- 1Department of Urology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Urology, Baoji Central Hospital, Baoji, China
- 3Department of Pathology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 4Department of Radiology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Prostate lymphoma (PL) is rarely observed and may be concurrently presented with prostate adenocarcinoma. Moreover, the appearance of PL on conventional imaging is similar with prostate adenocarcinoma. Thus, most of PL is diagnosed through prostate biopsy, or accidentally found in the specimens of surgery. Prostate-specific membrane antigen (PSMA) PET/CT has improved the management of prostate adenocarcinoma. While, the question regarding whether it benefits the discovery of the characteristics of PL is unknown. A 32-year-old man presented with worsening dysuria for 1 month, and the prostate-specific antigen (PSA) concentration was normal. While the pelvic MRI showed a mass in the prostate and multiple enlarged lymph nodes in the bilateral inguinal area. Then, the diagnosis of prostate adenocarcinoma was considered, but the serum PSA was normal and he was younger than most patients. So, 18F-PSMA PET/CT was then performed to further reveal the characteristics of the lesion and guide biopsy. However, there was no abnormal PSMA uptake in the lesion of the prostate and lymph nodes of the pelvic cavity and bilateral inguinal area. These lesions presented with increased glucose metabolism on fluorodeoxyglucose (FDG) PET/CT, and the prostate biopsy was then performed. PL was confirmed based on the results of the histopathologic examination, and the patient subsequently received systemic chemotherapy plus radiotherapy. Fortunately, the symptoms and the lesions completely disappeared after radiotherapy. The clinical symptoms of PL are atypical, and PL and adenocarcinoma may be concurrently presented. Moreover, distinguishing PL from prostate adenocarcinoma based on the appearance of conventional imaging is difficult. As opposed to prostate adenocarcinoma, a high FDG-avidity and low PSMA uptake by lymphoma either in the prostate or metastases are seen. So, PSMA PET/CT combined with FDG PET/CT can non-invasively identify the characteristics and origin of PL.
Introduction
Prostate lymphoma (PL) is rarely observed, accounting for 0.09% of all prostate neoplasms and 0.1% of all non-Hodgkin lymphomas (1). Because its clinical symptoms usually manifest as lower urinary tract symptoms, most patients are initially misdiagnosed as prostatitis or benign prostate hyperplasia and miss the optimal treatment time. Moreover, PL and adenocarcinoma may be concurrently present, and the appearance of PL on conventional imaging (i.e., MRI and CT) is similar with prostate adenocarcinoma (2, 3). Thus, most PL is diagnosed though prostate biopsy, or accidentally found in the specimens of transurethral resection of prostate or radical prostatectomy.
Prostate-specific membrane antigen (PSMA) is a type II transmembrane protein that is overexpressed on the cell membrane of nearly all prostatic cancer cells (4). Thus, PSMA is a promising and specific target for prostate adenocarcinoma imaging. In the past years, PSMA PET/CT has extremely improved the management of prostate adenocarcinoma when compared with conventional imaging, especially in the aspect of detecting metastatic spread and micrometastases (5, 6). Furthermore, as a molecular imaging examination, the expression level of PSMA on PET/CT is positively correlated with tumor stage and grade (7), which could help urologists identify patients who may need more intensive treatment and tailor treatment regimes. However, the appearance of PL on PSMA PET/CT and whether PSMA PET/CT benefits the discovery of the characteristics of PL are still unknown. Herein, we reported a case of a young man with PL who received both 18F-PSMA and 18F-fluorodeoxyglucose (FDG) PET/CT before diagnosis.
