- 1Guangdong Provincial Key Laboratory of Systems Biology and Synthetic Biology for Urogenital Tumors, Shenzhen Key Laboratory of Genitourinary Tumor, Department of Urology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital (Shenzhen Institute of Translational Medicine), Shenzhen, Guangdong, China
- 2Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, Shenzhen University Health Science Center, School of Biomedical Engineering, Shenzhen, Guangdong, China
- 3Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
- 4Department of Central Laboratory, Shenzhen Hospital, Beijing University of Chinese Medicine, Shenzhen, Guangdong, China
Background: Patients with severe acute kidney injury (AKI) may require renal replacement therapy (RRT), such as hemodialysis and peritoneal dialysis. Neutrophil gelatinase-associated lipocalin (NGAL) is a sensitive indicator for early diagnosis and recognition of AKI; however, its predictive value of AKI-associated need for RRT needs further evaluation.
Methods: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, relevant articles were systematically searched and selected from seven databases. The random effects model was applied to evaluate the predictive performance of NGAL for AKI requiring RRT. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of each included study.
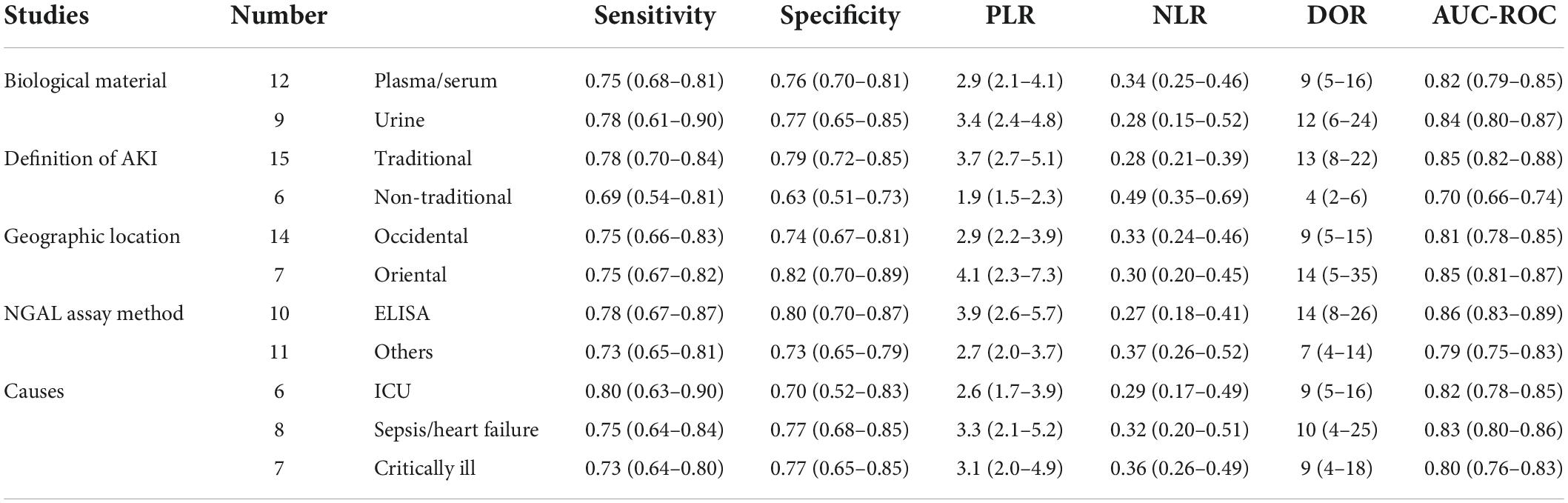
Results: A total of 18 studies including 1,787 patients with AKI and having an average NOS score of 7.67 were included in the meta-analysis. For plasma/serum NGAL, the pooled sensitivity and specificity with corresponding 95% confidence interval (CI) were 0.75 (95% CI: 0.68–0.81) and 0.76 (95% CI: 0.70–0.81), respectively. The pooled positive likelihood ratio (PLR) was 2.9 (95% CI: 2.1–4.1), and the pooled negative likelihood ratio (NLR) was 0.34 (95% CI: 0.25–0.46). Subsequently, the pooled diagnostic odds ratio (DOR) was 9 (95% CI: 5–16) using a random effects model, and the area under the curve (AUC) of summary receiver operating characteristic to summarize predictive accuracy was 0.82 (95% CI: 0.79–0.85). For urine NGAL, the pooled sensitivity, specificity, PLR, NLR, DOR, and AUC values were 0.78 (95% CI: 0.61–0.90), 0.77 (95% CI: 0.65–0.85), 3.4 (95% CI: 2.4–4.8), 0.28 (95% CI: 0.15–0.52), 12 (95% CI: 6–24), and 0.84 (95% CI: 0.80–0.87), respectively.
Conclusion: Plasma/serum and urine NGAL levels performed comparably well in predicting AKI requiring RRT. Our findings suggested that NGAL is an effective predictive biomarker for the AKI-associated need for RRT. Nevertheless, more pieces of high-quality evidence and future trials with larger sample sizes are needed for further improvement of patient outcomes.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022346595], identifier [CRD42022346595].
Introduction
Acute kidney injury (AKI) has been emerged as a crucial public health issue, which affects millions of patients worldwide and is a common complication in patients hospitalized in the intensive care unit (ICU), and AKI is independently associated with significant morbidity and mortality (1–3). Non-AKI patients usually have better clinical outcomes than patients with AKI (4–6). The management of AKI usually involves several conservative interventions, such as avoidance of nephrotoxins and prompt resuscitation of circulation (4, 7). However, patients with AKI with severe metabolic disorders, such as acidosis, hyperkalemia, uremia, and fluid disorders, whose kidney function does not recover after certain interventions have to undergo renal replacement therapy (RRT) with a possibility of eventual kidney transplantation (5, 8). Despite considerable research on RRT, it is still unclear if and when RRT should be commenced to improve the outcome of patients with AKI (5, 9). Although early initiation of RRT may reduce the mortality of patients with AKI (10), it may cause a higher risk of treatment-related complications, such as bloodstream infections (11). More importantly, few studies have specifically evaluated the value of various biomarkers to predict AKI that may persist or worsen and progress to a certain stage, resulting in a necessary reception of RRT (12, 13). Therefore, a new biomarker that serves as an early predictor of AKI and an indicator of the need to undergo RRT may play a critical role in improving the prognosis of AKI.
Neutrophil gelatinase-associated lipocalin (NGAL), a secretory protein with a molecular weight of 25 kDa, belongs to the lipocalin superfamily and is released from injured tubular epithelial cells in response to various insults (5, 14). NGAL has already been acknowledged by nephrologists as one of the most promising biomarkers of upcoming AKI. Recently, plasma/serum and urine NGAL levels have been investigated as biomarkers for early prognostication of AKI (9). It is well known that AKI and chronic kidney disease (CKD) are interconnected syndromes as AKI may exacerbate CKD progression and CKD increases the risk of AKI. Serum and urinary NGAL levels were significantly higher in patients with CKD than in the normal population and were negatively correlated with the glomerular filtration rate (GFR). Although NGAL cutoff values and kinetics are significantly altered in patients with CKD, NGAL is considered not only a better indicator of a GFR decline than serum creatinine (sCr) but also a potent marker of the degree of kidney injury. According to the multivariate Cox proportional risk regression model, serum and urinary NGAL levels were independent predictors of the risk of CKD progression (15). Furthermore, several studies have reported that an increase in NGAL levels can be detected well before an increase in plasma creatinine (pCr) levels, which highlights the sensitivity of the former as a biomarker for diagnosing AKI (11, 16). For instance, pCr did not increase until 24–72 h postoperatively in patients undergoing elective cardiac surgery; however, increases in urine and plasma NGAL levels were identified as soon as 2 h postoperatively, with areas under receiver operating characteristic curve of 0.99 and 0.91, respectively (16). However, because of the lack of corresponding statistical data for early prediction of AKI requiring RRT (17–19), it remains controversial whether NGAL is a predictive biomarker of AKI requiring RRT. Therefore, the potential of NGAL for early prediction of AKI-associated RRT remains to be established.
To illuminate this issue, a systematic review and meta-analysis was conducted to evaluate the ability of the available physiological and molecular biomarkers for predicting the initiation of RRT in patients with AKI. This systematic review and meta-analysis was performed to explore the predictive evidence of AKI requiring RRT.
Methods
Data sources and searches
We performed a systematic search of the following databases: PubMed, Embase, the Web of Science, Cochrane Library (in English), Chinese National Knowledge Infrastructure,1 and Wanfang Data (in Chinese).2 The search duration was from inception to November 2021. The following search terms were used: [“Biomarkers” (MeSH) OR biomarker OR marker OR neutrophil gelatinase associated lipocalin OR NGAL OR neutrophil gelatinase-associated lipocalin] and (AKI OR acute kidney injury OR acute kidney failure OR acute renal failure), and (RRT OR renal replacement therapy). Abstracts with a complete section “Results” were included in this study. There were no language restrictions. This meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement issued in 2009 (Checklist file) (20, 21).
