- 1Naval Medical Research Center, Silver Spring, MD, United States
- 2The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD, United States
- 3Departamento de Enfermedades Infecciosas e Inmunología Pediátrica, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
- 4Laboratorio Clínico, Clínica Alemana de Santiago, Facultad de Medicina Clínica Alemana, Universidad del Desarrollo, Santiago, Chile
- 5Instituto de Ciencias e Innovación en Medicina (ICIM), Facultad de Medicina Clínica Alemana, Universidad del Desarrollo, Santiago, Chile
- 6Instituto de Medicina Preventiva Veterinaria, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile
- 7Programa de Investigación Aplicada en Fauna Silvestre, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile
- 8Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
Scrub typhus is a potentially severe rickettsiosis, caused by Orientia tsutsugamushi in the Asia-Pacific region. Recently, however, two distinct pathogens, “Candidatus Orientia chuto” and “Candidatus Orientia chiloensis”, have been discovered in the Middle East and South America, respectively. Since the novel pathogens differ significantly from O. tsutsugamushi, many established diagnostic methods are unreliable. This work describes the development and validation of a new quantitative real-time PCR (qPCR) assay (Orien16S) for the detection of all known Orientia species. Based on a 94 bp sequence of the 16S rRNA gene (rrs), Orien16S recognized DNA samples from O. tsutsugamushi (n = 41), Ca. O. chiloensis (n = 5), and Ca. O. chuto (n = 1), but was negative for DNA preparations from closely related rickettsiae and other members of the order Rickettsiales (n = 22) as well as unrelated bacterial species (n = 11). After its implementation in Chile, the assay was verified, correctly identifying all tested eschar and buffy coat samples (n = 28) of clinical suspected cases. Furthermore, Orien16S detected Orientia DNA in trombiculid mites collected in endemic regions in southern Chile. The presented novel qPCR assay provides a useful tool for detecting Orientia and diagnosing scrub typhus from all geographical regions.
Introduction
Orientia, one of the two genera of the family Rickettsiaceae in the order Rickettsiales, was previously considered to contain only a single species, Orientia tsutsugamushi, and to be confined to an area designated as the tsutsugamushi triangle, which includes areas in Asia, Australia, and islands of the Indian and Pacific Oceans (1, 2). Until 2010, this obligate intracellular pathogen was considered the exclusive cause of greater than 1 million annual cases of scrub typhus, a severe rickettsiosis with significant mortality (1). However, several autochthonous cases of scrub typhus have recently been reported on Chiloé Island and in other parts of southern Chile (3–5). Based on genetic analyses of 18 DNA preparations of clinical samples from scrub typhus patients, the pathogen causing scrub typhus in South America was found to be genotypically distinct to O. tsutsugamushi and Candidatus Orientia chuto (6), therefore currently designated as “Candidatus Orientia chiloensis” (7). Ca. O. chuto was originally described from samples of a patient returning from the United Arab Emirates (6). Sequences related to this organism were detected in mite samples collected from a village in Kenya (8), where humans were also seroreactive to O. tsutsugamushi, suggesting a wider, possibly global risk of scrub typhus caused by different species of Orientia (2).
Molecular analyses are the key diagnostic tools for the identification of new species of the order Rickettsiales and other intracellular bacteria (9). For the initial detection of scrub typhus cases in Chile, established molecular markers for O. tsutsugamushi, targeting sequences of the genes for 16S rRNA (rrs), 56-kDa type-specific antigen (tsa), and 47-kDa high temperature requirement A antigen (htrA), were used (3, 4). However, results were inconsistent, and several probable cases showed negative results using these classical targets (authors’ unpublished observations). Therefore, in 2017, the Naval Medical Research Center and the Chilean Rickettsia and Zoonosis Research Group designed a novel quantitative real-time PCR (qPCR) assay named Orien16S targeting a fragment of the Orientia genus-specific rrs sequence. Since then, the new assay has been evaluated and successfully applied to diagnose further human scrub typhus cases in Chile (5, 10). Furthermore, the test was used to detect Orientia DNA in trombiculid mites collected from Chiloé Island (11). Herein we present the technical details of the development of this assay, the validation process using a broad DNA panel of Orientia, Rickettsia, and other bacteria species, the comparison to an established qPCR assay for O. tsutsugamushi (Otsu47) (12), and its real-world performance in research laboratories in Chile with clinical samples and mite specimens.
Materials and Methods
DNA Samples
The panel used to determine the analytical performance of the novel qPCR assay (Orien16S) included DNA samples from: (1) Orientia species [O. tsutsugamushi (n = 41), Ca. O. chiloensis (n = 5), and Ca. O. chuto (n = 1)]; (2) Rickettsia species (n = 18); (3) Anaplasmataceae species (n = 4), (Anaplasma phagocytophilum, Ehrlichia chaffeensis, Neorickettsia risticii, and Neorickettsia sennetsu); (4) unrelated bacterial species (n = 11); and (5) human DNA (n = 1) (Roche Applied Sciences, Indianapolis, IN, United States) and normal mouse DNA (n = 4) (Table 1). DNA samples of Ca. O. chiloensis were extracted from a serum sample of the first scrub typhus patient (3), and eschar material (biopsy or swab) of four further cases from Chiloé Island and continental Chile diagnosed in 2015 and 2016 (5). The presence of the Orientia DNA in Chilean patient samples was confirmed by PCR and sequencing of the rrs and htrA genes. BLAST™ searches in GenBank1 showed the closest matches to the sequences were Orientia species and subsequent phylogenetic analyses grouped them as Ca. O. chiloensis (7). The single Ca. O. chuto DNA sample was extracted from cell culture derived from a blood specimen of the unique scrub typhus case from the Arabian Peninsula (6); sources of the remaining DNA samples of O. tsutsugamushi and other microorganisms have been described previously (12–14).