Case Description
A 32-year-old man presented to our clinic with a 1-month history of worsening dysuria, without any fevers, night sweats, or weight loss. His past medical history included a 10-year history of smoking. His family history was unremarkable. The serum blood count was normal and the components of blood cell counts were as follows: white blood cell (WBC) count: 5.76 × 109 cells/L (normal range: 3.5–9.5 × 109 cells/L); red blood cell (RBC) count: 4.60 × 1012 cells/L (normal range: 3.8–5.1 × 1012 cells/L); platelet (PLT) count: 295 × 109 cells/L (normal range: 125–350 × 109 cells/L); neutrophil% (NEUT%): 82.4% (normal range: 40–75%); and lymphocyte% (LYMPH%): 10.8% (normal range: 20–50%). Furthermore, the serum prostate-specific antigen (PSA) concentration was 0.7 ng/ml (normal range <4 ng/mL). However, a digital rectal exam (DRE) showed a firm and enlarged prostate with normal consistency without nodule or induration, while there was not palpable lymphadenopathy either in the Troisier or inguinal areas on physical examination. Subsequently, multiparametric MRI (mp-MRI) was performed and revealed a heterogeneous mass in the right anterior lobe of the prostate. Moreover, the lesion was also extensively invading the bladder and the bilateral seminal vesicles. Meanwhile, multiple enlarged lymph nodes in the pelvic cavity and bilateral inguinal area were observed. Based on the results of imaging and physical examination, the diagnosis of prostate adenocarcinoma was subsequently considered. However, the serum PSA was normal and enlarged lymph nodes were present in the bilateral inguinal area, which is not the usual lymph node metastasis of prostate adenocarcinoma. So, 18F-PSMA PET/CT was performed to further reveal the characteristics of the lesion and guide biopsy after an informed consent form was obtained from the patient. However, there was no abnormal trace uptake in the lesion of the prostate and lymph nodes of the pelvic cavity and bilateral inguinal area on 18F-PSMA PET/CT (Figure 1A). Besides PSMA, FDG is another tracer used for PET/CT imaging examination to reveal the characteristics of prostate malignancies and obtain accurate disease staging. Therefore, after consulting with the patient, FDG PET/CT was freely performed to identify the metabolism of the prostate mass and enlarged lymph nodes. 18F-FDG PET/CT showed foci with increased glucose metabolism in the prostate and bilateral seminal vesicles. Furthermore, multiple lymph nodes in the hilum, retroperitoneum, and pelvic cavity presented with increased glucose metabolism to varying degrees, while there was no increased glucose metabolism in the liver, spleen, and bone (Figure 1B). Then, a prostate biopsy was performed and showed prostate parenchyma infiltrated by atypical medium-sized to large-sized lymphoid cells arranged in diffuse sheets. These lymphoid cells exhibited a small to moderate amount of eosinophilic cytoplasm and nuclei with irregular contours, which dispersed chromatin and occasional prominent nucleoli. As shown in Figure 2, the tumor cells were positive for leukocyte common antigen (LCA), CD20, CD19, and CD79a but negative for PSA, PSMA, androgen receptor (AR), Bcl6, Cyclin D1, CD5, and MUM1. Thus, based on the above results of the examination, diffuse large B-cell lymphoma (DLBCL) of the prostate was then diagnosed (stage IV according to the TNM classification).
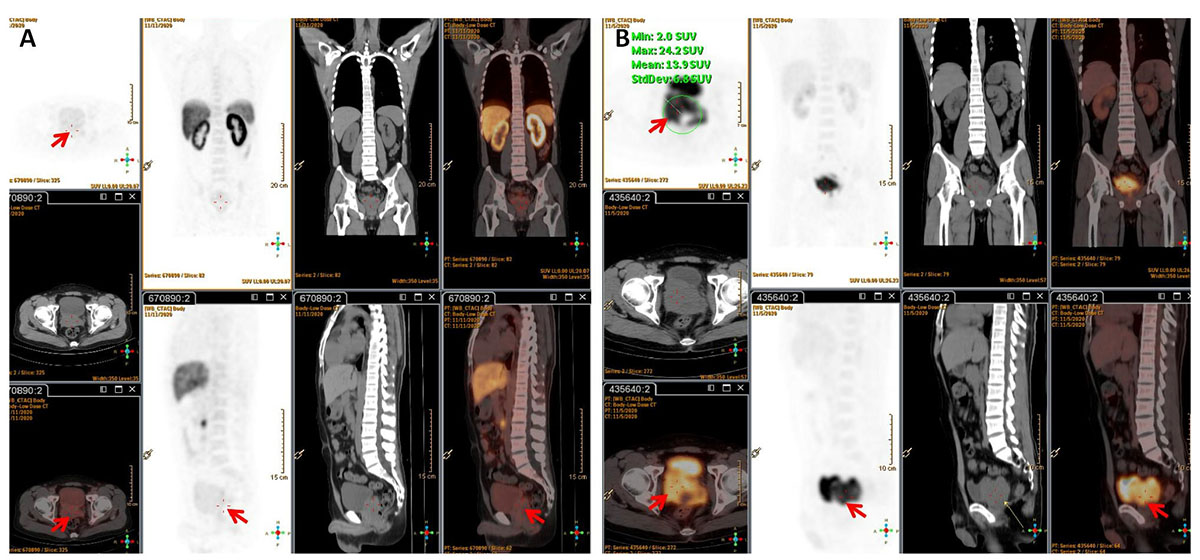
Figure 1. (A) 18F-PSMA PET/CT showed no trace PSMA uptake in the lesion (red arrow). (B) 18F-FDG PET/CT revealed foci with increased glucose metabolism in the prostate and bilateral seminal vesicles. Multiple enlarged lymph nodes with varying degrees of increased glucose metabolism were observed in the hilum, retroperitoneum, and pelvic cavity.