Study selection
All citations were reviewed, and the literature was retrieved by titles or abstracts, and subsequently, full texts were reviewed by two investigators (CX and SL) to determine the study eligibility. Any disagreements regarding study eligibility were resolved by consulting another investigator (ZL). Studies meeting the following inclusion criteria were included: (1) studies with participants aged ≥ 18 years; (2) studies using plasma/serum and/or urine NGAL for prediction of patients with AKI who might need RRT; (3) studies including AKI and non-AKI patients with sepsis who underwent RRT; (4) observational studies; and (5) studies with enough information to calculate true-positive, false-positive, false-negative, and true-negative values of NGAL as a predictor of AKI requiring RRT (contains AUC, sensitivity, and specificity values) or studies with these values provided. Studies were excluded if they met the following exclusion criteria: (1) studies with only animal or in vitro experiments; (2) studies lacking the information about predictive accuracy in control or experimental groups; (3) review articles, commentaries, poster presentations, letters, supplementary issues, and editorials; (4) studies with duplicate data or insufficient information; and (5) studies on individuals with prior kidney transplant, end-stage kidney disease, or prior RRT.
Data extraction and quality assessment
A total of two investigators (CX and SL) independently extracted the data from each trial, and any disagreements between them were resolved by consulting a third investigator (ZL) and reaching a consensus. Data on the following variables from each article were documented and recalculated: first author, year of publication, study location, population type, gender, total sample size, AKI definition, number of patients with AKI, number of patients undergoing RRT, age, NGAL assay results, sample type, AUC (95% CI), and NGAL cutoff, sensitivity, and specificity. Absolute data of true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) rates or equivalent data were calculated or extracted.
The two investigators (CX and SL) independently assessed the methodological quality of the studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool (22). This tool is based on four key domains: index test, reference standard, patient selection, and flow and timing. Each domain is evaluated in the aspect of “risk of bias,” and the first 3 domains are evaluated in the aspect of concern regarding applicability. The included studies collected response using “yes,” “no,” or “unclear” items. The responses of “yes” are considered positive responses for analysis herein.
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of each included study (23). Based on several aspects of the study, such as comparability (maximum points, 2), outcomes (maximum points, 3), and selection (maximum points, 4), the quality of the study was judged using a “star” scoring system of NOS. The scores range from 0 (for worst) to 9 (for best). A study with a score no less than 7 was considered a high-quality study.
Data synthesis and analysis
We used STATA version 12.0 (Stata Corp, College Station, TX) to perform all statistical analyses, which included TP, TN, FP, and FN rates for each test in every study, to assess the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) for each included study. Statistically significant heterogeneity was represented by P < 0.05 for Q statistic, and I2 > 50% was considered to indicate substantial heterogeneity (24). The degree of heterogeneity between multiple studies was measured using the I2 index, and I2 values of <25, 25–50, and >50% indicated modest, moderate, and substantial heterogeneity, respectively. A random effects model was chosen if I2 was greater than 50% (25). Any departure from the Hardy–Weinberg equilibrium (HWE) in the control group of each study was assessed using the χ2 test, and significant deviations were represented by P < 0.05 (26).
Forest plots of accuracy indices were constructed, and a summary receiver operating characteristic (SROC) curve was constructed on the basis of TP and FP rates to describe the relationship between test sensitivity and specificity. NGAL has been defined as a useful risk predictor when the AUC ≥ 0.70, and the predictive performance for the prediction of AKI in RRT by NGAL was measured by calculating the AUC as an overall summary index (27).
Furthermore, subgroup analyses were performed according to the biological material, definition of AKI, geographic location, NGAL assay method, and AKI causes. Finally, Begg’s and Egger’s measures were assessed and calculated using Begg’s funnel plots to detect publication biases (28, 29). A statistically significant difference was represented by P < 0.05 in the test results for the overall effect.
Results
Literature search
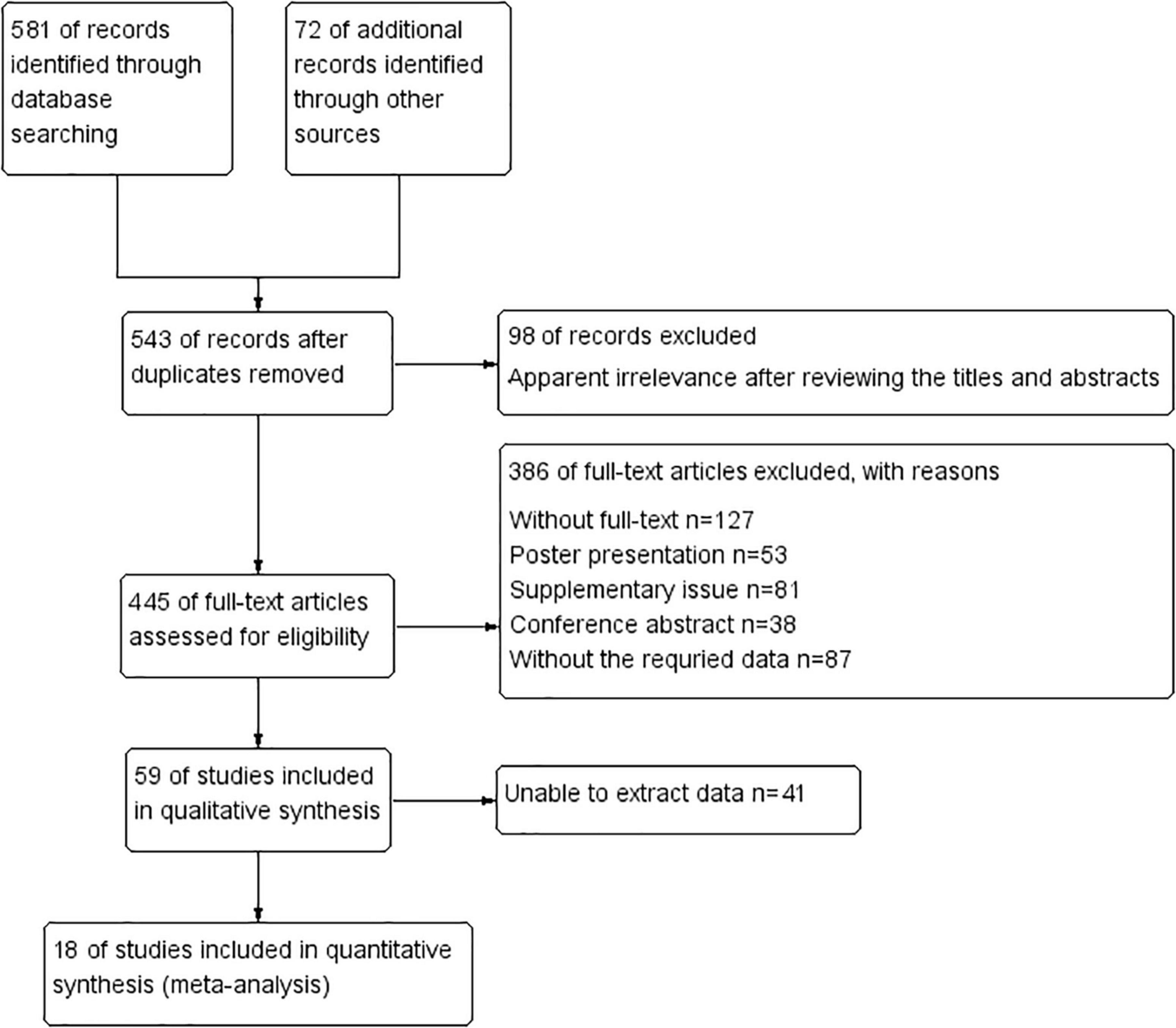
During the literature search, we initially identified 653 potentially relevant studies, and 543 studies remained after removing the duplicates found in electronic databases. Subsequently, 98 articles were identified as irrelevant after reviewing titles and abstracts and excluded. Then, 445 full-text articles were assessed for eligibility, and 386 of these articles were excluded as they did not meet the requirements of data extraction. After screening the full texts of the remaining articles, 41 studies were excluded as they did not meet our eligibility criteria. Finally, 18 articles that met the inclusion criteria were included in this meta-analysis. These studies encompassed a total of 5,441 participants who were included in the meta-analysis for prediction of AKI requiring RRT. The selection process of the included studies is shown in Figure 1.
