- 1School of Public Health, Medical College of Yangzhou University, Yangzhou University, Yangzhou, China
- 2Department of Geriatric Medicine, Huadong Sanatorium, Wuxi, China
- 3Joyfulway Clinic, Fosun Health Co., Ltd., Shanghai, China
- 4Department of Health Management, Huadong Sanatorium, Wuxi, China
- 5Department of Medical Record Statistics, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China
- 6Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
- 7Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China
Objective: China has established a goal of reducing adult smoking prevalence from 27.7% to 20% by 2030. Understanding the possible ongoing impairment in lung function in smokers, is critically important to encourage the populations to change their smoking behavior.
Methods: A total of 14,273 males joined the health examination at Huadong Sanatorium from Jan 2012 to Dec 2019 were included. In cross-sectional analysis, we used multiple linear regression to evaluate the association between baseline lung function and smoking status. Then, 3,558 males who received ≥2 spirometry exams were analyzed in longitudinal study. Annual lung function decline was compared using mixed linear models adjusted for confounders.
Results: In cross-sectional analysis, compared with never-smokers, decreases of −133.56 mL (95% CI: −167.27, −99.85) and −51.44 mL (−69.62, −33.26) in FEV1, −1.48% (−1.94, −1.02) and −1.29% (−1.53, −1.04) in FEV1/FVC were observed in former and current smokers. In longitudinal analysis, significant declines were observed in FEV1 [5.04 (2.30, 7.78) mL] and FEV1/FVC [0.09 (0.05, 0.13) %] in current smokers but not observed in former smokers after adjustment. Participants with long duration of smoking cessation had decelerate lung function than short duration. The annual decline rate of current smokers with high smoking intensity (≥30 cigarettes per day) was 13.80 and 14.17 times greater than that of never-smokers in FEV1 and FVC. Thus, early smoking cessation can slow down lung function decline trend for current smokers.
Conclusions: The harms of current smoking on lung function emphasize the necessity of smoking cessation, especially for those with comorbidities.
Introduction
Smoking is the major cause of premature death worldwide (1, 2). As the Global Adult Tobacco Survey (GATS) approximated in 2010, China is the largest producer and consumer of tobacco products in the world, with an estimation of 301 million current smokers (3–6). The current smoking prevalence among men was 52.9% and that among women was 2.4% in China (5). Such a high smoking rate of Chinese males shifts the negative effects on pulmonary health and accounts for nearly 20% of all-cause mortality during the past decades (7). The magnitude of tobacco related pulmonary disease has created a healthcare crisis in China (3).
Lung function is a critical measurement and early severity predictor for indicating cardio-pulmonary health (8). Decline of FEV1 indicates a higher risk of COPD (9); while the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC), also known as FEV1/FVC, is the primary index of airflow limitation or airway obstruction (10). Current smoking was found associated with accelerated age-related FEV1 decline (11, 12). While one meta-analysis showed a homogeneity effect of current and former smoking on FEV1 decline (9). However, former smokers having changed the smoking habits for part of the period during which the betas were estimated may lead to the non-significant estimates in this meta-analysis (9). Furthermore, prior studies always focused on the association between smoking status and FEV1 decline among COPD or asthma population (13–15). Besides, the mentioned studies were mainly conducted in the developed countries, e.g., the United States, Swedish, UK et.al. In these countries, workplace smoking cessation (SC) intervention is effective in increasing quit rate and more cases were voluntary SC promotion (16). In contrast, Chinese populations were less likely to promote voluntary SC, most of them quit smoking due to smoking-related diseases. Thus, it is essential to evaluate the association between different smoking status and lung function decline in the Chinese population.
As of the Health China 2030 strategy, the government has established a goal of reducing adult smoking prevalence from 27.7 to 20% by 2030 (17). Challenges remain in accomplishing the goal. Understanding the possible ongoing impairment of smoking in lung function, is increasingly important, to encourage the voluntary SC promotion. Hence, we conducted this retrospective study to evaluate the association between smoking exposure and changes of lung function (i.e., FEV1, FVC and FEV1/FVC) among Chinese males with repeated measure of the indicators.
Materials and Methods
Data Source
We used data from Huadong Sanatorium health examination database (HSHED) between Jan 1, 2012 and Dec 31, 2019. Huadong Sanatorium (HS) is a municipal medical institution integrating convalescence, rehabilitation and health care, which providing personalized health management services for the entire society. Most participants taking health examination in HS are employees of various employers from Shanghai aged 15–95 years old. HSHED was established based on hospital information system (HIS) in 2003. All the results of examination were recorded in the HSHED.
We extracted data from participants who volunteered to receive basic health examination and additional spirometry exams in HS. A total of 22,051 participants took spirometry exams were included. Date when participants first underwent a spirometry exam in HS was set as baseline. Female participants, with low smoking rate (< 1%), were excluded, left 14,273 males to evaluate the association between lung function and smoking status in the cross-sectional analysis phase (Substudy 1, Supplementary Figure 1). In order to examine the longitudinal association of lung function annual changes with smoking status, we restricted to the participants with valid spirometry at two or more exams. Then, 3,558 males were included in the longitudinal analysis (Substudy 2, Supplementary Figure 1).
The approval of this study was obtained from ethics committees at Huadong Sanatorium (No. 2020-01). Anonymized and de-identified information were used for analyses, and therefore informed consent was not required.
