- General ICU, Henan Key Laboratory of Critical Care Medicine, Zhengzhou Key Laboratory of Sepsis, Henan Engineering Research Center for Critical Care Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Aim: The purpose of this study was to explore prognostic factors of bloodstream infections (BSIs), a common severe infection and a major cause of mortality worldwide, so as to construct a prognosis model of patients with BSI.
Materials and Methods: Clinical and biochemical test data were obtained retrospectively from the medical records of 562 patients with BSI who had been treated at a single center; the end point was 60 days of all-cause death. The chi-square test was used to compare the mortality of patients grouped by the types of antibiotic treatment. The logistic regression analysis was adopted to identify prognostic factors; the Kaplan–Meier survival curve and log-rank test were conducted to compare the survival rate of patients with different prognostic factors; the receiver operating characteristic (ROC) curve was used to estimate the predictive value of different prognostic factors.
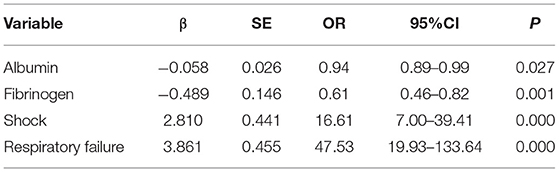
Results: Of the 562 patients, 455 survived (80.96%), and 107 died (19.04%). The mortality rate of patients treated with a combination of antibiotics (25.40%) was higher than that treated with a single antibiotic (15.82%). Univariate analysis identified 19 prognostic factors for patients with BSI, including gender, age, diabetes, malignant tumor (non-blood system), total hospitalization time, alanine aminotransferase, aspartate aminotransferase, total protein, albumin, total bilirubin, direct bilirubin, creatinine, ratio of granulocytes, fibrinogen, D-dimer, platelet, C-reactive protein, shock, and respiratory failure (P < 0.05). Multivariate analysis indicated that albumin (odds ratio [OR] = 0.94, 95% confidence interval [CI]: 0.89–0.99), fibrinogen (OR = 0.61, 95%CI: 0.46–0.82), shock (OR = 16.61, 95%CI: 7.00–39.41), and respiratory failure (OR = 47.53, 95%CI: 19.93–133.64) were independent factors. The combination of four indicators demonstrated a favorable predictive value for the 60-day outcome of patients with BSI, with an area under the ROC of 0.96 (95%CI: 0.94–0.99), sensitivity of 90.65%, specificity of 94.95%, and accuracy of 94.13%.
Conclusions: Shock, respiratory failure, albumin, and fibrinogen are potential independent prognostic factors for 60-day mortality.
Introduction
Bloodstream infection (BSI) is a common severe infection and a major cause of mortality worldwide; its morbidity is comparable to that of acute myocardial infarction and trauma (1, 2). BSIs can be caused by bacteria, fungi, viruses, and protozoa, but among the four groups of pathogens, bacteria account for most BSIs. Streptococci rank in the top 10 bacterial pathogens causing BSI. Streptococcus comprises common bacteria of pyogenic cocci and includes three different species: α-hemolytic streptococcus, β-hemolytic streptococcus, and γ-streptococcus (non-hemolytic). β-hemolytic streptococcus is an important invasive infection in humans, causing skin and soft tissue infection, infective endocarditis, arthritis, bacteremia, and so forth (3, 4). Streptococcus BSI has a poor prognosis. The most dangerous outcome is toxic shock syndrome complicated by multiple organ dysfunctions, which has a mortality rate of 50–70% (5, 6). Therefore, it is necessary to explore more reliable indicators for detecting and evaluating the inflammatory response, infection, and sepsis so that the appropriate treatment measures can be implemented, ultimately improving survival rates and prolonging the survival time of patients with streptococcus BSI.
An epidemiological study including 9,268 cases of BSI in southeast Sweden indicated that age, gender, and cancer were significantly associated with 30-day mortality (7). Based on an analysis of 147 patients with BSI, Samuel et al. reported that factors associated with the increased risk of BSI developing severe streptococcal infection risk included older age, neutropenia at the onset BSI, and source of respiratory infection (8). Alessander et al. revealed 13 potential proteins correlated with mortality and persistent bacteremia caused by Staphylococcus aureus and identified interleukin (IL)-8 and CCL2 as the strongest individual predictors of mortality (9). However, there have been few studies on prognostic factors (mainly including biochemical indicators and clinical characteristics) of patients with streptococcus-associated BSI.
