriorate the IBD, since it negatively affects M2 polarization of macrophages, which is induced by IL-4/IL-13 and promotes the activation of M1 macrophages that is caused by lipopolysaccharide (LPS) or interferon-γ (IFN-γ) (19).
There is an interplay among the proliferation signaling pathways, including Hippo, Wnt, and Notch, in intestinal regeneration (Figure 1) and imbalance among these pathways results in different problems, which are associated with different diseases, including IBD.
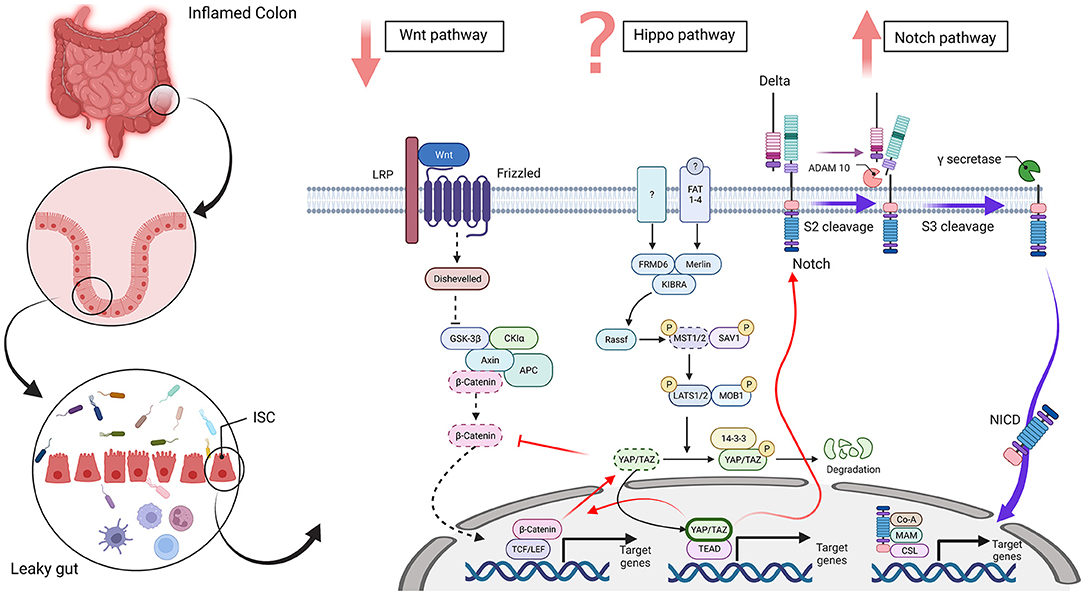
Figure 1. Wnt, Hippo, and Notch signaling in an intestinal stem cell [inflammatory bowel disease (IBD) condition]. The disrupted intestinal epithelial barrier promotes a leaky gut in inflammatory conditions, such as IBD. In this state, differentiation-related pathways, such as Wnt, Hippo, and Notch, are dysregulated in intestinal stem cells. The Wnt signaling pathway plays a vital role in the intestinal epithelium and downregulation of β-catenin disrupts the differentiation of secretory cells in IBD. The Hippo pathway regulates cell proliferation, survival, apoptosis, and stemness. An improved amount of nuclear YAP/TAZ occurred in IBD and increased the proliferation of undifferentiated intestinal stem cells (ISCs) and a lack of secretory cells. In contrast, cytoplasmic YAP/TAZ overexpression is seen in inflamed tissue. Also, MST1/2 is downregulated and disorganized villus structures and increased undifferentiated cells and dysplastic epithelia. However, it is controversial that Hippo pathway is upregulated or downregulated in IBD. The Notch pathway regulates the differentiation and development of cells, tissues, and organs by interactions among nearby cells. Upregulation of the Notch pathway in IBD inhibits differentiation of secretory cell lineages and abnormality in the expression of NICD in ulcers reduces the quantity of goblet cells in patients with ulcerative colitis (UC). Red arrows show crosstalk between these three pathways and dotted lines display downregulated pathways and molecules. Abbreviations: low-density-lipoprotein-related protein (LRP), adenomatosis polyposis coli (APC), glycogen synthase kinase 3 beta (GSK3β), casein kinase 1α (CK1α), T-cell factor (TCF), lymphoid enhancer factor (LEF), FERM domain-containing protein 6 (FRMD6), mammalian Sterile 20-related 1 and 2 kinases (MST1 and MST2), Salvador 1 (SAV1), Large tumor suppressor 1 and 2 kinases (LATS1 and LATS2), Mps One Binder Kinase Activator-Like 1A (MOB1), Yes-associated protein 1 (YAP), PDZ-binding motif (TAZ), TEA domain family member (TEAD), Fat-related atypical cadherins 1-4 (FAT 1-4), A Disintegrin and metalloproteinase domain-containing protein 10 (ADAM 10), Notch intracellular domain (NICD), meprin A-5 protein (MAM) and CBF1, Suppressor of Hairless, Lag-1 (CSL). The figure has drawn by BioRender (www.Biorender.com).