Measurements
Smoking status was self-reported as “never” “former” and “current” cigarettes smoking at each spirometry exam. Ever-smokers were defined as former and current smokers. In the cross-sectional analysis phase, all the 14,273 participants were divided into three groups according to baseline smoking status: never-smokers (N = 5,468), former smokers (N = 1,111), and current smokers (N = 7,694) (Table 1). In the longitudinal analysis phase, 3,268 participants reported smoking status unchanged across the follow-up period. These participants were classified as sustained never-smokers (N = 1,305), former smokers (N = 245), and current smokers (N = 1,718). Other 290 participants were classified as having variable smoking status (Supplementary Table 1).
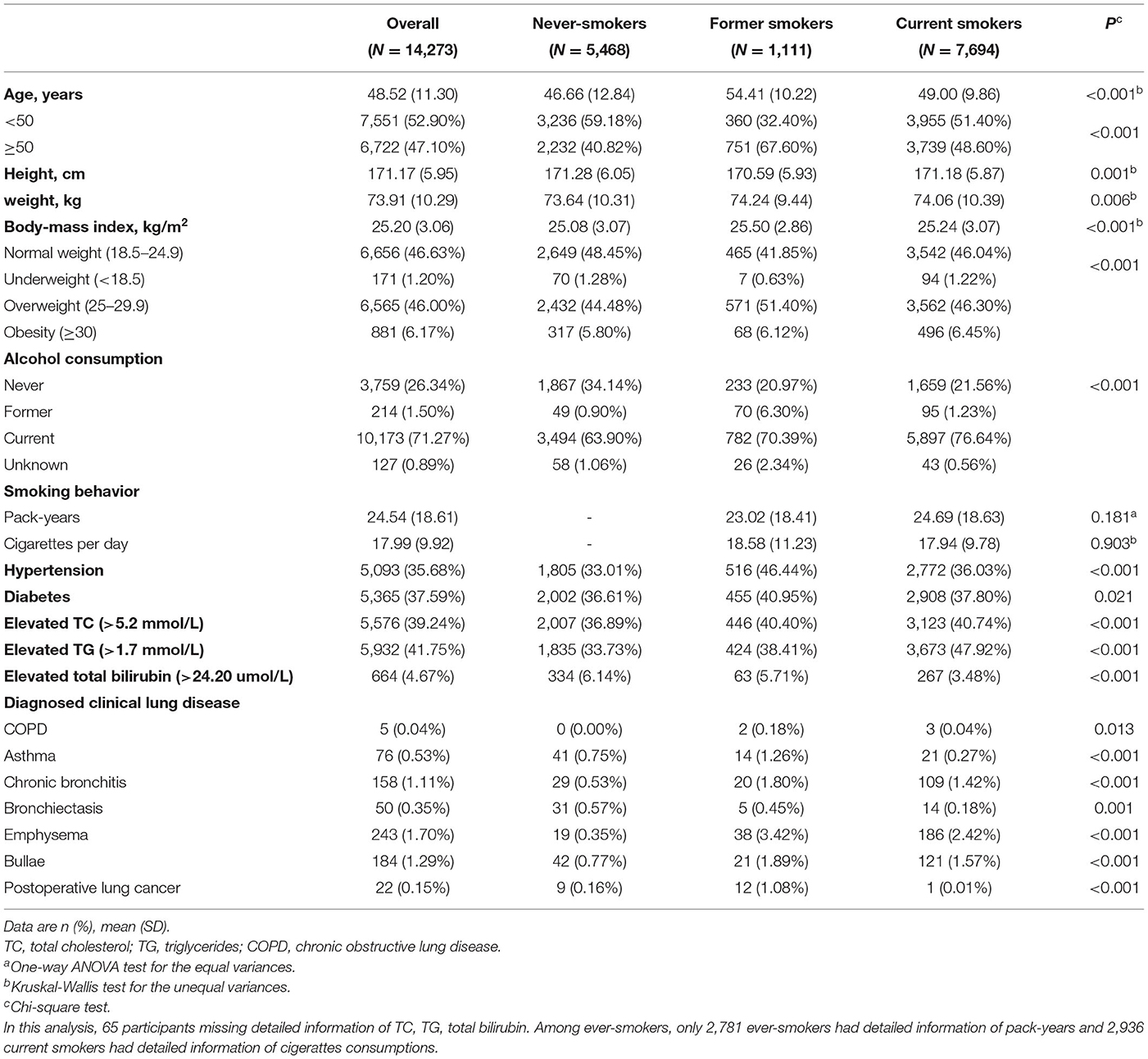
Table 1. Baseline characteristics of 14,273 male participants according to smoking status in the cross-section analysis.
Spirometry was performed using a MiniSpir spirometer at baseline and follow-up visits. A bronchodilator was not administered prior to spirometry. Lung function was measured with standardized protocols by the same equipment and acquired by the same investigators. To harmonize these data, we retrospectively did quality control checks according to the American Thoracic Society/European Respiratory Society 2005 standards, which define valid exams as two or more acceptable curves reproducible within 150 mL (18). Lung function outcomes were forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and their ratio (FEV1/FVC). The Global Lung Function Initiative equations (19) were used to define lower limit of normal (LLN).
Diagnosed clinical lung disease was defined as self-reported physician diagnosis of COPD, asthma, chronic bronchitis, bronchiectasis, emphysema, bullae and postoperative lung cancer. Airflow limitation was defined as FEV1/FVC lower than the LLN, defined by the NHANES III reference equations (20). Restrictive pattern was defined as FEV1/FVC≥LLN and FVC < LLN (21).
