
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 03 May 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.837092
This article is part of the Research Topic Updates in Ocular Therapeutics and Surgery View all 28 articles
 Shuang Liang1
Shuang Liang1 Shufan Ji2
Shufan Ji2 Xiao Liu2
Xiao Liu2 Min Chen3
Min Chen3 Yulin Lei4
Yulin Lei4 Jie Hou4
Jie Hou4 Mengdi Li1
Mengdi Li1 Haohan Zou1
Haohan Zou1 Yusu Peng3
Yusu Peng3 Zhixing Ma4
Zhixing Ma4 Yuanyuan Liu5
Yuanyuan Liu5 Vishal Jhanji6
Vishal Jhanji6 Yan Wang1,7*
Yan Wang1,7*Purpose: This retrospective study aimed to identify the key factors influencing postoperative refraction after small-incision lenticule extraction (SMILE) using information gain.
Methods: This study comprised 2,350 eyes of 1,200 patients who underwent SMILE using a Visumax 500-kHz femtosecond laser (Carl Zeiss Meditec AG) in three ophthalmic centers: Tianjin Eye Hospital (center A), Jinan Mingshui Eye Hospital (center B), and Qingdao Eye Hospital (center C). Anterior segment features, including corneal curvature and central corneal thickness (CCT), were obtained from Pentacam HR (Oculus, Wetzlar, Germany). Information gain was calculated to analyze the importance of features affecting postoperative refraction.
Results: Preoperative and postoperative mean spherical equivalent (SE) refraction were −5.00 (−6.13, −3.88) D and 0.00 (−0.25, 0.13) D, respectively. None of the patients lost more than two lines of corrected distance visual acuity. The safety index was 1.32 ± 0.24, 1.03 ± 0.08, and 1.13 ± 0.16 in centers A, B, and C, respectively. The efficacy index was 1.31 ± 0.25, 1.02 ± 0.08, and 1.13 ± 0.17 in centers A, B, and C, respectively. At least 95% of the eyes were within ±1.00 D of the attempted correction. Postoperative refraction was related to preoperative spherical diopter refraction (r = 0.369, p < 0.001), preoperative SE (r = 0.364, p < 0.001), maximum lenticule thickness (r = −0.311, p < 0.001), preoperative uncorrected distance visual acuity (r = 0.164, p < 0.001), residual stromal thickness (r = 0.139, p < 0.001), preoperative mean anterior corneal curvature (r = −0.127, p < 0.001), preoperative flattest anterior corneal curvature (r = −0.122, p < 0.001), nomogram (r = −0.100, p < 0.001) and preoperative CCT (r = −0.058, p = 0.005).
Conclusions: SMILE was considered a safe and effective procedure for correcting myopia. Based on information gain, postoperative refraction was influenced by preoperative mean anterior corneal curvature, CCT, refraction, and residual stromal thickness.
Small-incision lenticule extraction (SMILE) is a viable surgical option for the correction of myopia and astigmatism (1). Compared with laser-assisted in situ keratomileusis, the SMILE procedure was flapless. Because of no corneal flap, SMILE has the advantages of lower incidence of postoperative dry eye and better stability of corneal biomechanics (2, 3). There is a rising acceptance and recognition of SMILE surgery as a global surgical treatment option for refractive errors (4). Previous studies have reported that sex (5), age (6, 7), preoperative spherical equivalent (SE) (8), corneal curvature (9), optical zone (10), central corneal thickness (CCT) (11, 12), treatment nomogram (13), and laser energy (14, 15) affect visual outcomes after SMILE. While previous studies mostly analyzed the influence of a single factor, in this study, machine learning was used to analyze 20 different factors to determine the most important factors affecting SMILE.
Machine learning has been widely used for the diagnosis of corneal diseases (16), prediction of myopia progression (17), and diagnosis of keratoconus (18). Information gain allows the analysis of the correlation between different variables and their impact on outcomes. The impact of individual features on outcomes can be measured by the information gain (19). Information gain makes a comprehensive consideration of feature selection using the statistical properties of all samples and fitting non-linear data, while multiple linear regression is only capable of analyzing linear data. The purpose of this retrospective study was to explore the factors influencing postoperative refraction after SMILE in different ophthalmic centers using information gain.
This retrospective study included patients who underwent SMILE surgery in three ophthalmic centers, namely, Tianjin Eye Hospital (center A), Jinan Mingshui Eye Hospital (center B), and Qingdao Eye Hospital (center C). The inclusion criteria were as follows: age > 18 years, CCT > 450 μm, corrected distance visual acuity (CDVA) of 20/25 or better, stable refraction for the past 2 years and patients demonstrate a keen desire to remove their lenses. Patients stopped wearing soft contact lenses for at least 2 weeks and hard contact lenses for at least 4 weeks before examination. The exclusion criteria were active ocular disease, previous ocular surgery or ocular trauma, keratoconus, psychiatric disorders, and systemic diseases. Informed consent was obtained from all patients. The study protocol was approved by the ethics committee of the Tianjin Eye Hospital (TJYYLL-201914). The study design adhered to the tenets of the Declaration of Helsinki.
In machine learning applications, information gain is often used for feature selection by evaluating the gain of each feature in the context of the target outcome. The greater the value of the information gain of a feature, the greater the relevance of the feature to the target outcome. The feature with the highest information gain is considered the best feature to be chosen, as it affects the target outcome the most. Information gain can examine the contribution of features to the whole system. It is suitable for the so-called “global” feature selection. In our study, we employed information gain to measure the relevance of some SMILE features, such as residual stromal thickness (RST) and preoperative mean anterior curvature (Pre-Km), to the target SMILE outcome, that is, postoperative SE. The higher the information gain value, the more important the feature is to the SMILE outcome.