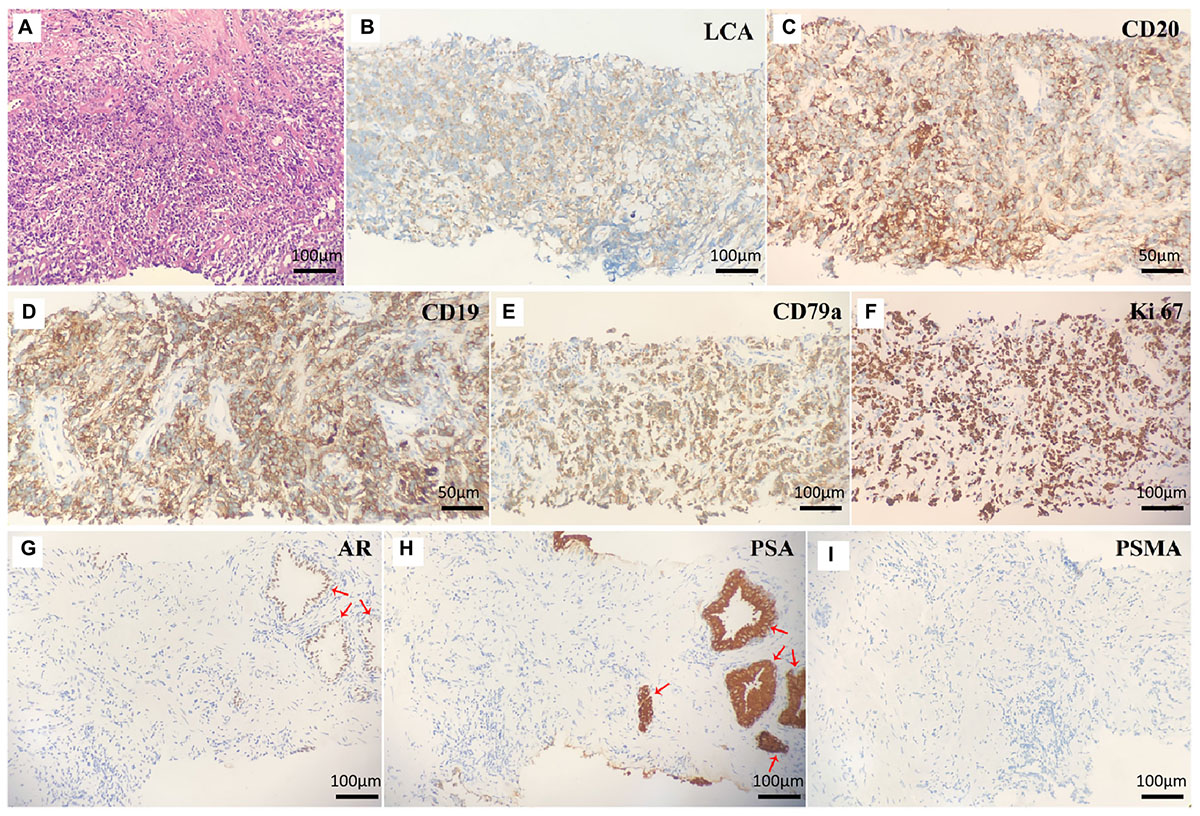
Figure 2. (A) Hematoxylin and eosin staining of prostate lesions showed diffuse large B-cell lymphoma. The eight micrographs illustrate (B) LCA+, (C) CD20+, (D) CD19+, (E) CD79a+, (F) Ki-67+, (G) AR–, (H) PSA–, and (I) PSMA– tumor cells. Normal prostate cells were positive for AR (G) and PSA (H) (red arrow).