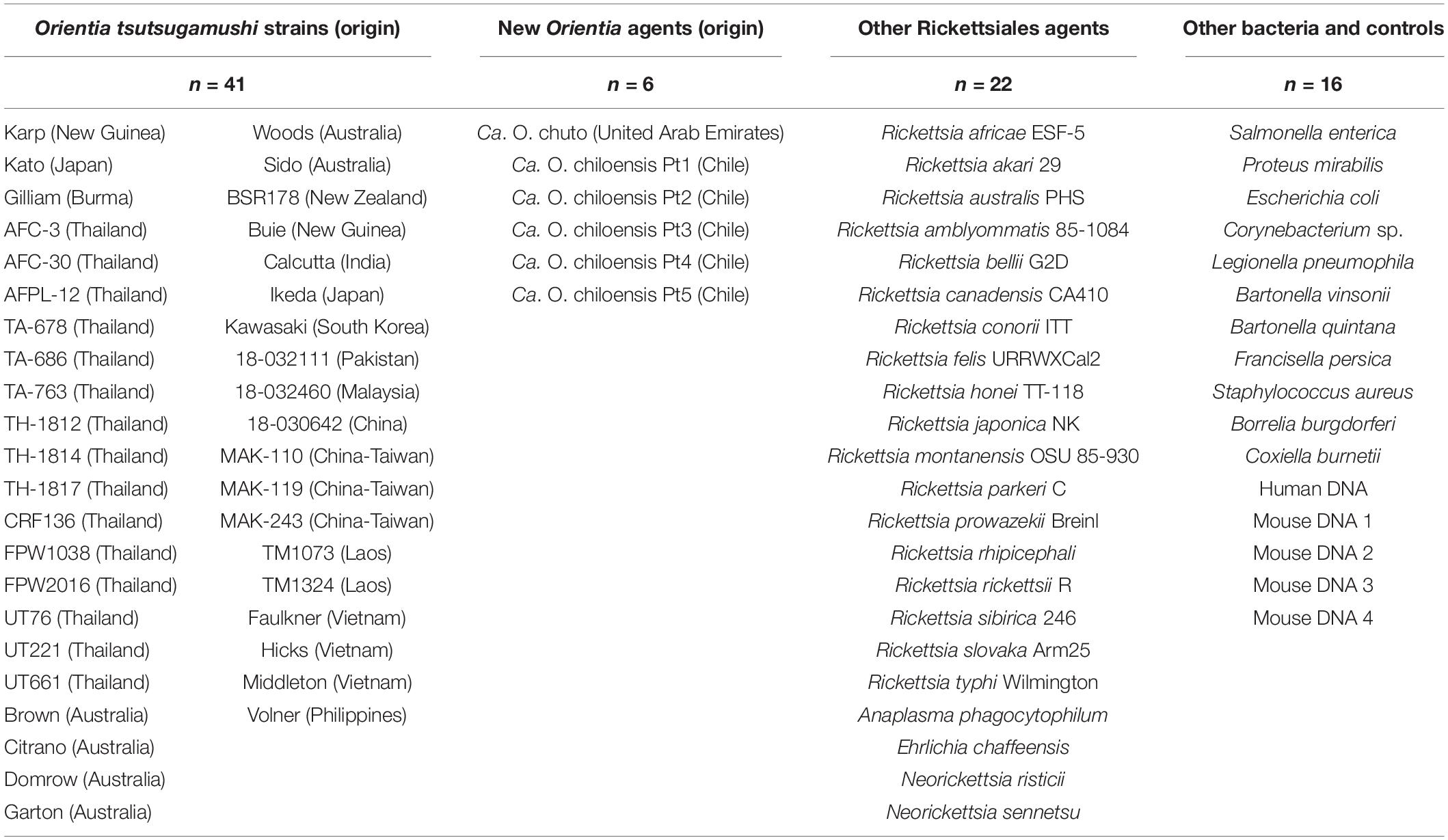
Table 1. DNA preparations from bacterial strains and other sources included in the validation of the novel qPCR assay, Orien16S.
A plasmid (pOrien6) with the target rrs sequence for Orien16S and htrA sequence for Otsu47 assays was designed using gene fragment synthesis and cloning (Eurofins Genomics, Louisville, KY, United States). We used pOrien6 not only as a positive control, but also to develop a standard curve to quantitate genome equivalents in samples and to define the limit of detection (LOD) of the Orien16S assay. The dried plasmid was resuspended in 1× TE buffer and the concentration of the plasmid was calculated. Tenfold serial dilutions were made ranging from 5 × 107 to 5 × 102 copies/μl, followed by half log serial dilutions down to 5 × 10–2 copies/μl.
Sequencing of the Chilean Orientia Isolates
Semi-nested PCR products from DNA preparations of four scrub typhus patients (Pt2–Pt5) were obtained using primers [16SO79F, 16SOR155F, and 16SOR1198R for rrs; and Otr47-263F, Otr47F, and Otr47-1404RL (GATTTACTTAT TAATGTTAGGTAAAGCAATGTAAAGCAT) for htrA] and amplification conditions described previously (7, 13). The amplicons of 986 bp of rrs and 1,421 bp of htrA were sequenced using Sanger method on a 3500 Genetic Analyzer (Thermo Fisher, Waltham, MA, United States) following procedures described previously (13). The sequences were submitted to GenBank with accession numbers: MZ773885 to MZ773888 for rrs from Pt2 to Pt5, respectively; and MZ773889 to MZ773891 for htrA from Pt2 to Pt4, respectively.
