- Department of Rheumatology and Clinical Immunology, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science and Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Although the dense fine speckled (DFS) immunofluorescence staining pattern has been studied by various researchers in recent years, its clinical associations remain unspecified. Thus, we performed a retrospective study in a non-selective population to explore the prevalence of this enigmatic antinuclear antibody (ANA) pattern and to determine its possible clinical associations with any identifiable pathology.
Methods: We retrieved the results of ANA testing ordered by various departments in 2019 to study the prevalence of DFS pattern. Demographic characteristics and clinical features of these participants were also collected from the electronic medical record system. Correlation analysis was made to study its clinical associations and a p-value < 0.05 was considered statistically significant.
Results: The prevalence of ANA positivity was 37.4% among 72,204 serum samples of which the median age was 44 (interquartile range: 31, 56) years old and 68.0% were women. The prevalence of the DFS staining pattern was 1.1% in the total population and accounted for 3.1% in the ANA-positive population. There were 97.6% of these cases displaying the DFS pattern with a low titer of ANA (≤1:320; starting serum dilution: 1:100). We found that this pattern correlated with several pathological conditions, such as skin disorders (25.1%), alopecia (4.6%), and obstetric complications (6.6%).
Conclusion: The presence of the DFS immunofluorescence staining pattern may accompany several pathological conditions and may be a signal of localized inflammation within certain organs or tissues, especially the skin.
Introduction
Autoantibodies have been proven to play a critical role in the pathogenesis of autoimmune diseases (AiDs). For example, antinuclear antibodies (ANAs) can be found in most patients with systemic lupus erythematosus, with varied sensitivities and specificities in the diagnostic test. Therefore, ANA testing is a prerequisite for the clinical diagnosis of ANA-associated rheumatic diseases, and a positive ANA result in this context tends to reveal a propensity of pathology with an autoimmune origin. In addition, ANAs could even be present several years before clinical onset (1–4). Aside from autoimmune-related diseases, ANAs can also be detected in people with cancer or infectious diseases (5–9). Moreover, it was reported that a sizable proportion of sera from apparently healthy individuals could be ANA-positive, which may arouse concern and complicate clinical diagnosis, thus making the interpretation of ANA-positive results extremely important (10–16).
The HEp-2 cell indirect immunofluorescence (IIF) assay is the standard test for ANA testing and is a key approach in the laboratory diagnosis of ANA-associated rheumatic diseases (17). According to the International Consensus of ANA Patterns, IIF staining patterns on HEp-2 cell substrates have 29 classifications (from AC-01 to AC-29), some of which have been reported to have clinical relevance associated with specific autoantibodies or diseases (14, 18–22). Among these, the nuclear dense fine speckled (DFS) pattern (AC-02) is characterized by unique speckled staining distributed in both the nucleoplasm of interphase cells and the metaphase chromosomal plate, with heterogeneous size, brightness, and density of the speckles (18, 23, 24). Initially, reported in patients with interstitial cystitis, later studies observed this pattern in a wide spectrum of clinical conditions, such as chronic inflammation, asthma, atopic dermatitis, autoimmune thyroiditis, as well as in apparently healthy individuals (25, 26). Despite sharing morphological similarity with the nuclear homogeneous pattern (AC-01), the DFS pattern has a completely different clinical significance that needs to be clarified (27). To date, the DFS pattern has mainly been linked to anti-DFS70 antibodies in various studies. In fact, discordant positivity of anti-DFS70 antibody has been reported among cases with the DFS IIF pattern by previous studies (19, 28–31). Therefore, discussion about the DFS pattern itself is warranted to further demonstrate its potential value.
The prevalence of the DFS pattern was reported to vary between 0.3 and 27.0% in cohorts of consecutive or randomly selected patients tested for ANA (Supplementary Table 1) (19, 29–34). Although this pattern could be detected in healthy individuals, as mentioned above, its presence does not necessarily indicate an absence of pathology. Thus, accurate identification and interpretation of the nuclear DFS pattern is of crucial importance to assist in diagnostic decision-making. Despite the fact that various studies have tried to interpret this pattern, heterogeneity among these researches lead to contradictory findings, continuing to make it difficult to interpret its clinical relevance. To determine this enigmatical issue, we investigated the frequency and clinical associations of this DFS pattern in a large-scale ANA-positive cohort.