Once the Hippo pathway gets activated, it negatively affects the Wnt signaling pathway by cytoplasmic and phosphorylated YAP/TAZ; however, deactivation of the Hippo pathway has a positive effect on the expression of Wnt target genes by nuclear and dephosphorylated YAP (20). Additionally, it is reported that β-catenin activates and upregulates YAP and TAZ (106, 107). Imajo et al. illustrated that the YAP/TAZ regulates Wnt signaling that relies on the state of phosphorylation and cellular localization of YAP/TAZ proteins (108). Cytoplasmic YAP/TAZ downregulates the Wnt signaling through the regulation of nuclear translocation and activation of β-catenin (108, 109). This is in contrast to nuclear YAP, which stabilizes β-catenin and resulting in the improved expression of Wnt target genes (110, 111). It is shown that YAP and β-catenin grow in nucleus within regeneration following inflammation. Once the nuclear YAP is overexpressed, it enhances Wnt/β-catenin signaling and significantly leads to the improvement of the IECs' healing ability, thereby demonstrating that nuclear YAP improves the IECs' proliferation by the activation of Wnt/β-catenin signaling pathways (112). In addition, within intestinal regeneration following tissue damage, cytoplasmic YAP restricts Wnt signals, interrupts the ISCs, and decreases the stem cells' growth, which induce abnormal migration of Paneth cells and reduction of ISCs (97).
In previous studies, it is reported that the Hippo pathway is able to regulate Notch signaling. In intestine, conditional knockout of MST1/2 leads to increased amount of NICD in nucleus (16). Reduction of MST1/2 results in activation of Notch signaling through decreasing of phosphorylation and increasing the abundance of nuclear accumulation of YAP (16). Intrinsically, the YAP molecules, which are located in nucleus, facilitate Notch signaling (98). Moreover, it is shown that administration of gamma-secretase inhibitors (GSIs), which restrict YAP-activating Notch, induce colitis (98, 113). In general, the Hippo pathway is able to downregulate Notch signaling via phosphorylation and suppression of the YAP.
Wnt and Notch signaling pathways are so intertwined that it has been suggested that they established an integrated signaling termed as “Wntch” (114). An accurate balance between Wnt and Notch is required for the homeostasis of intestine, as their dysregulation may result in inflammation, colitis, and tumorigenesis. It is demonstrated that activation of Notch pathway upregulates the expression of β-catenin (115). On the other hand, Kay et al., by utilizing chemical reaction network theory (CRNT), found that Wnt-mediated actions on Hes1 promoter are able to change dynamic transition of Notch signaling pathway from multistability to monostability, highlighting the role of β-catenin in modulating Notch signaling pathway (116).