Statistical Analysis
Baseline Characteristics of the Participants
Demographic characteristics of the study participants according to baseline smoking status were calculated and compared among groups. Baseline characteristics were assessed by one-way ANOVA, Chi-squared and Kruskal-Wallis test. Analyses were performed separately for Substudy 1 and Substudy 2.
Relationship Between Smoking Status and Lung Function at Baseline and Follow-Up
Firstly, we examined the relationship between smoking status and lung function at participants' first visit using cross-sectional analysis. We evaluated the mean differences in the lung function across different smoking exposure by multiple linear regression analysis.
To further evaluate the decline rate of lung function among different smoking status, longitudinal analysis was then performed. In this analysis, linear mixed models were used to test associations with repeated measures of lung function.
Sensitivity Analysis
Analyses were repeated in the participants without prevalent lung disease, with variable smoking status or aged older than 30 years to minimize the potential confounding effect.
Methods of smoking details, clinical and laboratory assessments, multiple linear regression, and linear mixed models were provided in the Supplementary Material. Data were analyzed using STATA software version 13 (STATA Corp, College Station, TX, USA). Statistical significance was defined as a two-tailed P < 0.05.
Results
Substudy 1 Cross-Sectional Associations of Lung Function With Smoking Exposures Among 14,273 Male Participants at Baseline
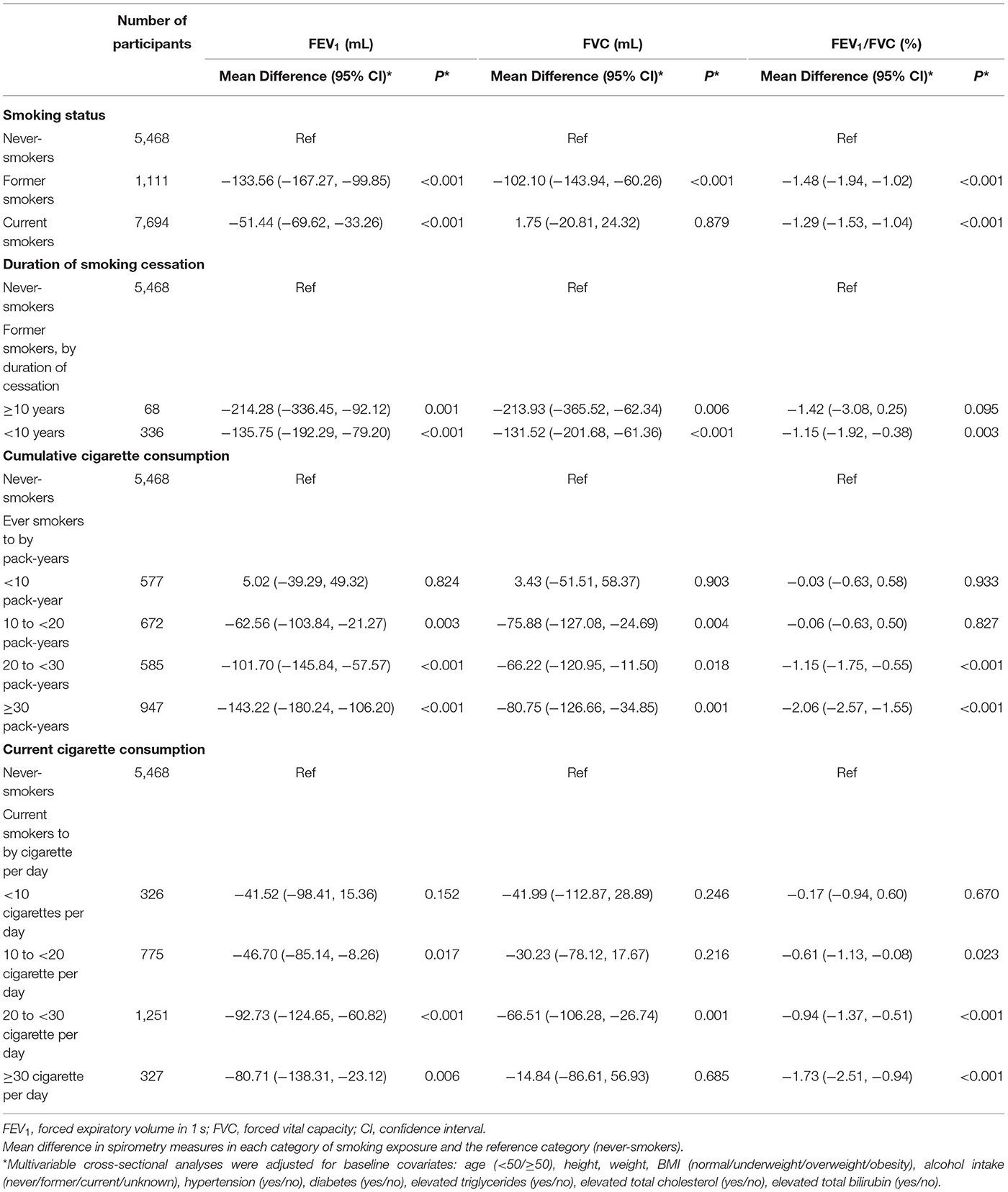
In the cross-sectional phase, baseline characteristics are shown in Table 1. Most of them (90.31%) were aged 30–70 years old. Former smokers and currents smokers were older than never-smokers (P < 0.001). Mean cumulative cigarette exposure of former smokers and current smokers were 23.02 ± 18.41 and 24.69 ± 18.63 pack-years (PYs), respectively. Current smokers consumed an average of 17.94 ± 9.78 cigarettes per day. Former smokers were more likely to have an underlying disease at the first visit, i.e., hypertension (46.44%), diabetes (40.95%), elevated total bilirubin (5.71%) and lung diseases (10.08%), when compared with never smokers and current smokers (P < 0.001).