Design of the Primers and the Probe for Orien16S Quantitative Real-Time PCR Assay
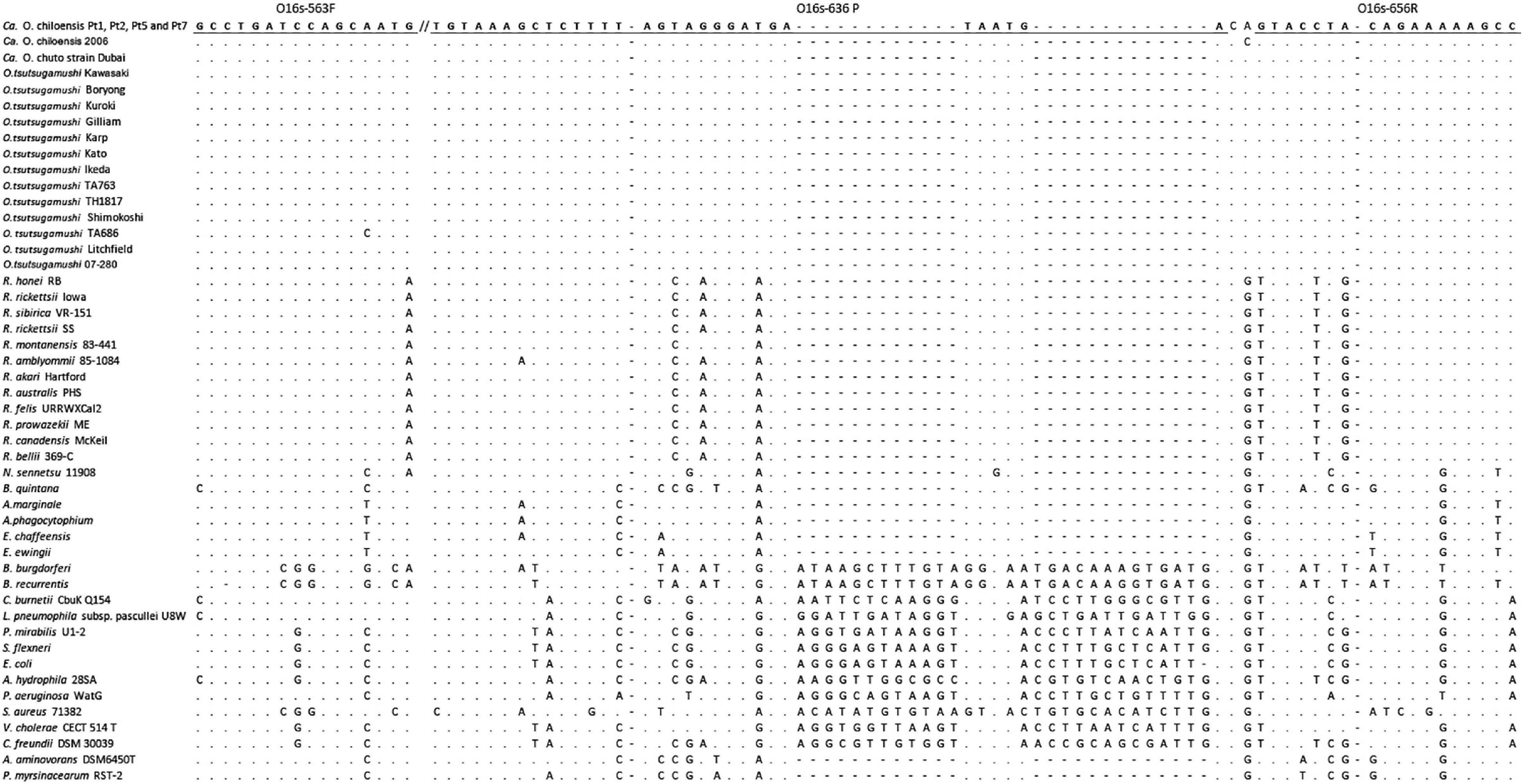
DNA sequences of rrs and htrA from various strains of O. tsutsugamushi, Ca. O. chuto, and Ca. O. chiloensis were studied to design the genus-specific qPCR assay; rrs was selected as the target gene due to the suitability of conserved sequences among all Orientia species assessed. The rrs sequences of 12 strains of O. tsutsugamushi from different geographical regions (Australia, New Guinea, Burma, Japan, Thailand, and South Korea), one strain of Ca. O. chuto (from United Arab Emirates) (6), and the initial isolate of Orientia species from Chile (3) were downloaded from GenBank. Sequences of the remaining four Chilean isolates used were obtained during this study. In addition, sequences of 12 Rickettsia species and nine other species of related Rickettsiales as well as 11 other bacteria were included. All sequences were aligned using the ClustalW within MEGA 7 (15). Then, the rrs conserved sequence fragments of Orientia species, which varied appreciably from other non-Orientia species, were used to design the primers and probe of the novel Orien16S qPCR assay (Figure 1). The suitability of the sequences as primers and probes were evaluated utilizing Beacon Designer software (Premier Biosoft, San Francisco, CA, United States). The selected primers (O16s-563F, 5′-GCCTGATCCAGCAATG-3′ and O16s-656R 5′-GGCTTTTTCTGTAGGTAC-3′) and the TaqMan probe (O16s-636 P 5′-FAM-TCATTATCAT CCCTACTAAAAGAGCTTTACA-BHQ-1-3′) were finalized after BLAST™ search for specificity.