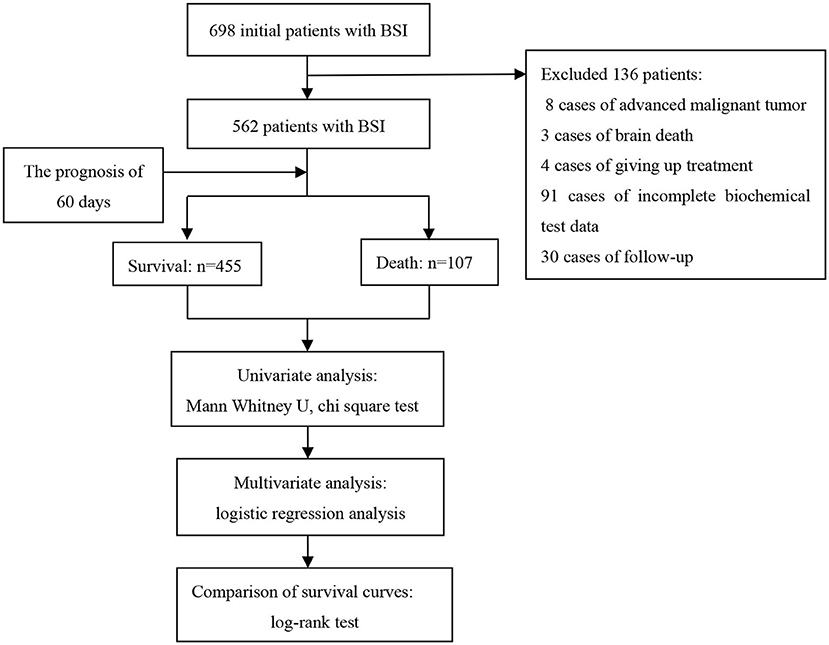
In this study, we explored the prognostic factors of patients with streptococcus BSI based on a prospective follow-up cohort and constructed a prognosis model of biochemical indicators and clinical characteristics. The study design is illustrated in Figure 1.
Materials and Methods
Patients in the Study
In this study, 698 patients with BSI treated in the First Affiliated Hospital of Zhengzhou University from January 2015 to December 2019 were selected as research objects. Finally, according to the inclusion and exclusion criteria, 562 patients with BSI were included in this study. The inclusion criteria were that blood culture and clinical symptoms (e.g., a clear source of infection, elevated body temperature, elevated infection indicators, and simultaneous culture of streptococcus in other parts) supported streptococcus BSI. Patients with a terminal-stage malignant tumor, brain death, loss of follow-up, abandonment of treatment, and/or data loss were excluded.
Information Collection
Patients with BSI were followed up by telephone from January 2020 to March 2020, and the end point was 60 days of all-cause death. According to a prognosis of 60 days, the patients were divided into death group and survival group. The clinical and biochemical test data of all subjects were collected from a comprehensive electronic medical record using standardized data collection tables, which mainly included gender, age, past history, respiratory failure, shock, hospitalization time, alkaline phosphatase, alanine aminotransferase, indirect bilirubin, fibrinogen, D-dimer, platelet, C-reactive protein, and other indicators within 48 h after positive blood culture. Approval for this study was obtained from the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Ethical number: 2020-KY-157).
Statistical Analysis
IBM SPSS statistics software (version 21.0) and GraphPad Prism 6.0 were used for data analysis. Categorical data were expressed as percentages. Continuous data were described using the mean and standard deviation or median and quartile. The Kolmogorov–Smirnova test was used to determine whether the data had a normal distribution. The t-test was employed to compare the data with a normal distribution, and the Mann–Whitney U test was applied for data that did not conform to the normal distribution. The classification data were compared using chi-square or Fisher's exact test. The logistic regression analysis was adopted to identify prognostic factors. The Kaplan–Meier survival curve and log-rank test were performed to compare the survival rate of patients with different prognostic factors. The receiver operating characteristic (ROC) curve was used to estimate the predictive value of different prognostic factors. All P values were determined based on the two-tailed test, and P < 0.05 was considered significant.