Multiple linear regression was used to evaluate the associations between smoking exposures with lung function (Table 2). After adjustment, ever-smoking was significantly related with lower FEV1 and FEV1/FVC at the first visit. Compared with never-smokers, current smokers had a −51.44 mL (95% CI: −69.62, −33.26, P < 0.001) decrease in FEV1 and a −1.29% (95% CI: −1.53, −1.04, P < 0.001) decrease in FEV1/FVC; and former smokers had an even lower level of lung function, with a −133.56 mL (95% CI: −167.27, −99.85, P < 0.001) decrease in FEV1 and a −1.48% (95% CI: −1.94, −1.02, P < 0.001) decrease in FEV1/FVC. For ever-smokers, greater cumulative cigarettes consumptions were associated with lower lung function, significantly when the cumulative pack-years exceeded to 20–30 or ≥30 PYs [Mean difference for FEV1: −101.70(−145.84, −57.57) and −143.22(−180.24, −106.20); FVC: −66.22(−120.95, −11.50) and −80.75(−126.66, −34.85); FEV1/FVC: −1.15(−1.75, −0.55) and −2.06(−2.57, −1.55) for 20–30 PYs and ≥30 PYs, when compared to never-smoking, Table 2]. For former smokers, longer durations of smoking cessation had a lower lung function [Mean difference for FEV1: −214.28 (−336.45, −92.12) vs. −135.75 (−192.29, −79.20); FVC: −213.93 (−365.52, −62.34) vs. −131.52 (−201.68, −61.36); FEV1/FVC: −1.42 (−3.08, 0.25) vs. −1.15 (−1.92, −0.38) for ≥10 and < 10 years cessation duration]. For current smokers, those with ≥10 cigarettes/day had significant FEV1 and FEV1/FVC decline compared with never-smokers [Mean difference for FEV1: −46.70 (−85.14, −8.26), −92.73 (−124.65, −60.82), −80.71 (−138.31, −23.12); FEV1/FVC: −0.61 (−1.13, −0.08), −0.94 (−1.37, −0.51), −1.73 (−2.51, −0.94) for 10–20, 20–30, and ≥30 cigarettes/day, respectively].
Substudy 2 Longitudinal Associations Between Smoking Exposures and Lung Function Among 3,558 Male Participants
In the previous step, former smokers were observed with a lower level of lung function than that of never or current smokers. We further explored whether persistent smoking would accelerate the declines of lung function along with age. In the current longitudinal analyses, 3,558 male participants with ≥2 valid spirometry exams contributed 8,935 spirometry exams during follow-up. The baseline characteristics of 3,558 males in this longitudinal analysis was similar with those of participants in the cross-sectional analysis (Table 1 and Supplementary Table 2). The mean pack-years of current smokers was 25.18 ± 18.73, which was greater than that of former smokers (21.97 ± 15.44) before baseline. The mean values of FEV1, FVC, and FEV1/FVC for former smokers at baseline were lower than that of other participants. Compared with never-smokers and current smokers, former smokers were older, had a higher proportion of drinkers and more likely to have underlying diseases.
Current smokers showed accelerated lung function decline compared with never-smokers. The unadjusted FEV1, FVC and FEV1/FVC decline among former smokers was 42.04 (36.77, 47.31), 47.36 (40.70, 54.03) mL and 0.16 (0.08, 0.24) % per year, compared to 33.99 (32.02, 35.97), 38.38 (35.90, 40.87) mL, and 0.09 (0.06, 0.11) % per year among never-smokers, and 39.26 (37.12, 41.40), 41.36 (38.55,44.17) mL, and 0.16 (0.14, 0.19)% per year among current smokers (Table 3). After adjusted for covariates, current smokers had an accelerated FEV1 decline of 5.04 ml (95% CI: 2.30, 7.78, P < 0.001) per year and FEV1/FVC decline of 0.09% (95% CI: 0.05, 0.13, P < 0.001) per year, when compared with never-smokers (Figure 1). Effect estimates were observed in participants with variable smoking status, with an accelerated FEV1 decline of 7.61 ml (95% CI: 3.04, 12.18, P = 0.001) per year and FVC decline of 6.25 ml (95%CI: 0.59, 11.90, P = 0.030) per year, compared to never-smokers. However, no significant estimates were analyzed for former smokers [FEV1, 3.90(−1.65, 9.44), P = 0.168; FVC, 3.38(−3.46, 10.22), P = 0.332; FEV1/FVC, 0.05(−0.03, 0.13), P = 0.224, Figure 1].
