- 1Department of Internal Medicine, Papageorgiou Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Postgraduate Course, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 3Department of Dermatology and Venereology, School of Medicine, Papageorgiou Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
Although psoriasis is predominantly a chronic inflammatory skin disorder, epidemiological data provide a solid link between psoriasis, especially in its more severe forms, and increased risk for cardiovascular morbidity and mortality. Apart from the increased prevalence of traditional cardiovascular risk factors, chronic inflammation appears to act synergistically with the underlying process of endothelial dysfunction toward the development of accelerated atherosclerosis, subclinical vascular injury and subsequently, clinically evident cardiovascular manifestations. Endothelial dysfunction is regarded as an early precursor of atherosclerosis with a predictive value for the development of future cardiovascular events. A thorough understanding of the mechanisms of endothelial dysfunction in psoriasis might pave the path for the development of more accurate cardiovascular risk prediction tools and possible therapeutic targets aiming to alleviate the increased cardiovascular burden associated with the disease. The present review summarizes the available evidence about the role of chronic inflammation and other important pathophysiological mechanisms involved in the development of endothelial dysfunction in psoriasis. An overview of studies implementing the most widely applied circulating and vascular biomarkers of endothelial dysfunction in psoriasis patients will be provided, and the impact of systemic psoriasis treatments on endothelial dysfunction and patients’ cardiovascular risk will be discussed.
Introduction
Psoriasis is a chronic inflammatory skin disease with a reported prevalence of approximately 2% in Europe and North America, triggered and preserved by dysregulated interactions between the innate and adaptive components of the immune system (1, 2). However, cutaneous manifestations are not the only clinical demonstration of the disease. Accelerated rates of cardiovascular complications and major adverse cardiovascular events have been consistently observed in patients with psoriasis (3). Although these patients are often characterized by an unfavorable cardiometabolic profile, increased cardiovascular risk in psoriasis appears to be directly associated with the disease per se (1, 2). Pathophysiologically, central to the inflammatory hypothesis of atherosclerosis is the mutual interplay between inflammation and endothelial dysfunction, as has been described in other chronic inflammatory autoimmune disorders (4). Their synergism triggers and augments the sequence of accelerated atherosclerosis, subclinical target organ damage and eventually, clinically evident cardiovascular manifestations (4, 5).
Keeping in mind that endothelial dysfunction is considered as the earliest precursor of cardiovascular disease (CVD) (6), a thorough understanding of this process in psoriasis might commence interventions for the early identification and more effective monitoring of high-risk individuals. Therefore, the present mini review aims to provide an overall assessment of endothelial dysfunction in psoriasis, with emphasis placed on mechanisms that promote endothelial dysfunction and subsequently lead to the increased cardiovascular risk associated with the disease. Studies providing evidence of endothelial dysfunction using established as well as novel markers of endothelial dysfunction in psoriasis will be discussed. Lastly, data regarding effects of psoriasis-specific systemic treatments on endothelial dysfunction will be provided. To this end, a PubMed search was performed to identify relevant articles published in English, using the following medical terms: “psoriasis,” “endothelial dysfunction,” “cardiovascular,” and “atherosclerosis.”
Cardiovascular Morbidity and Comorbidity in Psoriasis
Psoriasis is associated with multiple cardiometabolic diseases, which are divided into cardiovascular comorbidities and major adverse cardiovascular events (coronary heart disease, ischemic heart disease, myocardial infarction, congestive heart failure, and stroke) all of which contribute to increased cardiovascular morbidity and mortality (7, 8). The strength of this association appears to vary according to psoriasis disease severity (9). Table 1 summarizes relevant systematic reviews and meta-analyses, all of them published within the past decade, supporting the association of psoriasis with conventional cardiovascular risk factors and adverse cardiovascular events.
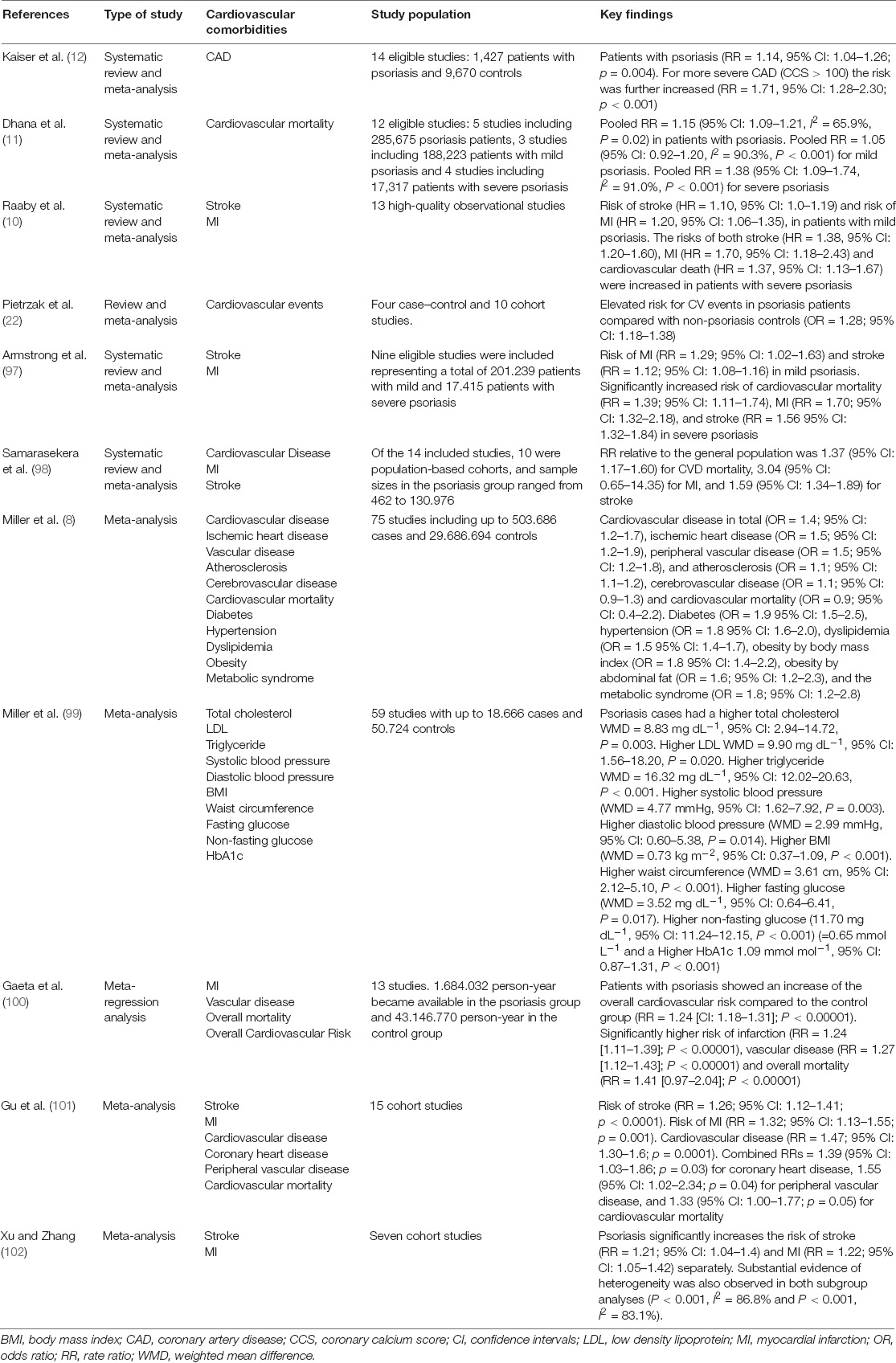
Table 1. A synopsis of systematic reviews and meta-analyses investigating the association of psoriasis with conventional cardiovascular risk factors and adverse cardiovascular events.