Results
Characteristics of the Study Population
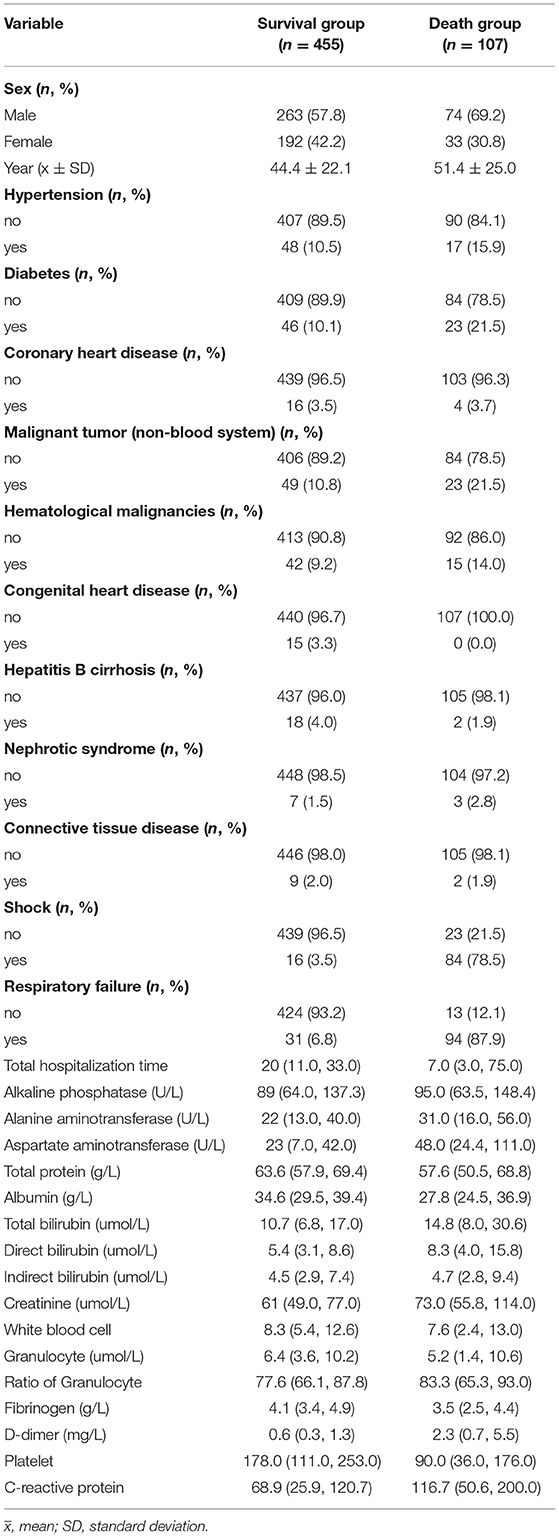
Among 698 patients with BSI, 136 cases were excluded, including 8 cases of end-stage malignant tumor, 3 cases of brain death, 4 cases of giving up treatment, 91 cases of incomplete biochemical test data, and 30 cases of patients due to loss of follow-up. The follow-up rate was 94.9% (562/592). Thus, 562 eligible patients were used for statistical analysis, including 337 males and 225 females. The patients were divided into survival group (n = 455 [80.96%]) and death group (n = 107 [19.04%]) based on the outcome of 60 days. The ages of the survival group and death group were 44.4 ± 22.1 and 51.4 ± 25.0 years, respectively. The basic characteristics of the 562 participants are summarized in Table 1.
Different Types of Antibiotics Used by Patients With BSI
Of the 562 patients with BSI, 373 patients were treated with a single antibiotic, and 189 patients were treated with a combination of antibiotics. The results of the chi-square test analysis showed that the mortality rate of patients using a combination of antibiotics was significantly higher than that of patients using a single antibiotic (25.40 and 15.82%, respectively; Table 2). This study did not support the combined treatment of streptococcus bloodstream infection. The seven most commonly used single antibiotics included the following: β-lactam addase inhibitor, carbapenems, cephalosporins, quinolones, glycopeptides, oxazolidinones, fourth generation cephalosporin, penicillins, and tetracyclines (Table 3). Analysis of the mortality of the three most commonly used single antibiotics revealed that the mortality of the β-Lactam addase inhibitor (10.29%) was significantly lower than that of carbapenems (25.49%; P = 0.002; Table 4). Among the combined antibiotics used, the top five most commonly used were carbapenem + glycopeptides, β- lactam plus enzyme inhibitor + quinolones, carbapenem + oxazolidinones, cephalosporins + quinolones and β-lactam plus enzyme inhibitor + glycopeptides (Table 4).