Information gain is calculated by the reduction of information entropy, which quantifies the amount of information present in the target outcome.
where IG(S, a) is the information gain for the outcome S with feature a, H(S) is the entropy for the outcome S without feature a, and H(S|a) is the conditional entropy for the outcome S given feature a. The entropy of S can be calculated from the probability distribution p_k, where k can be in K discrete states, and is written as the function H(S):
The conditional entropy H(S|a) can be calculated by splitting the dataset into groups for each observed value of a and calculating the sum of the ratio of examples in each group out of the entire dataset multiplied by the entropy of each group, that is,
where is the ratio of the number of examples in the dataset in which the variable a has the value v, and H(Sa(v)) is the entropy of the group of samples where the variable a has the value v.
In our data analysis, the postoperative SE at 3 months was discretized into three value ranges, 0, 1, and 2, defined as follows: 0:[ −0.25,0.25] D, 1:[ −0.50, −0.25) D or (0.25,0.50] D, and 2: <0.50 D or >0.50 D. Preoperative anterior segment features included flattest anterior corneal curvature (Pre-K1), steepest anterior corneal curvature (Pre-K2), mean anterior corneal curvature (Pre-Km), and preoperative CCT (Pre-CCT). The preoperative features included uncorrected distance visual acuity (UDVA), CDVA, intraocular pressure spherical diopter(Pre-SD), cylinder diopter, cylinder axis, SE, laterality, sex, and age. Surgical design parameters included RST, laser energy, maximum lenticule thickness (Max), cap thickness, optical zone, and treatment nomogram (Nomogram). Information gain values above 0.05 were considered significant.
The SMILE procedure was performed using a Visumax 500 kHz femtosecond laser (Carl Zeiss Meditec AG) under topical anesthesia in all patients. In centers A, B, and C, the surgical parameters were optical zone 6.2-7.0 mm, cap diameter 7.2-8.0 mm, cap thickness 110-140 μm, and laser energy 125-145 nJ. The SMILE surgery was performed using a standard surgical technique (20) by experienced surgeons at each of the centers.
All patients were prescribed 0.5% levofloxacin (Santen, Inc.) four times a day for 1 week, and 0.1% fluorometholone (Santen, Inc.) four times a day for 1-2 weeks postoperatively. UDVA, CDVA, manifest refraction, and corneal tomography (Pentacam HR, Oculus, Wetzlar, Germany) were performed. The follow-up period is 1 day, 1 week, 1 month, and 3 months after SMILE.
All analyses were performed using SPSS version 26.0 software (IBM Corp., Armonk, NY, USA) and SAS version 9.4 software (SAS Institute Inc., Cary, NC). The Kolmogorov-Smirnov test was used to test the normality of the data. The data that did not conform to the normal distribution were represented as median (P25, P75). The relationship between continuous variables, such as Pre-K1, Pre-Km, RST, Max, Pre-CCT, Pre-SD, Pre-SE, Pre-UDVA, Nomogram, and postoperative SE, were analyzed using the Spearman correlation analysis. A effect model was used to analyze the influencing factors. A p-value of < 0.05 was regarded as statistically significant.
A total of 1,200 subjects (2,350 eyes) were included in this study (60.8% male, 50.6% right eye). The average age of the patients was 20 (18, 21) years. The preoperative SE was −5.00 (−6.13, −3.88) D. Demographic data from the different ophthalmic centers are shown in Table 1. Information gain was used to determine the weight of the factors affecting surgical outcomes. Factors influencing postoperative SE are presented in Table 2. Pre-K1, Pre-Km, RST, Max, Pre-CCT, Pre-SD, Pre-SE, Pre-UDVA, and Nomogram were found to significantly impact postoperative SE in all three centers (Figure 1). The top common nine factors highlighted showed information gain values > 0.05 in all three centers. Other variables, such as thickness, sex, laterality (right/left), and Pre-CDVA, had a smaller effect on postoperative SE.
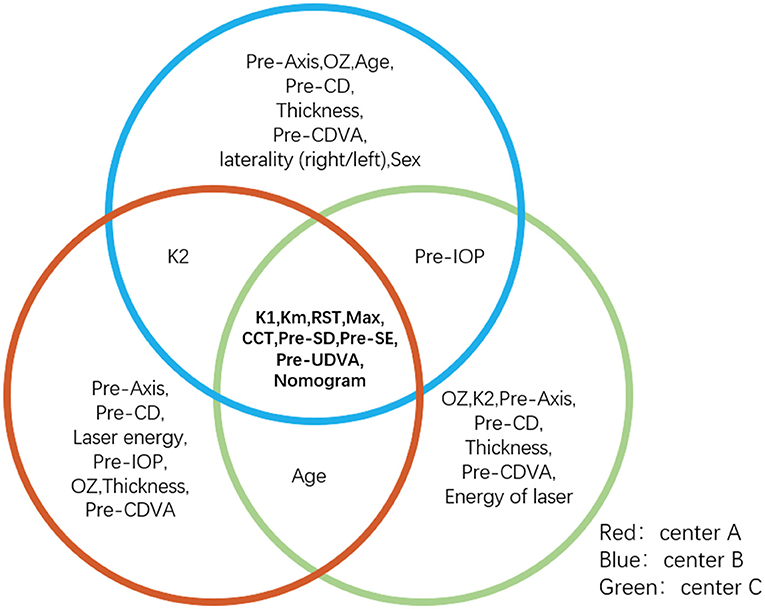
Figure 1. The overlap part of the circle is the feature with three ophthalmic centers information gain values > 0.05, which are considered important factors affecting postoperative SE. K1, Km, RST, Max, CCT, Pre-SD, Pre-SE, Pre-UDVA, and nomogram make a large contribution to postoperative refraction after SMILE. CCT, central corneal thickness; K1, flattest anterior corneal curvature; Km, mean anterior corneal curvature; Max, maximum lenticule thickness; Pre-UDVA, preoperative uncorrected distance visual acuity; Pre-SD, preoperative spherical diopter; Pre-SE, preoperative spherical equivalent; SMILE, small-incision lenticule extraction; RST, residual stromal thickness.