One crucial point is whether patients with psoriasis have an increased risk of cardiovascular events independently of -versus mediated by- their less favorable risk factor profile. At present, there is evidence for both directions. Prevalence of traditional cardiovascular risk factors is increased among patients with psoriasis compared to non-psoriasis individuals, such as ischemic heart disease, peripheral vascular disease, diabetes, hypertension, dyslipidemia, obesity and metabolic syndrome (8). In addition, several systematic reviews and meta-analyses have established the association of psoriasis with increased rates of atherosclerotic CVD. In a recent meta-analysis by Raaby et al., psoriatic patients, especially those with severe psoriasis, had an increased risk of CVDs (stroke, myocardial infarction, cardiovascular death) (10). Consistent with the above, Dhana et al. showed increased all-cause and cardiovascular mortality risk in psoriasis patients, especially those with severe psoriasis, compared to those without (11). Nevertheless, those with mild disease did not present an increased risk of cardiovascular mortality (11). More recently, increased risk of coronary artery disease was demonstrated in psoriasis by Kaiser et al., assessed by computed tomography and coronary calcium score (CCS) (12). Consequently, available epidemiological data provide solid evidence for a strong link between psoriasis and atherosclerotic CVD, which is at least partially mediated by their aggravated cardiovascular risk profile. Further experimental and clinical studies have attempted to shed light on the primary underlying pathophysiological processes eventually resulting in the establishment of clinically overt CVD independently of traditional cardiovascular factors, as described below.
Pathophysiology of Endothelial Dysfunction in Patients with Psoriasis: The Role of Chronic Inflammation
Endothelial dysfunction is defined as loss of vascular dilation in response to biological and mechanical stimuli owing to the pathologic transition of the endothelium into a non-adaptive state secondary to decreased nitric oxide (NO) bioavailability (13). It is considered as a key step in the initiation and progression of atherosclerosis, the development of which involves complex interactions between the endothelium, circulating lipids, platelets, and the immune system (14–19). Several factors, including circulating proinflammatory cytokines, reactive oxygen species, oxidized LDL-C, autoantibodies and traditional cardiovascular risk factors directly and indirectly activate endothelial cells and impair their function, resulting in impaired vascular relaxation, increased leukocyte adhesion, increased endothelial permeability and generation of a pro-thrombotic state (15, 20). Importantly, all of these factors are dysregulated in psoriasis. Due to activation of immune-mediated mechanisms, the vascular endothelium presents a pro-inflammatory phenotype in psoriasis with upregulation of chemotactic, proatherogenic and vascular adhesion molecules, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6 and the IL-17 family of cytokines, interferon, and vascular cell adhesion molecule 1 (VCAM1). The downstream consequences result in vascular arterial inflammation and direct cytokine-induced injury (20, 21). Mechanisms of endothelial dysfunction in psoriasis are presented in Figure 1.
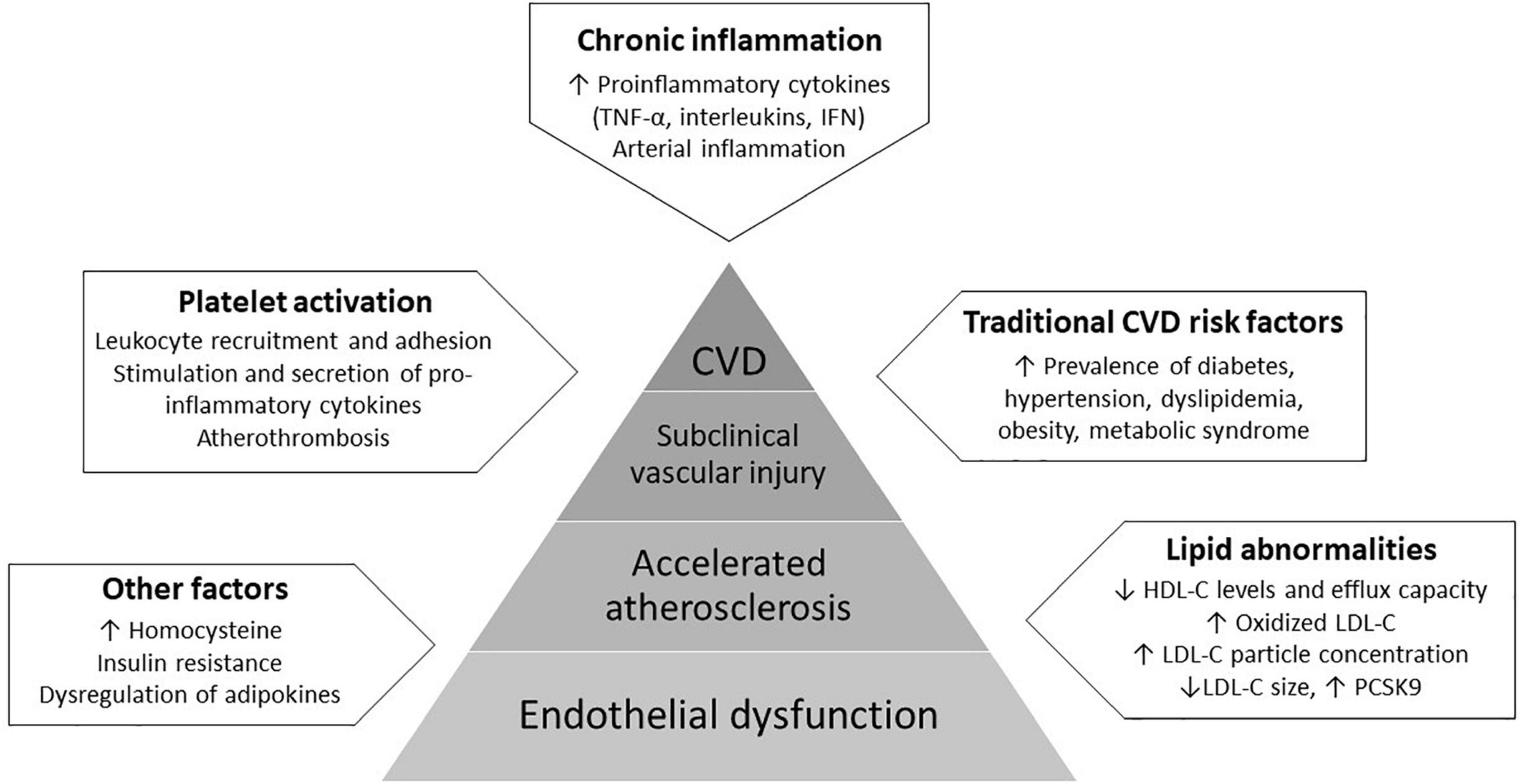
Figure 1. Mechanisms of endothelial dysfunction in psoriasis involve complex interactions between the endothelium, proinflammatory cytokines, circulating lipids and platelets, and the increased prevalence of traditional cardiovascular risk factors. These factors act synergistically directly and indirectly activate endothelial cells and impair their function, resulting in a pro-atherogenic state, the subsequent development of subclinical vascular injury and eventually, clinically evident cardiovascular disease (CVD).
