
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 24 May 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.837332
This article is part of the Research Topic Mechanisms and Novel Treatments of Pigmentary Disorders and Skin Regeneration View all 10 articles
 Xuelei Liang1†
Xuelei Liang1† Jiaying Li2†
Jiaying Li2† Yan Yan1
Yan Yan1 Yongsheng Xu1
Yongsheng Xu1 Xiujuan Wang1
Xiujuan Wang1 Haixuan Wu1
Haixuan Wu1 Yi Liu1
Yi Liu1 Linfeng Li1
Linfeng Li1 Fenglin Zhuo1*
Fenglin Zhuo1*Background: Fighting skin aging signs is one of the major challenges of the 21st century, recently, mesenchymal stem cells (MSCs) and microneedling (MN) have been applied for anti-aging. This study aims to evaluate the efficacy of the combination of MN and human umbilical cord-derived mesenchymal stem cells conditioned media (hUC-MSCs-CM) in skin brightness and rejuvenation.
Methods: Thirty volunteers with facial skin aging were recruited for the randomized, controlled split-face study. The left and right sides of the face were randomly applied with saline via MN or hUC-MSCs-CM via MN. Five sessions were performed for each volunteer at 2-week intervals. Two dermatologists evaluated the clinical improvement, in terms of skin brightness and texture. A satisfaction score based on a self-evaluation questionnaire was recorded at 2 weeks after the last treatment. The objective evaluation was recorded before the first treatment, and at 2 weeks after the last treatment.
Results: Twenty-eight volunteers with a mean (SD) age of 41 (6.54) years old completed the trial. The investigator’s assessment for skin brightness and texture, and the self-satisfaction score revealed statistically better effects in hUC-MSCs-CM -plus-MN group than in MN alone (MN saline) group. No severe side effects were reported during the whole study period. Compared to MN alone group, the objective assessment revealed significant improvements in skin brightness (reduced melanin index, ultraviolet spots, and brown spots) and skin texture (reduced wrinkles and pores, and increased skin elasticity) in hUC-MSCs-CM-plus-MN group, while there were no obvious differences in skin hydration, trans-epidermal water loss, and the erythema index.
Conclusion: The combination of hUC-MSCs-CM and MN exhibite anti-aging efficacy, and this could be used for facial rejuvenation in the future.
Skin aging is subject to both intrinsic (chronological) and extrinsic (environmental) factors, resulting in poor appearance and loss of functional capacity. Rejuvenating skin by fighting aging signs, such as wrinkles, enlarged pores, reduced resilience and irregular pigmentation, is one of the major challenges of the 21st century (1). Skin aging not only affects physiological skin function, but also has impacts on a person’s psychology and social life. As a result, several non-surgical treatments, such as oral treatments, ointments, dermabrasion, chemical peels, and laser therapy, have been developed over the past years, in order to counteract skin aging. However, these may be associated with prolonged recovery, dyspigmentation, and scarring.
Mesenchymal stem cells (MSCs) have the advantage of being easy to isolation, expansion, and multipotentiality, so they are popular in the field of skin rejuvenation therapy (2). These release several growth factors in autocrine and paracrine ways (3). There are various types of MSCs, including adipose-derived stem cells, bone marrow-derived stem cells (BMMSCs), and umbilical cord-derived mesenchymal stem cells. A recent study indicated that compared to BMMSCs-conditioned media, UC-MSCs-conditioned media (UC-MSCs-CM) can form premature adipocytes, collagen type 1 and collagen type 2 which have anti-aging effects (4).
Microneedling (MN) is a treatment widely applied for skin diseases and skin rejuvenation, due to its safety and efficacy. There are two main reasons for its medical use: (i) MN can accelerate the process of the skin’s natural healing. It penetrates the epidermis and papillary dermis (5), creating pores, and thereby triggering the skin’s repair mechanism (6). This can result in the short-term aggregation of inflammatory cells, fibroblasts proliferation, long-term remodeling, and the synthesis of collagen and elastin (5). (ii) MN can enhance the penetration of drugs (5). It is difficult for drugs to be absorbed through the skin due to the stratum corneum barrier (7). MN creates small transient holes that penetrate the stratum corneum barrier within a short period of time (8).
The present study aims to explore the synergistic effect of MN combined with hUC-MSCs-CM against aging skin. Non-invasive skin-measuring devices were used to objectively assess the changes in skin brightness and rejuvenation before and at 2 weeks after the final treatment.
A total of 30 volunteers with facial photo-aging (Fitzpatrick III 20, IV 10) were included for the present study. Two participants were withdrawn from the study due to job-related factors. The remaining 28 volunteers completed the study. These volunteers were within 35–60 years old, with a mean (SD) age of 41 (6.54) years old. The exclusion criteria included obvious inflammation, ulcer or effusion on the face, and previous skin rejuvenation treatment in the past 3 months.