Furthermore, since Table 2 incorporates too many parameters, and each parameter gets a small weight, we repeated the information gain analysis using the nine features found to be significant in Table 2 to obtain a greater weight (Table 3). In Table 3, we selected four out of the top six parameters (shown in bold), whose information gain values were higher than 0.10 in all the three centers. Finally, the result stated that Pre-Km, Pre-CCT, Pre-SD, and Pre-SE were the most influential features affecting postoperative refraction in all the three centers.
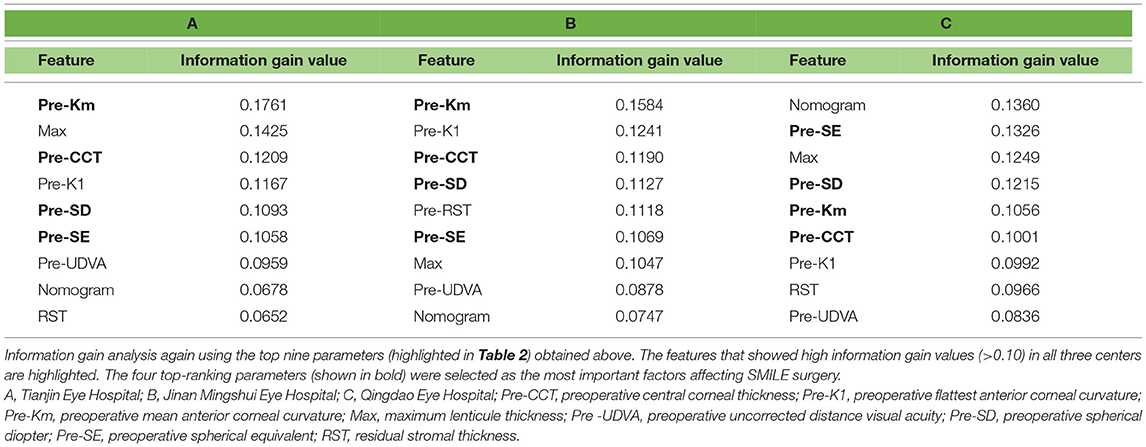
Table 3. Secondary information gain of the nine most influential feature (highlighted in Table 2) affecting postoperative refraction.
The result of the correlation analysis of the patients in all the three centers is displayed in Table 4. Postoperative SE was related to Pre-SD (r = 0.369, p < 0.001), Pre-SE (r = 0.364, p < 0.001), Max (r = −0.311, p < 0.001), Pre-UDVA (r = 0.164, p < 0.001), RST (r = 0.139, p < 0.001), Pre-Km (r = −0.127, p < 0.001), Pre-K1 (r = −0.122, p < 0.001), nomogram (r = −0.100, p < 0.001), and Pre-CCT (r = −0.058, p = 0.005).
The results of random effects estimation for the null model is shown in Table 5. The null model is the first step for building mixed effect model and is used to determine whether the construction of the mixed effect model is necessary. The results of null model indicate that the correlation in laterality is not statistically significant (p1 = 0.143, p2 = 0.106). It means that there is no significant difference between right eye, left eye and binocular.
Standardized graphs of surgical outcomes after SMILE are displayed in Figures 2–4. There was no intraoperative or postoperative complications in all centers.
