
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 02 March 2022
Sec. Hematology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.837253
This article is part of the Research Topic Women in Science - Hematology 2021 View all 12 articles
In this research, we described a very rare case of thrombotic microangiopathy induced by remethylation disorders. A 16-year-old boy presented to the emergency department with 5 months of weakness and fatigue. He was diagnosed with thrombotic microangiopathy based on clinical manifestation and laboratory information, which showed microangiopathic hemolytic anemia, renal impairment, and thrombocytopenia. After a complex diagnostic workup, the metabolite screening parameters and sequencing results guided us toward the diagnosis of remethylation disorders. The patient was diagnosed with thrombotic microangiopathy induced by remethylation disorders (cblC).
A hemolytic uremic syndrome is a form of thrombotic microangiopathy. Hemolytic uremic syndrome (HUS) encompasses several disorders: shiga toxin-induced HUS, and pneumococcus-induced HUS, HUS associated with complement dysregulation, HUS related to cobalamin C (cblC) defect, and HUS secondary to a heterogeneous group of causes (infections, drugs, cancer, and systemic diseases). Among these causes, cblC defect is one of the combined remethylation disorders with a genetically heterogeneous disorder of cbl metabolism. In this study, we presented a rare case with thrombotic microangiopathy (TMA) induced by remethylation disorders (cblC).
A 16-year-old boy presented to the emergency department with 5 months of weakness and fatigue. The patient was in good general health, had no relevant medical history, no abdominal pain, and diarrhea, and had a normal physical examination. Laboratory evaluation showed thrombocytopenia (platelet count, 58 × 109/L) and hemolytic anemia (hemoglobin, 77 g/L; reticulocyte, 199.9 × 109/L; lactate dehydrogenase, 609 IU/L). Additional laboratory tests showed an elevated creatinine level of 2.6 mg/dL and elevated 24 h proteinuria of 5.66 g/24 h. Serum vitamin B12 (348 nmol/L), folate (20.31 nmol/L), ferritin (169.2 ng/mL), iron (14.1 μmol/L), complement 3 (0.733 g/L), and complement 4 (0.325 g/L) were all within the normal range. Wright-stained peripheral smear (PS) showed schistocytes (Figure 1; original magnification × 1,000). Level of factor H and ADAMTS13 activity were normal. Anti-factor H-antibody and Coombs's test were negative. The autoantibodies, such as an anti-nuclear antibody, anti-dsDNA antibody, anti-phospholipase A2 receptor antibody, anti-phospholipid antibody, and anti-neutrophil cytoplasmic antibody were all negative. Renal pathology suggested that there were severe TMA with swelling endothelial cells, thickening arterial wall, thrombus in a capillary lumen, and IgM and C3 deposition. By screening for metabolic diseases, we found that the concentration of plasma total homocysteine (tHcy, 247.84 μmol/L), urinary methylmalonic acid (MMA, 62.29 mmol/mol creatinine), and blood propionyl carnitine (C3, 8.67 μmol/L) were increased obviously, and plasma methionine (Met, 7.8 μmol/L) was decreased. The whole exon sequencing and Sanger sequencing revealed a compound heterozygous MMACHC mutation (Figure 2; c.1A > G and c.80A > G).
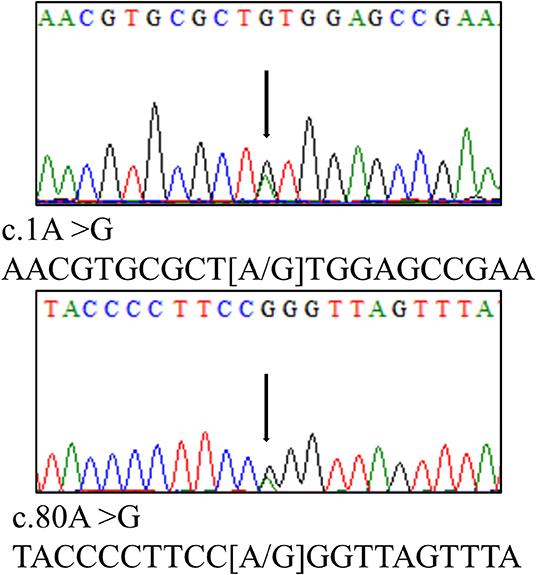
Figure 2. Genetic analysis revealed a compound heterozygous MMACHC mutation (c.1A > G and c.80A > G).