Characteristics and quality of the included studies
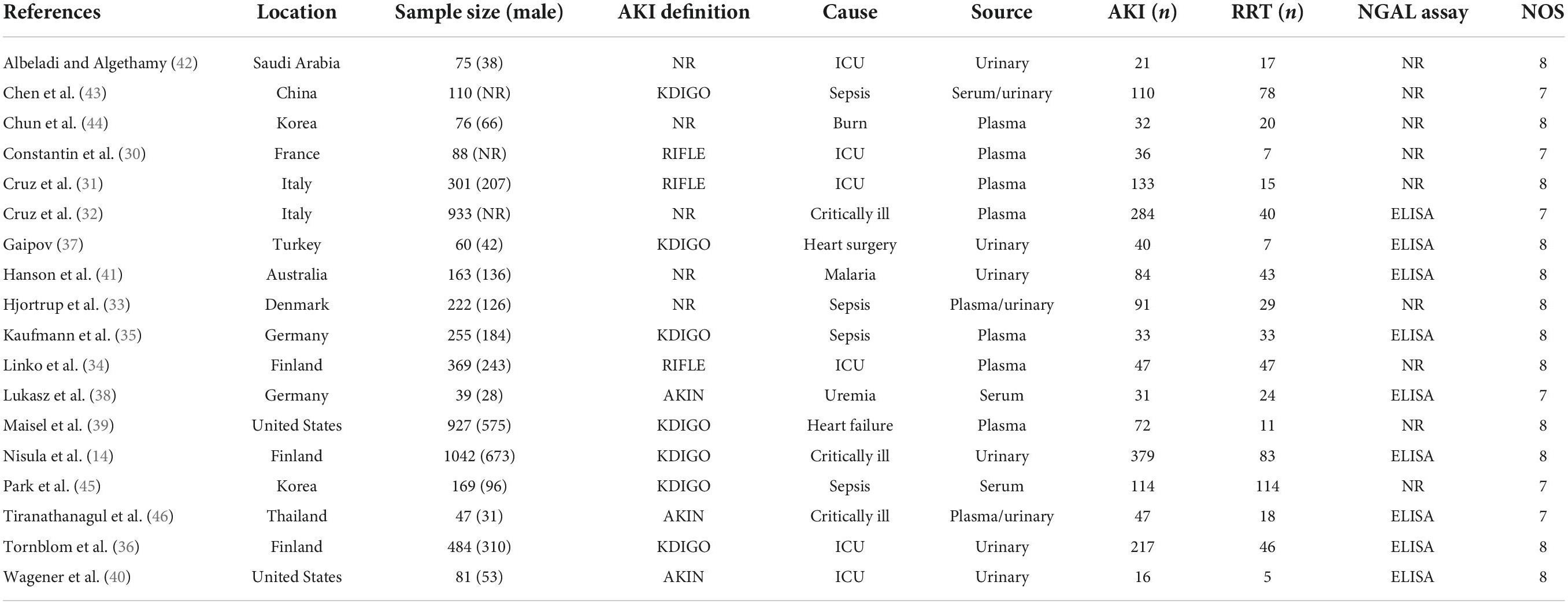
All 18 selected articles were written in English. To analyze the quality of the included studies, the main characteristics were extracted, as presented in Table 1. The included studies were geographically diverse: 10 studies were conducted in Europe (14, 30–38), two in America (39, 40), one in Australia (41), and the remaining five studies in Asia (42–46). The 18 observational studies involved a total of 5,441 patients from 12 countries. All studies were single-center trials published between 2006 and 2021. Overall, 1,787 patients developed AKI and 637 patients received RRT. The use of plasma/serum NGAL and urine NGAL was almost equal among the studies. The definitions of AKI varied among individual studies. A total of 13 studies used the traditional method to define AKI, and the remaining five studies used a non-traditional method. Most studies evaluated the NGAL level in the plasma and urine, rather than in the serum. Commercial enzyme-linked immune sorbent assay (ELISA) was frequently used for NGAL measurements. Among the 18 studies, nine used ELISA and nine used other methods to measure the NGAL level. The performance of NGAL for predicting AKI requiring RRT is summarized in Table 2.
Assessment of methodological quality and publication bias
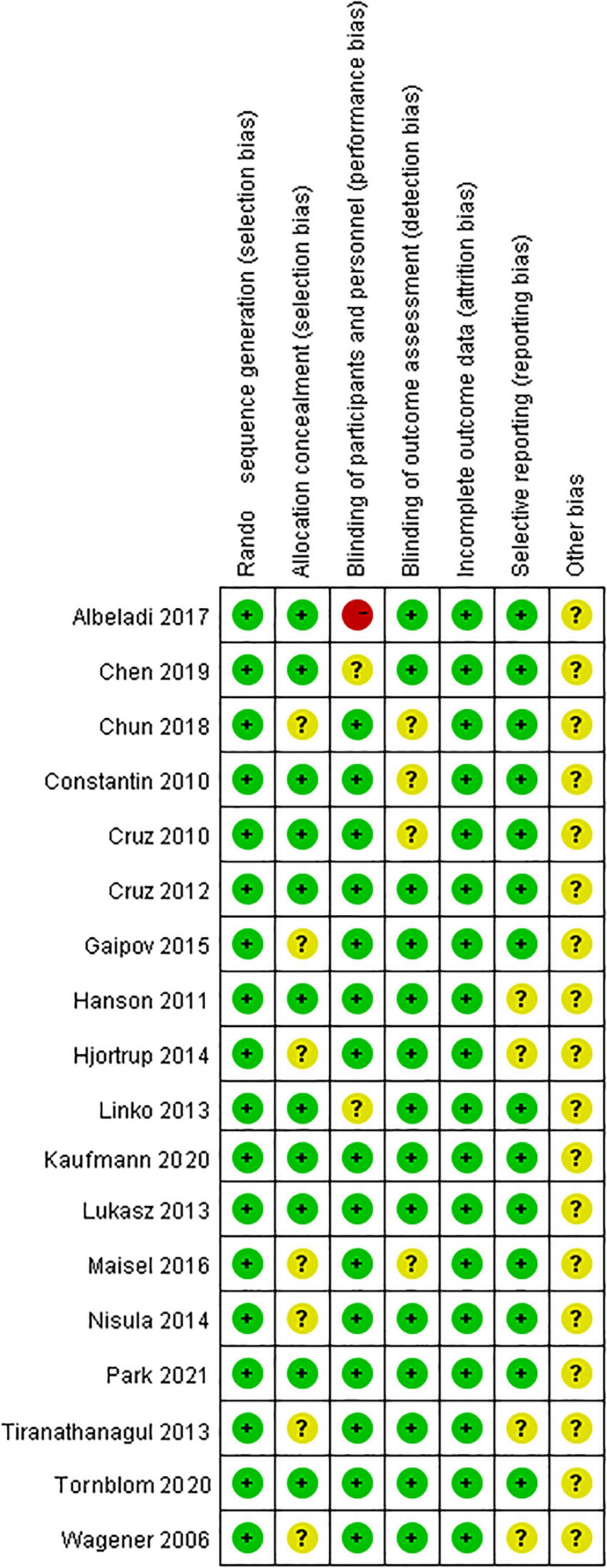
All studies were clearly defined with eligibility criteria and reasons for patient exclusion. The quality of each included study was assessed using the QUADAS tool, with all of them having high QUADAS scores (≥10). The overall quality of included trials was moderate. The results of the QUADAS-2 evaluation are shown in Figure 2.