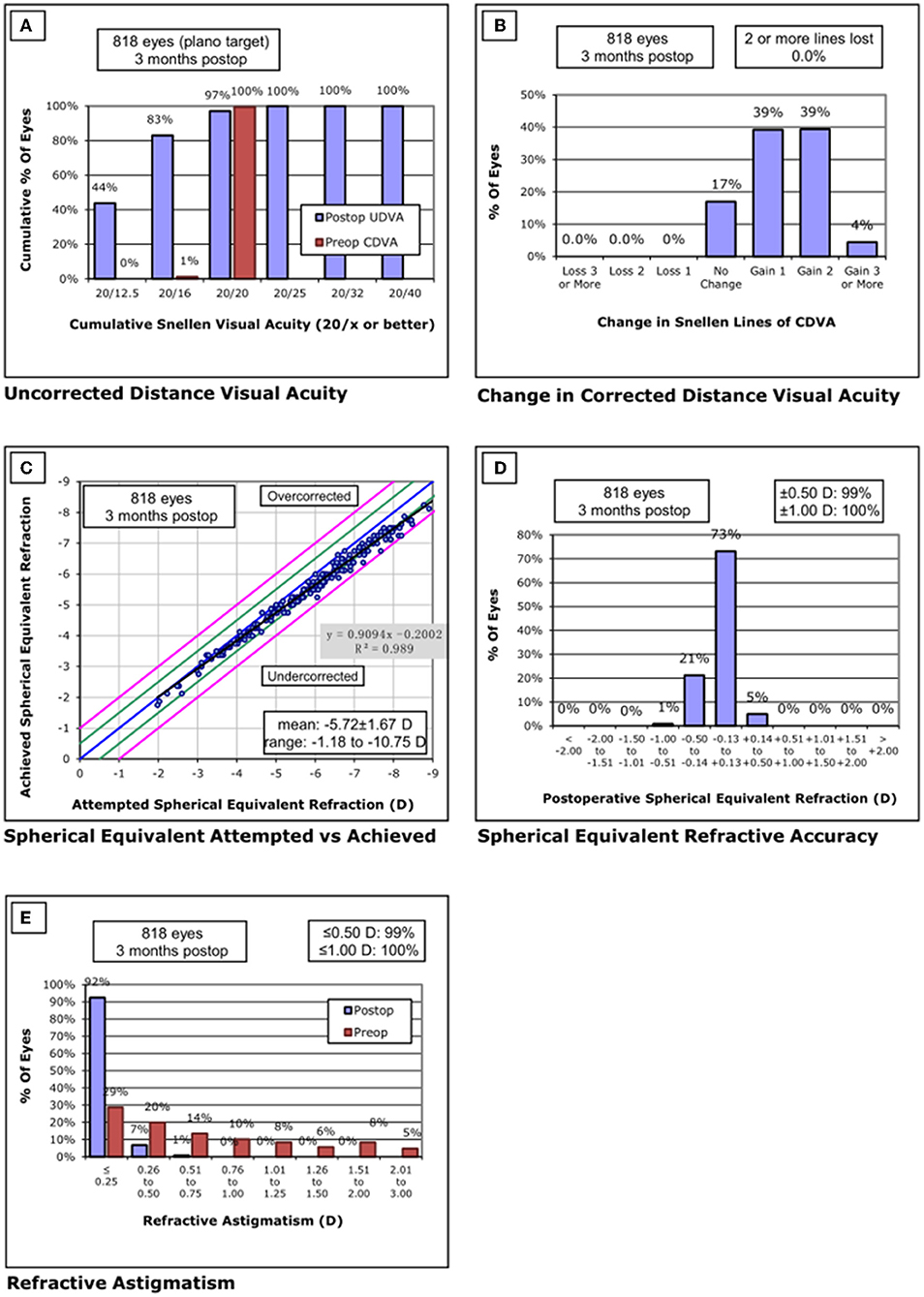
Figure 2. Standard graphs of refractive surgery visual and refractive outcomes for 830 eyes at 3 months post-SMILE in center A. (A) Uncorrected distance visual acuity. (B) Change in corrected distance visual acuity. (C) Spherical equivalent attempted vs. achieved. (D) Spherical equivalent refractive accuracy. (E) Refractive astigmatism. SMILE, small-incision lenticule extraction.
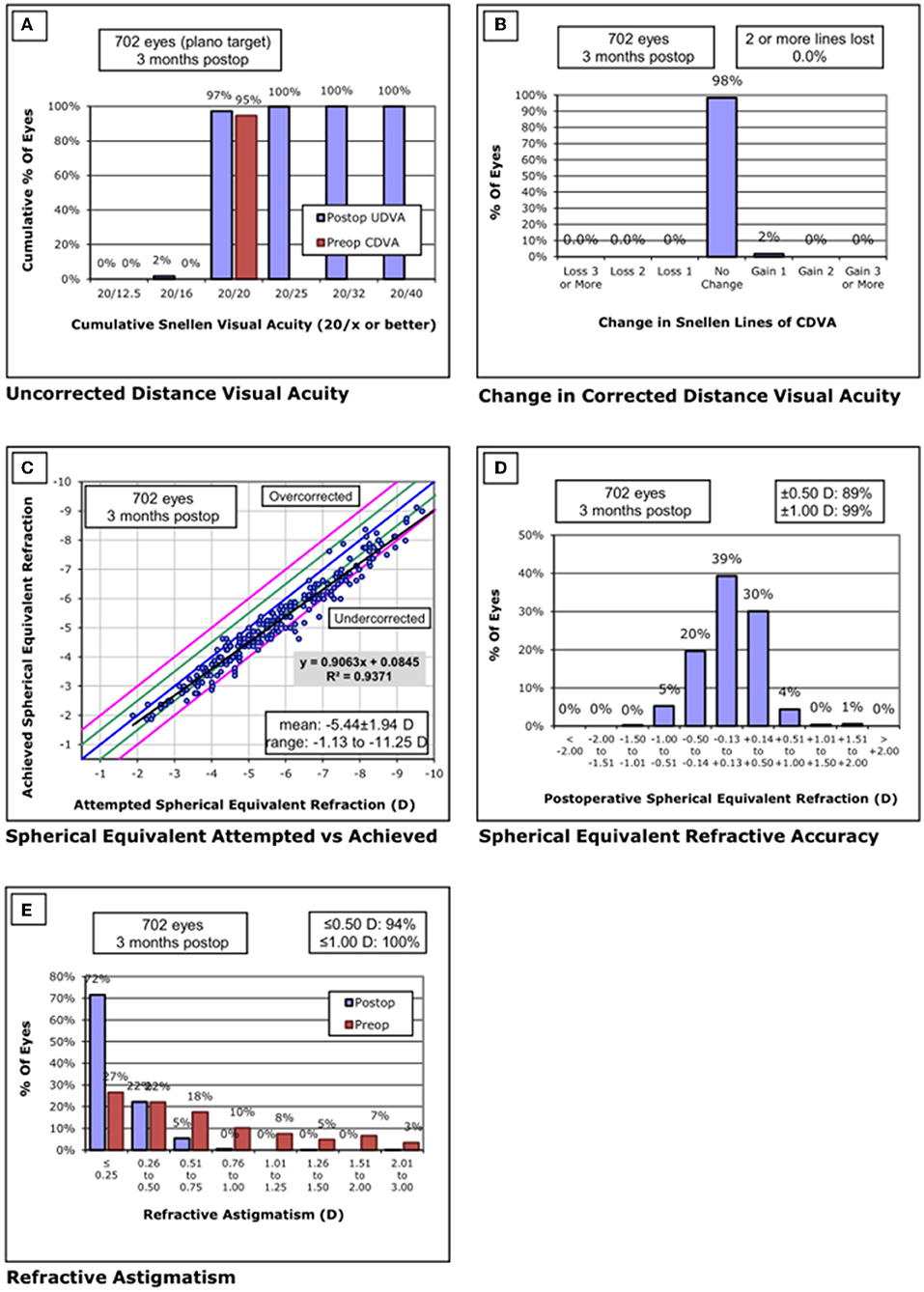
Figure 3. Standard graphs of refractive surgery visual and refractive outcomes for 702 eyes at 3 months post-SMILE in center B. (A) Uncorrected distance visual acuity. (B) Change