The present study was a randomized controlled split-face study. The Institutional Review Board of Beijing Friendship Hospital, Capital Medical University approved the study (Approval No. 2017-P2-086-01). All volunteers were thoroughly counseled on the potential risks and benefits before they signed the informed consent form, based on the 1975 Declaration of Helsinki. The registration number in http://www.chictr.org.cn is ChiCTR-INR-17013311.
MN plus hUC-MSCs-CM (Beijing Origife Health Care Co., Ltd., China) group and MN alone group (control) were randomly allocated to the left or right face side. Each participant was assigned a number, according to the random number table created by the computer software. If the number was odd, the left side was used as the test side. If the number was even, the right side was used as the test side. To ensure allocation concealment, the doctors in MN operation wore colorful glasses which are normally used to protect the eyes from Intense pulse Light treatment in our department. While each volunteer’s eyes were covered with eight layers of sterile gauze before treatment.
Prior to treatment, the faces of all participants were cleaned using facial cleanser (Nivea, Nivea Co., Ltd., Shanghai, China) and anesthetized with 5% compound lidocaine cream (Beijing Unisplendour Pharmaceutical Co., Ltd., China). For one side, 1.0 ml of saline or hUC-MSCs-CM was painted and the remaining 1.0 ml was added locally after dermaroller MN treatment, which was performed in eight rows, with a total of 192 needles, 0.5 mm in length (Suzhou Cynour Photoelectric Technology Co., Ltd., China)., the same method was performed on the other side. The endpoint of treatment was the presence of uniform erythema over the face. Generally, 2 ml saline or hUC-MSCs-CM could be completely absorbed. The participants received five sessions of treatment at 2-week intervals for a total of 10 weeks. Table 1 presents the densities of the main growth factors derived from hUC-MSCs-CM, which were measured using the Human ProcartaPlex Growth Factor Panel (11 plex) (Cat. No. EPX110-12170-901, Invitrogen, Thermo Fisher, United States).
Prior to all measurements and assessments, the volunteers underwent an acclimatization period of 30 min under controlled standardized conditions (20°C, 40% humidity). Skin measurements were performed at baseline and 2 weeks after the 5th treatment. Cutometer ® (MPA 580, Courage and Khazaka Electronic GmbH, Cologne, Germany) was used to test the hydration, transepidermal water loss (TEWL), elasticity, melanin index (MI), and erythema index. Photographs of both sides of the face, and the scores for skin spots, wrinkles, pores, ultraviolet spots, brown spots, and red spots were recorded by VISIA ® skin analysis (Canfield Scientific, Inc., New York, NY, United States).
At 2 weeks after the last treatment, the volunteers were instructed to evaluate their satisfaction with the efficacy, taking into account the adverse effects observed on both sides of the face, using the following scale: 0 = very dissatisfied, 1 = relatively dissatisfied, 2 = slightly dissatisfied, 3 = satisfied, 4 = relatively satisfied, and 5 = very satisfied.
Two dermatologists, who were blinded to the study design and treatment, evaluated the clinical improvement, and compared it to the baseline. The assessment was based on the photographs taken by VISIA ® skin analysis on both sides of the face at baseline and 2 weeks after the last treatment. The changes in skin brightness and rejuvenation were assessed, and the improvement was graded, as follows: 1 = 0–25% (no or minimal improvement), 2 = 26–50% (moderate improvement), 3 = 51–75% (marked improvement), and 4 = 76–100% (excellent improvement).
Adverse reactions were recorded by questioning and examining the subjects during the follow-up visits.
Statistical analysis was carried out by SPSS software 19.0 (IBM Corp., Armonk, NY, United States). Paired t test was used to compare the data of objective assessment between the test side and control side at every visit. The data of subjective evaluation was analyzed with the Wilcoxon signed-rank test. The measurements of both sides at baseline and the last visit were compared by paired t tests to see the effect of treatment. The statistically significant difference was set as P < 0.05.
A total of 28 volunteers completed the study. MN plus hUC-MSCs-CM side was more satisfied than MN alone side (P < 0.05, Table 2).
The physician’s global assessment of brightness and skin texture were greater improvements following hUC-MSCs-CM plus MN, compared to MN alone (P < 0.05, Table 3). The representative images revealed the larger improvement of pores and periocular wrinkles after MN plus hUC-MSCs-CM, compared to MN alone (Figures 1, 2).
