
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 21 June 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.852973
This article is part of the Research Topic Immune Response to Respiratory Viruses and Respiratory Viral Infections in Susceptible Populations View all 14 articles
 Biagio Pinchera1*
Biagio Pinchera1* Lorenzo Spirito2
Lorenzo Spirito2 Antonio Riccardo Buonomo1
Antonio Riccardo Buonomo1 Maria Foggia1
Maria Foggia1 Rosa Carrano3
Rosa Carrano3 Fabrizio Salemi3
Fabrizio Salemi3 Elisa Schettino3
Elisa Schettino3 Fortuna Papa3
Fortuna Papa3 Roberto La Rocca2
Roberto La Rocca2 Felice Crocetto2
Felice Crocetto2 Luigi Napolitano2
Luigi Napolitano2 Riccardo Villari1
Riccardo Villari1 Ivan Gentile1 on behalf of Federico II COVID Team
Ivan Gentile1 on behalf of Federico II COVID TeamIntroduction: In solid organ transplant recipients, COVID-19 is associated with a poor prognosis because of immunosuppression. Some studies suggest a potential therapeutic role of mammalian Target of Rapamycin (mTOR) inhibitors in SARS-CoV-2 infection. This study aimed to assess the impact of mTOR employment on the evolution and outcome of SARS-CoV-2 infection in solid organ transplant recipients.
Methods: We enrolled kidney transplant patients attending the Azienda Ospedaliera Universitaria Federico II in Naples and followed up on these patients from March 2020 to June 2021. We evaluated the risk of acquiring the SARS-CoV-2 infection, the clinical presentation of the disease, and its outcome together with the type of immunosuppressive therapy. Finally, we assessed the impact of mTOR inhibitors on relevant clinical metrics of SARS-CoV-2 infection.
Results: We enrolled 371 patients, of whom 56 (15.1%) contracted SARS-CoV-2 infection during the period of the study. There were no differences observed among the different immunosuppressive therapies concerning the risk of acquiring SARS-CoV-2 infection. In contrast, the type of immunosuppressive therapy had a significant impact on the outcome of the disease. In detail, patients who received mTOR inhibitors, as part of their immunosuppressive therapy, compared to other regimens had a lower chance of developing a moderate or severe form of the disease (OR = 0.8, 95, CI: (0.21–0.92), P = 0.041).
Conclusion: In kidney transplant patients, the use of mTOR inhibitors as part of an immunosuppressive regimen is associated with a better prognosis in the case of COVID-19.
Immunosuppressive therapy is a crucial aspect in a solid organ transplant patient. It is the mainstay of the prevention of rejection of the allograft, but at the same time, it contributes to determining the patient's susceptibility to several infections (1–3). Different immunosuppressive drugs, such as calcineurin inhibitors (tacrolimus and cyclosporine), corticosteroids, antimetabolite agents (mycophenolate and azathioprine), and the mammalian target Of rapamycin (mTOR) inhibitors (everolimus and sirolimus), are used to prevent rejection (4, 5). In particular, mTOR is a crucial pathway in many physiological processes (such as cell cycle progression, transcription, translation, differentiation, apoptosis, motility, and cell metabolism) and, therefore, plays a central role in the regulation of cell growth and proliferation, at the translational level, and in cell cycle progression. Moreover, as mTOR also modulates protein synthesis at ribosomal and transfer RNA transcription levels, it also plays a fundamental role in viral translation (6). It is already known that several viruses, such as adenovirus, cytomegalovirus, herpes simplex virus, and Middle East respiratory syndrome coronavirus (MERS – CoV), use the mTOR pathway to replicate (7, 8). The mTOR pathway is also involved in the life cycle of SARS-CoV-2 infection (9). The antiviral properties of mTOR have been known and ascribed to a variety of mechanisms (10). This aspect needs to be considered in relation to the pandemic impact (2–4). There are scarce data on the possible role of mTOR inhibitors vs. SARS-CoV-2 and their potential impact on the evolution of the disease; however, some studies support the potential therapeutic role of these drugs (11). Some reviews suggest the therapeutic potential of mTOR inhibitors, such as rapamycin, against COVID-19 both in vitro and in vivo (12–14).