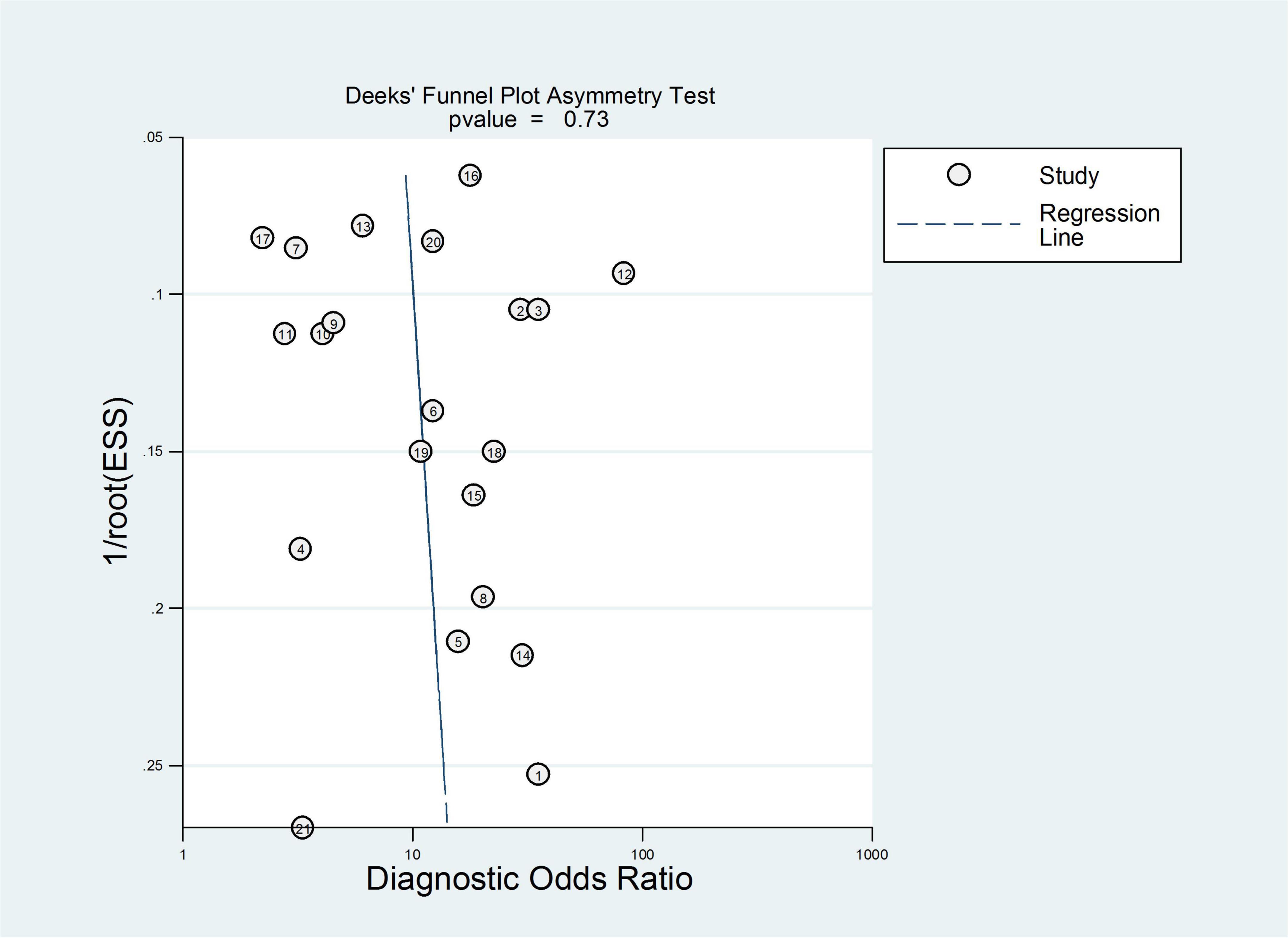
Subsequently, publication bias assessment was conducted using a funnel plot. The results of the funnel plot are shown in Figure 3, which indicated no significant threshold effect and no significant asymmetry, suggesting that there was no evident publication bias in the present meta-analysis. Therefore, it is unlikely that unpublished studies would substantially alter our findings.
Neutrophil gelatinase-associated lipocalin for prediction of acute kidney injury requiring renal replacement therapy
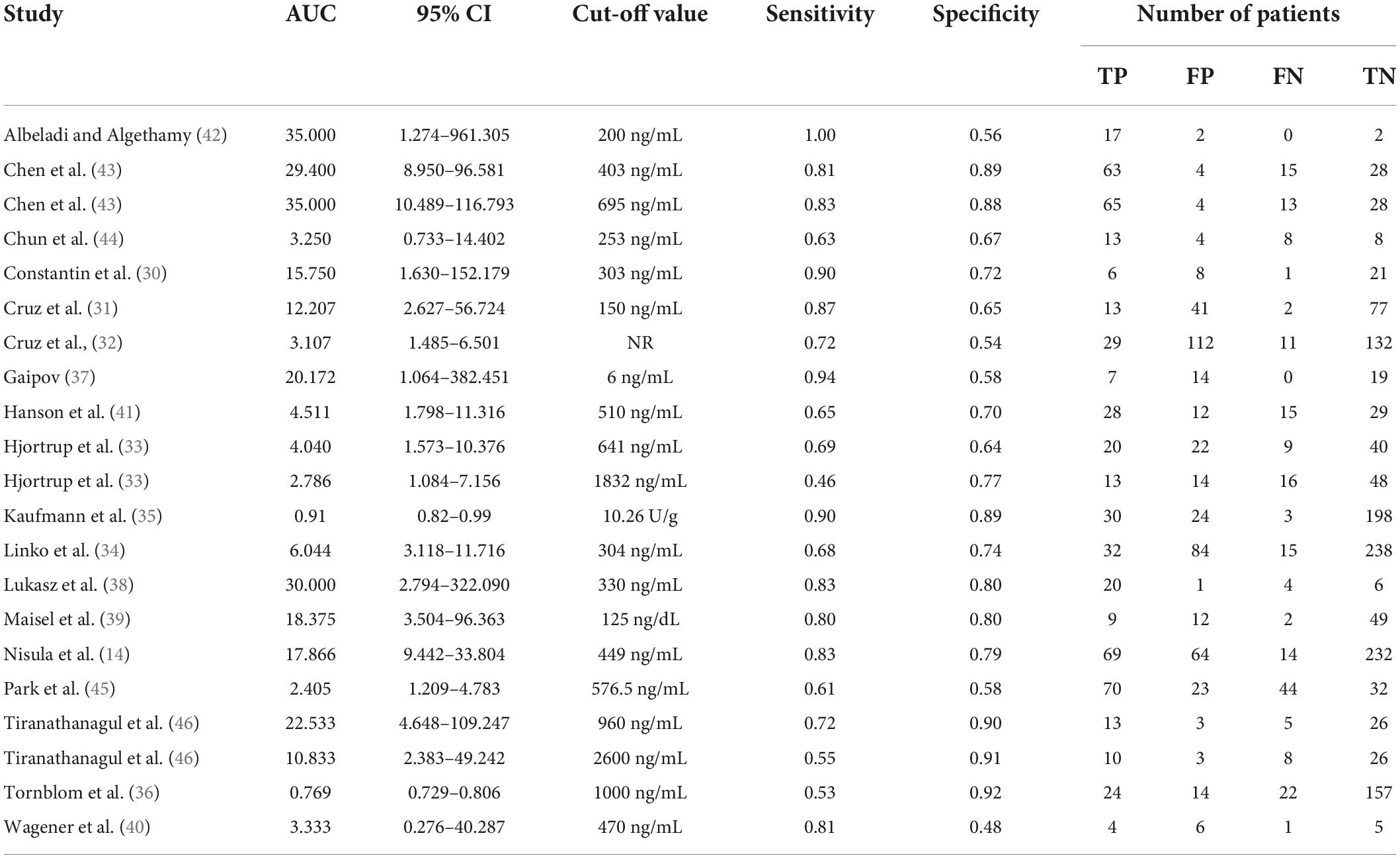
Table 2 shows a total of 21 sets of data extracted from 18 eligible studies, including TP/FP/FN/TN value, sensitivity, specificity, positive predictive value, negative predictive value (NPV), AUC, various optimal cutoff values for different sample types of NGAL, the NGAL assay method, and the definition of AKI. In the 18 studies, we investigated the predictive value of plasma NGAL as a biomarker of AKI requiring RRT in 1787/5441 patients who developed AKI. The pooled results of these studies are summarized in Table 2. Taken together, the predictive value of NGAL for AKI requiring RRT from plasma/serum and urine samples is shown in Figure 4. For summary performance estimates, the pooled sensitivity and specificity with corresponding 95% CI were 0.75 (95% CI: 0.68–0.81) and 0.76 (95% CI: 0.70–0.81), respectively. The pooled PLR was 2.9 (95% CI: 2.4–4.1), and the pooled NLR was 0.34 (95% CI: 0.25–0.46). The pooled DOR was 9 (95% CI: 5–16) using a random effects model (Figure 4). Moreover, the AUC for SROC to summarize predictive accuracy was 0.82 (95% CI: 0.79–0.85; Figure 5). Even though the result of SROC for AKI requiring RRT was worse than that for AKI, its clinical application was still of great value.