Table 2. Comparison of mortality of patients with blood stream infection treated with single and combined antibiotics.

Table 4. Comparison of mortality of patients with bloodstream infection treated with a common single antibiotic.
Prognostic Factors Associated With Streptococcus Bloodstream Infection
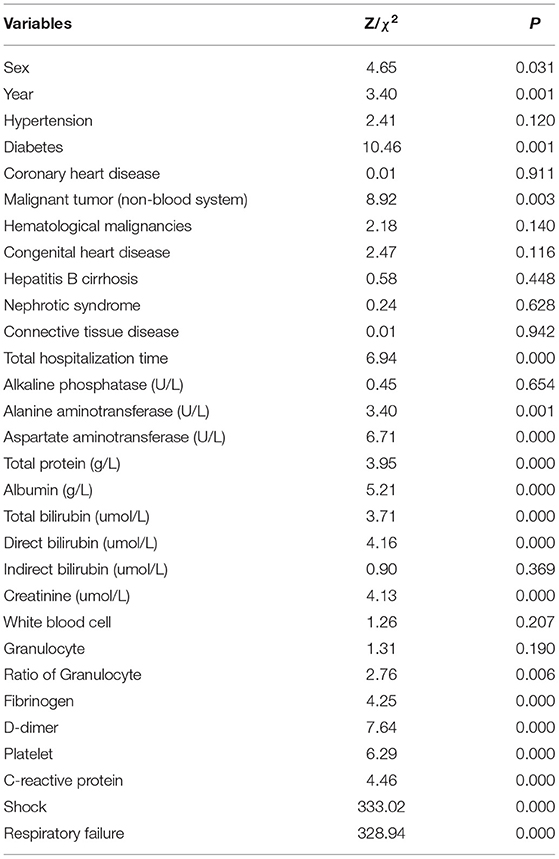
The Kolmogorov–Smirnova analysis was performed to test the normality of continuous data. The results showed that the data did not conform to the normal distribution (P < 0.05). Therefore, we applied univariate analysis to explore the potential prognostic factors of patients with BSI (Table 5). Compared with the survival group, patients in the death group were older (Z = 3.40, P = 0.001); with lower levels of total protein (Z = 3.95, P < 0.001), albumin (Z = 5.21, P < 0.001), fibrinogen (Z = 4.25, P < 0.001), and platelet (Z = 6.29, P < 0.001); with higher levels of alanine aminotransferase (Z = 3.40, P = 0.001), aspartate aminotransferase (Z = 6.71, P < 0.001), total bilirubin (Z = 3.71, P < 0.001), direct bilirubin (Z = 4.16, P < 0.001), creatinine (Z = 4.13, P < 0.001), ratio of granulocytes (Z = 2.76, P = 0.006), D-dimer (Z = 7.64, P < 0.001), and C-reactive protein (Z = 4.46, P < 0.001). Meanwhile, the incidence of diabetes (χ2 = 10.46, P = 0.001), non-hematological malignancies (χ2 = 8.92, P = 0.003), respiratory failure (χ2 = 328.94, P < 0.001), and shock (χ2 = 333.02, P < 0.001) was higher in the death group. Moreover, the total hospitalization time (Z = 6.94, P < 0.001) was shorter, and the proportion of men (χ2 = 4.65, P = 0.031) was higher in the death group. Furthermore, 19 factors with statistical significance in univariate analysis were used as independent variables, and the 60-day outcome of patients with BSI were used as dependent variables for the multivariate logistic regression analysis (Table 6). The results indicated that shock (OR = 16.61, 95%CI: 7.00–39.41) and respiratory failure (OR = 47.53, 95%CI: 19.93–133.64) were independent risk factors of 60-day death, and higher levels of albumin (OR = 0.94, 95%CI: 0.89–0.99) and fibrinogen (OR = 0.61, 95%CI: 0.46–0.82) were independent protective factors for patients with BSI.