For these reasons, blocking the mTOR signaling pathway could be a strategy to treat SARS-CoV-2 infection and its evolution. This study aimed to describe and assess the impact of the mTOR inhibitor therapy on the evolution and outcome of SARS-CoV-2 infection in solid organ transplant recipients followed in our center.
We conducted an observational retrospective cohort study. We enrolled patients with kidney transplants attending the Azienda Ospedaliera Universitaria Federico II in Naples from March 2020 to June 2021. Diagnosis of SARS-CoV-2 infection was obtained by positivity to the rhino-oropharyngeal swab for SARS-CoV-2 RNA research by reverse transcription-polymerase chain reaction (RT-PCR). For patients with COVID-19, we used the Henry Ford Hospital (HFH). COVID-19 severity scoring system to distinguish the disease's mild, moderate, and severe forms (15). In particular, the mild disease was defined as patients who had normal chest radiography and SpO2 of ≥94% without the need for supplemental oxygen. Patients with moderate disease were those who had abnormal chest radiography, SpO2 of <94%, and were in need of 1 and 5 L/min supplemental O2. Patients with severe disease were defined by abnormal chest radiography, SpO2 of <94%, and requiring ≥6 L/min of O2 (16). For each patient, we evaluated the epidemiological characteristics, the laboratory data, the data of radiological instrumental investigations, clinical characteristics, the time elapsed since the transplant, the type of immunosuppressive treatment, and their changes during SARS-CoV-2 infection, the need for treatment and the type of treatment for SARS infection -CoV-2, and the outcome. In particular, we evaluated the potential relationship between the use of mTOR vs. other immunosuppressive regimens and severity or clinical outcome.
Data were reported as the median and interquartile range (IQR) given their non-parametric distribution. For comparisons between continuous variables, the Mann-Whitney U test was performed. We used the Chi-square test to test if two categorical variables are associated. Co-variates significantly associated with death in the univariate analysis were also analyzed in a multivariate model. The P-value for statistical significance was set at <0.05 for all the tests.
With respect to the ethical issues, the study was conducted in compliance with the Declaration of Helsinki and the principles of good clinical practice. The authors confirm adherence to the ethical policies of the journal, as noted on the journal's author guidelines page.
We enrolled 371 patients with kidney transplant (229 men, 61.8%) with a median age of 49 (18–86) years and a mean age of 51.4 years. Of these, 56 (15.1%) became infected with SARS-CoV-2 during the period of the study. Of these 56 patients with SARS-CoV-2 infection, 30 (53.6%) showed symptoms of the disease (COVID-19) and 26 had an asymptomatic infection (Table 1). Of the 30 patients with COVID-19 symptoms, 15 (50%) had a mild form of the disease, 7 (24%) had a moderate form of the disease, and 8 (26%) had severe form of the disease. Hospitalization was necessary for 12 (21.4%) patients, eight with the severe form of the disease and four with the moderate one. Of the 12 patients admitted, five required oxygen supplementations, five required non-invasive/high flow ventilation, and two required invasive ventilation (Table 1). Of the enrolled patients, only 12 patients performed high-resolution lung computed tomography (HRCT); in particular, only hospitalized patients performed HRCT. The severity score index, as proposed by Chung et al. (17) was used for the analysis of each individual HRCT. The 12 patients had a median severity score index equal to 13/20 as proposed by Chung et al. (17). Of the 12 patients, only one was taking mTOR inhibitors, particularly sirolimus, and had a severity score index equal to 10/20, as proposed by Chung et al. (17). Distinguishing the severity score index, proposed by Chung et al. (17) between the group of mTOR inhibitors and the group without mTOR inhibitors (10/20 vs. 13/20), a severity score index, proposed by Chung et al. (17) was higher among patients without mTOR inhibitors.