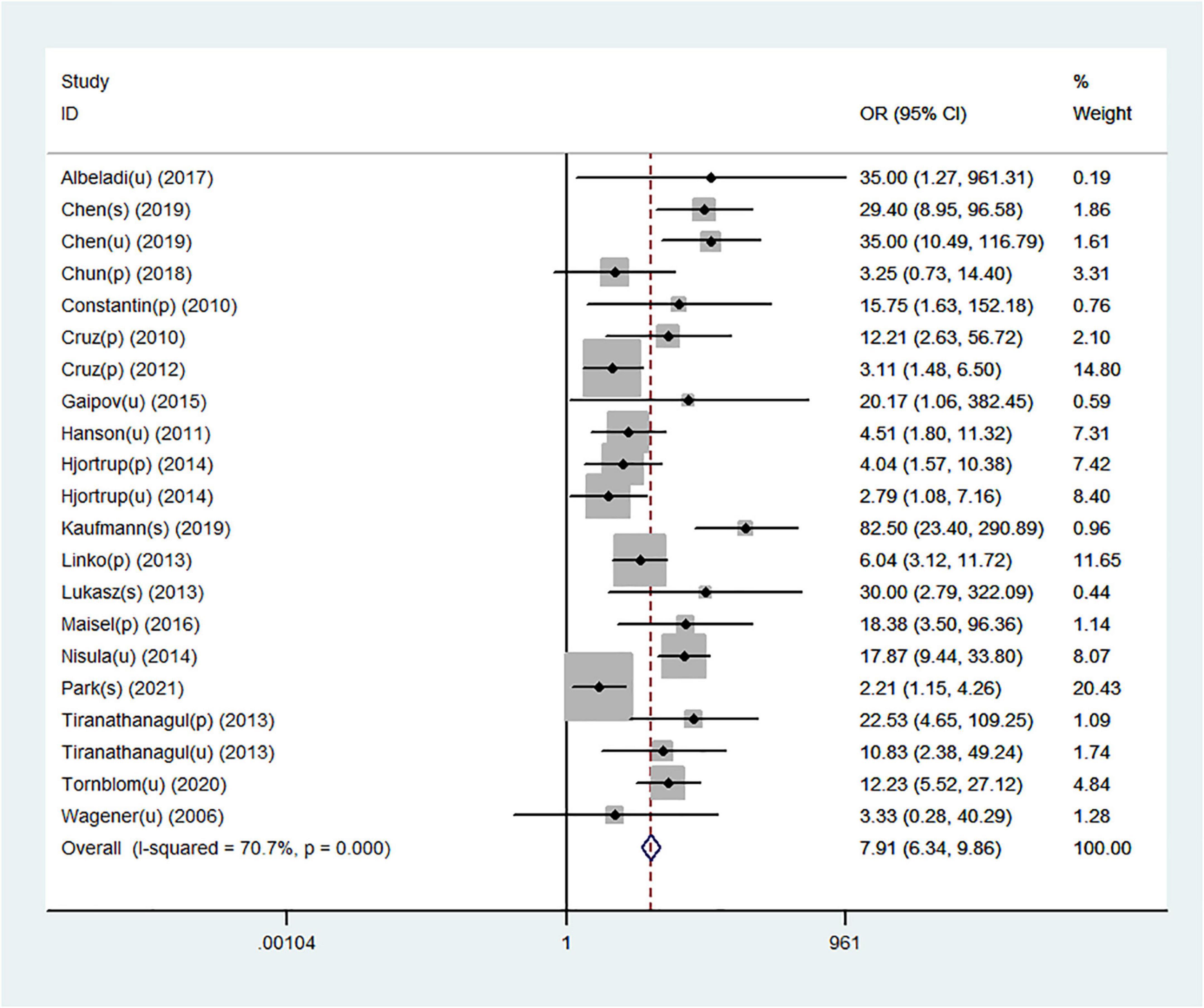
Figure 4. Forest plot for the predictive value of NGAL for AKI requiring RRT from plasma/serum and urine samples.
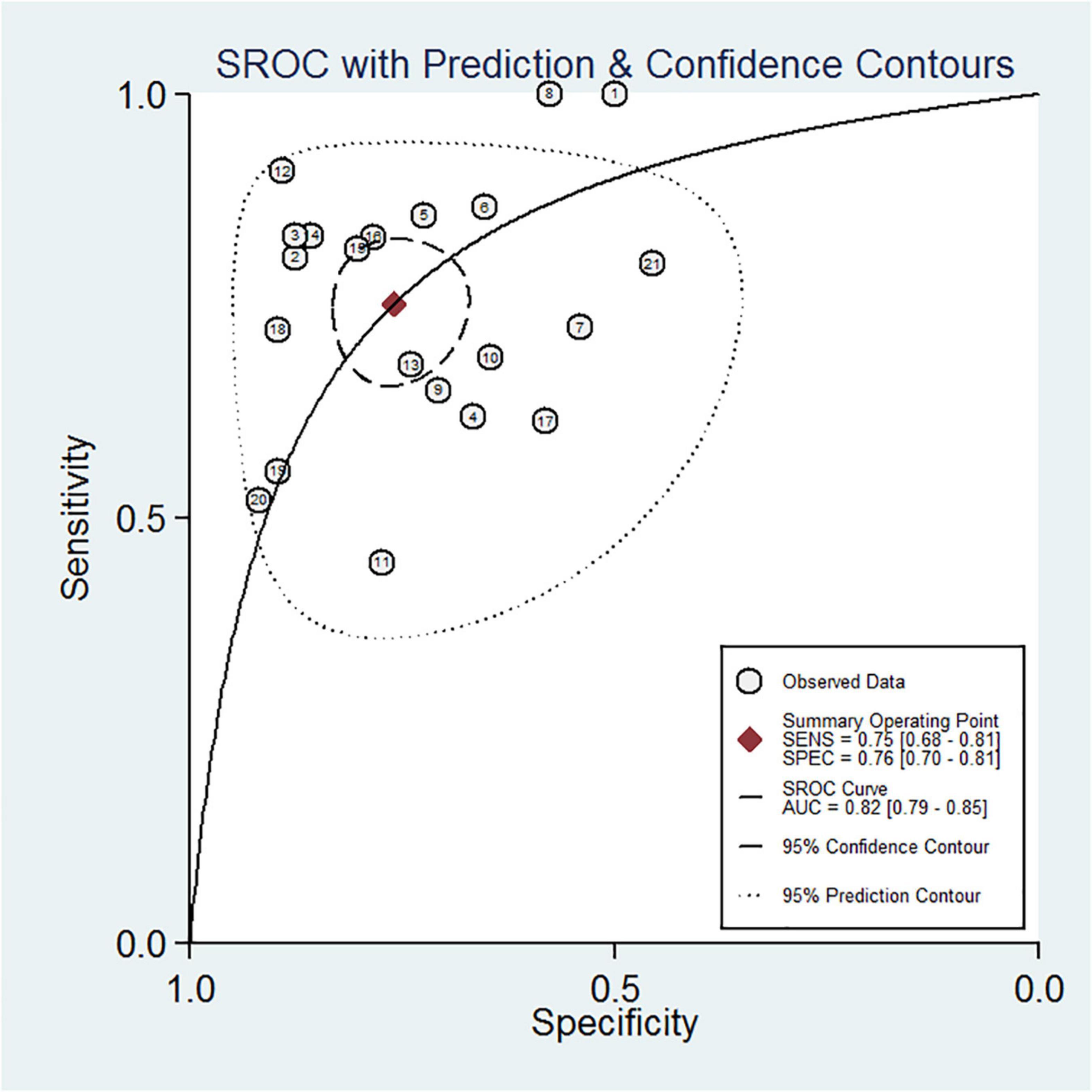
Figure 5. Summary receiver operating characteristic (SROC) curve of plasma/serum and urine NGAL for AKI requiring RRT.
Subgroup analysis
Subgroup analysis of this meta-analysis was performed, and the results are shown in Table 3. Based on the comparisons of DOR and AUC, the predictive value of NGAL for AKI requiring RRT showed some variability. For subgroup analysis of the biological material, the DOR and AUC of the urine NGAL level were significantly higher than those of the plasma/serum NGAL level in the prediction of AKI requiring RRT (DOR, 12; AUC, 0.84 vs. DOR, 9; AUC, 0.82). This showed that urine NGAL performed better for RRT prediction than plasma/serum NGAL. For subgroup analysis of geographic location, the value of the NGAL level to predict AKI requiring RRT in oriental was substantially higher than that in occidental (DOR, 14; AUC, 0.85 vs. DOR, 9; AUC, 0.81). Subsequently, for subgroup analysis of the NGAL assay method, we investigated the predictive accuracies of NGAL with and without ELISA. The findings revealed that the former had a higher predictive value for AKI requiring RRT (DOR, 14; AUC, 0.86 vs. DOR, 7; AUC, 0.79). For subgroup analysis of the definition of AKI, the traditional definition of AKI was associated with better DOR and AUC values (DOR, 13; AUC, 0.85) than the non-traditional definition of AKI (DOR, 4; AUC, 0.70). Moreover, subgroup analysis for different causes of AKI was conducted, and the results showed that AKI caused by sepsis/heart failure had the best predictive ability with optimal DOR and AUC (DOR, 10; AUC, 0.83), and AKI caused by ICU showed a similar predictive ability as AKI caused by critical illness (DOR, 9; AUC, 0.82 VS DOR, 9; AUC, 0.80). Based on the establishment of subgroup analyses of the 21 datasets from the 18 studies wherein multivariable analyses were provided, it would be reasonable to believe NGAL as an independent predictor of AKI requiring RRT.