Later, the patient was referred to the Department of Hematology and scheduled for bone marrow puncture in preparation for chemotherapy. The bone marrow cytology examination was normal, and fluorescence in situ hybridization revealed normal results for the B-cell lymphoma (BCL)-2, BCL-6, and cellular-myelocytomatosis viral oncogene (C-MYC) probes, ruling out “double-hit” or “three-hit” lymphoma. The patient chose to receive systemic chemotherapy (R-CHOP) owing to his personal preference, which consisted of a total of six cycles of three different drug regimens, including dexamethasone, cyclophosphamide, rituximab, vincristine, etoposide, and cytarabine. The patient independently completed the International Prostate Symptom Score (IPSS) patient symptom scale from before starting to after completing the treatment. Prior to chemotherapy, the patient had an IPSS score of 30. Dysuria was diminished with an IPSS score of 7 at the end of the third cycle of chemotherapy, and local radiotherapy (40 Gy, 25 cycles) was then preformed. 18F-FDG PET/CT at 3 and 6 months after radiotherapy demonstrated a complete metabolic response where the tumor mass of the prostate and enlarged lymph nodes disappeared (Figure 3). A CT scan and laboratory examination (i.e., blood routine examination, uric acid, and lactate dehydrogenase) were performed every 3 months during the follow-up period, and there was no sign of recurrence as of the 243th day after completing RT.
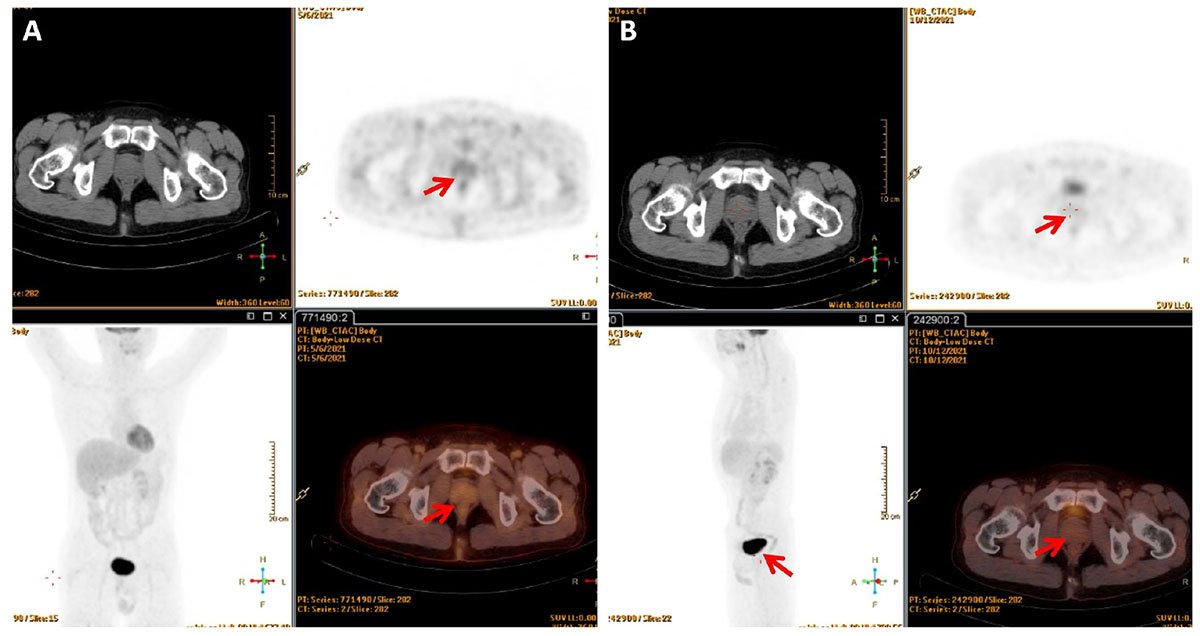
Figure 3. 18F-FDG PET/CT showed no abnormal increased glucose metabolism in the lesion (A) 3 months after radiotherapy; (B) 6 months after radiotherapy.