As the atherosclerotic procedure is being increasingly recognized as an inflammatory process, mechanisms of accelerated atherosclerosis in chronic autoimmune diseases have become a topic of growing interest (22). Pathogenetic mechanisms of CVD in psoriasis appear to be complex and have not yet been fully elucidated. However, psoriasis-triggered pathological pathways, in particular endothelial dysfunction, may activate or augment pre-existing atherosclerosis, which subsequently results in the development of clinically overt CVD (22–24).
Furthermore, chronic inflammation alters lipoproteins structurally and functionally in ways that cannot be captured through standard lipid measurements (25). Inflammation drives modification of LDL-C into small, dense particles that are known to exert pro-atherogenic effects (25). Dyslipidemia is independently associated with endothelial dysfunction, while (26, 27) lipoproteins are implicated in the generation of oxidative stress (26, 28). Oxidized LDL-C modulates NO availability through reduction of eNOS activity or by enhanced metabolism of NO by asymmetric dimethylarginine (ADMA), an endogenous competitive eNOS inhibitor (27, 29, 30). Abnormalities in lipid profile are common in psoriasis and considered to contribute substantially to endothelial dysfunction (31). Patients with psoriasis present reduced levels and efflux capacity of HDL-C, increased LDL-C particle concentration and decreased LDL-C size, as well as elevated levels of circulating PCSK9 when compared to non-psoriasis individuals (31–33).
Platelet activation is regarded as another potent mediator of endothelial dysfunction in psoriasis (34). Apart from their contribution to psoriatic skin lesions, platelets are key regulators of inflammation, immune function, and atherothrombosis. Activated platelets contribute substantially to psoriasis associated inflammation by stimulation and secretion of pro-inflammatory cytokines (18, 35). They perpetuate leukocyte recruitment, adhesion, and rolling along the activated endothelium, thereby promoting atherosclerosis and inducing endothelial dysfunction (36). Patients with psoriasis present increased circulating biomarkers of platelet activation, such as platelet-derived microvesicles, soluble p-selectin, platelet-lymphocyte and platelet-neutrophil aggregates (37). Platelets derived from psoriasis patients augment endothelial cell activation with up to a 20-fold increase in endothelial-derived cytokines such as IL-1β and IL-8 (35). In psoriasis, platelets promote IL-17 secretion from CD4+ lymphocytes, and induce in vitro endothelial injury and apoptosis when co-localized with neutrophil subtype granulocytes through the formation of neutrophil extracellular traps, a process known as NETosis (38, 39).
Lastly, although less established, other characteristic features of patients with psoriasis may contribute to the development of endothelial dysfunction. For instance, homocysteine levels are elevated in psoriasis and correlate with the severity of the disease (27). Increased homocysteine promotes oxidative stress and has been associated with the development of atherosclerosis and CVD (40, 41). In addition, insulin resistance is common in psoriasis. As a vasoactive hormone, insulin promotes vasodilation in a NO-dependent manner, increases vasodilation and presents anti-inflammatory effects (42). Likewise, adipokines appear dysregulated in patients with psoriasis, as a result of obesity which is a common comorbidity, but also independently, as they modulate cutaneous inflammation with a probable pathogenetic role in psoriasis (16). Pro-inflammatory adipokines in psoriasis may be released in the peripheral blood as a result of adipose tissue inflammation (16). Elevated adiponectin levels suppress inflammation and immune responses, whereas low adiponectin levels (including leptin, adiponectin, and resistin) are associated with endothelial dysfunction and the development of the metabolic syndrome or CVDs (16).
Evaluation of Endothelial Dysfunction in Psoriasis
Considering the pathophysiological and clinical significance of endothelial dysfunction, several studies have attempted to quantify the burden of endothelial dysfunction in psoriasis, using both circulating and vascular biomarkers.
Circulating Biomarkers of Endothelial Dysfunction in Psoriasis
Asymmetrical dimethylarginine (ADMA), oxidized LDL, endothelial progenitor cells (EPCs), endothelial glycocalyx and endothelial microvesicles (EMVs) represent reliable circulating biomarkers of endothelial dysfunction but are currently applied only as research tools (6).
Psoriasis patients present elevated levels of ADMA, a potent eNOS inhibitor of the L-arginine-NO pathway, that correlate with disease severity, suggesting an important role of endothelial dysfunction in the pathogenesis of psoriasis (27). By contrast, ADMA was not increased in a smaller study of mild-to-moderate plaque-type psoriatic patients with low-to-medium grade systemic inflammation (43). Few studies have examined oxidized LDL as a marker of endothelial dysfunction in psoriasis. In a large study of 252 psoriasis patients and controls, psoriasis subjects presented increased levels of lipoprotein (a), oxidized lipoprotein (a) and oxidized HDL (44), although a smaller study of 79 patients with psoriasis and 80 controls failed to reveal any differences in the levels of oxidized LDL (45). Remarkably, oxidized LDL in the former study was significantly associated with non-calcified coronary plaque burden assessed by coronary computed tomographic angiography (44).