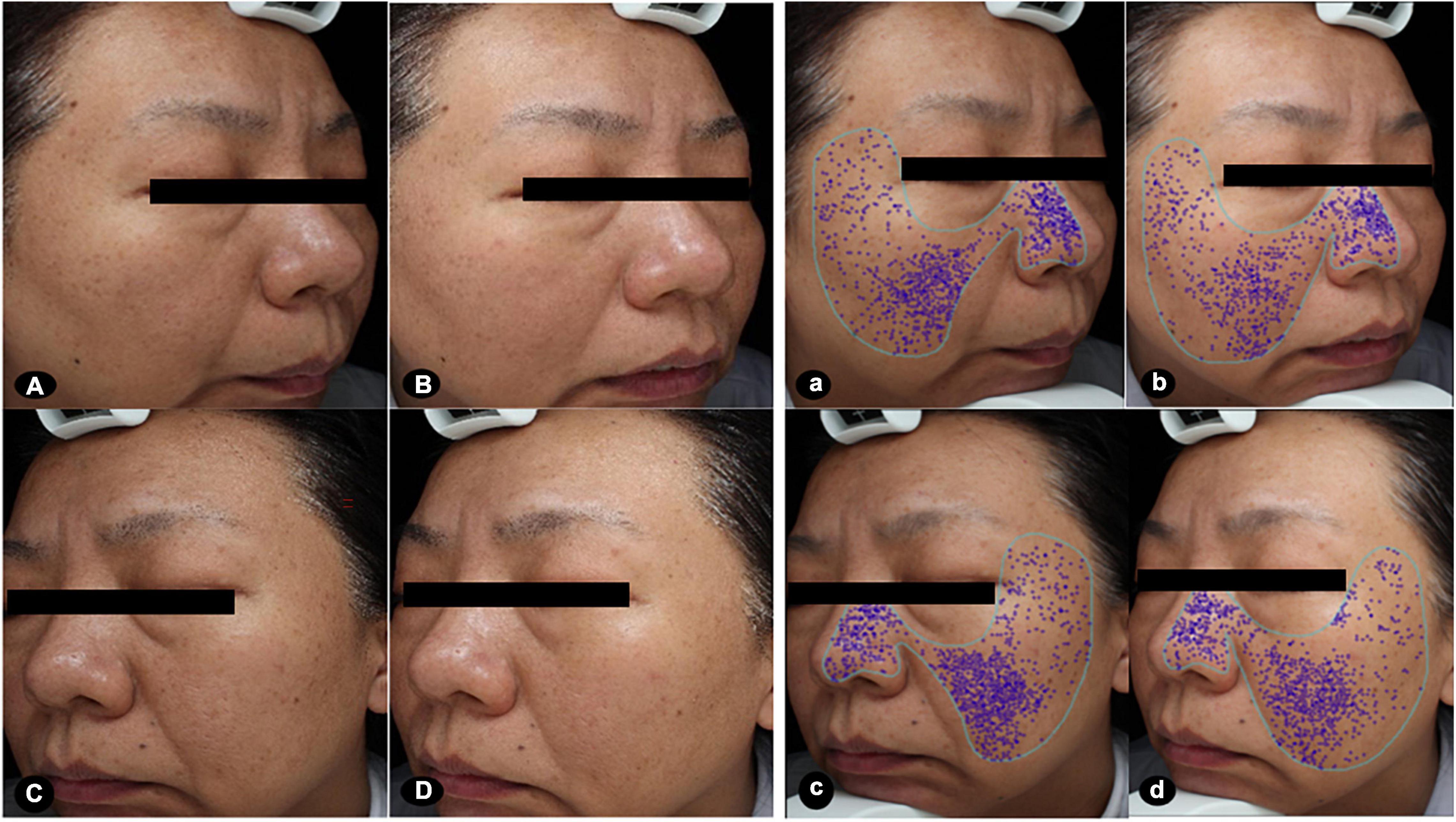
Figure 1. Images at baseline and after the treatments. The clinical images presented greater improvements in brightness and size of pores after MN plus hUC-MSCs-CM [(A,a) baseline; (B,b) after treatment], compared to MN alone [(C,c) baseline; (D,d) after treatment]. The score of pores at the side of MN via hUC-MSCs-CM decreased from 20.29 to 13.32 after treatment. While the score of pores at another side of MN alone decreased from 22.64 to 17.71 after treatment. MN: microneedling; hUC-MSCs-CM: human umbilical cord-derived mesenchymal stem cells conditioned media.
