
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 06 May 2022
Sec. Translational Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.828346
This article is part of the Research Topic Implementation of AI and Machine Learning Technologies in Medicine View all 21 articles
Background: Uterine cervical neoplasms is widely concerned due to its high incidence rate. Early diagnosis is extremely important for prognosis. The purpose of this article is evaluating the efficacy of Raman spectroscopy in the diagnosis of suspected uterine cervical neoplasms.
Methods: We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of science up to September 1, 2021. By analyzing the true positive (TP), false positive (FP), true negative (TN) and false negative (FN) of six included study, we evaluated the pooled and grouping sensitivity, specificity, positive, and negative likelihood ratios (LR), and diagnostic odds ratio (DOR), with 95% confidence intervals (CI), based on random effects models. The overall diagnostic accuracy of Raman spectrum was evaluated by SROC curve analysis and AUC.
Results: After screening with inclusion and exclusion criteria, a total of six study were included in the study. The pooled sensitivity and specificity was 0.98 (95% Cl, 0.93–0.99) and 0.95 (95% Cl, 0.89–0.98). The total PLR and NLR were 21.05 (95% CI, 8.23–53.86) and 0.03 (95% CI, 0.01–0.07), respectively. And the AUC of the SROC curve which show the overall diagnostic accuracy was 0.99 (0.98–1.00).
Conclusion: Through analysis, we confirmed the role of Raman spectroscopy (RS) in the diagnosis of suspected uterine cervical tumors.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42021284966].
The incidence rate of uterine cervical tumors is the fourth of female cancer. According to statistics, there were about 570,000 uterine cervical tumors patients and 310,000 deaths worldwide in 2018. Among them, China and India are the hardest hit areas of uterine cervical tumors, accounting for nearly two-thirds of the cases (1). Early diagnosis of cervical cancer and cervical intraepithelial neoplasia and early treatment are effective means to improve the survival rate of cervical cancer patients. Although there are screening tools such as cytological smear (TCT) and human papillomavirus (HPV) detection, the average sensitivity and specificity are not satisfactory (2).
More than 10 years ago, TCT was an effective tool for detecting and preventing uterine cervical tumors. However, the European guidelines for quality assurance of uterine cervical tumors screening (Abstract literature of the Second Edition) released in 2010 pointed out that the false positive rate of cytology is high, which will bring excessive medical treatment and additional economic losses (3). Therefore, HPV DNA detection was recommended due to its high sensitivity. But HPV DNA detection also had the problems of time-consuming and high price. Colposcopy had good sensitivity (>90%), but its specificity was poor (<50%), and the false positive rate was higher, which often lead to unnecessary biopsy. Histopathological examination is the gold standard for the evaluation and diagnosis of cancer, but it includes chemical fixation, dehydration, clearance, infiltration, paraffin embedding, sectioning, and hematoxylin eosin (H&E) staining. It takes about 1 week, which is time-consuming and expensive.
Raman spectroscopy is a new and reliable technology, which can analyze the molecular structure of substances and the chemical composition of human tissues (4). In medical research, Raman imaging has been successfully applied to nasopharyngeal carcinoma (5), gastric cancer (6), lung cancer (7), esophageal cancer (8), renal cell carcinoma (9), brain tumor (10) and so on. Raman technology has been used in the study of uterine cervical tumors for decades. The existing literature has proved that the specificity and accuracy of Raman spectroscopy in the diagnosis of uterine cervical tumors can reach more than 90%, which is no less than the traditional hematoxylin-eosin (HE) staining. Compared with HE staining, Raman technology has the advantages of no staining, no fixation, less demand for professionals, faster and so on, which provides another feasibility for the diagnosis of uterine cervical tumors (11). In conclusion, if Raman spectroscopy can be applied to cervical cancer, we have every reason to believe that it can carry out early diagnosis of cervical cancer and improve the screening rate of cervical cancer and the survival rate of patients. This Meta-analysis reviews the application of Raman spectroscopy in cervical cancer.