Methods
Study Design
This is a retrospective study conducted in the Chinese population. Results of consecutive samples under ANA testing ordered by various departments during 2019 were collected, which included immunofluorescence staining pattern and ANA titer. Besides, demographic characteristics and diagnoses or indications at the time of ANA testing were also retrieved from the electronic medical record system of Peking Union Medical College Hospital. With regard to duplicates, only the first record in the sampling time was included.
ANAs (IgG antibodies) were detected by the standard test HEp-2 immunofluorescence assay, which was performed on HEp-2 slides from EUROIMMUN AG (Lübeck, Germany) according to instructions with a starting serum dilution of 1:100. The visualization of ANA patterns was performed on EUROStar II microscope from EUROIMMUN AG (Lübeck, Germany) by two observers experienced in pattern reading. Samples displaying the DFS pattern were determined according to pattern-related characteristics (18, 23). Discordant readings of the slides were resolved by consensus or through a third observer.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital, and informed consent was waived due to the retrospective nature.
Statistical Analysis
Continuous variables with a non-normal distribution, such as age, are presented as medians with interquartile ranges (IQR). The chi-square test and Fisher's exact test were used to assess the association between unordered categorical variables, such as sex, age group, and ANA pattern. The Mann–Whitney U test was used to compare ANA titres between the AiDs group and the non-AiDs group. Statistical analyses were performed with SPSS-IBM v21, and the level of significance was set at p-value < 0.05.
Results
Prevalence of ANA Positivity and the DFS Pattern
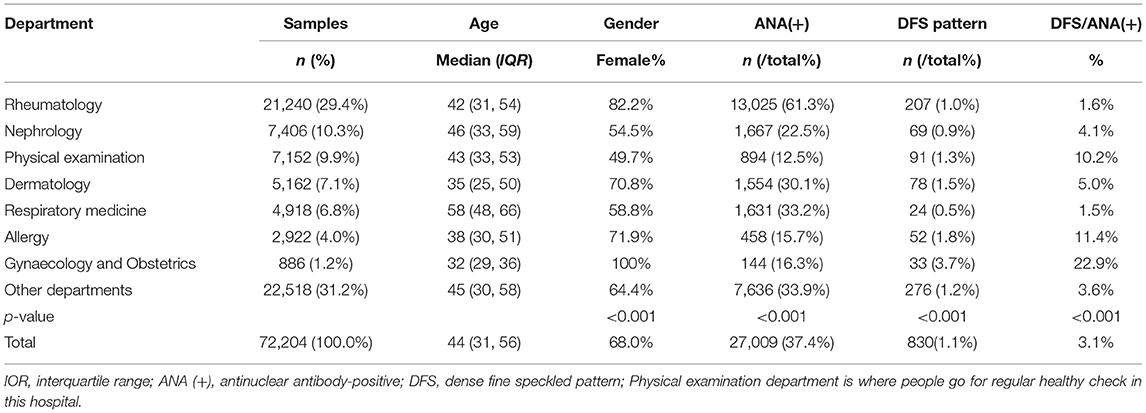
After eliminating redundancy, the total number of samples we took into analysis was 72,204, of which the median age was 44 years old and 68.0% were women (Table 1). The ages of samples from the Gynaecology and Obstetrics Department were generally younger while those from the Respiratory Medicine Department were older. Most ANA tests were ordered from the Rheumatology Department (21,240, 29.4%), Nephrology Department (7,406, 10.3%), and Physical Examination Department (7,152, 9.9%). The highest prevalence of ANA positivity (61.3%) was observed in the Rheumatology Department, followed by the Respiratory Medicine Department (33.2%), and the Dermatology Department (30.1%). The total number of cases that displayed the DFS pattern was 830, accounting for 1.1% of the total population and 3.1% of ANA-positive population, with 207 from the Rheumatology Department, 69 from the Nephrology Department, 91 from the Physical Examination Department, 78 from the Dermatology Department, 24 from the Respiratory Medicine Department, 52 from the Allergy Department, 33 from the Gynaecology and Obstetrics Department, and 276 from other departments. Notably, the prevalence of the DFS pattern varies by department and the Obstetrics and Gynaecology Department had the highest prevalence of the DFS pattern in ANA-positive population (22.9%, Table 1).