Heterogeneity analysis
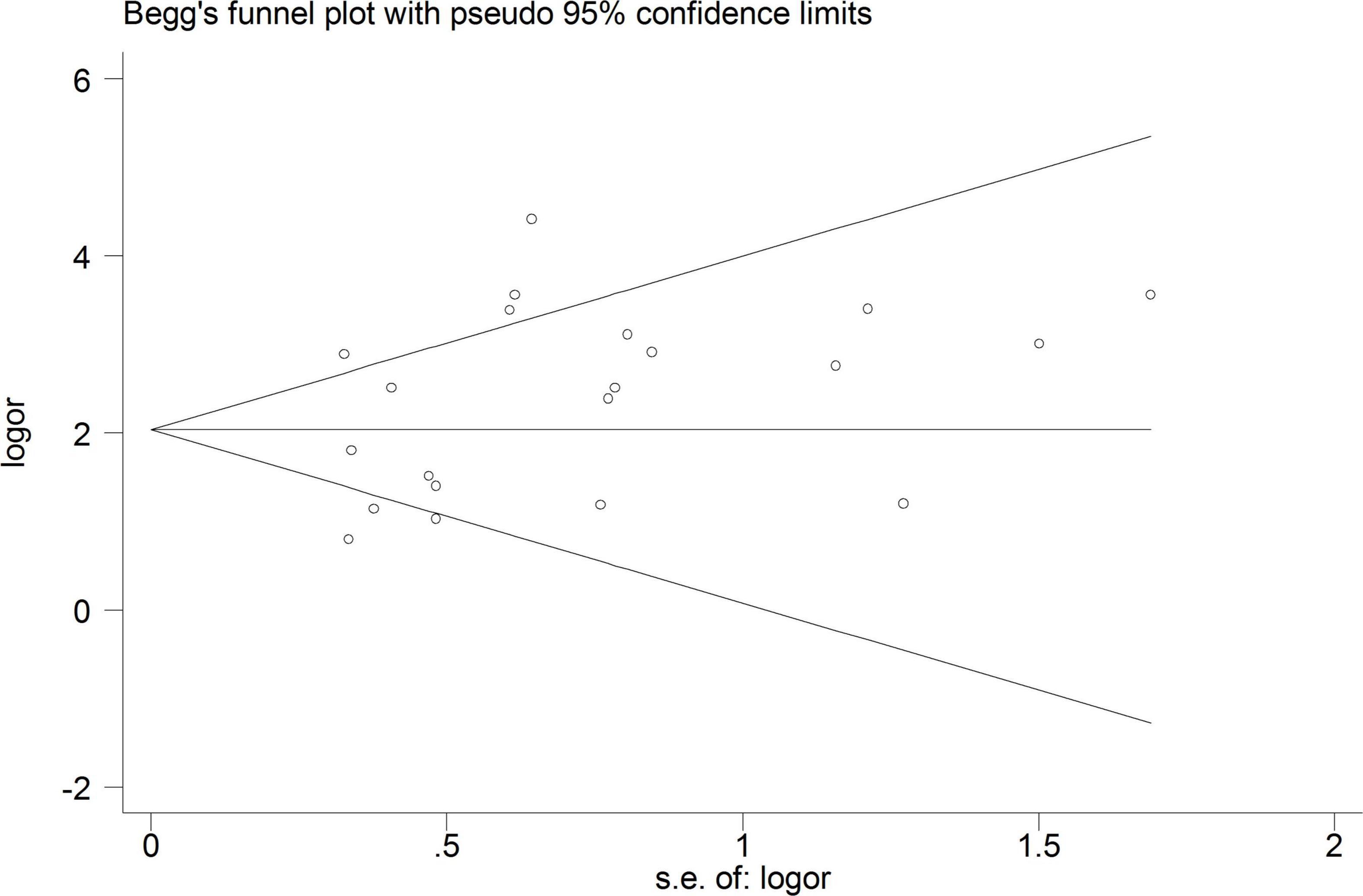
A total of 18 heterogeneous studies containing 21 datasets of plasma/serum NGAL and urine NGAL for the prediction of AKI requiring RRT were obtained. Overall, heterogeneity analyses were performed, and the SROC was constructed; the points in the plots did not show a “shoulder arm” pattern, which suggested no presence of the threshold effect. Subsequently, Begg’s funnel plot and Egger’s test were used to check for publication bias, and the funnel plots are shown in Figure 6. The results indicated a low probability of publication bias.
To explore other possible reasons for heterogeneity, meta-regression and subgroup analyses were performed. The main sources of heterogeneity could be the geographic location (occidental or oriental) and definition of AKI (traditional or non-traditional). However, the specimen type (urine or plasma/serum) and the NGAL assay (ELISA or others) may not be the sources of heterogeneity for NGAL.
Discussion
In the present systematic review and meta-analysis, we assessed the predictive accuracy of NGAL in 1,787 patients with AKI requiring RRT. The results of this meta-analysis revealed NGAL as a valuable renal biomarker to predict the need for RRT with high sensitivity, specificity, and DOR in patients scheduled to undergo AKI. Furthermore, subgroup analyses indicated that plasma/serum and urine NGAL had comparable predictive values for AKI requiring RRT. Moreover, the definition of AKI and/or the geographic location of the patients affected the efficiency of the NGAL level for predicting AKI requiring RRT. Of note, although the predictive value of NGAL has been shown in AKI requiring RRT, it is hard to suggest NGAL as a promising biomarker that may guide clinical decision regarding the timing of initiation of RRT among patients with AKI due to the lack of established cutoff values of NGAL for the initiation of RRT. In addition, whether mild AKI or severe AKI requiring RRT will be developed is difficult to be predicted only by a certain increase in NGAL levels. As a matter of fact, clinical manifestations and variables measured on clinical laboratory platforms are the common factors that should be taken into consideration in clinical practice to decide the initiation of RRT, such as severely decreased renal function indicated by sharply increased serum creatinine or dramatically decreased GFR, acidosis, severe edema, hyperkalemia, and so on. Furthermore, NGAL levels not only reflects kidney injury but may also be influenced by systemic conditions such as sepsis or originating from non-kidney tissues. A increase in NGAL indicates the possibility of AKI and provides appropriate preparations for subsequent possible therapies.
Neutrophil gelatinase-associated lipocalin has been implicated in a variety of processes, including cell differentiation, proliferation, and survival in renal epithelial cells, where it helps maintain the tubular structure and limits apoptosis (16). In addition, exogenous NGAL has been shown to have a dramatic renoprotective effect in the mouse models of renal ischemia–reperfusion injury (47). Generally, NGAL is among the most extensively studied biological markers for early prediction of AKI in both urine and blood specimens. A systematic review was performed by Haase-Fielitz et al. wherein they included 58 studies collectively encompassing >16,500 patients and reported that both plasma and urine NGAL were predictive of AKI, with overall AUCs ranging from 0.79 to 0.87 in different clinical settings (48). The predictive accuracy of plasma and urine NGAL for the prediction of AKI was systematically reviewed, and the pooled results indicated that plasma and urine NGAL precisely predicted AKI with sepsis (AUC = 0.86 and 0.90, respectively) (49). In addition, NGAL is considered to play a vital role in early prediction of AKI as its level can rapidly increase after contrast medium exposure (27). However, it remains controversial whether NGAL is predictive of AKI requiring RRT because of the lack of pertinent statistical data in this regard, and it remains unclear when and whether RRT should be commenced to improve the outcome of patients with AKI on the basis of NGAL levels. Currently, the information on NGAL for the prediction of RRT in patients with AKI is extremely limited.
Several studies have evaluated the predictive value of NGAL for AKI of different etiologies that requires RRT, and a wide range of predictive values of NGAL levels for AKI has been reported in observational cohort studies. However, few studies considered patients after AKI administration for RRT (50). Since it is still unclear whether and when to commence RRT, standards for prediction of AKI requiring RRT are considered as a major limitation of biomarker studies (51). Recently, two trials employed a preset NGAL threshold as an inclusion criterion for RRT prediction and used NGAL to guide the early initiation of RRT (9, 52). Nevertheless, NGAL was found to detect patients with AKI in the ELAIN trial, whereas it was universally elevated in the STARRT-AKI pilot trial and showed weak discriminative value between patients requiring and not requiring RRT (9). The results of our study were consistent with those of previous studies, and we found NGAL to be a useful early predictor of AKI requiring RRT; our sensitivity analyses also revealed that the findings were robust. The association between NGAL and AKI requiring RRT is further highlighted by a sensitivity of 75% and a specificity of 76%. By contrast, several studies have reported sensitivities of 40%–60% and specificities of 40%–55%. The observed differences may be attributed to variations in the definitions of AKI, the etiology of AKI, the NGAL assay, and the geographic location of patients. To evaluate the predictive value of the NGAL level in various conditions, our subgroup analysis had included these parameters.