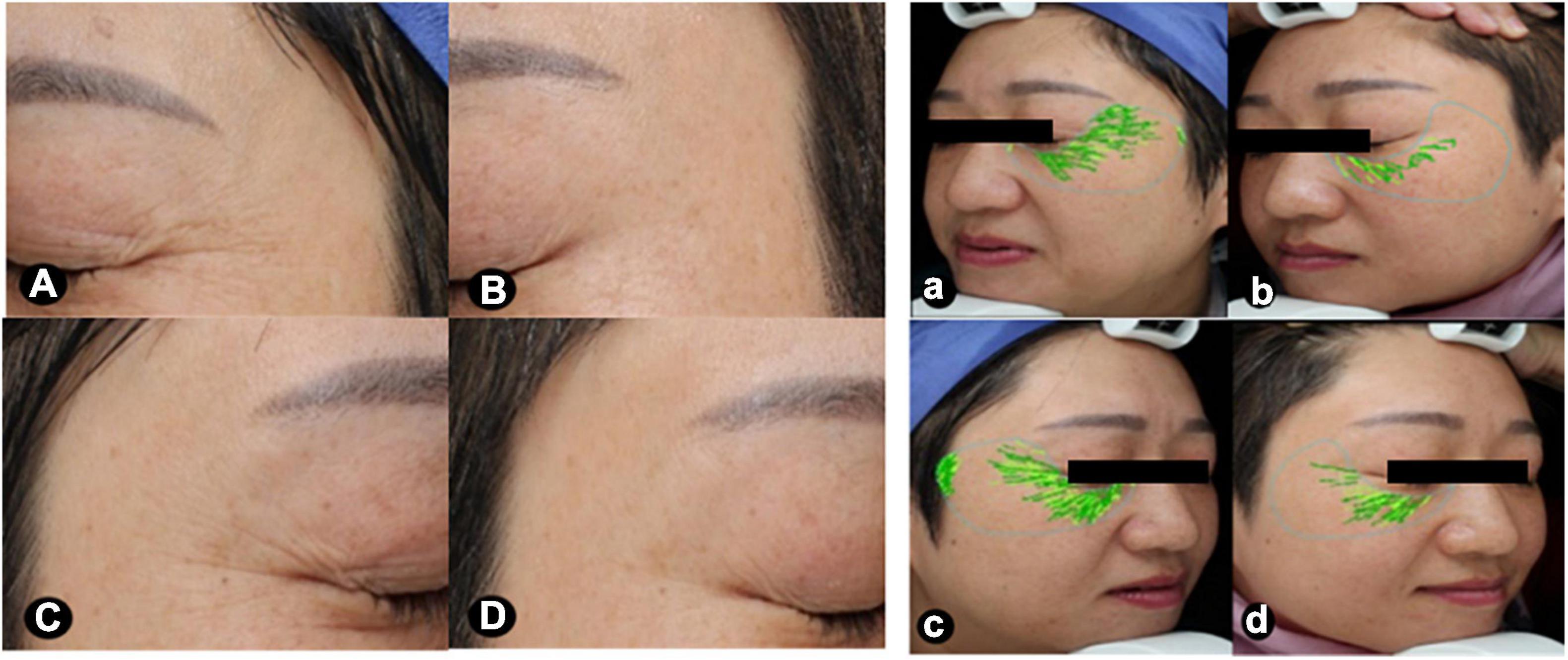
Figure 2. Images focusing on the periorbital areas at baseline and after treatment. The periorbital wrinkles exhibited greater improvements after MN plus hUC-MSCs-CM [(A,a) baseline; (B,b) after treatment], compared to MN alone [(C,c), baseline; (D,d) after treatment]. VISIA ® skin analysis assessment for periorbital wrinkles: a = 29.53, b = 8.39, c = 32.37, d = 15.63. MN: microneedling; hUC-MSCs-CM: human umbilical cord-derived mesenchymal stem cells conditioned media.
At baseline, there was no statistically significant difference for skin physiological parameters, skin brightness, and skin rejuvenation in the objective assessments between MN plus hUC-MSCs-CM group and MN alone group (Table 4).
Hydration and TEWL: The hydration content and TEWL are indicators for skin barrier function. There was no significant change in hydration content after treatment between MN plus hUC-MSCs-CM side [2.18 (5.80), P = 0.06] and MN alone side [2.07 (6.78), P = 0.12]. Furthermore, no significant change was found in the TEWL measurements between MN plus hUC-MSCs-CM side [1.96 (5.15), P = 0.05] and MN alone side [1.10 (3.79), P = 0.13], indicating that MN treatment did not damage the skin barrier.
At 2 weeks after the last treatment, the MI decreased more in MN plus hUC-MSCs-CM group than in MN alone group [24.25 (15.55) vs. 12.36 (16.38), P = 0.00; Figure 3A], indicating the efficacy of hUC-MSCs-CM for skin lightening (Figures 3A, 1A,B).