Discussion
The clinical symptoms of PL are atypical. After searching PubMed-indexed biomedical journals, we identified 20 individual cases with PL reported between January 2017 and November 2021. As shown in Table 1, it usually manifests with lower urinary tract symptoms (i.e., dysuria, urine frequency, and urgency) and, in some cases, hematuria. Moreover, the serum PSA is usually within 4 ng/ml, which was consistent with previously reported (8). Thus, PL is difficult to distinguish from other prostatic diseases, such as prostatitis and benign prostate hyperplasia before biopsy. The present patient presented to our clinic with a 1-month history of worsening dysuria, and his serum PSA level was normal. Thus, he was initially suspected to have benign prostate diseases. However, a DRE showed prostate hyperplasia, disappearance of the central fissure, and a hard consistency without tenderness, which are consistent with reports of PL in the literature (9). Furthermore, mp-MRI revealed the prostate mass and the enlarged lymph nodes of the bilateral inguinal area, which is not the common metastasis site of prostate adenocarcinoma. So, 18F-PSMA and FDG PET/CT were subsequently performed to identify the characteristics of the abnormally enlarged lymph nodes and the prostate lesion, and lymphoma was then confirmed though biopsy. Therefore, even if the patient’s PSA level is relatively low, when the DRE is abnormal and enlarged lymph nodes of the pelvic cavity are present, the possibility of lymphoma should not be ignored, and PSMA PET/CT combined with FDG PET/CT before biopsy may be helpful.
Prostate lymphoma tends to occur in older patients, with a mean age of 62 years, while secondary lymphoma occurs in relatively young people (10). As shown in Table 1, the mean age of patients with secondary PL was 59.8 ± 18.9, which was younger than patients with primary PL (59.8 ± 18.9 vs. 66.2 ± 8.8). Moreover, there are three conditions in which PL is determined to be primary (8): (1) presentation with symptoms attributable to prostatic enlargement; (2) involvement of the prostate gland predominantly, with or without involvement of adjacent tissue; and (3) absence of involvement of the liver, spleen, lymph nodes, or peripheral blood within 1 month of diagnosis of prostatic involvement. In the past, a needle biopsy was the standard method to identify whether extra-prostate tissue involvement was present, while it may lead to some complications (i.e., bleeding and infection). Recently, de Souza et al. pointed out that lymphoma may present as PSMA uptake after reviewing the data of 10 patients with different histological subtypes of lymphomas, and they subsequently found that the intensity of PSMA uptake was generally lower than FDG (11). Furthermore, several published case reports have also found that the PSMA expression of lymphoma was extremely low, or even had no expression (12–14). Thus, we deemed that the expression of PSMA on lymphoma was lower than adenocarcinoma, while the expression of FDG was higher. In our report, PSMA PET/CT was negative for lesions of the prostate gland and lymph nodes of the pelvic cavity and bilateral inguinal area, but FDG PET/CT showed higher glucose metabolism of lesions in the prostate gland with infiltration of the bladder wall and seminal vesicle. Moreover, multiple lymph nodes with increased glucose metabolism were observed, and the immunostaining further demonstrated that the lymphoma cells were negative for AR, PSA, and PSMA. Thus, our case did not satisfy these criteria based on the results of PSMA and FDG PET/CT and should be classified as secondary PL.