This meta-analysis searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of science to ensure that all potentially eligible articles are included (last search: September 1, 2021). We combined all the relevant medical subject heading (MeSH) terms of uterine cervical tumors and Raman spectrum: [(Uterine Cervical Neoplasms) OR (Cervical Neoplasm, Uterine) OR (Cervical Neoplasms, Uterine) OR (Neoplasm, Uterine Cervical) OR (Neoplasms, Uterine Cervical) OR (Uterine Cervical Neoplasm) OR (Neoplasms, Cervical) OR (Cervical Neoplasms) OR (Cervical Neoplasm) OR (Neoplasm, Cervical) OR (Neoplasms, Cervix) OR (Cervix Neoplasms) OR (Cervix Neoplasm) OR (Neoplasm, Cervix) OR (Cancer of the Uterine Cervix) OR (Cancer of the Cervix) OR (Cervical Cancer) OR (Uterine Cervical Cancer) OR (Cancer, Uterine Cervical) OR (Cancers, Uterine Cervical) OR (Cervical Cancer, Uterine) OR (Cervical Cancers, Uterine) OR (Uterine Cervical Cancers) OR (Cancer of Cervix) OR (Cervix Cancer) OR (Cancer, Cervix) OR (Cancers, Cervix)] AND [(Spectrum Analysis, Raman) OR (Raman Spectrum Analysis) OR (Raman Spectroscopy) OR (Spectroscopy, Raman) OR (Analysis, Raman Spectrum) OR (Raman Optical Activity Spectroscopy) OR (Raman Scattering) OR (Scattering, Raman)]. All potential studies were included with no other limitation. The meta-analysis has been registered in PROSPERO (CRD42021284966).
Articles like review articles, comments, report, letters will be eliminated from the study. Criteria as follows: (I) without animal tissues in the experiments; (II) reported the use of RS in uterine cervical tumors; (III) used histopathology to confirm the diagnosis; (V) reported the true positive (TP), false positive (FP), true negative (TN) and false negative (FN), based on which the sensitivity and specificity values can be calculated. After screening, a total of six study were included in the study.
Two independent investigators extracted a range of data from each study using a standardized data-collecting form: article title, first author, publication year, nationality. All relevant data is contained within the 6 included articles (12–17). Then the primary parameters, which mean the diagnostic value, including TP, FP, TN, and FN. And we can use these parameters to calculate the sensitivity and specificity values. The data obtained were summarized in Table 1.
We calculated the primary data of TP, FP, TN, FN from articles included, then calculated sensitivity, specificity, positive and negative likelihood ratios (LR), based on random effects models. We used Review Man 5.3 and Stata/SE 15.1 to generate the forest plots in order to show sensitivity and specificity.
Meanwhile, Summary Receiver Operator Characteristics (SROC) curves was generated to assess the combination of sensitivity and specificity by Stata/SE 15.1. To assess publication bias, we generated funnel plot using Stata/SE 15.1. In the meantime, we found that articles in uterine cervical tumors include in vivo and in vitro studies. Therefor we conducted a subgroup analysis according to these studies.