Survival Analysis of Patients With BSI in Prognostic Factors
Further, to test the four independent prognostic factors (shock, respiratory failure, albumin, and fibrinogen), we used the log-rank test to make a survival analysis on patients with BSI. The median survival time of 562 patients was 60 days, and the 60-day cumulative survival rate was 81%. We defined the albumin range of 35–55 g/L and the fibrinogen range of 2–4 g/L as normal. The survival analysis of patients with BSI in terms of prognostic factors is presented in Figure 2. At the time of this analysis, the cumulative survival rates of patients without and with shock were 95% (median OS, 60 days) and 16% (median OS, 5 days), respectively (χ2 = 478.04, P < 0.001). Patients without respiratory failure had a better prognosis than patients with respiratory failure (97 vs. 25%), and the difference was statistically significant (χ2 = 431.79, P < 0.001). For patients without and with respiratory failure, the median survival times were 60 and 8 days, respectively. In terms of albumin level, the cumulative survival rate of patients with a normal level was significantly higher than that of patients with an abnormal level (88 vs. 76%; median OS: 60 vs. 60 days; χ2 = 13.00, P < 0.001). As can be seen from the figure, the normal level of fibrinogen has a negative effect on prognosis, but there was no statistical significance in the difference (χ2 = 2.69, P = 0.101).
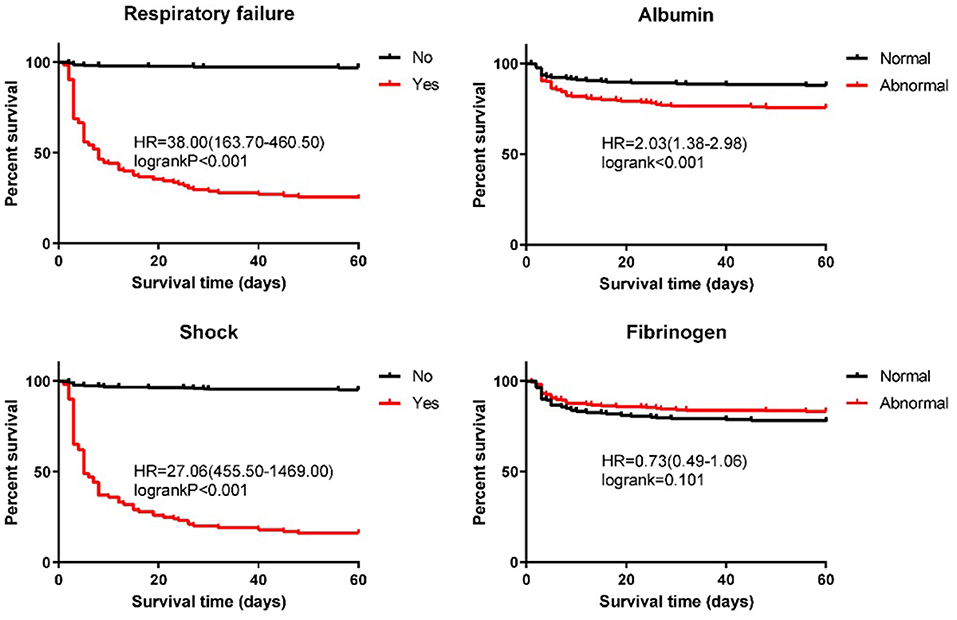
Figure 2. Kaplan–Meier estimates of the overall survival for patients with streptococcus bloodstream infection.
Predictive Value of Different Prognostic Factors for Patients With BSI
Since shock, respiratory failure, albumin, and fibrinogen were independent prognostic factors, we further analyzed the predictive value of four indicators and their combination for the prognosis of patients with BSI (Figure 3). The ranges of sensitivity and specificity of the four factors were 49.53–87.85% and 72.09–96.48%, respectively. The area under the ROC (AUC) is a comprehensive evaluation index to measure the accuracy of the binary classification model. The AUCs of the four factors ranged from 0.66 (95%CI: 0.60–0.72) to 0.90 (95%CI: 0.87–0.94). Moreover, the results demonstrated that the combination of the four prognostic indicators had a high predictive value for the 60-day outcome of patients with BSI, with an AUC of 0.96 (95%CI: 0.94–0.99), sensitivity of 90.65%, specificity of 94.95%, positive likelihood ratio of 17.95, negative likelihood ratio of 0.10, and accuracy of 94.13%.