Basic Characteristics of Cases With the DFS Pattern
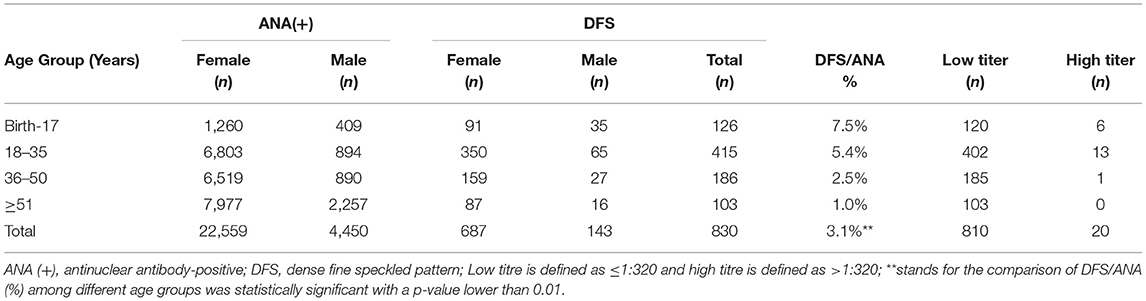
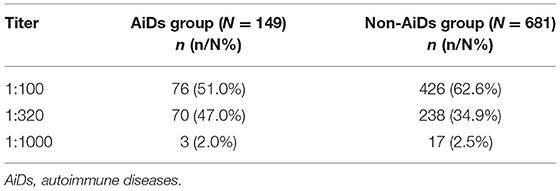
A total of 830 cases showed the DFS pattern on the IIF assay with a median age of 31 (IQR: 24, 39) years old, the gender ratio (F: M) of which was 4.8. The median age for women and men was 32 (IQR: 25, 40) and 28 (IQR: 18, 36) years old, respectively (p = 0.001). The majority of these cases were women between 18 and 35 years of age. The prevalence of the DFS pattern varied among different age groups and decreased with increasing age (Table 2). Most of these cases displayed a DFS pattern with a low titer of ANA (≤1:320, 97.6%), while the presence of a high titer of ANA (>1:320) was very rare (2.4%). Among 149 cases showing the DFS pattern diagnosed with AiDs, 146 had a titer lower than 1:320 or at 1:320, and the remaining 3 had a higher titer. There was statistical significance in titer distribution between AiDs and non-AiDs cases (p < 0.05), with the AiDs group at higher titers in general (Table 3).
Common Manifestations of Cases Showing the DFS Pattern
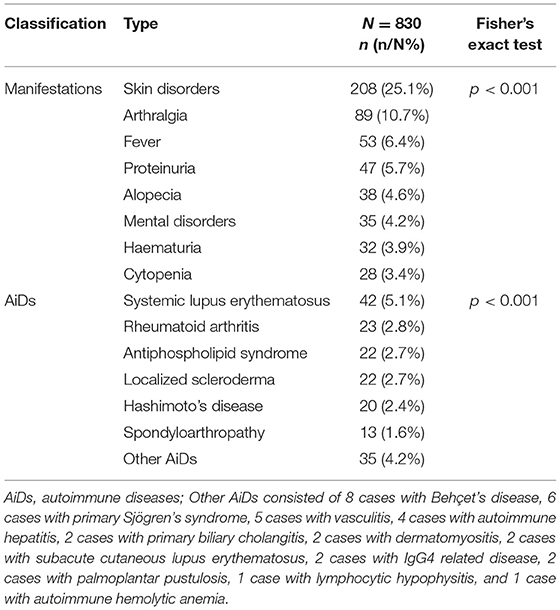
Skin disorder was the most prevalent manifestation in 830 cases with the DFS pattern (p < 0.001, Table 4), with 208 cases (25.1%) showing various types of skin disorders which included rashes, thickening and hardening of the skin, depigmentation, etc. (Supplementary Table 2). In addition, as shown in Table 4, arthralgia was the second most common manifestation in cohort with the DFS pattern (10.7%), followed by fever (6.4%), proteinuria (5.7%), alopecia (4.6%), mental disorders such as anxiety, depression and insomnia (4.2%), hematuria (3.9%), and cytopenia (3.4%) (detailed significance shown in Supplementary Figure 1).