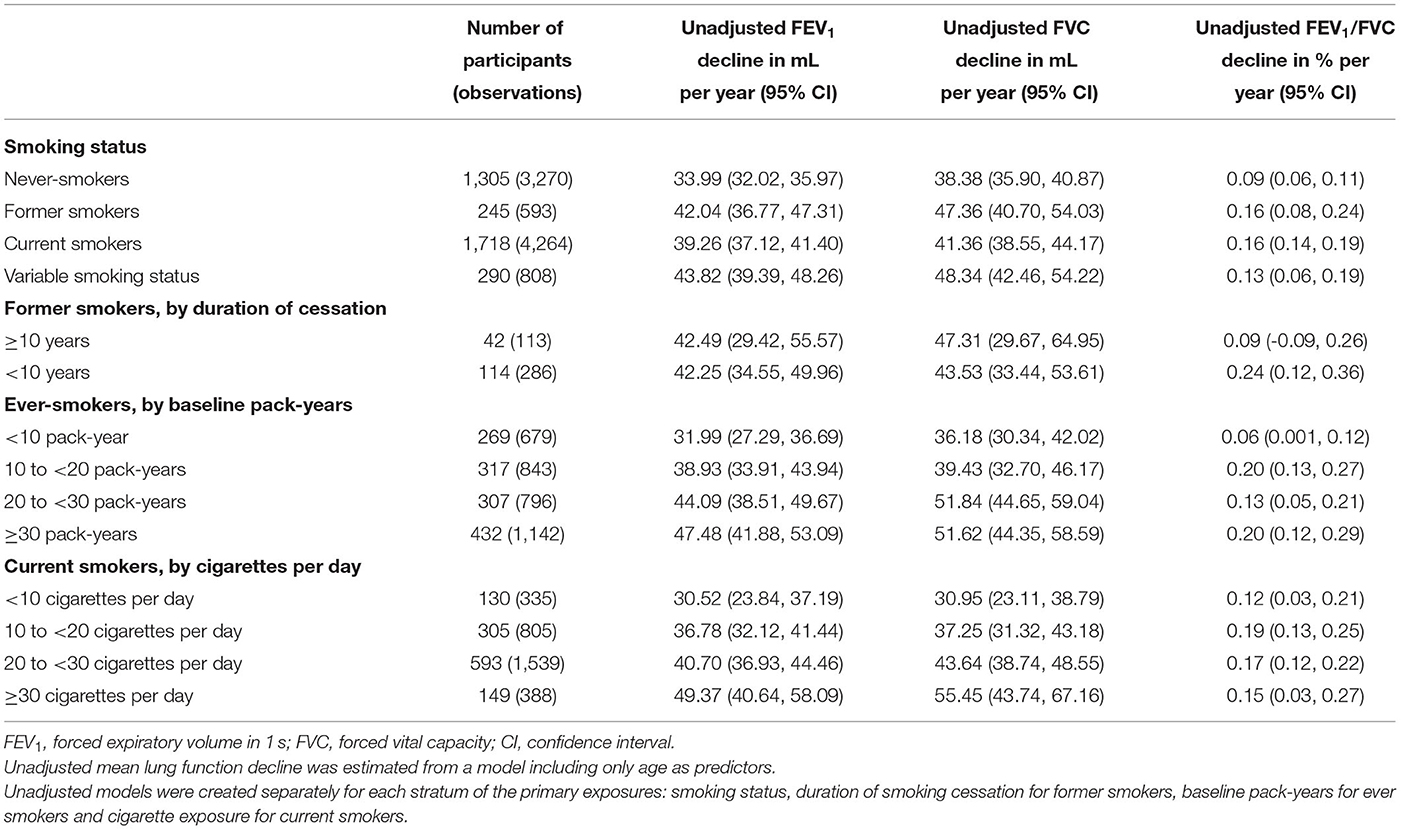
Table 3. Association between smoking status, duration of smoking cessation, cumulative and current cigarette consumption, and lung function decline in the longitudinal analysis.
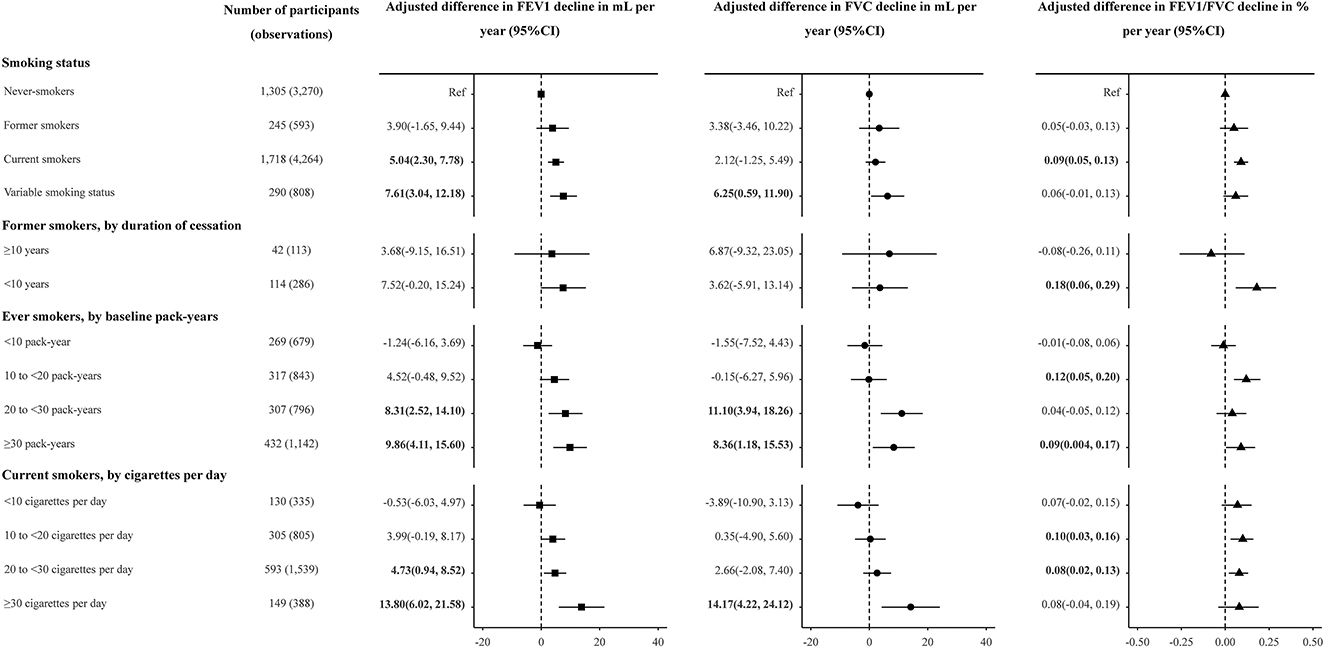
Figure 1. Adjusted association between smoking status, duration of smoking cessation, cumulative and current cigarette consumption, and lung function decline in the longitudinal analysis. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CI, confidence interval. Linear mixed models were used to test associations with repeated measures of FEV1, FVC and FEV1/FVC. Participants with variable smoking status were excluded from analyses of duration of smoking cessation and of cumulative and current cigarette consumption. Adjusted effect estimates for smoking exposures, relative to never-smoking, were generated with models adjusted for the smoking parameter, age, age2, height, weight, BMI, and alcohol consumption, hypertension, diabetes, TG, TC, total bilirubin at baseline. Multiplicative interactions with age were modeled for covariates. The effect estimate for smoking-exposure multiplied by age was interpreted as the association of the smoking exposure with annualized lung function decline.
For the former smokers, shorter durations of smoking cessation (< 10 years) were associated with more accelerated FEV1 decline than longer durations (≥10 years), when compared to never-smokers (3.68 vs. 7.52 mL, Figure 1). Compared with never-smokers, FEV1/FVC decline was accelerated by 0.18% per year (95% CI: 0.06, 0.29, P = 0.002) in former smokers with < 10 years of cessation, while the estimate was not obvious in former smokers with ≥10 years of cessation (P = 0.438) (Figure 1). Compared to never-smokers, decline was accelerated by 8.68 mL per year (P = 0.004) in FEV1 and by 0.10% per year (P = 0.029) in FEV1/FVC among 177 observed quitters (Supplementary Table 4).
For ever-smokers, the unadjusted estimates of declines in FEV1 accelerated with the increase of cumulative smoking pack-years (estimates for exposure with < 10, 10–20, 20–30, and ≥30 PYs were 31.99, 38.93, 44.09, and 47.48, respectively, Table 3). In the adjusted model, adjusted mean FEV1 decline accelerated with increased pack-years (estimates for exposure with < 10, 10–20, 20–30 and ≥30 PYs were −1.24, 4.52, 8.31, and 9.86, respectively, Figure 1). The adjusted effect estimate of FVC decline was significant but attenuated among participants with ≥20 PYs (estimates for exposure with 20–30 and ≥30 PYs were 11.10 and 8.36, respectively, Figure 1).