Optimization of Orien16S TaqMan Quantitative Real-Time PCR Assay
The concentrations of the primers, the probe, and MgCl2 were optimized using Platinum Quantitative PCR SuperMix-UDG (Thermo Fisher, Waltham, MA, United States) and run on a StepOne Plus (Thermo Fisher) thermocycler system. Primer concentrations were varied simultaneously from 0.2 to 0.7 μM with steps of 0.1 μM, probe concentrations were varied from 0.1 to 0.5 μM (in steps of 0.1 μM), and the concentrations of MgCl2 were varied from 3 to 7 mM (in steps of 1 mM). The annealing temperature was optimized in the range from 56 to 61°C (in steps of 1°C).
The optimized conditions used for the final evaluation of Orien16S qPCR assay were: primers and probe at 0.3 μM, MgCl2 at 6 mM, and annealing/elongation temperature at 58°C; each 20 μl reaction for all the qPCR assays contained 2 μl of template DNA. The cycler parameters included: incubation at 50°C for 2 min (to allow for UDG contained within the master mix to function); initial denaturation at 95°C for 2 min; 45 cycles of denaturation at 95°C for 15 s; and annealing/elongation at 58°C for 30 s.
Comparison of Orien16S Quantitative Real-Time PCR Assay With Otsu47 Quantitative Real-Time PCR Assay
The amplification results (Ct values) of Orien16S were compared to those of another Orientia qPCR assay, Otsu47, using identical Orientia DNA samples. Otsu47 is a qPCR assay developed for specifically detecting O. tsutsugamushi (12). This assay targets a portion of htrA and demonstrated high sensitivity and specificity detecting all O. tsutsugamushi strains tested in a previous study (12). The plasmid pOrien6, was used as positive control for both assays at 103 copies/μl concentration.
Application of Orien16S in Chile
After its development and validation in the Naval Medical Research Center, the novel Orien16S assay was implemented in the molecular laboratory of the Chilean Rickettsia and Zoonosis Research Group in Santiago, Chile, where it was adapted to the LightCycler 2.0 and LightCycler 480 platforms (Roche Life Science, Basel, Switzerland). The assay was applied within our ongoing surveillance project to detect Orientia DNA in suspected scrub typhus patients in Chile. The project was approved by the Comité Ético Científico, Pontificia Universidad Catolica de Chile in Santiago, Chile (#12–170 and #160816007) and the Naval Medical Research Center, Silver Spring, MD, United States (PJT-16–24). Orien16S was used to screen clinical samples and the positive samples were then confirmed by nested PCR assays using Orientia-specific targets, as previously described (7). All cases were acquired in southern Chile, except for one, which represented an imported scrub typhus case from South Korea (16). Further information on sample processing as well as clinical and epidemiological features of the cases can be found elsewhere (5, 16–18). Furthermore, the Orien16S assay was applied within a vector study on Chiloé Island, which collected and identified trombiculid mites. Mite pools were screened by Orien16S for the presence of Orientia DNA and subsequently confirmed by Orientia-specific nested PCR (11).
Results
The sequences of the forward primer (16 bp), reverse primer (17 bp), and probe (31 bp) of the Orien16S qPCR were 100% identical to all species/stains of Orientia used in the sequence alignment except for O. tsutsugamushi strain TA686, which had 1 bp difference in the forward primer site (Figure 1). Primers were designed to produce a 94 bp PCR product, and the TaqMan probe was created to detect this product as a reverse complimentary sequence. Under the optimized conditions described above, a standard curve was generated using serial dilutions of pOrien6, ranging from 108 to 0.1 copies/reaction (n = 13 points), which showed a R2 value of 0.996 and an assay performance efficiency of 100.026%. The LOD of Orien16S was assessed using pOrien6 at 1, 3.16, 5, and 10 copies/reaction. Since samples containing 10 copies/reaction were consistently positive (100% of 30 runs), 10 copies was determined as the LOD.
The validation of Orien16S utilized a panel of 85 DNA preparations, including a variety of rickettsial microorganisms (Table 1). The new assay correctly identified all 47 Orientia specimens, which included O. tsutsugamushi and the two newly described Candidatus species Ca. O. chiloensis and Ca. O. chuto, thus demonstrating a genus-specific sensitivity of 100%. The determination of specificity utilized a panel of 38 samples, consisting of 18 Rickettsia species, 4 species of Anaplasmataceae, and 11 other bacterial species, as well as human and mouse DNA (Table 1). No false positive reactions were observed in this panel.
The head-to-head comparison of Orien16S with a previously established qPCR assay for O. tsutsugamushi (Otsu47) demonstrated that both detected 41 O. tsutsugamushi strains with similar average replication cycle thresholds (Cts) of 27.9 and 28.7, respectively (range 18.8–36.3 and 20.5–37.6, respectively; Table 2). However, Orien16S assay detected Ca. O. chuto more efficiently (Ct 28.4 vs Ct 42.8) and identified all five Ca. O. chiloensis samples, which Otsu47 failed to detect (Table 2).