Expressed on the endothelial cell surface within blood vessels, the endothelial glycocalyx regulates blood vessel permeability and homeostasis. In a large study of 297 psoriatic patients and 150 controls, glycocalyx thickness in sublingual microvessels was reduced among patients, and correlated with disease activity, carotid atherosclerosis, impaired coronary flow reserve and markers of myocardial deformation assessed by speckle-tracking imaging (46). EPCs are the progenitor cells that are able to differentiate into functional endothelial cells, sustain vasculogenesis and promote vascular repair in ischemic diseases. Significantly reduced levels of circulating EPCs have been measured in psoriasis patients compared to controls, and an inverse correlation with disease severity was observed (47). Likewise, another study recruiting plaque-type psoriasis patients demonstrated decreased EPC levels, as well as an independent association with pulse wave velocity, a well-established marker of arterial stiffness (48). By contrast, these results were not confirmed in a more recent study (17). MicroRNA (miRNA) expression and especially circulating miR-200s were positively correlated with markers of cardiovascular dysfunction such as left ventricular mass (49).
Microvesicles are small vesicles (0.1–1 μm) released from plasma membrane as a result of cellular activation or apoptosis. EMVs display multivalent important biological properties and contribute to vascular homeostasis (50). Their levels increase substantially in patients with CVDs such as hypertension, diabetes mellitus, acute and chronic coronary artery disease (51–53), but also in patients with chronic autoimmune inflammatory diseases (54). Increased levels of EMVs have been found in patients with psoriasis (14, 55). Notably, increased EMVs concentrations in psoriasis were observed beyond cardiometabolic risk factors (55). Another study showed higher ratio of EMVs/EPCs in psoriasis patients, which independently correlated with higher carotid intima-media thickness, an established marker of subclinical atherosclerosis (19, 56). These findings suggest that increased EMVs in psoriasis might not simply represent a consequence of endothelial cell activation, but may also have a role in psoriasis pathophysiology leading to accelerated atherosclerosis.
Vascular Markers of Endothelial Dysfunction in Psoriasis
The most widely applied, non-invasive vascular methods for the functional assessment of endothelial dysfunction include laser Doppler flowmetry and imaging (LDF/LDI), and the gold-standard flow-mediated dilation (FMD) of the brachial artery, which is currently considered as the gold-standard non-invasive method, with a predictive value for future cardiovascular events especially in high-risk populations (57). Several studies have assessed endothelial dysfunction in psoriasis with FMD, summarized in a recent meta-analysis demonstrating lower FMD measurements among patients compared with controls (56). Although laser Doppler techniques have been mainly applied for the assessment of vascular perfusion within plaques rather than the evaluation of systemic microcirculation in psoriasis (58), NO-dependent vasodilation was attenuated in psoriasis patients in a small study using LDF, and correlated with the degree of psoriatic symptomatology (59). Other vascular methods that focus on the evaluation of endothelial function, such as quantitative coronary angiography and positron emission tomography (PET), are compromised by significant limitations including radiation, reproducibility and cost. However, a recent meta-analysis of 1,427 patients with psoriasis without prior coronary artery disease and 9,670 controls showed higher prevalence and burden of coronary artery disease among patients, detected by CCS with or without cardiac computed tomography angiography (12). Using 18F-fluorodeoxyglucose positron emission tomography imaging, a randomized placebo-controlled pilot study showed higher vascular inflammation in ascending aorta of patients with moderate-to-severe psoriasis as compared to controls (60). Similarly, arterial inflammation was more pronounced in patients with mild psoriasis compared to controls by use of the same method (61).
Collectively, available data regarding the above circulating and vascular biomarkers appear in line with the hypothesis that endothelial dysfunction is implicated in the development of accelerated atherosclerosis in psoriasis. Further extending this notion, the potential improvement of endothelial function following successful control of psoriasis-related inflammation has been the subject of several studies over the past years.
The Impact of Pharmacological Interventions on Endothelial Dysfunction in Psoriasis
To date, topical therapies are the cornerstone for managing mild psoriasis which typically covers less than 5% body surface area (62). However, patients with moderate and severe disease, who are presumably at higher cardiovascular risk based on the above, are candidates for newer systemic therapies as first-line treatment (63, 64). The observation that these therapies could target the accompanying vascular dysfunction and ameliorate biomarkers of cardiovascular risk (65), has several therapeutic implications for cardiovascular risk prevention in psoriasis that are currently under vigorous investigation.
Tumor Necrosis Factor-α Inhibitors
Tumor necrosis factor-α inhibitors (adalimumab, certolizumab, etanercept, or infliximab) showed a protective cardiovascular profile in multiple, mainly observational studies of psoriasis. It has been hypothesized that this action is probably mediated by their beneficial effect on endothelial cell function (66). There is at present no strong, definite evidence for a significant beneficial effect of anti-TNF-α biologics on endothelial function in psoriasis (66, 67), although some promising data do exist. TNF-α blockade has led to improvement of vascular function assessed through resting endothelium-dependent vascular tone by low-flow-mediated constriction, yet FMD values remained unchanged (68). In this context, treatment with TNF-a inhibitors was associated with significant reductions in endothelial and platelet microvesicles levels (69). A small cohort study detected a significant improvement of endothelial dysfunction markers (serum intercellular adhesion molecule 1 and FMD) after a short-period treatment with adalimumab (70). Adalimumab has shown anti-inflammatory effects and improved FMD in patients with psoriasis (71). Conversely, a recent RCT study, that used 18F-FDG PET-CT to assess vascular inflammation, did not detect any superiority of adalimumab over phototherapy or placebo (72). In a recent meta-analysis of RCTs, there was no beneficial effect on imaging biomarkers (aortic vascular inflammation or FMD) of cardiovascular risk in patients exposed to adalimumab (73). Treatment with etanercept increased the EPC count in a small double blind, placebo-controlled, cross-over study, indicating improved endogenous endothelial regenerative capacity, but brachial artery flow-mediated and nitroglycerin-mediated dilation was not modified with treatment (74).