At the levels of current smoking intensity, current smokers with greater smoking intensity had more accelerate in FEV1 and FVC decline (Table 3 and Figure 1). The adjusted effect estimates of FEV1 decline for those smoking ≥30 cigarettes per day (13.80, 95% CI: 6.02, 21.58, P < 0.001) was 2.92 times greater than that for those smoking 20–30 cigarettes per day (4.73, 95% CI: 0.94, 8.52, P = 0.015). Compared with never-smokers, FVC decline was accelerated by 14.17 mL per year (95% CI: 4.22, 24.12) in current smokers with ≥30 cigarettes per day (Figure 1).
Although there was statistical evidence of effect modification by age, height and weight (P < 0.05), effect sizes did not change considerably across strata of age, height and weight (Supplementary Figure 2). As former smokers had a higher prevalence of hypertension and diabetes, we further explored the associations stratifying by baseline diagnosis of hypertension and diabetes. For never-smokers, participants with baseline hypertension and diabetes had more accelerated FEV1 decline than those without underlying diseases (P = 0.008, data not shown in Supplementary Figure 3). Among participants without hypertension and diabetes, current smokers had accelerated FEV1 decline compared to never-smokers after adjustment (P = 0.004, data not shown in Supplementary Figure 3), which can also show that current smoking was an independent risk factor for lung function. Compared with never-smokers without underlying hypertension and diabetes, FEV1 decreased more rapidly in heavy smokers (≥20 pack-years for ever-smokers and ≥20 cigarettes per day for current smokers) with underlying hypertension and diabetes (P = 0.032 and < 0.001, data not shown in Supplementary Figure 3).
Sensitivity Analysis
After excluding the participants with prevalent lung diseases, the cross-sectional associations were slightly attenuated (Supplementary Table 3). The same longitudinal analyses were repeated among the participants without prevalent lung diseases (Supplementary Figure 4). The similar mean estimates were observed in these sensitivity analyses.
Discussion
Our study has documented the cross-sectional and longitudinal associations of smoking exposure and lung function in a general male population in China. Compared with never-smokers, we found that ever-smokers had a worse lung function. Current smokers, if not quit, would have an accelerated decline of lung function than former and never smokers. Furthermore, smokers with comorbid conditions, such as hypertension, elevated triglycerides and elevated total cholesterol, should raise more concerns about their lung health.