Recently, plasma and urine NGAL were reported to have relatively low predictive values for the requirement of RRT in ICU patients with severe sepsis and without CKD, and the AUCs were 0.73 (95% CI: 0.61–0.85; P = 0.64) and 0.68 (95% CI: 0.53–0.83; P = 0.64), respectively (33). In addition, in a study of 126 patients with sepsis, 23 of 58 patients with septic AKI received RRT (53). The results showed that the peak urine NGAL was higher in patients receiving hemodialysis than in those not receiving hemodialysis (median, 456 ng/mL vs. 341 ng/mL, P < 0.0001). The AUC of the peak urine NGAL for predicting the need for hemodialysis was 0.77 (95% CI: 0.64–0.83), with a cutoff value of 494 ng/mL; the sensitivity and specificity were 0.89 and 0.71, respectively. In addition, a study performed a subgroup analysis of AKI patients with community-acquired pneumonia who met the RIFLE-F criteria and showed that plasma NGAL was a poor predictor of the requirement of RRT (AUC, 0.62; 95% CI: 0.45–0.81) (54). Another study reported that the peak plasma NGAL showed fair discriminatory power for the prediction of AKI (AUC = 0.71) and need for RRT (AUC = 0.78); however, urine NGAL did not perform equally well for prediction of AKI (AUC = 0.70) and need for RRT (AUC = 0.70). Together, these findings are not comparable with our findings in terms of both plasma/serum NGAL and urine NGAL in the present meta-analysis for predicting AKI requiring RRT (55). Furthermore, a recent study conducted by Albert C. et al. also showed that urinary and plasma NGAL concentrations may identify patients at high risk for AKI and the associated need for dialysis therapy (56).
Currently, NGAL is the only biomarker that has been investigated in both plasma/serum and urine for its early predictive value of AKI and AKI requiring RRT. Therefore, herein, we assessed the predictive value of plasma/serum and urine NGAL and further examined the studies that conducted comparisons of both plasma/serum NGAL and urine NGAL for subgroup analysis. Regarding plasma/serum NGAL, many studies showed unsatisfactory results, and our subgroup analysis also showed that plasma/serum NGAL may have inferior performance to urine NGAL in prediction of AKI requiring RRT. Typically, plasma/serum NGAL is considered an indicator of systemic inflammation and not of renal injury. However, urine sample collection is non-invasive, and urine has reduced interfering proteins, thus making it an ideal fluid for kidney biomarker discovery. The present meta-analysis confirmed the superiority of urine NGAL for the prediction of AKI requiring RRT, suggesting urine NGAL levels should be quantified before plasma/serum NGAL levels. Conversely, it may be difficult to obtain urine samples from AKI patients with severe oliguria, which is common in cardiac surgery. Therefore, even though urine NGAL has a higher predictive value (DOR, 12; AUC, 0.84) than plasma/serum NGAL (DOR, 9; AUC, 0.82) for early predictive value of AKI requiring RRT, plasma/serum NGAL may be an alternative to urine NGAL in case urine is unobtainable. Subsequently, in the present study, subgroup analyses showed the definition of AKI as the main source of heterogeneity. Compared with the traditional definition of AKI (DOR, 13; AUC, 0.85), the non-traditional definition of AKI was found to be associated with an inferior predictive value of NGAL (DOR, 4; AUC, 0.70). To our knowledge, few studies have conducted a comparison of research-based assays. Therefore, prospective studies with geographic location or multiple RRT settings involving head-to-head comparisons may contribute to a more comprehensive evaluation of NGAL in prediction of AKI requiring RRT.
Nevertheless, this study has several limitations. First, the literature search strategy applied in this study was limited to open-access publications. In this case, the studies that may have met the inclusion criteria of this meta-analysis but were not published on open-access platforms were missed. Second, different cutoff values for NGAL were applied in the included studies. Furthermore, cutoff values were corrected by urine creatinine, and it was difficult to determine the optimized overall cutoff value for patients with AKI (57–59). Moreover, deeper investigations should be pursued incorporating a wide range of clinical settings of AKI. Third, the pooled ORs were calculated by the numbers of genotypes or alleles of controls and cases; however, no adjustment was performed for other confounding factors. Fourth, because of the limitation of statistical power, the results from subgroups analysis should be interpreted with caution. Finally, the included studies in the present meta-analysis had different match variables, and this may have affected the pooled results.
Conclusion
The present systematic review and meta-analysis showed a significant association between NGAL and AKI requiring RRT. Therefore, NGAL could be considered a useful marker with a high predictive value of AKI requiring RRT. Despite these encouraging findings, in further studies, a larger sample of homogeneous patients should be used, and different NGAL assay methods need to be unbiased to clarify this issue. Furthermore, similar relevant studies incorporating long-term follow-up studies are needed to confirm the role played by NGAL in the progression and development of AKI.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CX, SL, and ZL extracted the data and performed the initial analysis. CX and SL wrote the first draft, which has been carefully reviewed and edited by ZL. CX, SL, LM, and ZL performed further review and subsequent revisions. All authors have contributed to the conception and design of the study and agreed to the submission for publication.
Funding
This research was funded by the National Natural Science Foundation of China (82100706 and 81972366), Postdoctoral Science Foundation of China (2021M692219), Special Fund for Economic and Technological Development of Longgang District of Shenzhen City (LGKCYLWS2020064 and LGKCYLWS2020068), the National Science Foundation Projects of Guangdong Province (2022A1515012606), and the Special Project of Science and Technology for Sustainable Development, Shenzhen Municipal Science and Technology Innovation Committee (KCXFZ20211019143336002, JCYJ2018 0228163012046, and JCYJ20200109120208018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. (2019) 96:1083–99. doi: 10.1016/j.kint.2019.05.026
2. Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, et al. Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. (2020) 98:294–309. doi: 10.1016/j.kint.2020.04.020
3. Maggiore U. Incomplete recovery from COVID-19-associated acute kidney injury in kidney transplant recipients: prior graft injury matters the most. Transpl Int. (2021) 34:1002–4. doi: 10.1111/tri.13896
4. Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017. Intensive Care Med. (2017) 43:730–49. doi: 10.1007/s00134-017-4832-y
5. Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. (2016) 375:122–33. doi: 10.1056/NEJMoa1603017
6. Trongtrakul K, Sawawiboon C, Wang AY, Chitsomkasem A, Limphunudom P, Kurathong S, et al. Acute kidney injury in critically ill surgical patients: epidemiology, risk factors and outcomes. Nephrology (Carlton). (2019) 24:39–46. doi: 10.1111/nep.13192
7. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
8. Wang L, Wang T, Rushton SN, Parry G, Dark JH, Sheerin NS. The impact of severe acute kidney injury requiring renal replacement therapy on survival and renal function of heart transplant recipients - a UK cohort study. Transpl Int. (2020) 33:1650–66. doi: 10.1111/tri.13675
9. Zarbock A, Kellum JA, Schmidt C, Van AH, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. (2016) 315:2190–9. doi: 10.1001/jama.2016.5828
10. Chaudhuri D, Herritt B, Heyland D, Gagnon LP, Thavorn K, Kobewka D, et al. Early renal replacement therapy versus standard care in the ICU: a systematic review, meta-analysis, and cost analysis. J Intensive Care Med. (2019) 34:323–9. doi: 10.1177/0885066617698635
11. Forni LG, Darmon M, Ostermann M, Straaten OV, Pettilä V, Prowle JR, et al. Renal recovery after acute kidney injury. Intensive Care Med. (2017) 43:855–66. doi: 10.1007/s00134-017-4809-x
12. Beitland S, Joannidis M. Biomarkers of acute kidney injury – a mission impossible? Acta Anaesthesiol Scand. (2018) 62:2–5. doi: 10.1111/aas.13010
13. Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. (2018) 44:323–36. doi: 10.1007/s00134-018-5126-8
14. Nisula S, Yang R, Kaukonen KM, Vaara ST, Kuitunen A, Tenhunen J, et al. The urine protein NGAL predicts renal replacement therapy, but not acute kidney injury or 90-day mortality in critically ill adult patients. Anesth Analg. (2014) 119:95–102. doi: 10.1213/ANE.0000000000000243
15. Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. (2009) 4:337–44. doi: 10.2215/CJN.03530708
16. Macdonald SPJ, Bosio E, Neil C, Arendts G, Burrows S, Smart L, et al. Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis. Inflamm Res. (2017) 66:611–9. doi: 10.1007/s00011-017-1043-5