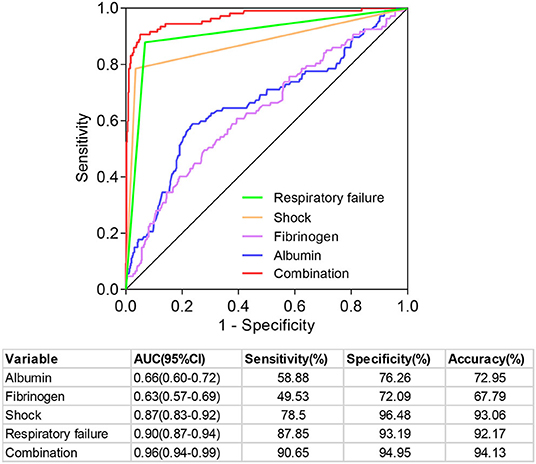
Figure 3. Predictive value of different prognostic indicators in predicting the prognosis of patients with bloodstream infection.
Discussion
Among the 562 BSI patients included in this study, more patients used a single antibiotic (66.37%) than the combined antibiotics (33.63%), and the most commonly used single antibiotics and combined antibiotics were β-lactam addase inhibitor and carbapenem + glycopeptides, respectively. It is worth noting that the mortality was higher in patients treated with a combination of antibiotics (25.40%) than patients treated with a single antibiotic (15.82%), perhaps because patients treated with combined antibiotics had more serious cases. Additionally, the physician in charge believed that the patients were co-infected with other bacterial infections, although there were no other positive etiological results.
In the present study, significant factors associated with the 60-day mortality of patients with BSI in the univariate analysis included older age, male, shorter hospitalization stay, diabetes, non-hematological malignancies, shock, respiratory failure; lower total protein, albumin, fibrinogen, and platelet levels; and higher alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, creatinine, ratio of granulocytes, D-dimer, and C-reactive protein levels. After adjusting for possible confounding factors, a prognostic model consisting of shock, respiratory failure, albumin, and fibrinogen was established for predicting the outcome of patients with BSI. Most diseases were age-related; in our current research, patients who died had a higher average age (51.4 ± 25.0 vs. 44.4 ± 22.1 years; P = 0.001), which is consistent with research by Kevin et al. (10). Cancer is the main cause of deterioration of clinical outcomes in patients with invasive streptococcosis (11, 12). Our data showed more patients with malignancy in the death group (21.5 vs. 10.8%; P = 0.003), which also supported previous reports. Notably, our results suggested that the proportion of patients with diabetes in the death group was higher than that in the survival group (21.5 vs. 10.1%; P = 0.001). In a multinational population-based assessment of group B streptococcus BSI, diabetes was the most common comorbidity (13).
Among these four factors that were determined to be crucial survival factors for patients with BSI, higher albumin and fibrinogen levels might be protective indicators for prognosis. A recent report on inflammatory biomarkers in patients with BSI demonstrated that lower albumin levels in neutropenic BSI indicate a poorer infection prognosis (14). Albumin, accounting for 55% of plasma protein, is completely synthesized by the liver and has a long half-life of ~20 days (15). Albumin has several important functions, including material binding, material transportation, enzyme activity, and antioxidation (16). It can regulate the main signal of inflammatory cells according to the redox state, which reduces body inflammation, protects organ function, and reduces acute kidney injury (17). Studies have confirmed that human serum albumin can achieve an ideal effect in the treatment of sepsis (18). Fibrinogen is a pleiotropic protein that plays an important role in blood coagulation, inflammation, and tissue repair and can alter many aspects of inflammatory cell function by involving white blood cells through various cell receptors and mechanisms (19, 20). Studies have shown that S. aureus can produce the virulence factor Efb in forming a “fibrinogen barrier” to inhibit platelet activation and phagocytosis of innate immune cells (21, 22). Mo et al. reported that pulmonary infection caused coagulation abnormality in the body, and various coagulation factors were gradually consumed in the process of thrombosis, so the levels of coagulation factors and fibrinogen decreased (23).
Sepsis and bacteremia are called BSIs. Severe sepsis can cause shock, disseminated intravascular coagulation, and multiple organ failure. In this study, shock and respiratory failure were risk indicators for prognosis in patients with BSI. A study on Klebsiella pneumoniae BSI and predictors of mortality pointed out that septic shock (OR:6.42, 95% CI: 1.34–30.69) was an independent risk factor for 28-day mortality of K. pneumoniae BSI (24). A report on risk factors and outcomes in K. pneumoniae BSIs of 370 patients revealed that respiratory failure (OR:5.27, 95% CI: 1.40–19.87) was independently associated with higher mortality risk (25).