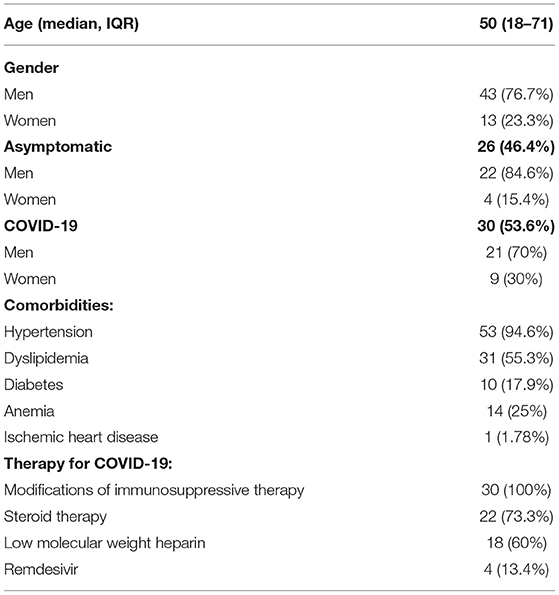
Table 1. Anagraphical and clinical features of patients with kidney transplant with SARS-CoV-2 infection.
All 371 patients underwent immunosuppressive therapy at the time of enrollment. In particular, 220 underwent triple immunosuppressive therapy, 142 dual therapy, and nine single immunosuppressant (Tables 2–4).
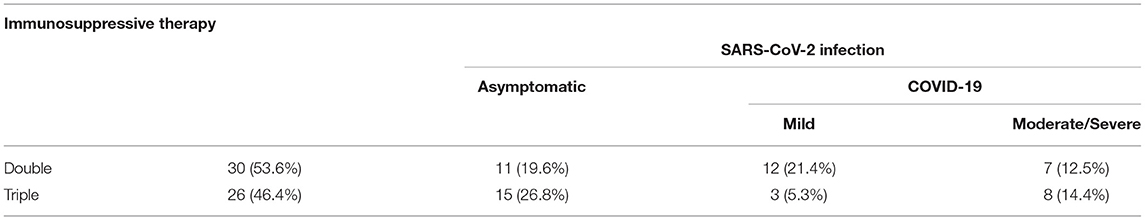
Table 4. Single vs. dual vs. triple immunosuppressive therapy in patients with SARS-CoV-2 infection.
Data concerning the different immunosuppressive regimens also in relation to clinical presentation and outcome are given in Tables 3, 6.
In relation to the different immunosuppressive therapies, 66 patients (17.8%) assumed the immunosuppressive therapy with mTOR inhibitors, 48 with everolimus, and 18 with sirolimus. Of these, 11 (16.6%) (eight treated with everolimus and three with sirolimus) acquired SARS-CoV-2 infection (OR for SARS-CoV-2 infection acquired vs. no SARS-CoV-2 infection acquired: 1.1, 95, CI: (0.25–2.8) mTOR inhibitors recipients vs. other regimens), p = 0.210). Of the 11 patients infected, 7 (63.6%) had COVID-19; in particular, six had a mild form of the disease, while 1 had a moderate form of the disease (OR for moderate/severe form vs. mild:0.8, 95, CI: (0.21–0.92) mTOR inhibitors recipients vs. other regimens; p = 0.041) (Tables 2, 5, 6). No patient treated with mTOR inhibitors presented a severe form of the disease.
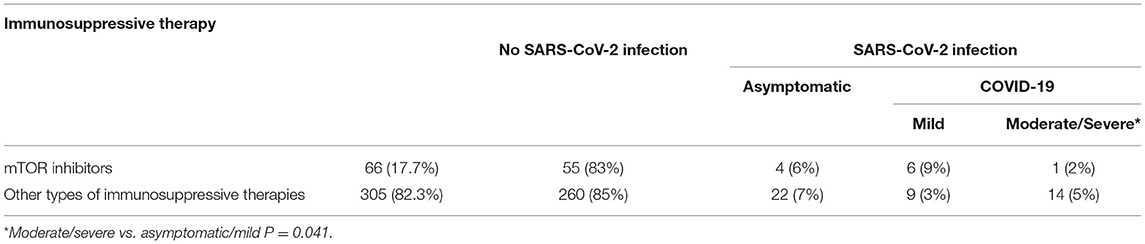
Table 6. Immunosuppressive therapy evaluation: mTOR inhibitors vs. other types of immunosuppressive therapies.