Cases With the DFS Pattern and Also Diagnosed With AiDs
In total, 149 cases with the DFS pattern were diagnosed with various AiDs, some of which had more than one kind of AiD simultaneously and were included in the repetitive analysis of AiDs. There were 42 cases diagnosed with systemic lupus erythematosus, 23 cases with rheumatoid arthritis, 22 cases with antiphospholipid syndrome (Supplementary Table 3), 22 cases with localized scleroderma, 20 cases with Hashimoto's disease, 13 cases withspondyloarthropathies, and 35 cases with other AiDs. Systemic lupus erythematosus was significantly most prevalent in cases with the DFS pattern (p < 0.001, Table 4, detailed significance shown in Supplementary Figure 1).
Clinical Associations of the DFS Pattern
Among 830 cases with the nuclear DFS pattern, there was a frequency of 2.7% for localized scleroderma characterized by localized thickening and hardening of the skin. Among ANA-positive cases showing other patterns, the frequency of localized scleroderma was 1.6%. The prevalence of localized scleroderma was significantly higher in cases with the DFS pattern than in those with other patterns (Table 5). Among 446 cases diagnosed as localized scleroderma, 22 cases displayed the DFS staining pattern, while no DFS pattern was observed in 588 patients with systemic sclerosis (Table 5). In total, 235 cases showing ANA positivity had alopecia, among which 38 cases presented the DFS pattern. The frequency of the DFS pattern in all ANA-positive cases with alopecia was higher than that in the Physical Examination Department (16.2% vs. 10.2%, p = 0.01). In addition, a significantly higher prevalence of alopecia was observed in cases with the DFS pattern than in those with other IIF patterns (Table 5).
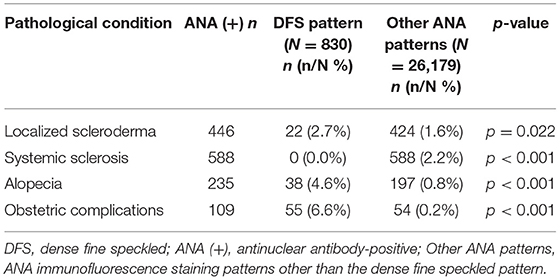
Table 5. Prevalence of several pathological conditions in cases with the DFS staining pattern vs. other ANA patterns.
It was also observed in our study that 55 patients with the DFS pattern (6.6%) had a history of obstetric complications, including spontaneous abortion, habitual abortion, fetal growth restriction, embryonic termination, pregnancy-induced hypertension syndrome, and infertility. Cases with the DFS pattern showed a significantly higher frequency of obstetric complications than cases with other patterns (Table 5).
Discussion
We conducted this research to demonstrate the prevalence and possible clinical associations of the DFS pattern in a large-scale ANA-positive cohort. A broad array of clinical conditions was sampled in our study with non-specific sera under ANA testing ordered by various departments, which reflected the real scenario in clinical practice. The prevalence of ANA positivity in our study was 37.4%. The prevalence of the DFS pattern was 1.1% in the total population and 3.1% in the ANA-positive population. In our study, it was observed that although Rheumatology Department ordered the most ANA tests and had the highest prevalence of ANA positivity (61.3%), the prevalence of the DFS pattern was relatively low, accounting for only 1.0% of the total population and 1.6% of the ANA-positive population. A higher prevalence of the DFS staining pattern was seen in the Obstetrics and Gynaecology Department, Allergy Department, Dermatology Department and Physical Examination Department. Another study also reported that the highest rate of the DFS pattern among the ANA-positive population was observed in an obstetrics and gynaecology hospital (35). This discrepancy between departments implies that the DFS pattern may have a stronger correlation with obstetric, allergic and dermal diseases, or these patients probably have a much low pretest probability for ANA-associated rheumatic diseases. ANA testing is increasingly used by clinical specialists other than rheumatologists as a screening method for the differential serological diagnosis of autoimmune rheumatic diseases. As shown in Table 1, 70.6% of ANA testing was ordered from non-rheumatology departments, and considerable proportions of these samples were ANA-positive. This is closely related to the deepening understanding of autoimmunity among other specialists. For routine autoimmune laboratories, it is necessary to have a deeper understanding of the clinical relevance of different ANA staining patterns, as well as the differences among detection methodologies. Only in this way can we give clinical specialists better advice on ANA testing and interpretation of results.