In this short article, we aimed to overlook on three proliferation pathways playing important roles in intestine and immune system. We also reviewed some of their impacts on pathogenesis of IBD. In the process of wound and mucosal healing in a healthy condition, adjacent cells to the lesion start to proliferate and migrate to retrieve the columnar polarity of the epithelial cells (24). This process is faulty in the intestine of patients with IBD, particularly those who have UC. Multiple factors contribute in this flaw, but it is shown that one of the most important factors is the dysregulation of proliferation pathways, such as Hippo, Wnt, and Notch, and also an imbalance among them (27). Moreover, dysregulation of these pathways leads to an imbalance of cell lineages in intestine. Importantly, this dysregulation results in depletion of goblet cells and Paneth cells, which leads to impaired secretion of mucus and defensins and invasion of various bacteria to epithelial cells (26). Accordingly, this results in massive responses of the immune system and inflammation that cause tissue damage and ulcers, which are the clinical symptoms of IBD (117). These signaling pathways also play important roles in various immune cells, including dendritic cells, macrophages, and T cells. These signaling pathways could impact immune cells to differentiate to a particular type that it could attenuate or strengthen immune responses (117). Moreover, there is a crosstalk between these signaling pathways and inflammatory signaling pathways, such as NF-κB, JAK-STAT3, and MAPK, that could influence an immune cell to produce proinflammatory or anti-inflammatory cytokines (104). Dysregulation in these pathways in immune cells is reported to be important in immune responses to inflammation in patients with IBD (117).
This study needs more investigation in different aspects. First, a precise analysis of the relationship among these three pathways needs to be more investigated. By doing so, we will be able to understand the impact of different molecules of signaling pathways on each other. In addition, current findings demonstrated the role of these pathways in several immune cells yet not all of them. More experiments could be done in finding their role in other immune cells. Finally, these proliferation pathways can be a potential target for medications. More studies are required to develop efficient drugs for triggering epithelial cells to regulate these pathways. The results of prospective studies can dwindle the morbidity and mortality linked to IBD, hence reduce the worldwide incidence of this disease.
SMK: literature search and writing. SBG, MF, SS, HA, GS, and MZ: literature search. NK: drawing of figure. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Endo K, Shiga H, Kinouchi Y, Shimosegawa T. Inflammatory bowel disease: IBD. Rinsho Byori Jpn J Clin Pathol. (2009) 57:527–32.
2. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. (2015) 12:205–17. doi: 10.1038/nrgastro.2015.34
3. Venkataraman GR, Rivas MA. Rare and common variant discovery in complex disease: the IBD case study. Hum Mol Genet. (2019) 28:R162–9. doi: 10.1093/hmg/ddz189
4. Kaplan GG. Global variations in environmental risk factors for IBD. Nat Rev Gastroenterol Hepatol. (2014) 11:708–9. doi: 10.1038/nrgastro.2014.182
5. Hammer T, Lophaven SN, Nielsen KR, Petersen MS, Munkholm P, Weihe P, et al. Dietary risk factors for inflammatory bowel diseases in a high-risk population: results from the Faroese IBD study. United Eur Gastroenterol J. (2019) 7:924–32. doi: 10.1177/2050640619852244
6. Putignani L, Del Chierico F, Vernocchi P, Cicala M, Cucchiara S, Dallapiccola B, et al. Gut Microbiota dysbiosis as risk and premorbid factors of IBD and IBS along the childhood–Adulthood transition. Inflamm Bowel Dis. (2016) 22:487–504. doi: 10.1097/MIB.0000000000000602