No significant differences were observed between those patients who received a triple vs. a mono/double immunosuppressive regimen in the risk of acquiring the infection (OR = 1.1, 95, CI: (0.60–2.5), p = 0.270) (Tables 3, 4).
All patients with symptoms underwent modifications of the immunosuppressive therapy. Regarding the therapeutic management of the infection, the first step was the reduction of immunosuppressive therapy, providing, in the first instance, the reduction or suspension of antimetabolites. In the case of severe forms of the disease, all immunosuppressive therapy was suspended, except for the steroid therapy, which was increased (OR for modification/suspension of immunosuppressive therapy vs. non-modification of immunosuppressive therapy in SOT with a moderate-severe form of COVID-19:0.7, 95, CI: (0.44–0.85), p = 0.048) (18–21). Only one patient experienced acute organ rejection, and two patients died.
We conducted a multivariate analysis of the possible variables that could impact the evolution of the COVID-19 disease, regardless of the presence or absence of mTOR inhibitors. We considered diabetes, BMI, duration of immunosuppressive treatment, duration of renal disease, and concomitant heart disease as variables. The multivariate analysis highlighted the values of diabetes [OR = 0.9, 95, CI: (0.85–1.4), P = 0.130], BMI [OR = 1.1, 95, CI: (0.92–1.3), P = 0.145], duration of immunosuppressive treatment [OR = 1.2, 95, CI: (0.72-1.8), P = 0.350], duration of renal disease [OR = 1.1, 95, CI: (0.52–2.1), P = 0.420], and concomitant heart disease [OR = 0.96, 95, CI: (0.88–1.7), P = 0.290].
Our study first shows that the use of mTOR inhibitors when compared with other immunosuppressive regiments was associated with a more favorable outcome of COVID-19 in a cohort of patients. Moreover, none of the patients undergoing immunosuppressive therapy with mTOR inhibitors (everolimus and sirolimus) presented a severe form of the disease.
In contrast, neither the number of immunosuppressive drugs nor their type was associated with the risk of acquiring the infection.
We underline that our data may add knowledge to the management of SARS-CoV-2 infection in patients who underwent solid organ transplant and, in particular, to the management of immunosuppressive therapy during this infection. Moreover, from our study, the role of mTOR inhibitors in COVID-19 treatment could be hypothesized even in a non-transplant setting. However, this hypothesis needs to be deepened and demonstrated with further studies with a different design (i.e., randomized controlled trial).
How can we explain these results? There are at least two possible explanations: an antiviral effect of mTOR inhibitors or an immunomodulant action. With respect to the first hypothesis, we underline that a potential positive impact of mTOR inhibitors in the course of several viral infections is already known in the literature (22, 23). However, to our best knowledge, our study is the first one to show a positive impact of mTOR inhibitors in the course of SARS-CoV-2 infection on the evolution of the disease. The results might be due to the wellknown immunomodulatory effect of these drugs that could reduce the cytokine storm typical of the immune activation phase of the disease. Alternatively, another possible reason could be due to the inhibitory action on the mTOR pathway, which could induce the inhibition of transcriptional processes and consequently induce a reduced viral replication.
By multivariate analysis, it was found that none of the variables considered (diabetes, BMI, duration of immunosuppressive treatment, duration of renal disease, and concomitant heart disease) showed a statistically significant impact regardless of the presence or absence of mTOR inhibitors. Furthermore, as reported in the meta-analysis by Gatti et al. (24) also in our case, there was no increased mortality risk in this category of patients compared to the general population.
We acknowledge that our study presents several limitations, such as the retrospective design, the small sample size, the monocentric cohort, the lack of data on dosages of immunosuppressive therapies, and changes in immunosuppressive therapy during SARS-CoV-2 infection.