It has been reported that the nuclear DFS pattern is more prevalent in young people (<35 years) (36–38). The prevalence of the DFS staining pattern observed in our study is lower than that in some previous studies (19, 31, 37), and this discrepancy probably derives from a selection bias since our study included a population consisting of a higher percentage of older people (>35 years old). In addition, the cohort our study included was consecutive and non-selective, while sera examined in previous studies were obtained from specific populations. Ethnicity may be another confounding factor accounting for this discrepancy since a varied prevalence of DFS patterns was reported among different regions (36). The use of different screening thresholds and commercial kits by studies results in a variation in reported positive rates. Hence, inevitable heterogeneity between studies must be taken into consideration in regard to the comparison and interpretation of these results. Among 830 cases in our study displaying a DFS staining pattern, the prevalence of the DFS pattern decreased with increasing age, and the majority of cases were women between 18 and 35 years old. Most cases with the DFS pattern showed a low titer of ANA (≤1:320), and cases diagnosed with AiDs had a significantly higher titer than cases without an autoimmune background. According to previous reports, the nuclear DFS pattern is not necessarily associated with a low titer, and some sera can have extremely high titers (14, 39). Usually, high-titer ANAs are more clinically significant than low-titer ANAs. In terms of the DFS pattern, titer has little bearing on diagnosis or disease activity after the screening threshold of 1:80 or 1:160 (14).
Previous findings indicated that the presence of the DFS pattern might correlate with several pathological conditions, such as atopic dermatitis, asthma, and interstitial cystitis (26). In our study, the most common manifestation in cases with the DFS pattern was various types of skin disorders (Supplementary Figure 1). Moreover, a significantly higher prevalence of alopecia was observed in cases with the DFS pattern compared to other ANA patterns, and the frequency of the DFS pattern in all ANA-positive cases with alopecia was higher than that in the Physical Examination Department. Interestingly, Okamoto et al. reported the localization of DFS70 in the outer root sheath cells and elevated anti-DFS70 antibodies in patients with alopecia (40). Combining evidence from these studies and our findings underpins a potential clinical association of the DFS pattern with pathological skin conditions.
In this study, 6.63% of cases with DFS staining patterns had a history of obstetric complications. Cases with the DFS pattern showed a significantly higher frequency of obstetric complications than cases with other ANA patterns. Besides, in the 22 patients with antiphospholipid syndrome, cases with the DFS pattern at a titer of 1:320 seem more likely to be accompanied with antiphospholipid antibody (Supplementary Table 3). However, the correlation between DFS pattern titer with antiphospholipid antibody levels could not be concluded due to limited sample size, which warrants further research. Notably, it was reported that a significant proportion of patients with the DFS pattern (13.1%) presented with a history of thrombosis or obstetric complications, and the prevalence of obstetric complications was 5.8% in female patients (41). The DFS pattern was also prevalent in patients with unexplained thrombosis and obstetric complications (41). Therefore, it is hypothesized that the presence of the DFS pattern may be associated with a high risk of thrombosis and obstetric complications.
Obviously, the role of anti-DFS70 antibodies is hard to avoid in the discussion of the DFS pattern. As the first and most widely reported autoantibody responsible for the presence of the DFS pattern, anti-DFS70 antibodies target the ~70 kd lens epithelium–derived growth Factor p75 (LEDGF/p75) protein (also designated DFS70). Notably, autoantibodies producing the DFS staining pattern do not exclusively target DFS70, and anti-DFS70 antibodies do not necessarily display this pattern, especially when they coexist with other autoantibodies (23, 24). For example, Bizzaro et al. observed a significantly higher prevalence of the DFS pattern in patients with thrombotic events or unexplained recurrent pregnancy loss than controls, while the results of anti-DFS70-specific antibodies showed no evidence of such an association (42). The involvement of other non-DFS70 reactive autoantibodies which could produce the DFS pattern, such as autoantibodies targeting JPO2/CDCA7L, may account for this discrepancy (24, 43). Thus, the relationship between the DFS pattern and its pathological roles could be better elucidated when all associated antigen-specific antibodies were also studied simultaneously.