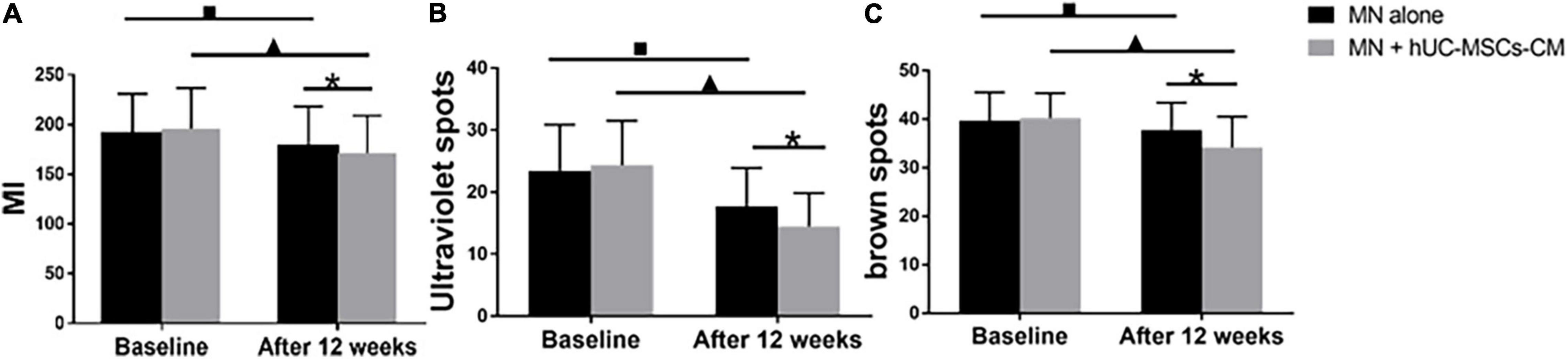
Figure 3. Objective non-invasive skin pigmentation measurements. (A) Melanin index (MI); (B) Ultraviolet spots; (C) Brown spots. MN: microneedling; hUC-MSCs-CM: human umbilical cord-derived mesenchymal stem cells conditioned media. *P < 0.05, post-treatment comparison between MN alone and MN plus hUC-MSCs-CM; ▲P < 0.05, pre-treatment and post-treatment in MN plus hUC-MSCs-CM. ■P < 0.05, pre-treatment and post-treatment in MN alone.
Ultraviolet spots are associated with exposure to ultraviolet sun radiation. The ultraviolet spot score of the side treated with MN plus hUC-MSCs-CM decreased from 24.39 (7.11) to 14.47 (5.38), P = 0.00, while the score for the side treated with MN alone decreased from 23.08 (7.58) to 17.72 (6.18), P = 0.00. There was no significant difference in ultraviolet spot score between the two sides before first treatment, but a significant difference was observed at the last measurement (P < 0.05, Figure 3B), indicating that hUC-MSCs-CM was effective in decreasing ultraviolet spots.
The formation of brown spots is related to dyspigmentation. Compared to baseline, either one side treated with MN plus hUC-MSCs-CM and MN presented with statistically significant changes in brown spot scores 2 weeks after the final treatment. The score for MN plus hUC-MSCs-CM side decreased from 40.21 (5.14) to 34.18 (6.32) (P < 0.05), while the score for MN alone side decreased from 39.71 (5.82) to 37.75 (5.63) (P < 0.05). At the last measurement, a significant change was observed between MN plus hUC-MSCs-CM side and MN alone side (P = 0.00, Figure 3C), indicating that MN plus hUC-MSCs-CM has a better effect in decreasing the brown scores.
There was no significant difference in erythema index (EI) after treatment between MN plus hUC-MSCs-CM side (P = 0.90) and MN alone side (P = 0.87). The EI score for the sides treated with MN plus hUC-MSCs-CM increased from 336.69 (63.08) at baseline to 336.85 (60.06) at 2 weeks after the final treatment. Similarly, the EI score for the sides treated with MN alone increased from 336.68 (60.80) at baseline to 337.29 (57.05) at 2 weeks following the final treatment. This indicates that MN and hUC-MSCs-CM does not induce skin inflammation.
Spots in the side treated with MN plus hUC-MSCs-CM decreased from 29.30 (5.58) to 28.20 (4.92) (P = 0.17), while spots on the other side decreased from 29.17 (5.93) to 28.08 (5.38) (P = 0.12). There was no statistically significant difference in red spot scores at the last measurement (P = 0.31).
Compared to baseline, the wrinkle score for MN plus hUC-MSCs-CM side decreased to 5.06 (3.44) (P = 0.00), while the score for MN side decreased to 10.73 (10.63) (P = 0.00). At the final measurement, MN plus hUC-MSCs-CM side exhibited a more significant decrease in wrinkle measurements than in MN alone side [12.15 (10.26) vs. 7.46 (6.07), P = 0.00; Figures 2, 4A].