in corrected distance visual acuity. (C) Spherical equivalent attempted vs. achieved. (D) Spherical equivalent refractive accuracy. (E) Refractive astigmatism. SMILE, small-incision lenticule extraction.
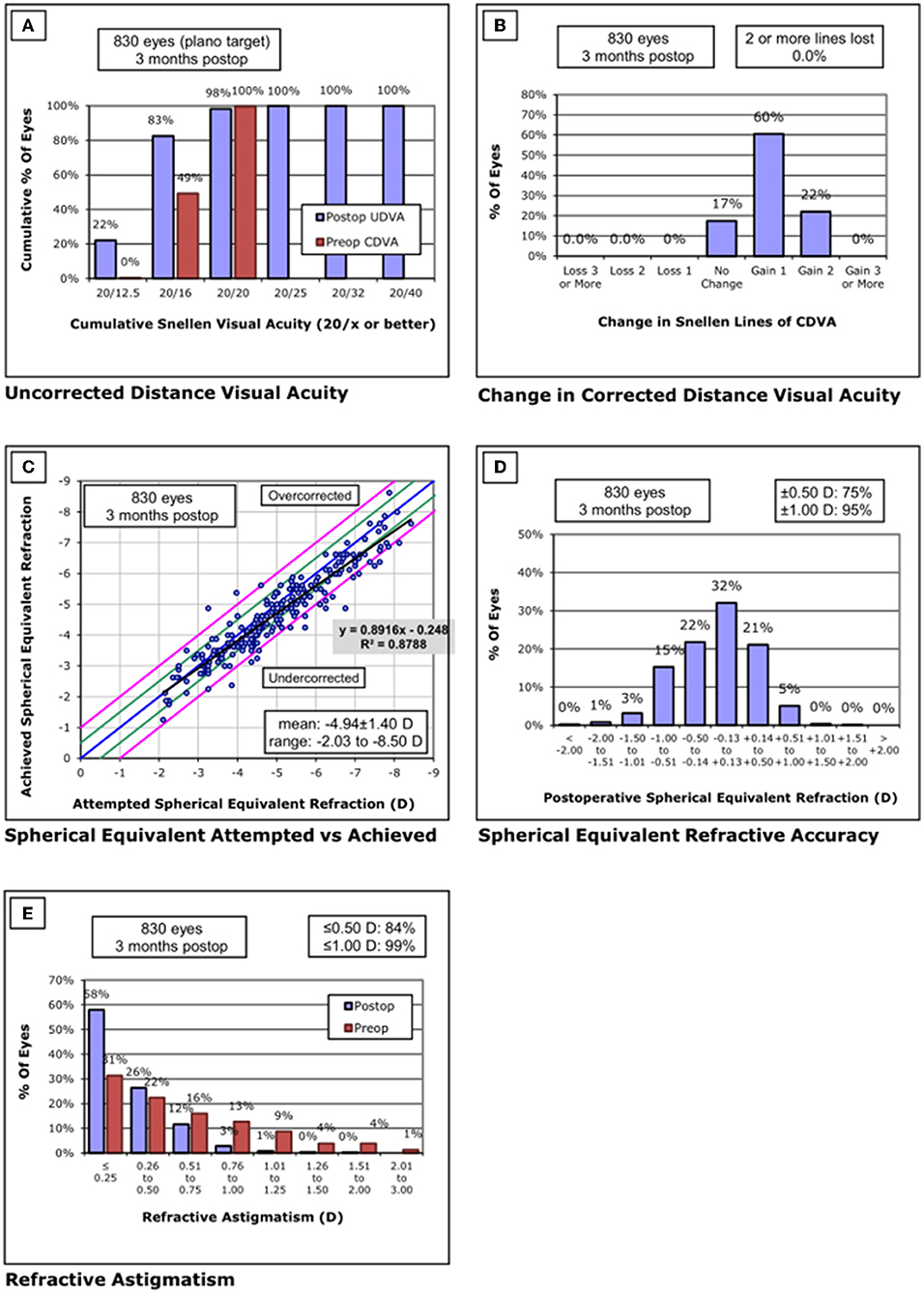
Figure 4. Standard graphs of refractive surgery visual and refractive outcomes for 830 eyes at 3 months post-SMILE in center C. (A) Uncorrected distance visual acuity. (B) Change in corrected distance visual acuity. (C) Spherical equivalent attempted vs. achieved. (D) Spherical equivalent refractive accuracy. (E) Refractive astigmatism. SMILE, small-incision lenticule extraction.