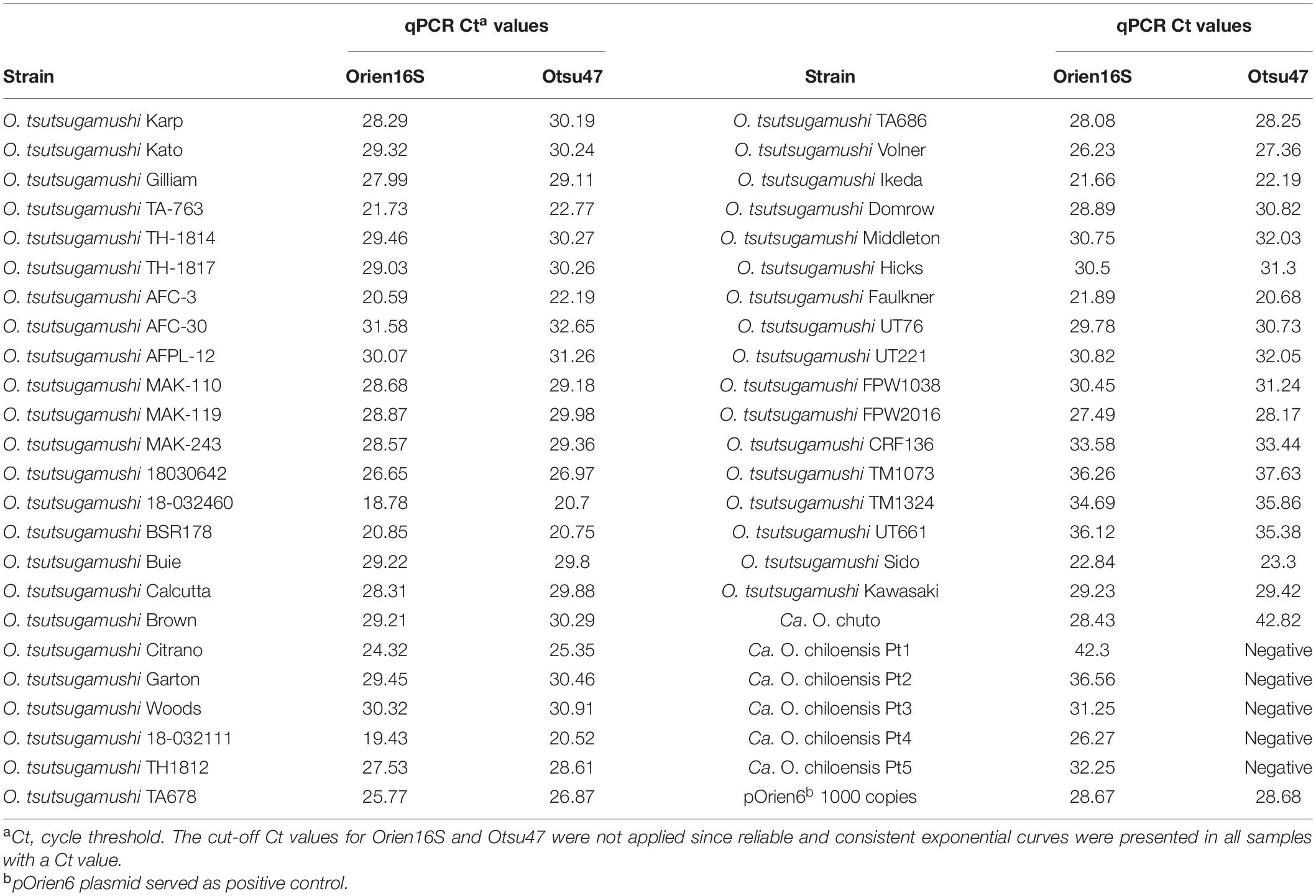
Table 2. Comparison of the amplification characteristics (Ct values) of Orien16S and Otsu47 qPCR assays with samples of Orientia tsutsugamushi, Ca. Orientia chuto, and Ca. Orientia chiloensis.
After its development, the new qPCR assay, Orien16S, was implemented in the molecular laboratory of the Chilean Rickettsia and Zoonosis Research Group, Santiago, Chile, and applied within our ongoing clinical surveillance and vector studies. In accordance with the validation data, Orien16S proved to be a reliable tool for the diagnosis of scrub typhus acquired in Chile and one imported O. tsutsugamushi case (4, 5, 7, 16–18). The new assay correctly identified 23 eschar samples and 5 buffy coat preparations, with Ct values ranging from 24.65 to 35.54 (Table 3, assay cut-off Ct = 36), all Orien16S positive samples were confirmed by Orientia-specific nested PCR protocols (7). The new assay was also used within a field project to investigate Orientia infection in chigger mites collected from captured rodents on Chiloé Island (11). As shown in Table 3, four mite pools confirmed by Orientia-specific nested PCR (targeting rrs) were identified by Orien16S with Ct values ranging from 30.94 to 33.48.