7. Lim JS, Lim MY, Choi Y, Ko G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol Brain. (2017) 10:1–12.
8. van der Sloot KWJ, Weersma RK, Dijkstra G, Alizadeh BZ. Development and validation of a web-based questionnaire to identify environmental risk factors for inflammatory bowel disease: the Groningen IBD Environmental Questionnaire (GIEQ). J Gastroenterol. (2019) 54, 238–48. doi: 10.1007/s00535-018-1501-z
9. Scoville DH, Sato T, He XC, Li LJG. Current view: intestinal stem cells and signaling. Gastroenterol. (2008) 134:849–64. doi: 10.1053/j.gastro.2008.01.079
10. Sancho R, Cremona CA, Behrens AJ. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep. (2015) 16, 571–81. doi: 10.15252/embr.201540188
11. Cui S, Chang PY. Current understanding concerning intestinal stem cells. Worlds J Gastroenterol. (2016) 22:7099. doi: 10.3748/wjg.v22.i31.7099
12. Gregorieff A, Wrana JL. Hippo signalling in intestinal regeneration and cancer. Curr Opin Cell Biol. (2017) 48:17–25. doi: 10.1016/j.ceb.2017.04.005
13. Xiao H, Xiong L, Song X, Jin P, Chen L, Chen X, et al. Angelica sinensis polysaccharides ameliorate stress-induced premature senescence of hematopoietic cell via protecting bone marrow stromal cells from oxidative injuries caused by 5-fluorouracil. Int J Mol Sci. (2017) 18:2265. doi: 10.3390/ijms18112265
14. Gassler NJ. Paneth cells in intestinal physiology and pathophysiology. World J Gastrointest Pathophysiol. (2017) 8:150. doi: 10.4291/wjgp.v8.i4.150
15. Gersemann M, Stange EF, Wehkamp JJ. From intestinal stem cells to inflammatory bowel diseases. World J Gastroenterol. (2011) 17:3198.
16. Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci. (2011) 108:E1312–20. doi: 10.1073/pnas.1110428108
17. Cahill EF, Tobin LM, Carty F, Mahon BP. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. (2015) 6:1–13. doi: 10.1186/s13287-015-0021-5
18. Chae WJ, Bothwell AL. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. (2018) 39:830–47. doi: 10.1016/j.it.2018.08.006
19. Zhou X, Li W, Wang S, Zhang P, Wang Q, Xiao J, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. (2019) 27, 1176–89. e1175. doi: 10.1016/j.celrep.2019.03.028
20. Xie Z, Wang Y, Yang G, Han J, Zhu L, Li L, et al. The role of the Hippo pathway in the pathogenesis of inflammatory bowel disease. Cell Death Dis. (2021) 12:1–14. doi: 10.1038/s41419-020-03229-8
21. Laukoetter MJR. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. (2006) 22:85–9. doi: 10.1097/01.mog.0000203864.48255.4f
22. Laukoetter MG, Nava P, Nusrat AJ. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. (2008)14, 401. doi: 10.3748/wjg.14.401
23. Watson AJ, Chu S, Sieck L, Gerasimenko O, Bullen T, Campbell FM, et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterol. (2005) 129:902–12. doi: 10.1053/j.gastro.2005.06.015
24. Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MBJ. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. (2007) 87:807–17. doi: 10.1038/labinvest.3700595
25. Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. (2011) 17:2161. doi: 10.3748/wjg.v17.i17.2161
26. Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, et al. Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation. (2009) 77:84–94. doi: 10.1016/j.diff.2008.09.008
27. Pu Z, Yang F, Wang L, Diao Y, Chen D. Advancements of compounds targeting Wnt and Notch signalling pathways in the treatment of inflammatory bowel disease and colon cancer. J Drug Target. (2021) 29:507–519. doi: 10.1080/1061186X.2020.1864741
28. Gracz AD, Magness ST. Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointestinal Liver Physiol. (2014) 307:G260–73. doi: 10.1152/ajpgi.00066.2014
29. Yeung TM, Chia LA, Kosinski CM, Kuo CJ. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. (2011) 68:2513–23. doi: 10.1007/s00018-011-0687-5
30. De Mey JR, Freund JN. Understanding epithelial homeostasis in the intestine: an old battlefield of ideas, recent breakthroughs and remaining controversies. Tissue Barriers. (2013) 1:e24965. doi: 10.4161/tisb.24965
31. Attisano L, Wrana JL. Signal integration in TGF-β, WNT, and Hippo pathways. F1000prime Reprts. (2013) 5:17. doi: 10.12703/P5-17
32. Nusse R, Brown A, Papkoff J, Scambler P, Shackleford GA, McMahon R, et al. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. (1991) 64:231–231. doi: 10.1016/0092-8674(91)90633-A
33. Sharma M, Pruitt KJ. Wnt pathway: an integral hub for developmental and oncogenic signaling networks. Int J Mol Sci. (2020) 21:8018. doi: 10.3390/ijms21218018
34. Bugter JM, Fenderico N, Maurice MMJ. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. (2021) 21:5–21. doi: 10.1038/s41568-020-00307-z
35. Giles RH, Van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et Biophysica Acta (BBA)-Rev Cancer. (2003) 1653: 1–24. doi: 10.1016/S0304-419X(03)00005-2
36. Clevers HJC. Wnt/β-catenin signaling in development and disease. Cell. (2006) 127:469–80. doi: 10.1016/j.cell.2006.10.018
37. Niehrs CJ. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. (2012) 13:767–79. doi: 10.1038/nrm3470
38. Li X, Ortiz MA, Kotula L. The physiological role of Wnt pathway in normal development and cancer. Exp Biol Med. (2020) 245:411–26.