There are several limitations in our study. On the one hand, the number of younger samples included is very limited, which may be partly responsible for the lower prevalence of the DFS pattern in our study compared with other studies. The decline in DFS pattern prevalence with increasing age implies that the DFS pattern may play a more important role in this population, which needs further investigation. On the other hand, although the DFS pattern could be detected in various AiDs such as systemic lupus erythematosus (Supplementary Figure 1), this retrospective study adds little value to the interesting on going discussion about the relationship between isolated DFS70 autoantibodies and systemic autoimmune rheumatic diseases since DFS70 autoantibodies were not detected in routine clinical laboratory tests.
Our findings contribute to a better understanding of the prevalence and characteristics of the DFS pattern in a large ANA-positive cohort. In addition, it will help interpret the DFS pattern in ANA testing for patients at risk to undergo subsequent investigation. In conclusion, although rare in autoimmune diseases, the presence of a nuclear DFS pattern indeed correlates with several pathological conditions, such as skin disorder, alopecia, and obstetric complications. It may be a signal of localized inflammation within certain organs or tissues, especially the skin. Further studies to investigate the mechanism by which antigen-specific autoantibodies produce this pattern are of great importance to shed light on this problem.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital. Informed consent was waived due to the retrospective nature of our study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 81971545 and 82172343) and CAMS Innovation Fund for Medical Sciences (CIFMS):2020-I2M-C&T-A-002.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the colleagues from the laboratory of the Department of Rheumatology and Immunology at Peking Union Medical College Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.829436/full#supplementary-material
References
1. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. (2003) 349:1526–33. doi: 10.1056/NEJMoa021933
2. Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapaa-Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. (2011) 13:R30. doi: 10.1186/ar3258
3. Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. (2007) 56:2344–51. doi: 10.1002/art.22665
4. McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. (2005) 11:85–9. doi: 10.1038/nm1167
5. Nisihara R, Machoski MCC, Neppel A, Maestri CA, Messias-Reason I, Skare TL. Anti-nuclear antibodies in patients with breast cancer. Clin Exp Immunol. (2018) 193:178–82. doi: 10.1111/cei.13136
6. Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. (1997) 40:1601–11. doi: 10.1002/art.1780400909
7. Yee LJ, Kelleher P, Goldin RD, Marshall S, Thomas HC, Alberti A, et al. Antinuclear antibodies (ANA) in chronic hepatitis C virus infection: correlates of positivity and clinical relevance. J Viral Hepatitis. (2004) 11:459–64. doi: 10.1111/j.1365-2893.2004.00530.x
8. Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb MJ, et al. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. (2005) 62:14–26. doi: 10.1002/pros.20112
9. Agustinelli RA, Rodrigues SH, Mariz HA, Prado MS, Andrade LEC. Distinctive features of positive anti-cell antibody tests (indirect immunofluorescence on HEp-2 cells) in patients with non-autoimmune diseases. Lupus. (2019) 28:629–34. doi: 10.1177/0961203319838348
10. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. (2012) 64:2319–27. doi: 10.1002/art.34380
11. Li XY, Liu X, Cui JJ, Song WJ, Liang Y, Hu Y, et al. Epidemiological survey of antinuclear antibodies in healthy population and analysis of clinical characteristics of positive population. J Clin Lab Anal. (2019) 33:e22965. doi: 10.1002/jcla.22965
12. Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. (2006) 27:153–60. doi: 10.1016/j.jaut.2006.09.001
13. Slight-Webb S, Smith M, Bylinska A, Macwana S, Guthridge C, Lu R, et al. Autoantibody-positive healthy individuals with lower lupus risk display a unique immune endotype. J Allergy Clin Immunol. (2020) 146:1419–33. doi: 10.1016/j.jaci.2020.04.047
14. Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. (2011) 63:191–200. doi: 10.1002/art.30084
15. Pisetsky DS. Antinuclear antibody testing - misunderstood or misbegotten? Nat Rev Rheumatol. (2017) 13:495–502. doi: 10.1038/nrrheum.2017.74
16. Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. (2020) 72:1026–35. doi: 10.1002/art.41214
17. Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. (2010) 69:1420–2. doi: 10.1136/ard.2009.127100
18. Chan EKL, Damoiseaux J, Carballo OG, Conrad K, Cruvinel WD, Francescantonio PLC, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front Immunol. (2015) 6:412. doi: 10.3389/fimmu.2015.00412
19. Dellavance A, Viana VST, Leon ER, Bonfa ESDO, Andrade LEC, Leser PG. The clinical spectrum of antinuclear antibodies associated with the nuclear dense fine speckled immunofluorescence pattern. J Rheumatol. (2005) 32:2144–9.