This study used the logistic regression to construct the prediction model of patients with BSI. It is a supervised statistical learning method and has the great advantage that the prediction results are output in the form of probability. In addition, the interpretability of the model is very simple. At present, there are many new modeling methods, such as ensemble modeling methods that can address non-linearity automatically without pre-specification (26). This study is the first to establish a prediction model related to the prognosis of patients with streptococcus BSI, which achieved high accuracy. This study also has certain limitations. Due to the existence of missing data, the blood lipid data of the study subjects were not included in the analysis. Additionally, this study was a single-center retrospective study. Therefore, sampling error and confounding factors may affect the outcome, which needs to be confirmed by further multicenter randomized controlled clinical studies.
In conclusion, this study demonstrated that shock, respiratory failure, albumin, and fibrinogen are independent prognostic factors for 60-day mortality, and the appropriate treatment measures should be taken in light of these prognostic factors to improve the survival rate of patients with BSI.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Written informed consent from the participants or their legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XD and TS contributed to conception and design of the study, wrote the first draft of the manuscript and wrote sections of the manuscript. RZ, XZ, and XD organized the database. XD performed the statistical analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the United Fund of National Natural Science Foundation of China (Grant No. U2004110), the National Natural Science Foundation of China (Grant No. 82172129).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Laupland KB, Gregson DB, Flemons WW, Hawkins D, Ross T, Church DL. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect. (2007) 135:1037–42. doi: 10.1017/S0950268806007631
2. Montassier E, Batard E, Gastinne T, Potel G, de La Cochetière MF. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol Infect Dis. (2013) 32:841–50. doi: 10.1007/s10096-013-1819-7
3. Chaiwarith R, Jullaket W, Bunchoo M, Nuntachit N, Sirisanthana T, Supparatpinyo K. Streptococcus agalactiae in adults at Chiang Mai University Hospital: a retrospective study. BMC Infect Dis. (2011) 11:149. doi: 10.1186/1471-2334-11-149
4. Farley MM, Harvey RC, Stull T, Smith JD, Schuchat A, Wenger JD, et al. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N Engl J Med. (1993) 328:1807–11. doi: 10.1056/NEJM199306243282503
5. Pandey M, Good MF. The quest for GAS vaccine. Oncotarget. (2015) 6:34063–4. doi: 10.18632/oncotarget.6140
6. Wang P, Yang Q, Zhou X, Xin-xin Z, Yao W, He W, et al. Clinical analysis of adults with toxic shock syndrome induced by Streptococcus pyogenes. Chin J Nosocomiol. (2016) 26:2251–2253+2259. doi: 10.11816/cn.ni.2016-160887
7. Holmbom M, Möller V, Nilsson LE, Giske CG, Rashid MU, Fredrikson M, et al. Low incidence of antibiotic-resistant bacteria in south-east Sweden: An epidemiologic study on 9268 cases of bloodstream infection. PLoS ONE. (2020) 15:e0230501. doi: 10.1371/journal.pone.0230501
8. Shelburne SA III, Tarrand J, Rolston KV. Review of streptococcal bloodstream infections at a comprehensive cancer care center, 2000–2011. J Infect. (2013) 66:136–46. doi: 10.1016/j.jinf.2012.11.007
9. Guimaraes AO, Cao Y, Hong K, Mayba O, Peck MC, Gutierrez J, et al. A prognostic model of persistent bacteremia and mortality in complicated Staphylococcus aureus bloodstream infection. Clin Infect Dis. (2019) 68:1502–11. doi: 10.1093/cid/ciy739
10. Laupland KB, Pasquill K, Parfitt EC, Steele L. Bloodstream infection due to β-hemolytic streptococci: a population-based comparative analysis. Infection. (2019) 47:1021–5. doi: 10.1007/s15010-019-01356-9