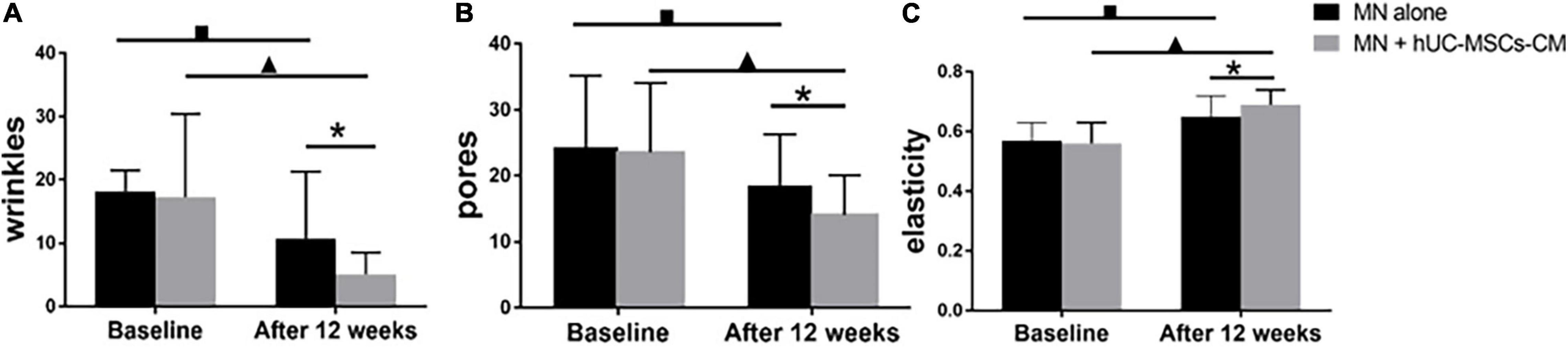
Figure 4. Objective non-invasive skin texture measurements: (A) wrinkles; (B) pores; (C) elasticity. MN: microneedling; hUC-MSCs-CM: human umbilical cord-derived mesenchymal stem cells conditioned media. *P < 0.05, post-treatment comparison between MN alone and MN plus hUC-MSCs-CM; ▲P < 0.05, pre-treatment and post-treatment in MN plus hUC-MSCs-CM; ■P < 0.05, pre-treatment and post-treatment in MN alone.
Significant changes were observed for pores in both MN plus hUC-MSCs-CM group (P = 0.00) and MN alone group (P = 0.00). The pores’ scores decreased from 23.55 (10.52) to 14.05 (6.11) in MN plus hUC-MSCs-CM group, while these decreased from 24.40 (10.77) to 18.59 (7.70) in MN side. Furthermore, there was a significantly better effect after treatment in hUC-MSCs-CM side, compared to MN alone side (P = 0.00; Figures 2, 4B), indicating that hUC-MSCs-CM can shrink the size of pores.
Compared to baseline, skin elasticity significantly improved in both groups (P = 0.00). At 2 weeks after the last treatment, there was a significantly higher increase in MN plus hUC-MSCs-CM group, compared to MN alone group (P = 0.00). Furthermore, the measurements of elasticity for MN alone group increased from 0.57 (0.06) to 0.65 (0.07), while the measurements for the other side increased from 0.56 (0.07) to 0.69 (0.05) (Figure 4C).
No severe side effects were reported during the whole study period. Two participants in MN plus hUC-MSCs-CM group reported dry skin and one reported erythema. Three patients reported dry skin and two patients reported erythema in MN alone group, respectively.
A total of 28 volunteers with a mean (SD) age of 41 (6.54) years old completed the trial. The investigator’s assessment of skin brightness and rejuvenation, and the self-satisfaction score revealed statistically better effects in MN plus hUC-MSCs-CM group, compared to MN alone group. No severe side effects were reported during the whole study period. The objective assessments revealed significantly more improvement in skin brightness (reduced MI, ultraviolet spots, and brown spots) and skin rejuvenation (reduced wrinkles and pores, and increased skin elasticity) in MN plus hUC-MSCs-CM group, compared to MN alone group, while there were no obvious differences in skin hydration, trans-epidermal water loss, and erythema index.
Microneedling stimulate the dermis, which increases new collagen and elastin, thereby rejuvenating the skin, improving wrinkles and increasing skin elasticity (9). Robati RM et al. compared MN to fractional Er:YAG laser in facial skin rejuvenation, and found a comparable efficacy. However, the slight “downtime” of MN makes it preferable for many patients (10). At present, MN is widely applied for various skin diseases, particularly in the skin-of-color population (i.e., Fitzpatrick skin types IV-VI) (11). Compared to laser treatments, MN is simple and inexpensive. Furthermore, the skin barrier disruption caused by MN resolves within 72 h (12). In the present study, the skin barrier signs, including skin hydration and TEWL, had no significant difference before and after treatment, in both MN plus hUC-MSCs-CM group and MN alone group. This finding indicates the safety of MN therapy.
The combination of MN with topical agents has successfully been used in the facial rejuvenation of aged skin (13). Most recently, stem cell extracellular vesicles or cell therapies, such as adipose-derived stem cells, MSCs and BMMSCs, have been used for skin rejuvenation due to the ability to repair and regenerate tissues and organs in cosmetic and reconstructive surgeries (14, 15). The long-term safety and controllability of cell-based therapies remain controversial, making cell-derived extracellular vesicles (exosomes or conditioned medium) preferable for most therapeutic applications. The effect of conditioned media from BMMSC- or adipose-derived stem cells on skin rejuvenation has been demonstrated (16, 17). In the present study, the participant’s satisfaction score and physician’s global assessments score for facial rejuvenation were higher in MN plus hUC-MSCs-CM group, compared to MN alone group. These present results support another study, which reported that amniotic membrane stem cell-conditioned medium can rejuvenate the skin (24).