20. Moroi Y, Peebles C, Fritzler MJ, Steigerwald J, Tan EM. Autoantibody to centromere (kinetochore) in scleroderma sera. P Natl Acad Sci Biol. (1980) 77:1627–31. doi: 10.1073/pnas.77.3.1627
21. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. (2013) 72:1747–55. doi: 10.1136/annrheumdis-2013-eular.238
22. Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis. (2019) 78:879–89. doi: 10.1136/annrheumdis-2018-214436
23. Infantino M, Carbone T, Manfredi M, Grossi V, Benucci M, Casiano CA, et al. Dense fine speckled (DFS) immunofluorescence pattern and anti-DFS70 antibodies: cleaning up the current concepts. Clin Chim Acta. (2020) 510:157–9. doi: 10.1016/j.cca.2020.07.001
24. Mahler M, Andrade LE, Casiano CA, Malyavantham K, Fritzler MJ. Anti-DFS70 antibodies: an update on our current understanding and their clinical usefulness. Expert Rev Clin Immunol. (2019) 15:241–50. doi: 10.1080/1744666X.2019.1562903
25. Ochs RL, Stein TW, Peebles CL, Gittes RF, Tan EM. Autoantibodies in interstitial cystitis. J Urol. (1994) 151:587–92. doi: 10.1016/S0022-5347(17)35023-1
26. Ochs RL, Muro Y, Si Y, Ge H, Chan EK, Tan EM. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol. (2000) 105(Pt. 1):1211–20. doi: 10.1067/mai.2000.107039
27. Van Hoovels L, Broeders S, Chan EKL, Andrade L, de Melo Cruvinel W, Damoiseaux J, et al. Current laboratory and clinical practices in reporting and interpreting anti-nuclear antibody indirect immunofluorescence (ANA IIF) patterns: results of an international survey. Auto Immun Highlights. (2020) 11:17. doi: 10.1186/s13317-020-00139-9
28. Hayashi N, Uto K, Imanishi A, Sugiyama D, Morinobu A, Saegusa J. Prevalence of anti-dense fine speckled 70 antibodies in healthy individuals and patients with antinuclear antibody-associated autoimmune rheumatic diseases in Japan. Medicine. (2021) 100:e24556. doi: 10.1097/MD.0000000000024556
29. Mutlu E, Eyigor M, Mutlu D, Gultekin M. Confirmation of anti-DFS70 antibodies is needed in routine clinical samples with DFS staining pattern. Cent Eur J Immunol. (2016) 41:6–11. doi: 10.5114/ceji.2016.58812
30. Lee H, Kim Y, Han K, Oh EJ. Application of anti-DFS70 antibody and specific autoantibody test algorithms to patients with the dense fine speckled pattern on HEp-2 cells. Scand J Rheumatol. (2016) 45:122–8. doi: 10.3109/03009742.2015.1060260
31. Carter JB, Carter S, Saschenbrecker S, Goeckeritz BE. Recognition and relevance of Anti-DFS70 autoantibodies in routine antinuclear autoantibodies testing at a community hospital. Front Med. (2018) 5:88. doi: 10.3389/fmed.2018.00088
32. Kang SY, Lee WI. [Clinical significance of dense fine speckled pattern in anti-nuclear antibody test using indirect immunofluorescence method]. Korean J Lab Med. (2009) 29:145–51. doi: 10.3343/kjlm.2009.29.2.145
33. Pazini AM, Fleck J, dos Santos RS, Beck ST. Clinical relevance and frequency of cytoplasmic and nuclear dense fine speckled patterns observed in ANA-HEp-2. Rev Bras Reumatol. (2010) 50:655–60. doi: 10.1590/S0482-50042010000600006