IL-17 and IL-23 Inhibitors
IL-17 inhibitors target either the IL-17 ligand (secukinumab, ixekizumab, and bimekizumab) or its receptor (brodalumab). Secukinumab did not show any clinically significant effect on aortic vascular inflammation (assessed by 18F-FDG PET/CT) in a placebo-controlled RCT either on short- or long-term follow-up (75). Nevertheless, the CARIMA (Evaluation of Cardiovascular Risk Markers in Psoriasis Patients Treated with Secukinumab) trial indicated the protective role of secukinumab on endothelial cell function measured with FMD (76). Recently, the potential benefit of a reduced dose interval has been examined in heavier patients and in those with suboptimal responses (77, 78). Ustekinumab inhibits both IL-12 and IL-23 by targeting their shared p40 subunit. Positive effects of ustekinumab impact on vascular inflammation were observed using 18F-FDG PET/CT (79). However, levels of circulating endothelial- and platelet-derived microvesicles remained unchanged in patients with psoriasis successfully treated with anti-IL-12/23, regardless of clinical improvement (80). In a recent meta-analysis of RCTs, ustekinumab, yet not secukinumab, induced short-term reductions in aortic vascular inflammation, whereas FMD remained unchanged. Nevertheless, these reductions were not sustained in the long-term (73). The hypothesis of cardiovascular risk reduction in psoriasis patients receiving ustekinumab (81) was further questioned by the observation of no substantially different risk of major adverse cardiovascular events among TNF-a inhibitors, ustekinumab or placebo therapy in several studies (82–85). The effect of newer IL-23 and other cytokines inhibitors recently approved (Guselkumab, Tildrakizumab, and Risankizumab) on endothelial dysfunction remains to be investigated (86–92).
Oral Systemic Treatments
In a prospective longitudinal pilot study, systemic therapy with fumaric acid esters resulted in an improvement of endothelial vasodilator function assessed by venous occlusion plethysmography (93). Methotrexate, apremilast, acitretin, and cyclosporine are oral available treatment options for psoriasis. Nevertheless, evidence regarding protective effects on cardiovascular disease in psoriasis exists mainly for methotrexate (94, 95), which has shown neutral short-term effects on endothelial function in patients with psoriasis (96). Further studies are warranted to determine the effects of these treatment modalities on endothelial dysfunction in patients with psoriasis.
Conclusion and Future Perspectives
Psoriasis, especially in its severe forms, is an independent risk factor for cardiovascular morbidity and mortality. Several psoriasis-induced mechanisms promote the development of endothelial dysfunction, which acts synergistically with chronic inflammation and activates or potentiates pre-existing atherosclerosis in psoriasis. Available human studies using the most widely applied circulating and vascular biomarkers of endothelial dysfunction provide clinical evidence of endothelial dysfunction in patients with psoriasis, that correlates with disease severity. However, the clinical utility of biomarkers of endothelial dysfunction in psoriasis patients in terms of cardiovascular risk prediction needs to be addressed further. Moreover, it is hypothesized that effective control of the disease might improve endothelial cell function and subsequently modulate cardiovascular risk. Although some encouraging data have been published, large, prospective, appropriately designed studies are urgently warranted to provide strong evidence regarding the possibility of sustained beneficial effects on endothelial function of such therapies in psoriasis. Last but not least, future studies need to investigate whether interventions specifically targeting at the improvement of endothelial function in patients with psoriasis might provide incremental benefits in the modulation of both chronic inflammation and risk of future CVD.
Author Contributions
EG, EL, and AP contributed to the conception and design of the study. PA and AM performed the literature searching and wrote the first draft of the manuscript. KG and MG wrote the sections of the manuscript. KG prepared the table. PA designed the figure and edited the final draft of the manuscript. EG reviewed and supervised the final version of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Ogdie A, Yu YD, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheumatic Dis. (2015) 74:326–32. doi: 10.1136/annrheumdis-2014-205675
3. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
4. Anyfanti P, Gavriilaki E, Douma S, Gkaliagkousi E. Endothelial dysfunction in patients with rheumatoid arthritis: the role of hypertension. Curr Hypertens Rep. (2020) 22:56. doi: 10.1007/s11906-020-01064-y
5. Aksu K, Donmez A, Keser G. Inflammation-induced management thrombosis: mechanisms, disease associations and management. Curr Pharm Des. (2012) 18:1478–93.
6. Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical significance of endothelial dysfunction in essential hypertension. Curr Hypertens Rep. (2015) 7:85. doi: 10.1007/s11906-015-0596-3
7. Yim KM, Armstrong AW. Updates on cardiovascular comorbidities associated with psoriatic diseases: epidemiology and mechanisms. Rheumatol Int. (2017) 37:97–105. doi: 10.1007/s00296-016-3487-2
8. Miller IM, Ellervik C, Yazdanyar S, Jemec GBE. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. (2013) 69:1014–24. doi: 10.1016/j.jaad.2013.06.053
9. Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. (2013) 149:1173–9. doi: 10.1001/jamadermatol.2013.5015
10. Raaby L, Ahlehoff O, de Thurah A. Psoriasis and cardiovascular events: updating the evidence. Arch Dermatol Res. (2017) 309:225–8. doi: 10.1007/s00403-016-1712-1
11. Dhana A, Yen H, Yen H, Cho E. All-cause and cause-specific mortality in psoriasis: a systematic review and meta-analysis. J Am Acad Dermatol. (2019) 80:1332–43. doi: 10.1016/j.jaad.2018.12.037
12. Kaiser H, Abdulla J, Henningsen KMA, Skov L, Hansen PR. Coronary artery disease assessed by computed tomography in patients with psoriasis: a systematic review and meta-analysis. Dermatology. (2019) 235:478–87. doi: 10.1159/000502138
13. Gkaliagkousi E, Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci (Landmark Ed). (2011) 16:1873–97. doi: 10.2741/3828
14. Pelletier F, Garnache-Ottou F, Angelot F, Biichlé S, Vidal C, Humbert P, et al. Increased levels of circulating endothelial-derived microparticles and small-size platelet-derived microparticles in psoriasis. J Invest Dermatol. (2011) 131:1573–6. doi: 10.1038/jid.2011.57
15. Steyers CM, Miller FJ. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. (2014) 15:11324–49.
16. Lynch M, Bao B, Ahern T, Sweeney CM, Malara A, Tobin AM, et al. Adipokines, psoriasis, systemic inflammation, and endothelial dysfunction. Int J Dermatol. (2017) 56:1103–18. doi: 10.1111/ijd.13699
17. Patschan D, Sugiarto N, Henze E, Mößner R, Mohr J, Müller GA, et al. Early endothelial progenitor cells and vascular stiffness in psoriasis and psoriatic arthritis. Eur J Med Res. (2018) 23:56. doi: 10.1186/s40001-018-0352-7
18. Garshick MS, Tawil M, Barrett TJ, Salud-Gnilo CM, Eppler M, Lee A, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol. (2020) 40:1340–51. doi: 10.1161/ATVBAHA.119.314008
19. Pirro M, Stingeni L, Vaudo G, Mannarino MR, Ministrini S, Vonella M, et al. Systemic inflammation and imbalance between endothelial injury and repair in patients with psoriasis are associated with preclinical atherosclerosis. Eur J Prevent Cardiol. (2015) 22:1027–35. doi: 10.1177/2047487314538858
20. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. (2009) 78:539–52.