The patient was diagnosed as TMA induced by remethylation disorders (cblC) according to the guideline (1). The patient was treated with betaine, hydroxycobalamin, and folic acid. After treatment, the tHcy level was normalized but hemolysis, thrombocytopenia, and acute kidney failure persisted. Because plasma exchange was ineffective, he required continued renal replacement treatment (CRRT) three times a week due to renal injury of TMA.
The study was in compliance with the Declaration of Helsinki and approved by the ethics committee of Peking University First Hospital. The significance of the research was explained to the patient and informed consent was obtained.
Remethylation disorders are rare inherited disorders in which impaired remethylation of homocysteine (Hcy) to Met. Met leads to accumulation of tHcy. Remethylation disorders can be classified into three groups (1). Combined remethylation disorders (cblC, cblD-MMA/HC, cblF, and cblJ) are alterations of the transport and intracellular metabolism of cobalamins, which causes a defect in the synthesis of the two functional forms of cobalamin (cbl; vitamin B12): methylcobalamin, cofactor of methionine synthase, and adenosylcobalamin, a cofactor for methylmalonyl-CoA mutase (MCM). Isolated remethylation disorders (cblD-HC, cblE, and cblG) are isolated deficits in the production of methylcobalamin. 5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency is an abnormality of the folate cycle.
The clinical diagnosis of remethylation disorders can be easily and rapidly diagnosed by biochemical investigation. Serum vitamin B12, serum folate, plasma tHcy, plasma Met, blood acylcarnitine profile, plasma, or urinary MMA are required. Combined remethylation disorders are associated with increased tHcy and MMA (2). In this case, Serum Vitamin B12 and folate were within the normal range. The concentration of plasma tHcy, urinary MMA, and blood C3 were increased obviously. The plasma Met was decreased. Therefore, this case was diagnosed as a combined remethylation disorder.
Combined remethylation disorders are genetically heterogeneous disorders of cbl metabolism. Different forms of the disorder have been classified as cblC, cblD-MMA/HC, cblF, and cblJ. Among these, cblC type is most frequent and caused by a mutation in the MMACHC gene on chromosome 1p34.1. The three most common mutations are c.271dupA, c.394C > T, and c.331C > T, However, in Chinese patients, the c.609G > A mutation accounts for 85% of the identified alleles in MMACHC mutation (1). In this case, heterozygous mutations of MMACHC (c.1A > G and c.80A > G) were found, which have been reported before (3), although the mutations are uncommon in Chinese patients.
cblC defect usually occurs during the 1st year of life, and late-onset is applicable to children older than 4 years. There is great variability in the clinical presentation of cblC defect, and more common in the blood and nervous system (3). In this study, we focus on the TMA, which is a rarely late-onset manifestation in cblC defect.
Hemolytic uremic syndrome (HUS) is a form of TMA, characterized by microangiopathic hemolytic anemia, renal impairment, and thrombocytopenia (4). Another form of TMA is thrombotic thrombocytopenic purpura (TTP) defined by a severe deficiency in ADAMTS13, the specific von Willebrand factor (vWF)-cleaving protease (5). It is worth noting that schistocytes are fragments of red blood cells produced by extrinsic mechanical damage and a diagnostic feature of TMA (6). A percentage of schistocytes above 1% is a robust indicator for the diagnosis of TMA (7). In this case, the thrombocytopenia, hemolytic anemia, renal dysfunction, schistocytes, and normal ADAMTS13 activity all support the diagnosis of TMA.
The HUS encompasses a heterogeneous group of disorders, including typical HUS due to an infection from shiga toxin-producing Escherichia coli (STEC), and atypical hemolytic uremic syndrome (aHUS), caused by dysregulation of the alternative pathway of complement secondary to complement gene mutations or anti-complement factor H antibodies (8). cblC is a genetic disease that can lead to HUS (9). So far, only a few cases of HUS have been triggered by a metabolic disorder of cbl (10). Although HUS can be caused by cblC defect accompanied by complement dysregulation (11), dysregulation of the complement alternative pathway was not found with the whole-exome sequencing and ADAMTS13 test in this case. This patient is not complement-driven and, therefore, cannot benefit from complement inhibitors (12). Based on the diagnoses of cblC defect, the patient was diagnosed as TMA induced by combined remethylation disorders (cblC).