Prostate lymphoma is an uncommon entity in surgical practice and its diagnosis often poses considerable difficulty as it often mimics or is concurrent with carcinoma (15–17). Ultrasound, CT, and mp-MRI were the most commonly used imaging methods to detect PL and guide biopsy in the past. PSMA is a transmembrane protein that is considerably overexpressed in most prostate adenocarcinoma tumor cells (18). PSMA PET/CT was originally used to assess biochemical recurrence, and its clinical use has been recently extended to detection, restaging, and therapy response assessment of prostate adenocarcinoma. However, the diagnostic value of PSMA PET/CT in PL remains unclear. Miceli et al. reported a 38-year-old man with concomitant prostate adenocarcinoma and mediastinal lymphoma, and they found a high FDG-avidity and low PSMA uptake by mediastinal lymphadenopathies as opposed to the prostate adenocarcinoma (13). To our knowledge, this is the first report to clarify the appearance of PL on PSMA PET/CT and FDG PET/CT. Furthermore, we also found that PL was negative for PSMA, AR, and PSA, which also indicates that malignant neoplasms develop from lymph nodes or lymphatic tissues. Although PSMA had normal uptake in PL, PSMA PET/CT could non-invasively assist urologists in identifying the origin of lesions, revealing the characteristics of disease. Thus, we deduced that the combination of PSMA PET/CT and FDG PET/CT will benefit the discovery of the characteristics of PL. Because our report is a single case, further studies are needed to confirm this conclusion.
Conclusion
In summary, secondary PL mostly occurs in relatively young patients, and its clinical symptoms are atypical. In addition to a DRE, PSMA PET/CT combined with FDG PET/CT may non-invasively help urologists identify the characteristics and origin of PL. Furthermore, systemic chemotherapy combined with local radiotherapy may be an optimal treatment regime to achieve more complete remission.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this case report (including all data and images).
Author Contributions
FJ: data and material collection, literature search, and manuscript writing. JF: date collection, literature search, and manuscript writing. HL: literature search, pathological specimens review, and manuscript writing. XD: PET/CT data collection and manuscript writing. DH: project development and manuscript editing. KW: project development, operation performance, and manuscript editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Rong Wang, Department of Radiology, for her help with the preparation of the radiological materials. We also thank Guanjun Zhang, Department of Pathology, for his help in this manuscript.
References
1. Warrick JI, Owens SR, Tomlins SA. Diffuse large B-cell lymphoma of the prostate. Arch Pathol Lab Med. (2014) 138:1286–9. doi: 10.5858/arpa.2014-0276-CC
2. Terris MK, Freiha FS. Transrectal ultrasound appearance of hematolymphoid malignancies involving the prostate. Urology. (1998) 51:339–41. doi: 10.1016/s0090-4295(97)00504-9
3. Taleb A, Ismaili N, Belbaraka R, Bensouda A, Elghissassi I, Elmesbahi O, et al. Primary lymphoma of the prostate treated with rituximab-based chemotherapy: a case report and review of the literature. Cases J. (2009) 2:8875. doi: 10.1186/1757-1626-0002-0000008875
4. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. (2016) 13:226–35. doi: 10.1038/nrurol.2016.26
5. Wang Z, Zheng A, Li Y, Dong W, Liu X, Yuan W, et al. (18)F-PSMA-1007 PET/CT performance on risk stratification discrimination and distant metastases prediction in newly diagnosed prostate cancer. Front Oncol. (2021) 11:759053. doi: 10.3389/fonc.2021.759053
6. Chang SS, Gaudin PB, Reuter VE, Heston WD. Prostate-specific membrane antigen: present and future applications. Urology. (2000) 55:622–9. doi: 10.1016/s0090-4295(99)00600-7
7. Jiao J, Kang F, Zhang J, Quan Z, Wen W, Zhao X, et al. Establishment and prospective validation of an SUVmax cutoff value to discriminate clinically significant prostate cancer from benign prostate diseases in patients with suspected prostate cancer by (68)Ga-PSMA PET/CT: a real-world study. Theranostics. (2021) 11:8396–411. doi: 10.7150/thno.58140
8. Bostwick DG, Iczkowski KA, Amin MB, Discigil G, Osborne B. Malignant lymphoma involving the prostate: aeport of 62 cases. Cancer-Am Cancer Soc. (1998) 83:732–8. doi: 10.1002/(sici)1097-0142(19980815)83:43.0.co;2-t
9. Antunes AA, Dall’Oglio M, Srougi M. Primary lymphoma of the prostate: a rare cause of urinary obstruction. Int Braz J Urol. (2004) 30:410–2. doi: 10.1590/s1677-55382004000500011
10. Alvarez CA, Rodriguez BI, Perez LA. Primary diffuse large B-cell lymphoma of the prostate in a young patient. Int Braz J Urol. (2006) 32:64–5. doi: 10.1590/s1677-55382006000100010
11. de Souza S, Tobar N, Frasson F, Perini EA, de Souza CA, Delamain MT, et al. Head-to-head comparison between 68Ga-PSMA and 18F-FDG-PET/CT in lymphomas: a preliminary analysis. Nucl Med Commun. (2021) 42:1355–60. doi: 10.1097/MNM.0000000000001465
12. Hanna F, Prakash A, Allan E, Khalafallah AA. Successful treatment of concomitant metastatic prostate cancer and B-cell non-hodgkin’s lymphoma with R-EPOCH chemotherapy regimen and antiandrogen therapy. BMJ Case Rep. (2018) 2018:bcr2017223637. doi: 10.1136/bcr-2017-223637
13. Miceli A, Riondato M, D’Amico F, Donegani MI, Piol N, Mora M, et al. Concomitant prostate cancer and hodgkin lymphoma: a differential diagnosis guided by a combined 68Ga-PSMA-11 and 18F-FDG PET/CT approach. Medicina (Kaunas). (2021) 57:975. doi: 10.3390/medicina57090975
14. Dendl K, Merkel A, Kratochwil C, Choyke PL, Kleist C, Cardinale J, et al. Positive multifocal PSMA PET/CT in a patient with prostate cancer and follicular lymphoma. Clin Nucl Med. (2021) 47:e47–8. doi: 10.1097/RLU.0000000000003828
15. Tlili G, Ammar H, Majdoub W, Dziri S, Farhat W, Acacha E, et al. Incidental chronic lymphocytic leukemia diagnosed following radical prostatectomy for prostate cancer: a case report. Ann Med Surg (Lond). (2021) 68:102516. doi: 10.1016/j.amsu.2021.102516
16. Wong TH, Ambinder D, Flusberg M, Shakil F, Phillips JL, Wong NC. Small lymphocytic lymphoma of the prostate mimicking a PIRADS 5 lesion that resolved after systemic treatment. Urol Case Rep. (2021) 39:101775. doi: 10.1016/j.eucr.2021.101775
17. Karademir B, Kisa E, Ozbilen MH, Bildirici C, Karabacakoglu B, Suelozgen T, et al. Unexpected hematologic malignancies after prostatectomy: case report and literature review. Urologia. (2021) 88:382–5. doi: 10.1177/0391560321993596
18. Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. (1999) 59:3192–8.