21. von Stebut E, Boehncke WH, Ghoreschi K, Gori T, Kaya Z, Thaci D, et al. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol. (2020) 10:3096. doi: 10.3389/fimmu.2019.03096
22. Pietrzak A, Bartosińska J, Chodorowska G, Szepietowski JC, Paluszkiewicz P, Schwartz RA, et al. Cardiovascular aspects of psoriasis: an updated review. Int J Dermatol. (2013) 52:153–62.
24. Schmitt J, Ford DE. Psoriasis is independently associated with psychiatric morbidity and adverse cardiovascular risk factors, but not with cardiovascular events in a population-based sample. J Eur Acad Dermatol Venereol. (2010) 24:885–92. doi: 10.1111/j.1468-3083.2009.03537.x
25. Borén J, John Chapman M, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
26. Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. (2001) 303:33–9. doi: 10.1016/s0009-8981(00)00358-2
27. Bilgiç O, Altınyazar HC, Baran H, Unlu A. Serum homocysteine, asymmetric dimethyl arginine (ADMA) and other arginine–NO pathway metabolite levels in patients with psoriasis. Arch Dermatol Res. (2015) 307:439–44. doi: 10.1007/s00403-015-1553-3
28. Afsin A, Kaya H, Suner A, Uzel KE, Bursa N, Hosoglu Y, et al. Plasma atherogenic indices are independent predictors of slow coronary flow. BMC Cardiovasc Disord. (2021) 21:608. doi: 10.1186/s12872-021-02432-5
29. van der Zwan LP, Scheffer PG, Dekker JM, Stehouwer CDA, Heine RJ, Teerlink T. Systemic inflammation is linked to low arginine and high ADMA plasma levels resulting in an unfavourable nos substrate-to-inhibitor ratio: the Hoorn study. Clin Sci. (2011) 121:71–8. doi: 10.1042/CS20100595
30. Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. (2011) 164:213–23. doi: 10.1111/j.1476-5381.2010.01196.x
31. Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res. (2012) 53:1618–24. doi: 10.1194/jlr.M027367
32. Garshick MS, Baumer Y, Dey AK, Grattan R, Ng Q, Teague HL, et al. Characterization of PCSK9 in the blood and skin of psoriasis. J Invest Dermatol. (2021) 141:308–15. doi: 10.1016/j.jid.2020.05.115
33. Mehta NN, Li R, Krishnamoorthy P, Yu YD, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. (2012) 224:218–21. doi: 10.1016/j.atherosclerosis.2012.06.068
34. Fan Z, Wang L, Jiang H, Lin Y, Wang Z. Platelet dysfunction and its role in the pathogenesis of psoriasis. Dermatology. (2021) 237:56–65. doi: 10.1159/000505536
35. Weber B, Merola JF, Husni ME, di Carli M, Berger JS, Garshick MS. Psoriasis and cardiovascular disease: novel mechanisms and evolving therapeutics. Curr Atheroscler Rep. (2021) 23:67. doi: 10.1007/s11883-021-00963-y
36. Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. (2015) 147:123–35. doi: 10.1016/j.pharmthera.2014.11.008
37. Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol. (2010) 62:621–6. doi: 10.1016/j.jaad.2009.06.053
38. Teague HL, Varghese NJ, Tsoi LC, Dey AK, Garshick MS, Silverman JI, et al. Neutrophil subsets, platelets, and vascular disease in psoriasis. JACC Basic Transl Sci. (2019) 4:1–14. doi: 10.1016/j.jacbts.2018.10.008
39. Sanz-Martínez MT, Moga E, Sánchez Martínez MA, Zamora Atenza C, Vidal S, Juárez C, et al. High levels of platelet-lymphocyte complexes in patients with psoriasis are associated with a better response to Anti–TNF-α Therapy. J Investigat Dermatol. (2020) 140:1176–83. doi: 10.1016/j.jid.2019.08.457
40. Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. (2016) 17:1733. doi: 10.3390/ijms17101733
41. Graham IM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, et al. Plasma homocysteine as a risk factor for vascular disease. The European concerted action project. JAMA. (1997) 277:1775–81. doi: 10.1001/jama.1997.03540460039030
42. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. (2005) 11:1448–54. doi: 10.1161/01.CIR.0000158483.13093.9D
43. Usta M, Yurdakul S, Aral H, Turan E, Oner E, Inal BB, et al. Vascular endothelial function assessed by a noninvasive ultrasound method and serum asymmetric dimethylarginine concentrations in mild-to-moderate plaque-type psoriatic patients. Clin Biochem. (2011) 44:1080–4. doi: 10.1016/j.clinbiochem.2011.06.007
44. Sorokin AV, Kotani K, Elnabawi YA, Dey AK, Sajja AP, Yamada S, et al. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis an observational cohort study. Circ Res. (2018) 123:1244–54.
45. Gerdes S, Osadtschy S, Buhles N, Baurecht H, Mrowietz U. Cardiovascular biomarkers in patients with psoriasis. Exp Dermatol. (2014) 23:322–5.
46. Ikonomidis I, Pavlidis G, Lambadiari V, Rafouli-Stergiou P, Makavos G, Thymis J, et al. Endothelial glycocalyx and microvascular perfusion are associated with carotid intima-media thickness and impaired myocardial deformation in psoriatic disease. J Hum Hypertens. (2021) [Epub ahead of print].