17. Bagshaw SM, Darmon M, Ostermann M, Finkelstein FO, Wald R, Tolwani AJ, et al. Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med. (2017) 43:841–54. doi: 10.1007/s00134-017-4762-8
18. Siddiqui WJ, Alvarez C, Aslam M, Bakar A, Khan MH, Aslam A, et al. Meta-analysis comparing outcomes and need for renal replacement therapy of transcatheter aortic valve implantation versus surgical aortic valve replacement. Am J Cardiol. (2018) 122:468–76. doi: 10.1016/j.amjcard.2018.04.030
19. Ragland C, Ochoa L, Hartjes T. Early mobilisation in intensive care during renal replacement therapy: a quality improvement project. Intensive Crit Care Nurs. (2019) 52:22–7. doi: 10.1016/j.iccn.2018.12.005
20. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
21. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
22. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Wu H, Zhang H, Li P, Gao T, Lin J, Yang J, et al. Association between dietary carbohydrate intake and dietary glycemic index and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. (2014) 55:3660–8. doi: 10.1167/iovs.13-13695
25. Dong F, Yang G, Pan HW, Huang WH, Jing LP, Liang WK, et al. The association of PTPN22 rs2476601 polymorphism and CTLA-4 rs231775 polymorphism with LADA risks: a systematic review and meta-analysis. Acta Diabetologica. (2014) 51:691–703. doi: 10.1007/s00592-014-0613-z
26. Xia C, Amador C, Huffman J, Trochet H, Campbell A, Porteous D, et al. Pedigree- and SNP-associated genetics and recent environment are the major contributors to anthropometric and cardiometabolic trait variation. PLos Genetics. (2016) 12:e1005804. doi: 10.1371/journal.pgen.1005804
27. Macdonald SP, Stone SF, Neil CL, van Eeden PE, Fatovich DM, Arendts G, et al. Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS One. (2014) 9:e110678. doi: 10.1371/journal.pone.0110678
28. Cantoni V, Green R, Acampa W, Petretta M, Bonaduce D, Salvatore M, et al. Long-term prognostic value of stress myocardial perfusion imaging and coronary computed tomography angiography: a meta-analysis. J Nucl Cardiol. (2016) 23:185–97. doi: 10.1007/s12350-015-0349-3
29. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
30. Constantin JM, Futier E, Perbet S, Roszyk L, Lautrette A, Gillart T, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. (2010) 25:e1–6. doi: 10.1016/j.jcrc.2009.05.010
31. Cruz DN, De MC, Garzotto F, Perazella MA, Lentini P, Corradi V, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. (2010) 36:444–51. doi: 10.1007/s00134-009-1711-1
32. Cruz D, Geus HRD, Ronco C, Groeneveld ABJ, Kulkarni OP, Hagemann JH, et al. New advances in the pathophysology of AKI. Nephrol Dial Transplant. (2012) S2:ii31. doi: 10.1093/ndt/gfs190
33. Hjortrup PB, Haase N, Treschow F, Møller MH, Perner A. Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiol Scand. (2015) 59:25–34. doi: 10.1111/aas.12427
34. Linko R, Pettilä V, Kuitunen A, Korhonen A-M, Nisula S, Alila S, et al. Plasma neutrophil gelatinase-associated lipocalin and adverse outcome in critically ill patients with ventilatory support. Acta Anaesthesiol Scand. (2013) 57:855–62. doi: 10.1111/aas.12112
35. Kaufmann M, Schlossbauer M, Hubauer U, Stadler S, Fischer M, Wallner S, et al. N-acety-b-D-glucosaminidase: a potential biomarker for early detection of acute kidney injury in acute chest pain. Nephrology (Carlton). (2020) 25:135–43. doi: 10.1111/nep.13664
36. Tornblom S, Nisula S, Petaja L, Vaara ST, Haapio M, Pesonen E, et al. Urine NGAL as a biomarker for septic AKI: a critical appraisal of clinical utility-data from the observational FINNAKI study. Ann Intensive Care. (2020) 10:51. doi: 10.1186/s13613-020-00667-7
37. Abduzhappar G, Yalcin S, Kultigin T, Aysun T, Ahmet Nihat B, Humeyra C, et al. Serum uric acid may predict development of progressive acute kidney injury after open heart surgery. Ren Fail. (2015) 37:96–102. doi: 10.3109/0886022X.2014.976130
38. Lukasz A, Beneke J, Menne J, Vetter F, Schmidt BM, Schiffer M, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) in patients with Shiga toxin mediated haemolytic uraemic syndrome (STEC-HUS). Thromb Haemost. (2014) 111:365–72. doi: 10.1160/TH13-05-0387
39. Maisel AS, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, et al. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: the AKINESIS study. J Am Coll Cardiol. (2016) 68:1420–31. doi: 10.1016/j.jacc.2016.06.055
40. Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. (2006) 105:485–91. doi: 10.1097/00000542-200609000-00011
41. Hanson J, Hasan MMU, Royakkers AA, Alam S, Charunwatthana P, Maude RJ, et al. Laboratory prediction of the requirement for renal replacement in acute falciparum malaria. Malar J. (2011) 10:217. doi: 10.1186/1475-2875-10-217
42. Albeladi FI, Algethamy HM. Urinary neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury, severe kidney injury, and the need for renal replacement therapy in the intensive care unit. Nephron Extra. (2017) 7:62–77. doi: 10.1159/000477469
43. Chen X, Chen Z, Wei T, Li P, Zhang L, Fu P. The effect of serum neutrophil gelatinase-associated lipocalin on the discontinuation of continuous renal replacement therapy in critically ill patients with acute kidney injury. Blood Purif. (2019) 48:10–7. doi: 10.1159/000499026
44. Chun W, Kim Y, Yoon J, Lee S, Yim H, Cho YS, et al. Assessment of plasma neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury and prediction of mortality in severely burned patients. J Burn Care Res. (2018) 39:387–93. doi: 10.1097/BCR.0000000000000605
45. Park Y, Ban TH, Kim HD, Ko EJ, Lee J, Kim SC, et al. Mortality prediction of serum neutrophil gelatinase-associated lipocalin in patients requiring continuous renal replacement therapy. Korean J Intern Med. (2021) 36:392–400. doi: 10.3904/kjim.2019.446
46. Tiranathanagul K, Amornsuntorn S, Avihingsanon Y, Srisawat N, Susantitaphong P, Praditpornsilpa K, et al. Potential role of neutrophil gelatinase-associated lipocalin in identifying critically ill patients with acute kidney injury stage 2-3 who subsequently require renal replacement therapy. Ther Apher Dial. (2013) 17:332–8. doi: 10.1111/1744-9987.12004
47. Hong SN, Medeiros P, Menezes P, Bridi R, Balbi A, Ponce D. Sepsis and AKI in clinical emergency room patients: the role of urinary NGAL. Biomed Res Int. (2015) 2015:413751. doi: 10.1155/2015/413751
48. Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. (2014) 51:335–51. doi: 10.1177/0004563214521795
49. Zhang A, Cai Y, Wang PF, Qu JN, Luo ZC, Chen XD, et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Critical Care. (2016) 20:41. doi: 10.1186/s13054-016-1212-x
50. Bouman CSC, Forni LG, Joannidis M. Biomarkers and acute kidney injury: dining with the Fisher King? Intensive Care Med. (2010) 36:381–4. doi: 10.1007/s00134-009-1733-8
51. Parikh CR, Heather TP. Key concepts and limitations of statistical methods for evaluating biomarkers of kidney disease. J Am Soc Nephrol. (2014) 25:1621–9. doi: 10.1681/ASN.2013121300
52. Wald R, Adhikari NK, Smith OM, Weir MA, Pope K, Cohen A, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. (2015) 88:897–904. doi: 10.1038/ki.2015.184
53. Fan H, Zhao Y, Zhu J, Song F. Urine neutrophil gelatinase-associated lipocalin in septic patients with and without acute kidney injury. Ren Fail. (2014) 36:1399–403. doi: 10.3109/0886022X.2014.945184
54. Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. (2011) 80:545–52. doi: 10.1038/ki.2011.160
55. Bagshaw SM, Michael B, Michael H, Anja HF, Moritoki E, Hiroshi M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. (2010) 36:452–61. doi: 10.1007/s00134-009-1724-9
56. Albert C, Zapf A, Haase M, Rover C, Pickering JW, Albert A, et al. Neutrophil gelatinase-associated lipocalin measured on clinical laboratory platforms for the prediction of acute kidney injury and the associated need for dialysis therapy: a systematic review and meta-analysis. Am J Kidney Dis. (2020) 76:826–41.e1. doi: 10.1053/j.ajkd.2020.05.015
57. Che M, Xie B, Xue S, Dai H, Qian J, Ni Z, et al. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following elective cardiac surgery. Nephron Clin Pract. (2010) 115:c66–72. doi: 10.1159/000286352
58. Liu S, Che M, Xue S, Xie B, Zhu M, Lu R, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers. (2013) 18:95–101. doi: 10.3109/1354750X.2012.740687
Keywords: neutrophil gelatinase-associated lipocalin, acute kidney injury, renal replacement therapy, predictive value, systematic review and meta-analysis
Citation: Xu C, Lin S, Mao L and Li Z (2022) Neutrophil gelatinase-associated lipocalin as predictor of acute kidney injury requiring renal replacement therapy: A systematic review and meta-analysis. Front. Med. 9:859318. doi: 10.3389/fmed.2022.859318
Received: 21 January 2022; Accepted: 30 August 2022;
Published: 21 September 2022.
Edited by:
Kathrin Eller, Medical University of Graz, AustriaReviewed by:
Werner Ribitsch, Medical University of Graz, AustriaAndrea Angeletti, Giannina Gaslini Institute (IRCCS), Italy
Copyright © 2022 Xu, Lin, Mao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zesong Li, THpzc2NAZW1haWwuc3p1LmVkdS5jbg==
†These authors have contributed equally to this work
 Chunhua Xu
Chunhua Xu Shan Lin
Shan Lin Longyi Mao1
Longyi Mao1 Zesong Li
Zesong Li