Skin brightness and texture are more correlated to skin rejuvenation. The less pigmented the skin is, the brighter it becomes. Objective pigmentation parameters, including the MI, ultraviolet spots, and brown spots, were explored in the present study. Compared to MN alone group, all three pigmentation parameters significantly decreased in combination group at 2 weeks after the final treatment. Compared to baseline, MN alone side presented with a significant decrease in MI. However, in a Korean study, the MI merely significantly decreased in subjects treated with MN combined with secretory factors of endothelial precursor cells derived from human embryonic stem cells (19). This difference may be attributed to the different length of microneedling (0.25 mm in the Korean study vs. 0.5 mm in this study). A previous study revealed that the single-session MN-treatment for melasma with a needle length of 0.5 mm resulted in significantly reduced melanin density, pendulous melanocytes, and a pathological basement membrane (20).
Except for pigmentation, the skin textures, such as wrinkles, pores and elasticity, tested by VISIA ® skin analysis were also explored in the present study. Compared to baseline, wrinkles, pores, and elasticity improved in MN plus hUC-MSCs-CM group and MN alone, and the effect of the combined group was significantly better than that of MN alone group. These results can be attributed to the various growth factors of hUC-MSCs-CM, such as EGF, VEGF, PDGF-BB. For example, EGF stimulates epidermal cell proliferation (21). VEGF facilitates skin angiogenesis and increases blood vessel permeability to improve tissue nutrition. PDGF-BB is involved in fibroblast proliferation and regulates cell growth (22). According to the present findings, the changes in the skin micro-environment may lead to the greater improvement in wrinkles, pores and elasticity in combination group, compared to MN alone. A recent study concluded that the remodeling of dermal structures in skin biopsy pathology can be observed mainly on the combined side (23). Furthermore, the histometry of the epidermis revealed a significant increase in epidermal thickness on both the skin-needled and combined side (i.e., skin needling plus amniotic fluid mesenchymal stem cell derived conditioned media). This is consistent with the present results, in which the improvement in skin rejuvenation was greater in the combined side, compared to MN alone side.
In the present study, merely minor treatment-related side effects were observed. Two patients reported dry skin and one patient reported erythema in MN plus hUC-MSCs-CM group, while three patients reported dry skin and two patients reported erythema in MN alone group the dry skin was likely due to a temporary breakdown in skin barrier function caused by microneedling.
The small sample size and lack of pathological assessment were the main limitations of this study, despite the fact that great improvement was observed in the images taken by VISIA ® test. However, the split—face study design presented the reliable comparison between the two groups with such a relatively small number of volunteers. In the future, a larger sample size will be considered and more objective evaluations will be applied. The repeatability and stability of hUC-MSCs-CM are always the focus when performing the project.
Up to now, only two randomized controlled trials and one control trial was published in the application of MN with and without MSCs-CM or extracts from media of MSCs in facial rejuvenation (Table 5), and these studies provide strong evidence for the effective treatment of facial aging by this method. The data from the three articles supported that combination therapy with MN via MSCs derivative improve significantly than MN alone.
In conclusion, MN combined with hUC-MSCs-CM is a safe and effective treatment modality for facial rejuvenation, and may potentially be used as a novel method for anti-aging.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Beijing Friendship Hospital affiliated to Capital Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by Beijing Natural Science Foundation (No. 7222040).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Boisma F, Serror K, Dobos G, Zuelgaray E, Bensussan A, Michel L. Skin aging: pathophysiology and innovative therapies. Med Sci (Paris). (2020) 36:1163–72. doi: 10.1051/medsci/2020232
2. Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. (2021) 22:2410. doi: 10.3390/ijms22052410
3. Wang JV, Schoenberg E, Zaya R, Rohrer T, Zachary CB, Saedi N. The rise of stem cells in skin rejuvenation: a new frontier. Clin Dermatol. (2020) 38:494–6. doi: 10.1016/j.clindermatol.2020.04.003
4. Yang S, Huang S, Feng C, Fu X. Umbilical cord-derived mesenchymal stem cells: strategies, challenges, and potential for cutaneous regeneration. Front Med. (2012) 6:41–7. doi: 10.1007/s11684-012-0175-9
5. Yang D, Chen M, Sun Y, Jin Y, Lu C, Pan X, et al. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. (2021) 121:119–33. doi: 10.1016/j.actbio.2020.12.004
6. Soliman YS, Horowitz R, Hashim PW, Nia JK, Farberg AS, Goldenberg G. Update on acne scar treatment. Cutis. (2018) 102:21, 25, 47–8.