47. Batycka-Baran A, Paprocka M, Krawczenko A, Kantor A, Duś D, Szepietowski JC. Reduced number of circulating endothelial progenitor cells (CD133+/KDR+) in patients with plaque psoriasis. Dermatology. (2012) 225:88–92. doi: 10.1159/000341534
48. Liu JH, Chen Y, Zhen Z, Yeung CK, Chan J, Chan HH, et al. Relation between endothelial progenitor cells and arterial stiffness in patients with psoriasis. J Dermatol. (2016) 43:888–93. doi: 10.1111/1346-8138.13235
49. Magenta A, D’Agostino M, Sileno S, di Vito L, Abeni D, Martino F, et al. The oxidative stress Induced-MIR-200C IS UP-regulated in psoriasis and correlates with disease severity and determinants of cardiovascular risk. Atherosclerosis. (2020) 315:77–8. doi: 10.1155/2019/8061901
50. Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat ML, et al. Microvesicles in vascular homeostasis and diseases position paper of the european society of cardiology (ESC) working group on atherosclerosis and vascular biology. Thromb Haemostasis. (2017) 117:1296–316. doi: 10.1160/TH16-12-0943
51. Gkaliagkousi E, Gavriilaki E, Vasileiadis I, Nikolaidou B, Yiannaki E, Lazaridis A, et al. Endothelial microvesicles circulating in peripheral and coronary circulation are associated with central blood pressure in coronary artery disease. Am J Hypertens. (2019) 32:1199–205. doi: 10.1093/ajh/hpz116
52. Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P, et al. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc Dis Res. (2019) 16:458–65. doi: 10.1177/1479164119844691
53. Gkaliagkousi E, Gavriilaki E, Yiannaki E, Vasileiadis I, Nikolaidou B, Lazaridis A, et al. Platelet microvesicles are associated with the severity of coronary artery disease: comparison between peripheral and coronary circulation. J Thromb Thrombol. (2021) 51:1138–43. doi: 10.1007/s11239-020-02302-5
54. Anyfanti P, Gavriilaki E, Nikolaidou B, Yiannaki E, Lazaridis A, Papadopoulos N, et al. Patients with autoimmune chronic inflammatory diseases present increased biomarkers of thromboinflammation and endothelial dysfunction in the absence of flares and cardiovascular comorbidities. J Thromb Thrombol. (2021) 53:10–6. doi: 10.1007/s11239-021-02517-0
55. Takeshita J, Mohler ER, Krishnamoorthy P, Moore J, Rogers WT, Zhang L, et al. Endothelial cell-, platelet-, and monocyte/macrophage-derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J Am Heart Assoc. (2014) 3:e000507. doi: 10.1161/JAHA.113.000507
56. Fang N, Jiang M, Fan Y. Association between psoriasis and subclinical atherosclerosis. Medicine. (2016) 95:e3576.
57. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. (2013) 168:344–51. doi: 10.1016/j.ijcard.2012.09.047
58. Lacarrubba F, Pellacani G, Gurgone S, Verzì AE, Micali G. Advances in non-invasive techniques as aids to the diagnosis and monitoring of therapeutic response in plaque psoriasis: a review. Int J Dermatol. (2015) 54:626–34. doi: 10.1111/ijd.12870
59. Alba BK, Greaney JL, Ferguson SB, Alexander LM. Endothelial function is impaired in the cutaneous microcirculation of adults with psoriasis through reductions in nitric oxide-dependent vasodilation. Am J Physiol Heart Circ Physiol. (2018) 314:343–9. doi: 10.1152/ajpheart.00446.2017
60. Kaur S, Shafiq N, Dogra S, Mittal BR, Attri SV, Bahl A, et al. 18F-fluorodeoxyglucose positron emission tomography-based evaluation of systemic and vascular inflammation and assessment of the effect of systemic treatment on inflammation in patients with moderate-to-severe psoriasis: a randomized placebo-controlled pilot study. Ind J Dermatol Venereol Leprol. (2018) 84:660–6. doi: 10.4103/ijdvl.IJDVL_717_17
61. Youn SW, Kang SY, Kim SA, Park GY, Lee WW. Subclinical systemic and vascular inflammation detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with mild psoriasis. J Dermatol. (2015) 42:559–66. doi: 10.1111/1346-8138.12859
62. Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. (2013) 2013:CD005028.
63. Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. (2019) 80:1029–72.
64. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. (2011) 65:137–74. doi: 10.1016/j.jaad.2010.11.055
65. Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. (2011) 25:1187–93. doi: 10.1111/j.1468-3083.2010.03947.x
66. Knowles L, Nadeem N, Chowienczyk PJ. Do anti-tumour necrosis factor-α biologics affect subclinical measures of atherosclerosis and arteriosclerosis? A systematic review. Br J Clin Pharmacol. (2020) 86:837–51. doi: 10.1111/bcp.14215
67. Eder L, Joshi AA, Dey AK, Cook R, Siegel EL, Gladman DD, et al. Association of tumor necrosis factor inhibitor treatment with reduced indices of subclinical atherosclerosis in patients with psoriatic disease. Arthritis Rheumatol. (2018) 70:408–16. doi: 10.1002/art.40366
68. Wegner J, Karbach S, Drosos I, Schnorbus B, Muxel S, Schmidt F, et al. TNF-α blockade may lead to improvement of vascular function in psoriasis patients. Exp Dermatol. (2021) 31:237–41. doi: 10.1111/exd.14452
69. Avgerinou G, Tousoulis D, Siasos G, Oikonomou E, Maniatis K, Papageorgiou N, et al. Anti-tumor necrosis factor alpha treatment with adalimumab improves significantly endothelial function and decreases inflammatory process in patients with chronic psoriasis. Int J Cardiol. (2011) 151:382–3. doi: 10.1016/j.ijcard.2011.06.112
70. Pelletier F, Garnache-Ottou F, Biichlé S, Vivot A, Humbert P, Saas P, et al. Effects of anti-TNF-α agents on circulating endothelial-derived and platelet-derived microparticles in psoriasis. Exp Dermatol. (2014) 23:924–5. doi: 10.1111/exd.12551
71. Holzer G, Hoke M, Sabeti-Sandor S, Perkmann T, Rauscher A, Strassegger B, et al. Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: results from a prospective, randomized, observer-blinded head-to-head trial. J Eur Acad Dermatol Venereol. (2021) 35:441–9. doi: 10.1111/jdv.16635
72. Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. (2018) 11:e007394. doi: 10.1161/CIRCIMAGING.117.007394
73. González-Cantero A, Ortega-Quijano D, Álvarez-Díaz N, Ballester MA, Jimenez-Gomez N, Jaen P, et al. Impact of biological agents on imaging and biomarkers of cardiovascular disease in patients with psoriasis: a systematic review and meta-analysis of randomized placebo-controlled trials. J Invest Dermatol. (2021) 141:2402–11. doi: 10.1016/j.jid.2021.03.024