Evidence of significant decline of lung function in smokers has been present (21, 22). The unadjusted mean decline in FEV1 in healthy male never-smokers in our study were similar to that in the European Community Respiratory Health Survey (22). In our cross-sectional study, former smokers had a worse lung function than never smokers, even than current smokers. An older age, with more comorbid conditions including hypertension, diabetes, and lung diseases in those former smokers may account for this phenomenon. In the longitudinal assessment, current cigarette smokers showed a more rapid decline in lung function than never-smokers and former smokers. Greater pack-years and cigarette consumptions have been associated with accelerated lung function decline in our study, which was consistent with previous study (23). Several previous studies have indicated that smoking cessation has a beneficial effect on FEV1 decline (12, 21, 24). Our findings showed that although the former smokers had worse lung function at baseline, their annual declines in lung function were approximately identical to those of never-smokers during the follow-up. All the results above reinforce the importance of smoking cessation.
Cigarette smoking leads to numerous pulmonary and systemic immunological changes (25). Previous studies have indicated that smoking increases the number of macrophages, neutrophils, eosinophils, and mast cells in the lung, and decreases the number of airway dendritic cells, and alters macrophage and neutrophil function (26, 27). These pathways of inflammation and immunity making the lung dysregulation have been observed to be associated with smoking-related lung function decline. Additionally, smoking can decelerate the lung function along with epigenetic alterations (28), airway hyper-responsiveness (29), mucous hypersecretion (30), and altered airway dimensions (31).
One strength of the present study was that we conducted two sub-studies to evaluate the association of smoking exposure and lung function among healthy subjects in China. In addition, the dynamic data of smoking status and lung function can be collected during the follow-up. However, several limitations of this study should not be ignored. Firstly, the sample size of participant received two or more spirometry exams was relatively short. Due to lack of standard questionnaire, part of the participants did not report the detailed information of smoking exposure such as duration of cessation, cigarette consumptions. Secondly, China is both the world's largest producer and consumer of tobacco products, with 52.9% of men and 2.4% of women being current smokers in 2010 (3, 4, 6). Regarding this situation, we only restricted male subjects to samples in this study. The number of former smokers was significantly less than never-smokers and current smokers. Thirdly, despite the large sample size, the included participants were limited in the single center. Further robust epidemiological evidence and functional study is urgently needed to better understand the biological mechanism of smoking exposure on lung function. Moreover, some potential confounders, such as physical activities, exercises and second-hand smoke exposure, cannot be collected.
In 2016, President Xi Jinping announced the Healthy China (HC2030) blueprint. According to the blueprint, a target to reduce the smoking rate among people ≥15 years of age to 20% by 2030 from the current 27.7% has been set (17, 32). To achieve this goal, more and more current smokers should participate in quitting smoking. Our data suggest that smoking cessation can slow the lung function decline even if the initial state of lung function is poor. It is essential for current smokers to quit smoking as soon as possible, especially for those with comorbidities.
Conclusion
Our results therefore reinforce the view that acceleration of decline in lung function must be added to the long list of negative health consequences of smoking and that smoking cessation is the most effective means of harm reduction. Our findings about the harms of current smoking also raise concerns about lung health, especially for those with comorbid conditions, which can further encourage people to quit smoking.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Data of the present research is available from the corresponding author on reasonable request. Requests to access these datasets should be directed to OTA2OTIxNTMyQHFxLmNvbQ==.
Ethics Statement
The approval of this study was obtained from Ethics Committees at Huadong Sanatorium (No. 2020-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XJ: full access to all of the data in the study, takes responsibility for the integrity of the data, and the accuracy of the data analysis. TT and CS: concept and design and obtain funding. XJ and RQ: acquisition of data. TT and YD: drafting of the manuscript and statistical analysis. CS: critical revision of the manuscript for important intellectual content. CY and XX: administrative, technical, or material support. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Nature Science Foundation of China (81903382); China Postdoctoral Science Foundation (General Program, 2019M651900); the Nature Science Foundation of Jiangsu Province (BK20190652); the Graduate Research and Innovation Program of Jiangsu Province (KYCX20_1413).
Acknowledgments
The authors thank the patients and the supporting staff in this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.843162/full#supplementary-material
References
1. Zhao L, Song Y, Xiao L, Palipudi K, Asma S. Factors influencing quit attempts among male daily smokers in China. Prev Med. (2015) 81:361–6. doi: 10.1016/j.ypmed.2015.09.020
2. World Health Organization. WHO Report on the Global Tobacco Epidemic. Geneva, Switzerland (Published July 25, 2019). Available online at: https://www.who.int/tobacco/global_report/en/.