None of the eyes lost two or more lines of CDVA. The safety index was 1.32 ± 0.24. Throughout the follow-up, the UDVA was 20/20 or better in 794/818 eyes (97%) and equal to or better than the preoperative CDVA in 818/818 eyes (100%). The efficacy index was 1.31 ± 0.25. The postoperative SE was within ±0.50 D of the attempted correction in 99% of eyes and within ±1.00 D in all the eyes.
None of the eyes lost two or more lines of CDVA. The safety index was 1.03 ± 0.08. Throughout the follow-up, the UDVA was 20/20 or better in 682/702 eyes (97%) and equal to or better than the preoperative CDVA in 702/702 eyes (100%). The efficacy index was 1.02 ± 0.08. Postoperative SE was within ±0.50 D of the attempted correction in 89% of the eyes and within ±1.00D in 99% of the eyes.
None of the eyes lost two or more lines of CDVA. The safety index was 1.13 ± 0.16. Throughout the follow-up, the UDVA was 20/20 or better in 816/830 eyes (98%) and equal to or better than the preoperative CDVA in 830/830 eyes (100%). The efficacy index was 1.13 ± 0.17. Postoperative SE was within ±0.50 D of the attempted correction in 75% of the eyes and within ±1.00D in 95% of the eyes.
The safety, efficacy, and predictability of SMILE were confirmed in all the patients in our study. After analyzing a total of 20 parameters, including anterior segment features, preoperative parameters, and surgical design parameters, valuable and interesting results were obtained. Corneal curvature, CCT, SD, SE, UDVA, RST, maximum lenticule thickness, and nomogram were the factors affecting postoperative refraction after SMILE. In addition, mean anterior corneal curvature, CCT, SD, and SE were the most influential features of postoperative refraction among the nine common features.
There are various factors that impact the SMILE procedure in order to obtain better vision outcome. In the study, the contribution of each parameter was obtained by combining the data in multicenter, so that the top factors influencing the surgery were acquired. The factors that influence the postoperative refraction of SMILE include not only corneal parameters but also preoperative refraction and surgical parameters. Our findings indicate that preoperative corneal parameters, including Pre-Km (r = −0.127, p < 0.001), Pre-K1 (r = −0.122, p < 0.001), and Pre-CCT(r = −0.058, p = 0.005), play a crucial role in postoperative refraction after SMILE. The diverse ocular biometric parameters are interactive. This result is consistent with that of a previous study in which in the eyes with low myopia, a steeper corneal curvature could lead to a greater undercorrections after SMILE (9), suggesting that a steeper corneal curvature is often associated with high myopia, which tends to be undercorrected after SMILE (22, 23). In the current study, the entire corneal thickness was negatively correlated with the postoperative SE. This might be attributed to the differences in corneal biomechanics based on corneal thickness (24).
Our study showed that preoperative refraction parameters, including Pre-SD (r = 0.369, p < 0.001), Pre-SE (r = 0.364, p < 0.001), and Pre-UDVA (r = 0.164, p < 0.001), had a positive correlation with postoperative SE after SMILE. A higher preoperative SD or SE is associated with a greater postoperative SE after photorefractive keratectomy, laser-assisted in situ keratomileusis, or SMILE (21, 25, 26). In addition, in our study, the higher the preoperative UDVA, the greater the postoperative SE, demonstrating that preoperative UDVA is somewhat predictive of postoperative surgical outcomes. Cui et al. (13) indicated that preoperative UDVA can affect the nomogram in SMILE, which may explain why preoperative UDVA plays a role in postoperative SE. Much more attention should be paid to the patient's preoperative UDVA in future studies to improve surgical outcomes.
Among surgical parameters, RST (r = 0.139, p < 0.001), Max (r = −0.311, p < 0.001), and nomogram (r = −0.100, p < 0.001) were noted to influence postoperative SE after SMILE. Ogasawara et al. (27) suggested that RST correlated with regression of myopia after laser-assisted in situ keratomileusis during long-term follow-up and that adequate RST is important to preserve a good UDVA. Nevertheless, there was no obvious correlation between UDVA and postoperative SE. In this study, preserving more RST was beneficial in obtaining a greater postoperative SE. It is worth noting that the maximum lenticule thickness represents the actual corneal ablation depth. A tendency for undercorrection after surgery for high myopia compared to mild to moderate myopia is well documented (21). Evidence indicates that the nomogram plays an important role in the safety, efficacy, and predictability of corneal refractive surgery (28). In the eyes with high myopia 1 year after SMILE, the SE was significantly worse. Adjustment of the nomogram to 0.13 × attempted SE (D)-0.66 D has been suggested (23). In summary, more degrees need to be added in high myopia for correction.