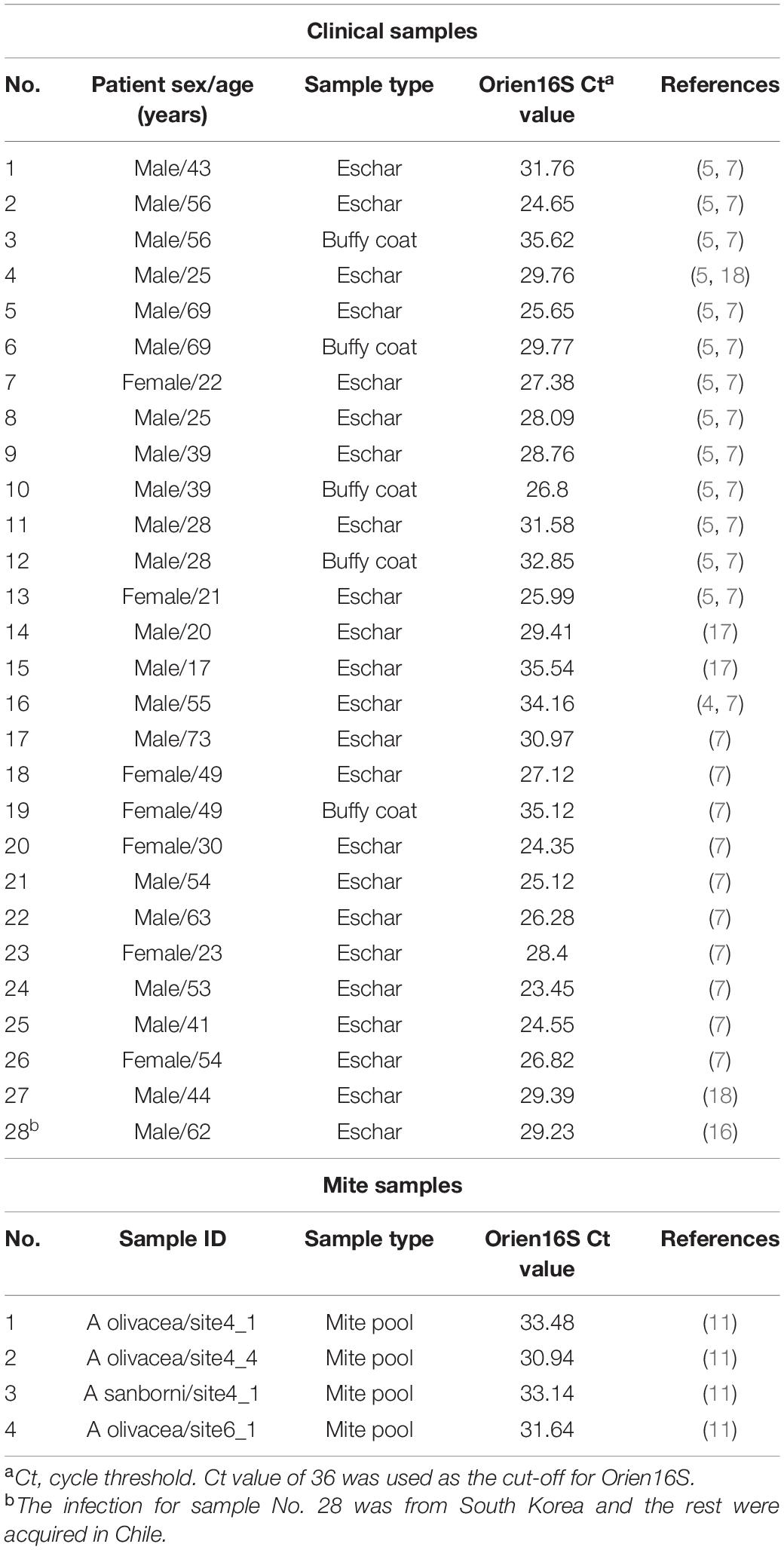
Table 3. Amplification results (Ct values) of new Orien16S qPCR assay in specimens from human scrub typhus cases and mite pools from Chiloé Island.
Discussion
Similar to other rickettsial diseases, scrub typhus can be a severe and potentially life-threatening infection and therefore requires rapid and effective treatment. However, The generalized flu-like symptoms are clinically indistinguishable from many febrile illnesses, such as dengue fever and other rickettsioses; the characteristic eschar for scrub typhus can also be caused by other bacterial infections (19); in addition, the strictly intracellular nature of these causative pathogens and the requirement of BSL3 labs make the isolation and culture impossible in most areas of the world. Due to these facts, timely and reliable identification is challenging. Molecular methods are nowadays considered the indispensable tool and are becoming a standard for the detection and identification of this group of microorganisms (20).
The Naval Medical Research Center has developed a qPCR assay, Otsu47, to detect O. tsutsugamushi (12). This assay has been used successfully around the world both in clinical and vaccine studies within the tsutsugamushi triangle (21, 22). However, Otsu47 performed poorly with the detection of Ca. O. chuto (13), and it did not recognize Orientia DNA from the first reported scrub typhus patient in Chile (3). Subsequent cases from Chile confirmed that Otsu47 and other established O. tsutsugamushi-specific PCR assays failed to consistently identify Orientia samples from this region (authors’ unpublished observations). The unsatisfactory performance of these detection methods targeting O. tsutsugamushi became comprehensible, when the cause of scrub typhus in Chile was identified as a novel Orientia agent distinct from O. tsutsugamushi and Ca. O. chuto (7).
In response to this diagnostic gap, we designed and validated a novel qPCR assay, Orien16S, which is based on genus-specific rrs sequences including those of Ca. O. chiloensis. After optimization, the LOD of this assay was determined to be 10 copies/reaction. Using a broad panel of O. tsutsugamushi samples of different geographical origins as well as samples of the two newly designated Orientia species (Ca. O. chuto and Ca. O. chiloensis), Orien16S demonstrated an excellent analytical sensitivity (100%). The assay also showed a high level of genus-specificity among a broad control panel of 22 species within the Rickettsiaceae and Anaplasmataceae families.