Our real-life study showed a positive impact of therapy with mTOR inhibitors in SARS-CoV-2 infection occurring in patients who underwent kidney transplant. Due to potential antiviral or immunomodulant properties, this class of drugs might be considered a possible weapon in the fight against COVID-19, both in transplant and non-transplant settings. These hypotheses need to be explored in randomized controlled trials.
Ametrano Luigi, Amicone Maria, Borrelli Francesco, Buonomo Antonio Riccardo, Cattaneo Letizia, Conte Maria Carmela Domenica, Cotugno Mariarosaria, Di Filippo Giovanni, Di Filippo Isabella, Esposito Nunzia, Festa Lidia, Fusco Ludovica, Foggia Maria, Gallicchio Antonella, Gentile Ivan, Giaccone Agnese, Iuliano Antonio, Lanzardo Amedeo, Licciardi Federica, Mercinelli Simona, Minervini Fulvio, Nobile Mariano, Piccione Amerigo, Pinchera Biagio, Reynaud Laura, Salemi Fabrizio, Sardanelli Alessia, Sarno Marina, Schiano Moriello Nicola, Scordino Fabrizio, Scotto Riccardo, Stagnaro Francesca, Tosone Grazia, Vecchietti Ilaria, Viceconte Giulio, Zappulo Emanuela, and Zotta Irene.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee Federico II. Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional Requirements.
BP: conceptualization, investigation, writing—original draft, writing—review and editing, and project administration. IG: writing—original draft, writing—review and editing, and supervision. RV: resources, data curation, and validation. LN: data curation, software, and project administration. FC: validation, investigation, and writing—review and editing. RL: formal analysis, data curation, and resources. FP: data curation, software, and resources. ES: formal analysis, data curation, and project administration. FS: software, data curation, and investigation. RC: methodology, resources, and supervision. MF: validation, resources, and project administration. AB: methodology, writing—review and editing, and visualization. LS: methodology, formal analysis, and data curation. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fishman JA. Infection in organ transplantation. Am J Transplant. (2017) 17:856–79. doi: 10.1111/ajt.14208
2. Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and solid organ transplantation: a review article. Transplantation. (2021) 105:37–55. doi: 10.1097/TP.0000000000003523
3. Nair V, Jandovitz N, Hirsch JS, Nair G, Abate M, Bhaskaran M, et al. COVID-19 in kidney transplant recipients. Am J Transplant. (2020) 20:1819–25. doi: 10.1111/ajt.15967
4. Scientific Registry of Transplant Recipients (SRTR) and Organ Procurement and Transplantation Network (OPTN). SRTR/OPTN 2010 annual data report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. Am J Transplant. (2012) 12(Suppl. 1):553–86. doi: 10.1111/ajt.16983
5. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. (2007) 357:2601–14. doi: 10.1056/NEJMra064928
6. Castle BT, Dock C, Hemmat M, et al. Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets. bioRxiv. (2020). doi: 10.1101/2020.05.22.111237
7. Le Sage V, Cinti A, Amorim R, Mouland AJ. Adapting the stress response: viral subversion of the mTOR signaling pathway. Viruses. (2016) 8:152. doi: 10.3390/v8060152
8. Karam BS, Morris RS, Bramante CT, Puskarich M, Zolfaghari EJ, Lotfi-Emran S, et al. mTOR inhibition in COVID-19: a commentary and review of efficacy in RNA viruses. J Med Virol. (2021) 93:1843–46. doi: 10.1002/jmv.26728
9. Terrazzano G, Rubino V, Palatucci AT, Giovazzino A, Carriero F, Ruggiero G. An open question: is it rational to inhibit the mTor-dependent pathway as COVID-19 therapy? Front Pharmacol. (2020) 11:e856. doi: 10.3389/fphar.2020.00856
10. Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-celldifferentiation. Nature. (2009) 460:108–12. doi: 10.1038/nature08155