34. Sener AG, Afsar I. Frequency of dense fine speckled pattern in immunofluorescence screening test. Eur J Rheumatol. (2015) 2:103–5. doi: 10.5152/eurjrheum.2015.0003
35. Zheng B, Wang Z, Mora RA, Liu A, Li C, Liu D, et al. Anti-DFS70 antibodies among patient and healthy population cohorts in China: results from a multicenter training program showing spontaneous abortion and pediatric systemic autoimmune rheumatic diseases are common in anti-DFS70 positive patients. Front Immunol. (2020) 11:562138. doi: 10.3389/fimmu.2020.562138
36. Albesa R, Sachs U, Infantino M, Manfredi M, Benucci M, Baus Y, et al. Increased prevalence of anti-DFS70 antibodies in young females: experience from a large international multi-center study on blood donors. Clin Chem Lab Med. (2019) 57:999–1005. doi: 10.1515/cclm-2018-1233
37. Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. (2004) 50:892–900. doi: 10.1002/art.20096
38. Ganapathy V, Casiano CA. Autoimmunity to the nuclear autoantigen DFS70 (LEDGF): what exactly are the autoantibodies trying to tell us? Arthritis Rheum. (2004) 50:684–8. doi: 10.1002/art.20095
39. Carbone T, Pafundi V, Tramontano G, Gilio M, Padula MC, Padula AA, et al. Prevalence and serological profile of anti-DFS70 positive subjects from a routine ANA cohort. Sci Rep. (2019) 9:2177. doi: 10.1038/s41598-019-38686-5
40. Okamoto M, Ogawa Y, Watanabe A, Sugiura K, Shimomura Y, Aoki N, et al. Autoantibodies to DFS70/LEDGF are increased in alopecia areata patients. J Autoimmun. (2004) 23:257–66. doi: 10.1016/j.jaut.2004.07.004
41. Marlet J, Ankri A, Charuel JL, Ghillani-Dalbin P, Perret A, Martin-Toutain I, et al. Thrombophilia associated with anti-DFS70 autoantibodies. PLoS ONE. (2015) 10:e0138671. doi: 10.1371/journal.pone.0138671
42. Bizzaro N, Pesce G, Trevisan MT, Marchiano M, Cinquanta L, Infantino M, et al. Anti-DFS70 antibodies detected by specific methods in patients with thrombosis or recurrent pregnancy loss: no evidence of an association. Sci Rep. (2020) 10:7748. doi: 10.1038/s41598-020-64550-y
Keywords: antinuclear antibody, indirect immunofluorescence, prevalence, dense fine speckled pattern, pathological conditions
Citation: Deng C, Wang A, Hu C, Zhang W, Zeng X and Fei Y (2022) The Prevalence and Clinical Relevance of the DFS Immunofluorescence Staining Pattern in a Large ANA-Positive Cohort. Front. Med. 9:829436. doi: 10.3389/fmed.2022.829436
Received: 13 January 2022; Accepted: 17 March 2022;
Published: 10 May 2022.
Edited by:
Sophia Adamia, DFCI/HCC, United StatesReviewed by:
Christine Gibson Parks, National Institute of Environmental Health Sciences (NIH), United StatesMarvin Fritzler, University of Calgary, Canada
Dirk Roggenbuck, Brandenburg University of Technology Cottbus-Senftenberg, Germany
Copyright © 2022 Deng, Wang, Hu, Zhang, Zeng and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunyun Fei, ZmVpeXVueXVuQHB1bWNoLmNu; Xiaofeng Zeng, eGlhb2ZlbmcuemVuZ0Bjc3Rhci5vcmcuY24=
†These authors have contributed equally to this work
 Chuiwen Deng
Chuiwen Deng Anqi Wang†
Anqi Wang† Chaojun Hu
Chaojun Hu Wen Zhang
Wen Zhang Xiaofeng Zeng
Xiaofeng Zeng Yunyun Fei
Yunyun Fei