19. Rallabandi HB, Thirukovela J, Swain M, Meeramira D, Gowrishankar S. Intravascular large B cell lymphoma of prostate, a rare entity. Indian J Pathol Microbiol. (2021) 64:575–8. doi: 10.4103/IJPM.IJPM_324_20
20. Nerli RB, Sanikop A, Sharma M, Patel P, Kangale R, Patil S, et al. Primary non-hodgkin lymphoma of prostate: a case report. Indian J Surg Oncol. (2020) 11:274–7. doi: 10.1007/s13193-020-01190-8
21. Nusrat A, Nazim SM. Primary diffuse large B cell lymphoma (DLBCL) of the prostate presenting with lower urinary tract symptoms (LUTS). BMJ Case Rep. (2020) 13:e236280. doi: 10.1136/bcr-2020-236280
22. Derigs M, Pehl A, Riera-Knorrenschild J, Hofmann R, Hegele A. Burkitt’s lymphoma of the prostate presenting as acute urinary retention: a case report. Bmc Urol. (2020) 20:53. doi: 10.1186/s12894-020-00616-3
23. Mansbridge MM, Parker JR, Francisco S, McClintock ST. Diagnosis of monoclonal B cell lymphocytosis (MBL) through transurethral resection of prostate for obstructive lower urinary tract symptoms. Urol Case Rep. (2020) 29:101086. doi: 10.1016/j.eucr.2019.101086
24. El-Taji O, Omer A, Al-Mitwalli AA, Agarwal S, Sharma A, Vasdev N. Incidental lymphoplasmacytic lymphoma diagnosed following robotic-assisted laparoscopic prostatectomy for prostate cancer. Curr Urol. (2019) 13:166–8. doi: 10.1159/000499275
25. Wang K, Wang N, Sun J, Fan Y, Chen L. Primary prostate lymphoma: a case report and literature review. Int J Immunopathol Pharmacol. (2019) 33:1681100465. doi: 10.1177/2058738419863217
26. Galante A, Asirvatham RJ, Yeung LL. Treatment-refractory non-hodgkin lymphoma of the prostate: a case report and review of the literature. Urol Case Rep. (2019) 24:100867. doi: 10.1016/j.eucr.2019.100867
27. Jafari A, Mofid B, Tabibi A, Kowsari F. Primary prostate lymphoma managed with combined modality treatment: a case report. Urol J. (2019) 16:412–4. doi: 10.22037/uj.v0i0.4294
28. Zhu F, Pan H, Xiao Y, Li Q, Liu T, Liu X, et al. A case report of primary prostate intravascular large B cell lymphoma presenting as prostatic hyperplasia. Medicine (Baltimore). (2019) 98:e18384. doi: 10.1097/MD.0000000000018384
29. Yasuoka S, Kimura G, Toyama Y, Moriya K, Takahashi K, Matsuoka R, et al. A case of primary malignant lymphoma of the prostate gland presenting as right lower back pain and dysuria. J Nippon Med Sch. (2018) 85:236–40. doi: 10.1272/jnms.JNMS.2018_85-37
30. Martin OD, Wadskier LA, Quiroz Y, Bravo HP, Cacciamani G, Umana P, et al. Primary non-hodgkin lymphoma of the prostate: a case report. Ecancermedicalscience. (2017) 11:789. doi: 10.3332/ecancer.2017.789
31. Tamang T, Singh P, Garellek J, Malhotra S, Chandra AB, Solomon W. Prostatic lymphoma masquerading as urinary retention and hematuria with review of literature. World J Oncol. (2017) 8:132–5. doi: 10.14740/wjon1055w
32. Milburn PA, Cable CT, Trevathan S, El TM. Mantle cell lymphoma of the prostate gland treated with holmium laser enucleation. Proc (Bayl Univ Med Cent). (2017) 30:338–9. doi: 10.1080/08998280.2017.11929640
33. Hashemzadeh S, Farrokhi F, Hozhabrossadaty A, Ghafarzadegan K, Ashraf H. A case of recurrent hematuria in primary prostatic low grade mucosa associated lymphoid tissue. J Nephropathol. (2017) 6:49–52. doi: 10.15171/jnp.2017.08
Keywords: prostate lymphoma, PSMA, PET/CT, clinical symptom, management
Citation: Jiang F, Fan J, Liang H, Duan X, He D and Wu K (2022) 18F-Prostate-Specific Membrane Antigen and 18F-Fluorodeoxyglucose PET/CT Unmasked the Characteristics of Prostate Lymphoma: A Case Report and Literature Review. Front. Med. 9:842093. doi: 10.3389/fmed.2022.842093
Received: 23 December 2021; Accepted: 21 February 2022;
Published: 06 April 2022.
Edited by:
Chander Singh Digwal, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Nguyen Minh Duc, Pham Ngoc Thach University of Medicine, VietnamGhassen Tlili, University of Sousse, Tunisia
Copyright © 2022 Jiang, Fan, Liang, Duan, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijie Wu, S2FpamllX3d1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Fan Jiang
Fan Jiang Junjie Fan
Junjie Fan Hua Liang3
Hua Liang3 Dalin He
Dalin He Kaijie Wu
Kaijie Wu