39. Hale R, Strutt D. Conservation of planar polarity pathway function across the animal kingdom. Ann Rev Gene. (2015) 49:529–51. doi: 10.1146/annurev-genet-112414-055224
40. Widera D, Papaccio G, Cantù C, James AW, Houschyar KS, Houschyar K, et al. Wnt Pathway in Bone Repair and Regeneration–What Do We Know So Far. Front Cell Develop Biol. (2019) 6:170. doi: 10.3389/fcell.2018.00170
41. Lu H, Zhang R, Haydon R, Rayburn E, Kang Q, Si W, et al. Wnt/β-Catenin signaling pathway as novel cancer drug targets. Curr Cancer Drug Targets. (2004) 4:653–71. doi: 10.2174/1568009043332709
42. Flanagan DJ, Austin CR, Vincan E, Phesse TJJ. Wnt signalling in gastrointestinal epithelial stem cells. Genes. (2018) 9:178. doi: 10.3390/genes9040178
43. Yang S, Yu M. Role of goblet cells in intestinal barrier and mucosal immunity. J Inflamm Res. (2021) 14:3171. doi: 10.2147/JIR.S318327
44. Kurokawa K, Hayakawa Y, Koike KJ. Plasticity of intestinal epithelium: stem cell niches and regulatory signals. Int J Mol Sci. (2020) 22:357. doi: 10.3390/ijms22010357
45. Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. (2005) 132:1443–51. doi: 10.1242/dev.01700
46. Wang Y, He K, Sheng B, Lei X, Tao W, Zhu X, et al. The RNA helicase Dhx15 mediates Wnt-induced antimicrobial protein expression in Paneth cells. Proc Natl Acad Sci. (2021) 118:e2017432118. doi: 10.1073/pnas.2017432118
47. Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, et al. The Paneth cell α-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol. (2007) 179:3109–18. doi: 10.4049/jimmunol.179.5.3109
48. Krausova M, Korinek VJ. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. (2014) 26:570–9. doi: 10.1016/j.cellsig.2013.11.032
49. Rajamäki K, Taira A, Katainen R, Välimäki N, Kuosmanen A, Plaketti RM, et al. Genetic and Epigenetic Characteristics of Inflammatory Bowel Disease–Associated Colorectal Cancer. Gastroenterol. (2021)161, 592–607. doi: 10.1053/j.gastro.2021.04.042
50. Suryawanshi A, Hussein MS, Prasad PD, Manicassamy SJ. Wnt signaling cascade in dendritic cells and regulation of anti-tumor immunity. Front Immunol. (2020) 122. doi: 10.3389/fimmu.2020.00122
51. Tan K, Xie X, Shi W, Miao L, Dong X, Yang W, et al. Deficiency of canonical Wnt/β-catenin signalling in hepatic dendritic cells triggers autoimmune hepatitis. Liver Int. (2020) 40:131–40. doi: 10.1111/liv.14246
52. Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. (2010) 329:849–853. doi: 10.1126/science.1188510
53. Vannella KM, Wynn T. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. (2017) 79:593–617. doi: 10.1146/annurev-physiol-022516-034356
54. Feng Y, Ren J, Gui Y, Wei W, Shu B, Lu Q, et al. Wnt/β-catenin–promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. (2018) 29:182–93. doi: 10.1681/ASN.2017040391
55. Shao Y, Zheng Q, Wang W, Xin N, Song X, Zhao CJO. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget. (2016) 7:67674. doi: 10.18632/oncotarget.11874
56. Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J. (2019) 17:1–13. doi: 10.1016/j.csbj.2018.11.004