11. Ghasemnejad-Berenji M. mTOR inhibition: a double-edged sword in patients with COVID-19? Hum Cell. (2021) 34:698–9. doi: 10.1007/s13577-021-00495-2
12. Husain A, Byrareddy SN. Rapamycin as a potential repurpose drug candidate for the treatment of COVID-19. Chem Biol Interact. (2020) 331:109282. doi: 10.1016/j.cbi.2020.109282
13. Omarjee L, Janin A, Perrot F, Laviolle B, Meilhac O, Mahe G. Targeting T-cell senescence and cytokine storm with rapamycin to prevent severe progression in COVID-19. Clin Immunol. (2020) 216:108464. doi: 10.1016/j.clim.2020.108464
14. Zheng Y, Li R, Liu S. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategybeyond vaccines and specifc antiviral medicines. J Med Virol. (2020) 92:1495–500. doi: 10.1002/jmv.26009
15. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. (2020) 383:1813-26. doi: 10.1056/NEJMoa2007764
16. Chaudhry ZS, Williams JD, Vahia A, Fadel R, Acosta TP, Prashar R, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a case-control study. Am J Transplant. (2020) 20:3051–60. doi: 10.1111/ajt.16188
17. Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. (2020) 295:202–7. doi: 10.1148/radiol.2020200230
18. Di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: the multicenter Italian CORIST Study. Thromb Haemost. (2021) 121:1054-65. doi: 10.1055/a-1347-6070
19. COVID-19 RISk and Treatments (CORIST) Collaboration. RAAS inhibitors are not associated with mortality in COVID-19 patients: findings from an observational multicenter study in Italy and a meta-analysis of 19 studies. Vascul Pharmacol. (2020) 135:106805. doi: 10.1016/j.vph.2020.106805
20. Sagnelli C, Sica A, Gallo M, Peluso G, Varlese F, D'Alessandro V, et al. Renal involvement in COVID-19: focus on kidney transplant sector. Infection. (2021) 49:1265-75. doi: 10.1007/s15010-021-01706-6
21. Desmazes-Dufeu N, Coltey B, Amari L, Gouitaa M, Touzery C, Reynaud-Gaubert M, et al. Discordant courses of COVID-19 in a cohabiting couple of lung transplant recipients. Case Reports Transpl Infect Dis. (2021) 23:e13410. doi: 10.1111/tid.13410
22. Bowman LJ, Brueckner AJ, Doligalski CT. The role of mTOR inhibitors in the management of viral infections: a review of current literature. Transplantation. (2018) 102(2 Suppl. 1):S50-59. doi: 10.1097/TP.0000000000001777
23. Paoletti E, Citterio F, Corsini A, Potena L, Rigotti P, Sandrini S, et al. Everolimus in kidney transplant recipients at high cardiovascular risk: a narrative review. J Nephrol. (2020) 33:69-82. doi: 10.1007/s40620-019-00609-y
24. Gatti M, Rinaldi M, Bussini L, Bonazzetti C, Pascale R, Pasquini Z, et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect. (2022). S1198-743X(22)00116-1. doi: 10.1016/j.cmi.2022.02.039
Keywords: kidney transplant, SARS-CoV-2, COVID-19, mTOR inhibitors, immunosuppressive therapy
Citation: Pinchera B, Spirito L, Buonomo AR, Foggia M, Carrano R, Salemi F, Schettino E, Papa F, La Rocca R, Crocetto F, Napolitano L, Villari R and Gentile I (2022) mTOR Inhibitor Use Is Associated With a Favorable Outcome of COVID-19 in Patients of Kidney Transplant: Results of a Retrospective Study. Front. Med. 9:852973. doi: 10.3389/fmed.2022.852973
Received: 11 January 2022; Accepted: 29 April 2022;
Published: 21 June 2022.
Edited by:
Farid Rahimi, Australian National University, AustraliaReviewed by:
Manfred Johannes Stangl, Ludwig Maximilian University of Munich, GermanyCopyright © 2022 Pinchera, Spirito, Buonomo, Foggia, Carrano, Salemi, Schettino, Papa, La Rocca, Crocetto, Napolitano, Villari and Gentile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biagio Pinchera, biapin89@virgilio.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.