In this study, we presented a case of combined remethylation disorder, which is cblC defect and MMACHC-related, is the most common inborn error of cbl metabolism and it is a rare cause of TMA. Combined remethylation disorders should be considered in those patients with unclear microangiopathic hemolytic anemia. Therefore, plasma tHcy and MMA measurement should be included in the workup of renal TMA children. Plasma tHcy is readily available in clinical laboratories and easily ruled the suspicion of a cbl metabolism defect, so it is the first biochemical parameter to assess when a remethylation disorder is suspected. Thereafter, the combination of methylmalonic aciduria, homocystinuria, and normal serum vitamin B12 concentration distinguish patients with a genetic defect of Cbl from vitamin B12 deficiency due to nutritional deficiency or acquired malabsorption (1).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University First Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
LP, JC, and HL conceived the manuscript, performed analysis, and wrote the manuscript. HY, HH, BJ, XW, LP, and HL performed interpretation of clinical data and contributed to manuscript writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
aHUS, atypical hemolytic uremic syndrome; C3, propionyl carnitine; cbl, cobalamin; HUS, hemolytic uremic syndrome; Met, methionine; MMA, methylmalonic acid; MTHFR, 5, 10-methylenetetrahydrofolate reductase; PS, peripheral smear; STEC, shiga toxin-producing Escherichia coli; tHcy, total homocysteine; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura; vWF, von Willebrand factor.
1. Huemer M, Diodato D, Schwahn B, Schiff M, Bandeira A, Benoist JF, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. (2017) 40:21–48. doi: 10.1007/s10545-016-9991-4
2. Huemer M, Diodato D, Martinelli D, Olivieri G, Blom H, Gleich F, et al. Phenotype, treatment practice and outcome in the cobalamin-dependent remethylation disorders and MTHFR deficiency: data from the E-HOD registry. J Inherit Metab Dis. (2019) 42:333–52. doi: 10.1002/jimd.12041
3. Chen RY, Li XZ, Lin Q, Zhu Y, Shen YY, Xu QY, et al. Proteinuria as a presenting sign of combined methylmalonic acidemia and homocysteinemia: case report. BMC Med Genet. (2020) 21:183. doi: 10.1186/s12881-020-01122-x
4. Cody EM, Dixon BP. Hemolytic uremic syndrome. Pediatr Clin North Am. (2019) 66:235–46. doi: 10.1016/j.pcl.2018.09.011
5. Joly BS, Coppo P, Veyradier A. An update on pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev Hematol. (2019) 12:383–95. doi: 10.1080/17474086.2019.1611423
6. Bhandari S, Kumar R. Thrombotic thrombocytopenic purpura. N Engl J Med. (2019) 380:e23. doi: 10.1056/NEJMicm1813768
7. Zini G, d'Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, et al. ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol. (2012) 34:107–16. doi: 10.1111/j.1751-553X.2011.01380.x
8. Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. (2017) 390:681–96. doi: 10.1016/S0140-6736(17)30062-4
9. Adrovic A, Canpolat N, Caliskan S, Sever L, Kiykim E, Agbas A, et al. Cobalamin C defect-hemolytic uremic syndrome caused by new mutation in MMACHC. Pediatr Int. (2016) 58:763–5. doi: 10.1111/ped.12953
10. Martinez de Companon Z, Poblet-Puig M, Valles G, Del Toro M, Vilalta R, Moreno A, et al. Cobalamin disorder CblC presenting with hemolytic uremic syndrome and pulmonary hypertension. Nefrologia. (2018) 38:333–5. doi: 10.1016/j.nefro.2017.03.019
11. Barlas UK, Kihtir HS, Goknar N, Ersoy M, Akcay N, Sevketoglu E. Hemolytic uremic syndrome with dual caution in an infant: cobalamin C defect and complement dysregulation successfully treated with eculizumab. Pediatr Nephrol. (2018) 33:1093–6. doi: 10.1007/s00467-018-3941-3
Keywords: hemolytic uremic syndrome, schistocytes, remethylation disorders, cblC, MMACHC
Citation: Pang L, Chen J, Yu H, Huang H, Jin B, Wang X and Li H (2022) Case Report: A Rare Case of Thrombotic Microangiopathy Induced by Remethylation Disorders. Front. Med. 9:837253. doi: 10.3389/fmed.2022.837253
Received: 16 December 2021; Accepted: 31 January 2022;
Published: 02 March 2022.
Edited by:
Roberta Fenoglio, Ospedale San Giovanni Bosco, ItalyReviewed by:
Ioanna Sakellari, G. Papanikolaou General Hospital, GreeceCopyright © 2022 Pang, Chen, Yu, Huang, Jin, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haixia Li, YmR5eWxoeEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.