57. Gattinoni L, Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. (2009) 15:808–13. doi: 10.1038/nm.1982
58. Driessens G, Zheng Y, Gajewski TF. β-catenin does not regulate memory T cell phenotype. Nat Med. (2010) 16:513–4. doi: 10.1038/nm0510-513
59. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell–like properties. Nat Med. (2011) 17:1290–7. doi: 10.1038/nm.2446
60. Arens R, Staal FJ, van Eggermond MC, van den Elsen PJ, Tiemessen MM, Baert MR, et al. T Cell Factor 1 Represses CD8. J Immunol. (2014) 193:5480–7. doi: 10.4049/jimmunol.1303417
61. Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. (2008) 14:162–169. doi: 10.1038/nm1707
62. Graham JA, Fray M, Haseth SD, Lee KM, Lian M-M, Chase CM, et al. Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3β inhibition. J Biol Chem. (2010) 285:32852–9. doi: 10.1074/jbc.M110.150904
63. Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. (2018). Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke 13, 612–632. doi: 10.1177/1747493018778713
64. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. (2006) 7:678–89. doi: 10.1038/nrm2009
65. Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Develop. (1998) 12:1751–62. doi: 10.1101/gad.12.12.1751
66. Tsakonas SA, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. (1999) 284:770–6. doi: 10.1126/science.284.5415.770
67. Canalis E. Notch in skeletal physiology and disease. Osteoporosis Int. (2018) 29:2611–21. doi: 10.1007/s00198-018-4694-3
68. Kumar R, Juillerat-Jeanneret L, Golshayan D. Notch antagonists: potential modulators of cancer and inflammatory diseases. J Med Chem. (2016) 59:7719–37. doi: 10.1021/acs.jmedchem.5b01516
69. Soumelis V, Reche P, Kanzler H, Yuan W, Edard G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. (2000) 3:673–80. doi: 10.1038/ni805
70. Kaemmerer E, Jeon MK, Berndt A, Liedtke C, Gassler N. Targeting Wnt Signaling via Notch in Intestinal Carcinogenesis. Cancers. (2019) 11:555. doi: 10.3390/cancers11040555
71. Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas SJN. Notch signals control the fate of immature progenitor cells in the intestine. Nature. (2005) 435:964–8. doi: 10.1038/nature03589
72. van Es JH, Van Gijn ME, Riccio O, Van Den Born M, Vooijs M, Begthel H, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. (2005) 435:959–63. doi: 10.1038/nature03659
73. Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. (2015) 402:98–108. doi: 10.1016/j.ydbio.2015.03.012
74. Sueda R, Kageyama R. Regulation of active and quiescent somatic stem cells by Notch signaling. Develop Growth Diff. (2020) 62:59–66. doi: 10.1111/dgd.12626
75. Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. (2017) 97:1235–94. doi: 10.1152/physrev.00005.2017
76. Ghorbaninejad M, Heydari R, Mohammadi P, Shahrokh S, Haghazali M, Khanabadi B, et al. Contribution of NOTCH signaling pathway along with TNF-α in the intestinal inflammation of ulcerative colitis. Gastroenterol Hepatol Bed Bench. (2019) 12:S80.