Corneal cap thickness, sex, laterality (right/left), laser energy, and preoperative CDVA did not affect postoperative refraction in our cohort. Liu et al. (12) have demonstrated that a 110-μm cap thickness had better visual outcomes postoperatively compared with a 150-μm cap thickness. In contrast, another study found that postoperative refraction was not significantly affected by cap thickness of 100 and 120 μm in SMILE (11). In our study, cap thickness ranged from 110 to 140 μm, which may have led to different results. In contrast with a previous study on the impact of the energy setting on visual outcomes after SMILE (14), the influence of laser energy was clinically insignificant in our study. Although a Visumax 500 kHz femtosecond laser was used in all patients, the temperature or humidity settings might have been different in the three centers. In addition, the large number of parameters analyzed might explain why laser energy contributed less to postoperative refraction.
The current study has both strengths and limitations. Due to the strong covariance of the data, the linear model is not effective at the beginning of this study. However, Applying information gain, a ranking of the importance of 20 features affecting postoperative SE was derived in the study. In particular, although its design was retrospective, this study included a large number of eyes from three ophthalmic centers. However, different surgical setups may result in measurement errors. In addition, further statistical analysis in the study revealed that no correlation was found between monocular and binocular, which may reduce the possible risk of wrong results due to the violation of the assumption of independence. Finally, the outcomes in center A varied widely compared to those of centers B and C. The reason for this is that the surgeon in center A has extensive experience and has been performed more than 10,000 SMILE procedures since 2011.
In summary, our study assessed factors affecting postoperative refraction after SMILE. Among 20 parameters evaluated in three ophthalmic centers, preoperative mean anterior corneal curvature, CCT, SD, and SE significantly affected postoperative refraction. A larger preoperative Km and CCT is associated with a smaller preoperative SD and RST and a smaller postoperative SE. These findings can be used to optimize the outcomes of SMILE surgery. The refractive surgery surgeons should pay more attention to the patient's preoperative Km, CCT, SD and RST in the daily routine to obtain great outcome for postoperative refraction.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethical Committee of Tianjin Eye Hospital. The patients/participants provided their written informed consent to participate in this study.
YW, SL, SJ, MC, and YLe designed and performed the research. SL and XL organized the manuscript writing. XL, SJ, and YLi analyzed the data. JH, ML, HZ, YP, and ZM collected the data. VJ and YW reviewed the manuscript. YW obtained funding. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (No. 81873684). The funding organization had no role in the design or conduct of this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors treasured the comments and suggestions from reviewers sincerely.
SMILE, small-incision lenticule extraction; CCT, central corneal thickness; SE, spherical equivalent; CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity; K1, flattest anterior corneal curvature; Km, mean anterior corneal curvature; Max, maximum lenticule thickness; Pre-UDVA, preoperative uncorrected distance visual acuity; Pre-SD, preoperative spherical diopter; Pre-SE, preoperative spherical equivalent; Pre-Km, preoperative mean anterior curvature; Pre-K1, preoperative flattest anterior corneal curvature; Pre-K2, preoperative steepest anterior corneal curvature, RST, residual stromal thickness.
1. Sánchez-González JM, Alonso-Aliste F. Visual and refractive outcomes of 100 small incision lenticule extractions (SMILE) in moderate and high myopia: a 24-month follow-up study. Graefes Arch Clin Exp Ophthalmol. (2019) 257:1561–7. doi: 10.1007/s00417-019-04349-4
2. Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, Stobrawa G, et al. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg. (2008) 34:1513-20. doi: 10.1016/j.jcrs.2008.05.033
3. Yu M, Chen M, Dai J. Comparison of the posterior corneal elevation and biomechanics after SMILE and LASEK for myopia: a short- and long-term observation. Graefes Arch Clin Exp Ophthalmol. (2019) 257:601-6. doi: 10.1007/s00417-018-04227-5
4. Reinstein DZ. The time has come for refractive surgery to be included in the fight against global visual impairment due to uncorrected refractive error. J Refract Surg. (2022) 38:6-8. doi: 10.3928/1081597X-20211109-03
5. Hjortdal JØ, Vestergaard AH, Ivarsen A, Ragunathan S, Asp S. Predictors for the outcome of small-incision lenticule extraction for myopia. J Refract Surg. (2012) 28:865–71. doi: 10.3928/1081597X-20121115-01
6. Primavera L, Canto-Cerdan M, Alio JL, Alio Del Barrio JL. Influence of age on small incision lenticule extraction outcomes. Br J Ophthalmol. (2020) 106:341-8. doi: 10.1136/bjophthalmol-2020-316865