3. Flenaugh EL. Tobacco smoking in China: a pulmonary health crisis. Curr Opin Pulm Med. (2019) 25:188–91. doi: 10.1097/MCP.0000000000000556
4. Zheng Y, Ji Y, Dong H, Chang C. The prevalence of smoking, second-hand smoke exposure, and knowledge of the health hazards of smoking among internal migrants in 12 provinces in China: a cross-sectional analysis. BMC Public Health. (2018) 18:655. doi: 10.1186/s12889-018-5549-8
5. Chinese Center for Disease Control and Prevention. Global Adult Tobacco Survey (GATS) China 2010 Country Report. Beijing: China Sanxia Press(Published 2011. pp. 7–8). Available online at: www.notc.org.cn/newjcpg/201304/W020121108628365808856.pdf
6. Liu Y, Song H, Wang T, Wang T, Yang H, Gong J, et al. Determinants of tobacco smoking among rural-to-urban migrant workers: a cross-sectional survey in Shanghai. BMC Public Health. (2015) 15:131. doi: 10.1186/s12889-015-1361-x
7. Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. (2015) 386:1447–56. doi: 10.1016/S0140-6736(15)00340-2
8. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. (2005) 127:1952–9. doi: 10.1378/chest.127.6.1952
9. Lee PN, Fry JS. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med. (2010) 8:84. doi: 10.1186/1741-7015-8-84
10. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. (2005) 26:948–68. doi: 10.1183/09031936.05.00035205
11. Islam SS, Schottenfeld D. Declining FEV1 and chronic productive cough in cigarette smokers: a 25-year prospective study of lung cancer incidence in Tecumseh, Michigan. Cancer Epidemiol Biomarkers Prev. (1994) 3:289–98.
12. Omori H, Nonami Y, Morimoto Y. Effect of smoking on FEV1 decline in a cross-sectional and longitudinal study of a large cohort of Japanese males. Respirology. (2005) 10:464–9. doi: 10.1111/j.1440-1843.2005.00727.x
13. Toren K, Bake B, Olin AC, Engstrom G, Blomberg A, Vikgren J, et al. Measures of bronchodilator response of FEV1, FVC and SVC in a Swedish general population sample aged 50-64 years, the SCAPIS Pilot Study. Int J Chron Obstruct Pulmon Dis. (2017) 12:973–80. doi: 10.2147/COPD.S127336
14. Chou KT, Su KC, Hsiao YH, Huang SF, Ko HK, Tseng CM, et al. Post-bronchodilator Reversibility of FEV1 and Eosinophilic Airway Inflammation in COPD. Arch Bronconeumol. (2017) 53:547–53. doi: 10.1016/j.arbres.2017.01.014
15. Wain LV, Shrine N, Miller S, Jackson VE, Ntalla I, Soler Artigas M, et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med. (2015) 3:769–81. doi: 10.1016/S2213-2600(15)00283-0
16. Wang MP, Li WHC, Suen YN, Cheung KC, Lau OS, Lam TH, et al. Association between employer's knowledge and attitude towards smoking cessation and voluntary promotion in workplace: a survey study. Tob Induc Dis. (2017) 15:44. doi: 10.1186/s12971-017-0149-4
17. Tan X, Liu X, Shao H. Healthy China 2030: a vision for health care. Value Health Reg Issues. (2017) 12:112–4. doi: 10.1016/j.vhri.2017.04.001
18. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
19. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. (2012) 40:1324–43. doi: 10.1183/09031936.00080312
20. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. (1999) 159:179–87. doi: 10.1164/ajrccm.159.1.9712108
21. Oelsner EC, Balte PP, Bhatt SP, Cassano PA, Couper D, Folsom AR, et al. Lung function decline in former smokers and low-intensity current smokers: a secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir Med. (2020) 8:34–44. doi: 10.1016/S2213-2600(19)30276-0
22. Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Anto JM, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. (2005) 365:1629–35. doi: 10.1016/S0140-6736(05)66511-7
23. Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med. (2017) 196:1021–30. doi: 10.1164/rccm.201703-0506OC
24. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. (1994) 272:1497–505. doi: 10.1001/jama.1994.03520190043033
25. Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. (2014) 106:dju294. doi: 10.1093/jnci/dju294
26. Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res. (2008) 57:497–503. doi: 10.1007/s00011-008-8078-6
27. Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. (2002) 2:372–7. doi: 10.1038/nri803
28. Belinsky SA, Palmisano WA, Gilliland FD, Crooks LA, Divine KK, Winters SA, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. (2002) 62:2370–7. doi: 10.1016/S0165-4608(01)00623-9
29. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. (1998) 339:1194–200. doi: 10.1056/NEJM199810223391703
30. Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. (1996) 153:1530–5. doi: 10.1164/ajrccm.153.5.8630597
31. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. (2004) 350:2645–53. doi: 10.1056/NEJMoa032158
32. Liu W. Health sector, the next big industry. Chinadaily. Available online at: https://www.chinadaily.com.cn/china/2016-10/26/content_27181681.htm (accessed: October 26, 2016).
Keywords: smoking cessation, FEV1, FVC, FEV1/FVC, health examination
Citation: Tian T, Jiang X, Qin R, Ding Y, Yu C, Xu X and Song C (2023) Effect of Smoking on Lung Function Decline in a Retrospective Study of a Health Examination Population in Chinese Males. Front. Med. 9:843162. doi: 10.3389/fmed.2022.843162
Received: 24 December 2021; Accepted: 11 April 2022;
Published: 06 January 2023.
Edited by:
Shu-Chuan Ho, Taipei Medical University, TaiwanReviewed by:
Gunnar N. Hillerdal, Karolinska University Hospital, SwedenPai-Chien Chou, Taipei Medical University Hospital, Taiwan
Copyright © 2023 Tian, Jiang, Qin, Ding, Yu, Xu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueqin Jiang, OTA2OTIxNTMyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ting Tian
Ting Tian Xueqin Jiang
Xueqin Jiang Rujie Qin
Rujie Qin Yuqing Ding
Yuqing Ding Chengxiao Yu6,7
Chengxiao Yu6,7 Ci Song
Ci Song