77. Zheng X, Tsuchiya K, Okamoto R, Iwasaki M, Kano Y, Sakamoto N, et al. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm Bowel Dis. (2011) 17:2251–60. doi: 10.1002/ibd.21611
78. Zha J-M, Li H-S, Lin Q, Kuo W-T, Jiang Z-H, Tsai P-Y, et al. Interleukin 22 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of Wnt and notch signaling. Cell Mol Gastroenterol Hepatol. (2019) 7:255–74. doi: 10.1016/j.jcmgh.2018.09.006
79. Ahmed I, Roy BC, Raach R-MT, Owens SM, Xia L, Anant S, et al. Enteric infection coupled with chronic Notch pathway inhibition alters colonic mucus composition leading to dysbiosis, barrier disruption and colitis. PLoS One. (2018)13:e0206701. doi: 10.1371/journal.pone.0206701
80. Tyagi A, Sharma AK, Damodaran C. A Review on Notch Signaling and Colorectal Cancer. Cells. (2020) 9:1549. doi: 10.3390/cells9061549
81. Hue S, Kared H, Mehwish Y, Mouhamad S, Balbo M, Levy Y. Notch activation on effector T cells increases their sensitivity to T reg cell-mediated suppression through upregulation of TGF-β RII expression. Eur J Immunol. (2012) 42:1796–803. doi: 10.1002/eji.201142330
82. Levi B. Macrophages take rheumatoid arthritis up a Notch. Sci Transl Med. (2017) 9. doi: 10.1126/scitranslmed.aan3022
83. Singh SB, Coffman CN, Carroll-Portillo A, Varga MG, Lin HC. Notch Signaling Pathway Is Activated by Sulfate Reducing Bacteria. Front Cell Infect Microbiol. (2021) 11:695299. doi: 10.3389/fcimb.2021.695299
84. Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Progr Clin Biol Res. (1987) 249:251–62.
85. Wu Z, Guan KL. Hippo signaling in embryogenesis and development. Trends Biochem Sci. (2021) 46:51–63. doi: 10.1016/j.tibs.2020.08.008
86. Ma S, Meng Z, Chen R, Guan K-L. The Hippo pathway: biology and pathophysiology. Ann Rev Biochem. (2019) 88:577–604. doi: 10.1146/annurev-biochem-013118-111829
87. Misra JR, Irvine KD. The Hippo Signaling Network and Its Biological Functions. Annu Rev Genet. (2018) 52:65–87. doi: 10.1146/annurev-genet-120417-031621
88. Nterma P, Panopoulou E, Papadaki-Petrou E, Assimakopoulou M. Immunohistochemical profile of tumor suppressor proteins RASSF1A and LATS1/2 in relation to p73 and YAP expression, of human inflammatory bowel disease and normal intestine. Pathol Oncol Res. (2020) 26:567–74. doi: 10.1007/s12253-018-00575-z
89. Hong W, Guan K-L. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Sem Cell Develop Biol. (2012) 23:785–93. doi: 10.1016/j.semcdb.2012.05.004
90. Han Y. Analysis of the role of the Hippo pathway in cancer. J Transl Med. (2019) 17:116. doi: 10.1186/s12967-019-1869-4
91. Raj N, Bam R. Reciprocal Crosstalk Between YAP1/Hippo Pathway and the p53 Family Proteins: Mechanisms and Outcomes in Cancer. Front Cell Develop Biol. (2019) 7:159. doi: 10.3389/fcell.2019.00159
92. Cai J, Zhang N, Zheng Y, De Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Develop. (2010) 24:2383–8. doi: 10.1101/gad.1978810
93. Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell. (2018) 22:35–49. e37. doi: 10.1016/j.stem.2017.11.001
94. Kriz V, Korinek V. Wnt, RSPO and Hippo Signalling in the Intestine and Intestinal Stem Cells. Genes. (2018) 9:20. doi: 10.3390/genes9010020
95. Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. (2015) 17:7–19. doi: 10.1038/ncb3084
96. Fu V, Plouffe SW, Guan KL. The Hippo pathway in organ development, homeostasis, and regeneration. Curr Opin Cell Biol. (2017) 49:99–107. doi: 10.1016/j.ceb.2017.12.012
97. Barry ER, Morikawa T, Butler BL, Shrestha K, de La Rosa R, Yan KS, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. (2013) 493:106–10. doi: 10.1038/nature11693
98. Zhou D, Conrad C, Xia F, Park J-S, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. (2009) 16:425–38. doi: 10.1016/j.ccr.2009.09.026
99. Taniguchi K, Wu L-W, Grivennikov SI, Jong PR, Lian I, Yu F-X, et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature. (2015)519:57–62. doi: 10.1038/nature14228