7. Choy YB, Prausnitz MR. The rule of five for non-oral routes of drug delivery: ophthalmic, inhalation and transdermal. Pharm Res. (2011) 28:943–8. doi: 10.1007/s11095-010-0292-6
8. Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. (2011) 154:148–55. doi: 10.1016/j.jconrel.2011.05.021
9. Alessa D, Bloom JD. Microneedling options for skin rejuvenation, including non-temperature-controlled fractional microneedle radiofrequency treatments. Facial Plast Surg Clin North Am. (2020) 28:1–7. doi: 10.1016/j.fsc.2019.09.001
10. Robati RM, Hamedani B, Namazi N, Niknejad N, Gheisari M. Efficacy of microneedling versus fractional Er: YAG laser in facial rejuvenation. J Cosmet Dermatol. (2020) 19:1333–40. doi: 10.1111/jocd.13440
11. Cohen BE, Elbuluk N. Microneedling in skin of color: a review of uses and efficacy. J Am Acad Dermatol. (2016) 74:348–55. doi: 10.1016/j.jaad.2015.09.024
12. Han TY, Park KY, Ahn JY, Kim SW, Jung HJ, Kim BJ. Facial skin barrier function recovery after microneedle transdermal delivery treatment. Dermatol Surg. (2012) 38:1816–22. doi: 10.1111/j.1524-4725.2012.02550.x
13. Kim JH, Park HY, Jung M, Choi EH. Automicroneedle therapy system combined with topical tretinoin shows better regenerative effects compared with each individual treatment. Clin Exp Dermatol. (2013) 38:57–65. doi: 10.1111/j.1365-2230.2012.04405.x
14. Zarei F, Abbaszadeh A. Application of cell therapy for anti-aging facial skin. Curr Stem Cell Res Ther. (2019) 14:244–8. doi: 10.2174/1574888X13666181113113415
15. Suh A, Pham A, Cress MJ, Pincelli T, TerKonda SP, Bruce AJ, et al. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. (2019) 54:100933. doi: 10.1016/j.arr.2019.100933
16. Kim SN, Lee CJ, Nam JH, Choi B, Chung E, Song SU. The effects of human bone marrow-derived mesenchymal stem cell conditioned media produced with fetal bovine serum or human platelet lysate on skin rejuvenation characteristics. Int J Stem Cells. (2021) 14:94–102. doi: 10.15283/ijsc20070
17. Li L, Ngo HTT, Hwang E, Wei X, Liu Y, Liu J, et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int J Mol Sci. (2019) 21:49. doi: 10.3390/ijms21010049
18. Prakoeswa CRS, Pratiwi FD, Herwanto N, Citrashanty I, Indramaya DM, Murtiastutik D, et al. The effects of amniotic membrane stem cellconditioned medium on photoagi. J Dermatolog Treat. (2019) 30:478–82. doi: 10.1080/09546634.2018.1530438
19. Lee HJ, Lee EG, Kang S, Sung JH, Chung HM, Kim DH. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: a randomized, controlled, blinded split-face study. Ann Dermatol. (2014) 26:584–91. doi: 10.5021/ad.2014.26.5.584
20. Cassiano DP, Espósito ACC, Hassun KM, Lima EVA, Bagatin E, Miot HA. Early clinical and histological changes induced by microneedling in facial melasma: a pilot study. Indian J Dermatol Venereol Leprol. (2019) 85:638–41. doi: 10.4103/ijdvl.IJDVL_44_19
21. Zhang XL, Zhang LT. Clinical study on the efficiency of rhEGFgel to skin function of facial hormone-dependent dermatitis. Tian Jin Med J. (2016) 44:629–31. doi: 10.11958/20150434
22. Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. (2014) 30:157–71. doi: 10.1055/s-0034-1372423
23. El-Domyati M, Mofta NH, Nasif GA, Ameen SW, Ibrahim MR, Ragaie MH. Facial rejuvenation using stem cell conditioned media combined with skin needling: a split-face comparative study. J Cosmet Dermatol. (2020) 19:2404–10. doi: 10.1111/jocd.13594
Keywords: microneedling, human umbilical cord-derived mesenchymal stem cells, conditioned media, skin rejuvenation, skin brightness, efficacy
Citation: Liang X, Li J, Yan Y, Xu Y, Wang X, Wu H, Liu Y, Li L and Zhuo F (2022) Efficacy of Microneedling Combined With Local Application of Human Umbilical Cord-Derived Mesenchymal Stem Cells Conditioned Media in Skin Brightness and Rejuvenation: A Randomized Controlled Split-Face Study. Front. Med. 9:837332. doi: 10.3389/fmed.2022.837332
Received: 16 December 2021; Accepted: 14 April 2022;
Published: 24 May 2022.
Edited by:
Xing-Hua Gao, The First Affiliated Hospital of China Medical University, ChinaReviewed by:
Saritha Mohanan, Indira Gandhi Medical College and Research Institute, IndiaCopyright © 2022 Liang, Li, Yan, Xu, Wang, Wu, Liu, Li and Zhuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenglin Zhuo, emZsc3VubnlAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.