7. Luger MHA, Ewering T, Arba-Mosquera S. Influence of patient age on high myopic correction in corneal laser refractive surgery. J Cataract Refract Surg. (2013) 39:204–10. doi: 10.1016/j.jcrs.2012.07.032
8. Tay E, Bajpai R. Visual recovery after small incision lenticule extraction (SMILE) in relation to pre-operative spherical equivalent. Graefes Arch Clin Exp Ophthalmol. (2021) 259:1053–60. doi: 10.1007/s00417-020-04954-8
9. Liu J, Wang Y. Influence of preoperative keratometry on refractive outcomes for myopia correction with small incision lenticule extraction. J Refract Surg. (2020) 36:374–9. doi: 10.3928/1081597X-20200513-01
10. Wu Y, Huang Z. Comparison of early visual quality in patients with moderate myopia using different optical zones in small incision lenticule extraction (SMILE). BMC Ophthalmol. (2021) 21:46. doi: 10.1186/s12886-020-01798-y
11. Taneri S, Arba-Mosquera S, Rost A, Hansson C, Dick HB. Results of thin-cap small-incision lenticule extraction. J Cataract Refract Surg. (2021) 47:439–44. doi: 10.1097/j.jcrs.0000000000000470
12. Liu T, Yu T, Liu L, Chen K, Bai J. Corneal cap thickness and its effect on visual acuity and corneal biomechanics in eyes undergoing small incision lenticule extraction. J Ophthalmol. (2018) 2018:6040873. doi: 10.1155/2018/6040873
13. Cui T, Wang Y, Ji S, Li Y, Hao W, Zou H, et al. Applying machine learning techniques in nomogram prediction and analysis for SMILE treatment. Am J Ophthalmol. (2020) 210:71–7. doi: 10.1016/j.ajo.2019.10.015
14. Li L, Schallhorn JM, Ma J, Cui T, Wang Y. Energy setting and visual outcomes in SMILE: a retrospective cohort study. J Refract Surg. (2018) 34:11–6. doi: 10.3928/1081597X-20171115-01
15. Donate D, Thaëron R. SMILE with low-energy levels: assessment of early visual and optical quality recovery. J Refract Surg. (2019) 35:285–93. doi: 10.3928/1081597X-20190416-01
16. Gu H, Guo Y, Gu L, Wei A, Xie S, Ye Z, et al. Deep learning for identifying corneal diseases from ocular surface slit-lamp photographs [Sci. rep.:17851]. Sci Rep. (2020) 10:17851. doi: 10.1038/s41598-020-75027-3
17. Yang X, Chen G, Qian Y, Wang Y, Zhai Y, Fan D, Xu Y. Prediction of myopia in adolescents through machine learning methods. Int J Environ Res Public Health. (2020) 17:463. doi: 10.3390/ijerph17020463
18. Cao K, Verspoor K, Sahebjada S, Baird PN. Evaluating the performance of various machine learning algorithms to detect subclinical keratoconus. Transl Vis Sci Technol. (2020) 9:24. doi: 10.1167/tvst.9.2.24
19. Tabares-Soto R, Orozco-Arias S, Romero-Cano V, Segovia Bucheli V, Rodríguez-Sotelo JL, Jiménez-Varón CF. A comparative study of machine learning and deep learning algorithms to classify cancer types based on microarray gene expression data. PeerJ Comput Sci. (2020) 6:e270. doi: 10.7717/peerj-cs.270
20. Chuck RS, Jacobs DS, Lee JK, Afshari NA, Vitale S, Shen TT, et al. Refractive errors and refractive surgery preferred practice Pattern®. Ophthalmology. (2018) 125:P1–104. doi: 10.1016/j.ophtha.2017.10.003
21. Jin HY, Wan T, Wu F, Yao K. Comparison of visual results and higher-order aberrations after small incision lenticule extraction (SMILE): high myopia vs. mild to moderate myopia. BMC Ophthalmol. (2017) 17:118. doi: 10.1186/s12886-017-0507-2
22. Muthu Krishnan V, Jayalatha K, Vijayakumar C. Correlation of central corneal thickness and keratometry with refraction and axial length: a prospective analytic study. Cureus. (2019) 11:e3917. doi: 10.7759/cureus.3917
23. Wu W, Wang Y, Zhang H, Zhang J, Li H, Dou R. One-year visual outcome of small incision lenticule extraction (SMILE) surgery in high myopic eyes: retrospective cohort study. BMJ Open. (2016) 6:e010993. doi: 10.1136/bmjopen-2015-010993
24. Fu D, Zhao Y, Zhou X. Corneal biomechanical properties after small incision lenticule extraction surgery on thin cornea. Curr Eye Res. (2021) 46:168–73. doi: 10.1080/02713683.2020.1792507
25. Mimouni M, Vainer I, Shapira Y, Levartovsky S, Sela T, Munzer G, et al. Factors predicting the need for retreatment after laser refractive surgery. Cornea. (2016) 35:607–12. doi: 10.1097/ICO.0000000000000795
26. Yan MK, Chang JS, Chan TC. Refractive regression after laser in situ keratomileusis. Clin Exp Ophthalmol. (2018) 46:934–44. doi: 10.1111/ceo.13315
27. Ogasawara K, Onodera T. Residual stromal bed thickness correlates with regression of myopia after LASIK. Clin Ophthalmol. (2016) 10:1977–81. doi: 10.2147/OPTH.S116498
Keywords: myopia, small-incision lenticule extraction, contributing factors, information gain, multicenter
Citation: Liang S, Ji S, Liu X, Chen M, Lei Y, Hou J, Li M, Zou H, Peng Y, Ma Z, Liu Y, Jhanji V and Wang Y (2022) Applying Information Gain to Explore Factors Affecting Small-Incision Lenticule Extraction: A Multicenter Retrospective Study. Front. Med. 9:837092. doi: 10.3389/fmed.2022.837092
Received: 16 December 2021; Accepted: 28 March 2022;
Published: 03 May 2022.
Edited by:
Georgios Panos, Nottingham University Hospitals NHS Trust, United KingdomCopyright © 2022 Liang, Ji, Liu, Chen, Lei, Hou, Li, Zou, Peng, Ma, Liu, Jhanji and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wang, d2FuZ3lhbjcxNDNAdmlwLnNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.