100. Ou W, Xu W, Liu F, Guo Y, Huang Z, Feng T, et al. Increased expression of yes-associated protein/YAP and transcriptional coactivator with PDZ-binding motif/TAZ activates intestinal fibroblasts to promote intestinal obstruction in Crohn's disease. EBioMedicine. (2021) 69:103452. doi: 10.1016/j.ebiom.2021.103452
101. Tang F, Gill J, Ficht X, Barthlott T, Cornils H, Schmitz-Rohmer D, et al. The kinases NDR1/2 act downstream of the Hippo homolog MST1 to mediate both egress of thymocytes from the thymus and lymphocyte motility. Sci Signal. (2015) 8:ra100–ra100. doi: 10.1126/scisignal.aab2425
102. Li C, Bi Y, Li Y, Yang H, Yu Q, Wang J, et al. Dendritic cell MST1 inhibits Th17 differentiation. Nat Commun. (2017) 8:1–13. doi: 10.1038/ncomms14275
103. De Nehme NT, Schmid JP, Debeurme F, André-Schmutz I, Lim A, Nitschke P, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. (2012) 119:3458–68. doi: 10.1182/blood-2011-09-378364
104. Yamauchi T, Moroishi T. Hippo Pathway in Mammalian Adaptive Immune System. Cells. (2019) 8:398. doi: 10.3390/cells8050398
105. Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH 17 cells and T reg cells. Nat Immunol. (2017) 18:800–12. doi: 10.1038/ni.3748
106. Konsavage WM, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. (2012) 287:11730–9. doi: 10.1074/jbc.M111.327767
107. Tsai BP, Hoverter NP, Waterman ML. Blending hippo and WNT: sharing messengers and regulation. Cell. (2012) 151:1401–3. doi: 10.1016/j.cell.2012.12.007
108. Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. (2012) 31:1109–22. doi: 10.1038/emboj.2011.487
109. Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev Cell. (2010) 18:579–91. doi: 10.1016/j.devcel.2010.03.007
110. Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. (2011) 332:458–61. doi: 10.1126/science.1199010
111. Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. (2012) 151:1457–73. doi: 10.1016/j.cell.2012.11.026
112. Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, et al. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. (2018) 9:1–16. doi: 10.1038/s41419-017-0244-8
113. Jeon MK, Klaus C, Kaemmerer E, Gassler N. Intestinal barrier: molecular pathways and modifiers. World J Gastrointest Pathophysiol. (2013) 4:94. doi: 10.4291/wjgp.v4.i4.94
114. Hayward P, Kalmar T, Martinez Arias A. Wnt/Notch signalling and information processing during development. Development. (2008) 135:411–24. doi: 10.1242/dev.000505
115. Patni AP, Harishankar MK, Joseph JP, Sreeshma B, Jayaraj R, Devi A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—clinical implications. Cell Oncol. (2021) 44:473–94. doi: 10.1007/s13402-021-00591-3
116. Kay SK, Harrington HA, Shepherd S, Brennan K, Dale T, Osborne JM, et al. The role of the Hes1 crosstalk hub in Notch-Wnt interactions of the intestinal crypt. PLoS Comput Biol. (2017) 13:e1005400. doi: 10.1371/journal.pcbi.1005400
Keywords: inflammatory bowel disease, Wnt signaling, Notch signaling, Hippo signaling, immune system
Citation: Khoramjoo SM, Kazemifard N, Baradaran Ghavami S, Farmani M, Shahrokh S, Asadzadeh Aghdaei H, Sherkat G and Zali MR (2022) Overview of Three Proliferation Pathways (Wnt, Notch, and Hippo) in Intestine and Immune System and Their Role in Inflammatory Bowel Diseases (IBDs). Front. Med. 9:865131. doi: 10.3389/fmed.2022.865131
Received: 29 January 2022; Accepted: 14 April 2022;
Published: 23 May 2022.
Edited by:
Alain Pierre Gobert, Vanderbilt University Medical Center, United StatesReviewed by:
Harpreet Kaur, Vanderbilt University Medical Center, United StatesCopyright © 2022 Khoramjoo, Kazemifard, Baradaran Ghavami, Farmani, Shahrokh, Asadzadeh Aghdaei, Sherkat and Zali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaghayegh Baradaran Ghavami, U2guYmdoYXZhbWlAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.