11. Lamagni TL, Neal S, Keshishian C, Powell D, Potz N, Pebody R, et al. Predictors of death after severe Streptococcus pyogenes infection. Emerg Infect Dis. (2009) 15:1304–7. doi: 10.3201/eid1508.090264
12. Blancas D, Santin M, Olmo M, Alcaide F, Carratala J, Gudiol F. Group B streptococcal disease in nonpregnant adults: incidence, clinical characteristics, and outcome. Eur J Clin Microbiol Infect Dis. (2004) 23:168–73. doi: 10.1007/s10096-003-1098-9
13. Ballard MS, Schønheyder HC, Knudsen JD, Lyytikäinen O, Dryden M, Kennedy KJ, et al. The changing epidemiology of group B streptococcus bloodstream infection: a multi-national population-based assessment. Infect Dis. (2016) 48:386–91. doi: 10.3109/23744235.2015.1131330
14. Chen S, Liu S, Yuan X, Wang H, Wen F. Evaluation of inflammatory biomarkers in pediatric hematology-oncology patients with bloodstream infection. J Pediatr Hematol Oncol. (2021) 23:e596–e600. doi: 10.1097/MPH.0000000000001935
15. Røsjø H, Masson S, Caironi P, Stridsberg M, Magnoli M, Christensen G, et al. Prognostic value of secretoneurin in patients with severe sepsis and septic shock: data from the Albumin Italian Outcome Sepsis Study. Crit Care Med. (2018) 46:e404–e410 doi: 10.1097/CCM.0000000000003050
16. Das P, Chaudhari SK, Das A, Kundu S, Saha C. Interaction of flavonols with human serum albumin: a biophysical study showing structure-activity relationship and enhancement when coated on silver nanoparticles[J]. J Biomol Struct Dyn. (2019) 37:1414–26. doi: 10.1080/07391102.2018.1462732
17. Zhang Y, Lee P, Liang S, Zhou Z, Wu X, Yang F, et al. Structural basis of non-steroidal anti-inflammatory drug diclofenac binding to human serum albumin. Chem Biol Drug Des. (2015) 86:1178–84. doi: 10.1111/cbdd.12583
18. Sun X, Lu Y, Li X, Chen Y, Guo C. Albumin vs.Crystalloids in fluid resuscitation for adults with severe spesis and septic shock:a meta-analysis. Herald Med. (2017) 36:804–8. doi: 10.3870/j.issn.1004-0781.2017.07.021
19. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. (2019) 133:511–20. doi: 10.1182/blood-2018-07-818211
20. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) 34:43–62. doi: 10.1007/s00281-011-0290-8
21. Ko YP, Kuipers A, Freitag CM, Jongerius I, Medina E, van Rooijen WJ, et al. Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface. PLoS Pathog. (2013) 9:e1003816. doi: 10.1371/journal.ppat.1003816
22. Ko YP, Liang X, Smith CW, Degen JL, Höök M. Binding of Efb from Staphylococcus aureus to fibrinogen blocks neutrophil adherence. J Biol Chem. (2011) 286:9865–74 doi: 10.1074/jbc.M110.199687
23. Mo M, Pan L, Yan G, Jiang L, Yang Z, Wu Q, et al. Association between coagulation indicators and all-cause mortality in sepsis-related acute kidney injury patients. Chin J Nephrol. (2019) 35:758–64. doi: 10.3760/cma.j.issn.1001-7097.2019.10.006
24. Zhang Y, Guo LY, Song WQ, Wang Y, Dong F, Liu G. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. (2018) 18:248. doi: 10.1186/s12879-018-3160-3
25. Xiao T, Yu W, Niu T, Huang C, Xiao Y. A retrospective, comparative analysis of risk factors and outcomes in carbapenem-susceptible and carbapenem-nonsusceptible Klebsiella pneumoniae bloodstream infections: tigecycline significantly increases the mortality. Infect Drug Resist. (2018) 11:595–606. doi: 10.2147/IDR.S153246
Keywords: streptococcus, bloodstream infection, prognostic, antibiotics, 60-day mortality
Citation: Duan X, Zhang R, Zhang X, Ding X and Sun T (2022) Identification of Prognostic Factors in Patients With Streptococcus Bloodstream Infection. Front. Med. 9:832007. doi: 10.3389/fmed.2022.832007
Received: 21 December 2021; Accepted: 25 March 2022;
Published: 26 April 2022.
Edited by:
Yong Jiang, Southern Medical University, ChinaReviewed by:
You Shang, Huazhong University of Science and Technology, ChinaZhongheng Zhang, Sir Run Run Shaw Hospital, China
Copyright © 2022 Duan, Zhang, Zhang, Ding and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongwen Sun, c3VudG9uZ3dlbkAxNjMuY29t
 Xiaoguang Duan
Xiaoguang Duan Xianfei Ding
Xianfei Ding Tongwen Sun
Tongwen Sun