74. Hayek SS, Neuman R, Kavtaradze N, Sher S, Jones D, Li Q, et al. Tumor necrosis factor-alpha antagonism with etanercept improves endothelial progenitor cell counts in patients with psoriasis: etanercept, vascular function and endothelial progenitor cells in psoriasis. Int J Cardiol. (2015) 182:387–9. doi: 10.1016/j.ijcard.2014.12.093
75. Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S). J Invest Dermatol. (2020) 140:1784–93.e2. doi: 10.1016/j.jid.2020.01.025
76. von Stebut E, Reich K, Thaçi D, Koenig W, Pinter A, Körber A, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. (2019) 139:1054–62. doi: 10.1016/j.jid.2018.10.042
77. Augustin M, Reich K, Yamauchi P, Pinter A, Bagel J, Dahale S, et al. Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial. Br J Dermatol. (2022): [Epub ahead of print]. doi: 10.1111/bjd.20971
78. Reich K, Körber A, Mrowietz U, Sticherling M, Sieder C, Früh J, et al. Secukinumab 2-weekly vs. 4-weekly dosing in patients with plaque-type psoriasis: results from the randomized GAIN study*. Br J Dermatol. (2021) 184:849–56. doi: 10.1111/bjd.19398
79. Kim BS, Lee WK, Pak K, Han J, Kim GW, Kim HS, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: feasibility study using 18 F-fluorodeoxyglucose PET/CT. J Am Acad Dermatol. (2019) 80:1322–31. doi: 10.1016/j.jaad.2018.03.011
80. Ho JC, Lee CH, Lin SH. No significant reduction of circulating endothelial-derived and platelet-derived microparticles in patients with psoriasis successfully treated with anti-IL12/23. BioMed Res Int. (2016) 2016:3242143. doi: 10.1155/2016/3242143
81. Langley RG, Papp K, Gottlieb AB, Krueger GG, Gordon KB, Williams D, et al. Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J Eur Acad Dermatol Venereol. (2013) 27:1252–61. doi: 10.1111/j.1468-3083.2012.04705.x
82. Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. (2013) 27:622–7. doi: 10.1111/j.1468-3083.2012.04500.x
83. Papp KA, Griffiths CEM, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. (2013) 168:844–54. doi: 10.1111/bjd.12214
84. Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of Ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol. (2019) 155:700–7.
85. Ryan C, Leonardi CL, Krueger JG, Kimball AB, Bruce Strober ME, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events a meta-analysis of randomized controlled trials. JAMA. (2011) 306:864–71. doi: 10.1001/jama.2011.1211
86. Lebwohl MG, Leonardi CL, Mehta NN, Gottlieb AB, Mendelsohn AM, Parno J, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. (2021) 84:398–407. doi: 10.1016/j.jaad.2020.09.047
87. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. (2018) 392:650–61. doi: 10.1016/S0140-6736(18)31713-6
88. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. (2017) 390:276–88. doi: 10.1016/S0140-6736(17)31279-5
89. Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy–assessor-blinded clinical trial*. Br J Dermatol. (2021) 184:50–9. doi: 10.1111/bjd.19341
90. Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. (2021) 397:475–86. doi: 10.1016/S0140-6736(21)00126-4
91. Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. (2019) 394:831–9. doi: 10.1016/S0140-6736(19)31773-8
92. Reich K, Armstrong AW, Foley P, Song M, Miller M, Shen YK, et al. Maintenance of response through up to 4 years of continuous guselkumab treatment of psoriasis in the VOYAGE 2 Phase 3 study. Am J Clin Dermatol. (2020) 21:881–90. doi: 10.1007/s40257-020-00555-7
93. Boehncke S, Fichtlscherer S, Salgo R, Garbaraviciene J, Beschmann H, Diehl S, et al. Systemic therapy of plaque-type psoriasis ameliorates endothelial cell function: results of a prospective longitudinal pilot trial. Arch Dermatol Res. (2011) 303:381–8. doi: 10.1007/s00403-010-1108-6
94. Prodanowich S, Ma F, Taylor J, Pezon C, Fasihi T, Kirsner R. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. (2005) 52:262–7. doi: 10.1016/j.jaad.2004.06.017
95. Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld LE, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. (2015) 29:1128–34. doi: 10.1111/jdv.12768
96. Gyldenløve M, Jensen P, Løvendorf MB, Zachariae C, Hansen PR, Skov L. Short-term treatment with methotrexate does not affect microvascular endothelial function in patients with psoriasis. J Eur Acad Dermatol Venereol. (2015) 29:591–4. doi: 10.1111/jdv.12385
97. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. (2013) 2:e000062. doi: 10.1161/JAHA.113.000062
98. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol. (2013) 133:2340–6. doi: 10.1038/jid.2013.149
99. Miller IM, Skaaby T, Ellervik C, Jemec GBE. Quantifying cardiovascular disease risk factors in patients with psoriasis: a meta-analysis. Br J Dermatol. (2013) 169:1180–7. doi: 10.1111/bjd.12490
100. Gaeta M, Castelvecchio S, Ricci C, Pigatto P, Pellissero G, Cappato R. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. (2013) 168:2282–8. doi: 10.1016/j.ijcard.2013.01.197
101. Gu WJ, Weng CL, Zhao YT, Liu QH, Yin RX. Psoriasis and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol. (2013) 168:4992–6. doi: 10.1016/j.ijcard.2013.07.127
Keywords: endothelial dysfunction, psoriasis, cardiovascular risk, atherosclerosis, circulating biomarkers, vascular biomarkers
Citation: Anyfanti P, Margouta A, Goulas K, Gavriilaki M, Lazaridou E, Patsatsi A and Gkaliagkousi E (2022) Endothelial Dysfunction in Psoriasis: An Updated Review. Front. Med. 9:864185. doi: 10.3389/fmed.2022.864185
Received: 28 January 2022; Accepted: 24 May 2022;
Published: 10 June 2022.
Edited by:
Müzeyyen Gönül, Dışkapı Yildirim Beyazit Training and Research Hospital, TurkeyReviewed by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyCristina M. Failla, Institute of Immaculate Dermatology (IRCCS), Italy
Copyright © 2022 Anyfanti, Margouta, Goulas, Gavriilaki, Lazaridou, Patsatsi and Gkaliagkousi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panagiota Anyfanti, cGFueWZhbkBob3RtYWlsLmNvbQ==
 Panagiota Anyfanti
Panagiota Anyfanti Anastasia Margouta1
Anastasia Margouta1 Kyriakos Goulas
Kyriakos Goulas Maria Gavriilaki
Maria Gavriilaki Aikaterini Patsatsi
Aikaterini Patsatsi