After its implementation in Chile, the Orien16S successfully detected Orientia DNA extracted from clinical samples of new or previously confirmed scrub typhus cases, including serum from the first Chilean case from 2006 (3) and a variety of sample types (blood, eschar material, and eschar swabs) from all other confirmed Chilean patients as well as the first known imported scrub typhus case in Chile caused by O. tsutsugamushi (Table 3). Due to its reliability and rapid turnaround time, it became the primary diagnostic tool within our ongoing scrub typhus surveillance in Chile. In the future, this new molecular tool may help to detect cases in other parts of Latin America, where scrub typhus caused by Ca. O. chiloensis might be present (23). Given that the Orien16S assay also detects Ca. O. chuto, it should be useful in the Middle East and Africa, where this species and/or related species may be endemic (2). Further evaluation of Orien16S by other research groups is needed to conclusively prove its utility for identifying orientiae in clinical samples.
In addition to clinical diagnosis, Orien16S was applied to identify Orientia DNA in chigger mites, collected in endemic areas in southern Chile (11); the assay reliably identified four Orientia-infected mite pools and the positive results were confirmed by nested PCR. The observed Ct values of those samples were within the same range as in clinical samples. However, we observed some pooled mite samples that produced higher Ct values by Orien16S but were not amplified by nested-PCR assays (data not shown). Some of those samples would be interpreted as positive, if their Ct values were within the range of the LOD (Ct < 36) and repeatedly demonstrating a consistent melting curve. Ct values well above 35 have been treated as positive as reported in a study on O. tsutsugamushi in chigger mites in Asia (22). Since trombiculid mites are less studied than other arthropod vectors, methodological standards for sample preparation and DNA extraction are lacking (10), higher Ct values from those mites might be due to methods used were not optimal. Interestingly, all Orientia-infected mites from our field study belonged to a newly described trombiculid species, Herpetacarus eloisae (24). Orien16S was also successfully applied to demonstrate the presence of Orientia DNA in rodent tissues from Chiloé Island (authors’ unpublished observations).
Conclusion
In conclusion, the new qPCR assay Orien16S is a highly sensitive and specific tool for the genus-specific detection of Orientia DNA. In our clinical and epidemiological studies in Chile, it has proven its usefulness to identify Ca. O. chiloensis in different types of patient samples and in trombiculid mites. The ability of the assay to detect all known Orientia species suggests that it might help to clarify the possible existence of scrub typhus in regions outside the tsutsugamushi triangle including Latin America, the Middle East, and Africa.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Comité Ético Científico, Pontificia Universidad Catolica de Chile in Santiago, Chile (#12–170 and #160816007) and the Naval Medical Research Center, Silver Spring, MD, United States (PJT-16–24). Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
Author Contributions
AR and JJ: conceptualization. JJ: methodology and visualization. JJ, CM-V, and GA-J: validation. JJ and TW: formal analysis and writing—original draft preparation. JJ, KA, and GA-J: investigation. AR and CF: resources. JJ and CM-V: data curation. AR, CF, CM-V, KA, and GA-J: writing—review and editing. AR, CF, and TW: supervision. CF and KA: project administration and funding acquisition. All authors have read and agreed to the final version of the manuscript.
Funding
This work was supported by the Global Disease Detection program of the US Centers for Disease Control and Prevention and the Armed Forces Health Surveillance Branch and its Global Emerging Infections Surveillance and Response (GEIS) Section (ProMIS ID P0071_19_NM_03), NMRC work unit number A1402, and by Fondo Nacional de Desarrollo Científico y Tecnologico (FONDECYT No. 1170810).
Author Disclaimer
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. CF is an employee of the U.S. Government and this work was prepared as part of her official duties. Title 17 U.S.C. §105 provides that copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Conflict of Interest
JJ and AR are employed by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographical distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. (2009) 48:S203–30. doi: 10.1086/596576
2. Jiang J, Richards AL. Scrub typhus: no longer restricted to the Tsutsugamushi Triangle. Trop Med Infect Dis. (2018) 3:11. doi: 10.3390/tropicalmed3010011
3. Balcells ME, Rabagliati R, García P, Poggi H, Oddó D, Concha M, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. (2011) 17:1659–63. doi: 10.3201/eid1709.100960
4. Weitzel T, Dittrich S, Lopez J, Phuklia W, Martinez-Valdebenito C, Velasquez K, et al. Endemic scrub typhus in South America. N Engl J Med. (2016) 375:954–61. doi: 10.1056/NEJMoa1603657
5. Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, Jiang J, Richards AL, Abarca K. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. (2019) 25:1214–7. doi: 10.3201/eid2506.181860
6. Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. (2010) 48:4404–9. doi: 10.1128/JCM.01526-10
7. Abarca K, Martínez-Valdebenito C, Angulo J, Jiang J, Farris CM, Richards AL, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. (2020) 26:2148–56. doi: 10.3201/eid2609.200918
8. Masakhwe C, Linsuwanon P, Kimita G, Mutai B, Leepitakrat S, Yalwala S, et al. Identification and characterization of Orientia chuto in trombiculid chigger mites collected from wild rodents in Kenya. J Clin Microbiol. (2018) 56:e01124–18. doi: 10.1128/JCM.01124-18
9. Richards AL. Worldwide detection and identification of new and old rickettsiae and rickettsial diseases. FEMS Immunol Med Microbiol. (2012) 64:107–10. doi: 10.1111/j.1574-695X.2011.00875.x
10. Weitzel T, Makepeace BL, Elliott I, Chaisiri K, Richards AL, Newton PN. Marginalized mites: neglected vectors of neglected diseases. PLoS Negl Trop Dis. (2020) 14:e0008297. doi: 10.1371/journal.pntd.0008297
11. Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, Silva-de La Fuente MC, Jiang J, Richards AL, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species. PLoS Negl Trop Dis. (2020) 14:e0007619. doi: 10.1371/journal.pntd.0007619
12. Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching W-M, Richards AL. Development of a quantitative real-time PCR assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. (2004) 70:351–6. doi: 10.4269/ajtmh.2004.70.351
13. Jiang J, Paris DH, Blacksell SD, Aukkanit N, Newton PN, Phetsouvanh R, et al. Diversity of the 47-kD HtrA nucleic acid and translated amino acid sequences from 17 recent human isolates of Orientia. Vector Borne Zoonotic Dis. (2013) 13:367–75. doi: 10.1089/vbz.2012.1112
14. Jiang J, An H, Lee JS, O’Guinn ML, Kim HC, Chong ST. Molecular characterization of Haemaphysalis longicornis-borne rickettsiae, republic of Korea and China. Ticks Tick Borne Dis. (2018) 9:1606–13. doi: 10.1016/j.ttbdis.2018.07.013
15. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. (2016) 33:1870–4. doi: 10.1093/molbev/msw054
16. Weitzel T, Aylwin M, Martínez-Valdebenito C, Jiang J, Munita JM, Thompson L, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. (2018) 4:10. doi: 10.1186/s40794-018-0070-8
17. Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, Richards AL, Grobusch MP, Abarca K. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. (2019) 29:78–9. doi: 10.1016/j.tmaid.2019.01.004
18. Weitzel T, Aylwin M, Martínez-Valdebenito C, Acosta-Jamett G, Abarca K. Scrub typhus in Tierra del Fuego: a tropical rickettsiosis in a Subantarctic region. Clin Microbiol Infect. (2021) 5:793–4. doi: 10.1016/j.cmi.2020.11.023
19. Mediannikov O, Socolovschi C, Million M, Sokhna C, Bassene H, Diatta G, et al. Molecular identification of pathogenic bacteria in eschars from acute febrile patients, Senegal. Am J Trop Med Hyg. (2014) 91:1015–9. doi: 10.4269/ajtmh.13-0629
20. Luce-Fedrow A, Mullins K, Jiang J, Richards AL. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol. (2015) 10:537–64. doi: 10.2217/fmb.14.141
21. Sunyakumthorn P, Paris DH, Chan TC, Jones M, Luce-Fedrow A, Chattopadhyay S, et al. An intradermal inoculation model of scrub typhus in Swiss CD-1 mice demonstrates more rapid dissemination of virulent strains of Orientia tsutsugamushi. PLoS One. (2013) 8:e54570. doi: 10.1371/journal.pone.0054570
22. Elliott I, Thangnimitchok N, de Cesare M, Linsuwanon P, Paris DH, Day NPJ, et al. Targeted capture and sequencing of Orientia tsutsugamushi genomes from chiggers and humans. Infect Genet Evol. (2021) 91:104818. doi: 10.1016/j.meegid.2021.104818
23. Silva-Ramos CR, Jacinavicius FC, Weitzel T, Walker DH, Faccini-Martínez ÁA. Scrub typhus: a new cause of acute undifferentiated febrile illness in Latin America? Travel Med Infect Dis. (2021) 43:102138. doi: 10.1016/j.tmaid.2021.102138
24. Silva-de la Fuente MC, Stekolnikov AA, Weitzel T, Beltrami E, Martínez-Valdebenito C, Abarca K, et al. Chigger mites (Acariformes: Trombiculidae) of Chiloé Island, Chile, with descriptions of two new species and new data on the genus Herpetacarus. J Med Entomol. (2021) 58:646–57. doi: 10.1093/jme/tjaa258
Keywords: scrub typhus, Orientia, Candidatus Orientia chiloensis, molecular diagnostic techniques, quantitative real-time PCR (qPCR), Orien16S, South America, Chile
Citation: Jiang J, Martínez-Valdebenito C, Weitzel T, Farris CM, Acosta-Jamett G, Abarca K and Richards AL (2022) Development of a New Genus-Specific Quantitative Real-Time PCR Assay for the Diagnosis of Scrub Typhus in South America. Front. Med. 9:831045. doi: 10.3389/fmed.2022.831045
Received: 07 December 2021; Accepted: 05 April 2022;
Published: 27 April 2022.
Edited by:
Sunil Dhiman, Defence Research and Development Establishment (DRDE), IndiaReviewed by:
John Antony Jude Prakash, Christian Medical College and Hospital, IndiaSmita Kulkarni, National AIDS Research Institute (ICMR), India
Lucas Blanton, University of Texas Medical Branch at Galveston, United States
Copyright © 2022 Jiang, Martínez-Valdebenito, Weitzel, Farris, Acosta-Jamett, Abarca and Richards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ju Jiang, amppYW5nQGhqZnJlc2VhcmNoLm9yZw==
 Ju Jiang
Ju Jiang Constanza Martínez-Valdebenito
Constanza Martínez-Valdebenito Thomas Weitzel
Thomas Weitzel Christina M. Farris
Christina M. Farris Gerardo Acosta-Jamett
Gerardo Acosta-Jamett Katia Abarca
Katia Abarca Allen L. Richards
Allen L. Richards