Two independent investigators used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) guidelines by Review Manager 5.3 to evaluate the quality of included studies. And the risk of bias of included studies was shown in Figures 1A,B. To assess publication bias, we plotted funnel plots and Egger’s regression test using Stata/SE 15.1. The funnel plots and Egger’s regression test included in the study are shown in Figures 1C,D. As shown in the Figure 1D, P = 0.420, less than 0.05, and Egger’s regression test indicates that there is no publication bias.
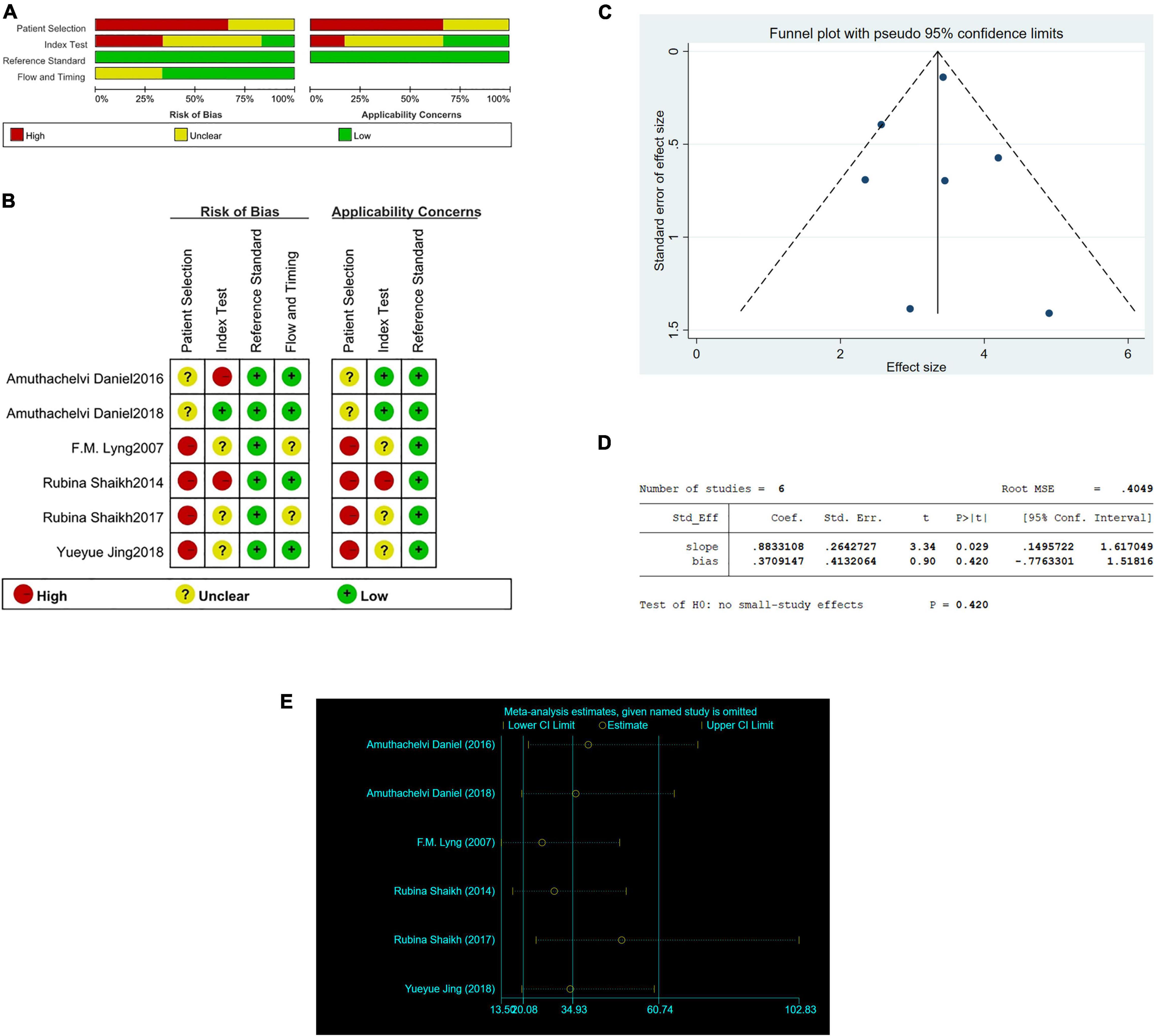
Figure 1. The graphical display of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) of the included studies. (A) Risk of bias and applicability concerns evaluation of included studies in pool. (B) Risk of bias and applicability concerns evaluation of included studies individually. (C) Funnel plot of publication bias in Raman diagnosis of cervical cancer. (D) Egger’s regression test of publication bias in Raman diagnosis of cervical cancer. (E) Sensitivity analysis in Raman diagnosis of cervical cancer.
And we conducted a sensitivity analysis. In Figure 1E, the results showed that none of the studies had an impact on this meta-analysis.
The process of included articles screening was presented in Figure 2. 403 potential articles were searched at first (including PubMed, n = 106, Web of science, n = 186, Embase, n = 111), in which included 198 duplicate records. Among the rest of 205 articles, 38 articles excluded due to: they were review, meeting or letters. Go a step further by browsing the 167 potentially relevant studies, 126 records excluded due to they were cytological study (n = 79), serological research (n = 28), medicine efficacy study (n = 11), animals research (n = 8). By reading the rest of 41 articles, 24 reports excluded due to they were biochemical assessment (n = 12), failed to give concrete date (n = 6) and irrelevant to the subject (n = 6). After careful perusing, 5 articles excluded due to failed to mention TN, FN, TP, FP and 6 excluded because of cervical precancer. Ultimately, 6 studies included in this review.
Table 1 carefully described the particular characteristics of the 6 included articles. Among the 6 articles, 5 were published between 2014 and 2018, the rest of article was published in 2007. There are a total of 242 patients and 167 normal people in the included articles, and the total number of spectra incorporated was 720 (two articles didn’t provide the number of spectra). In terms of the nationalities, four studies were from India, other two studies were from China and Ireland, respectively. As for diagnostic algorithm, one article calculated ORR (NADH/FAD), another article used linear discriminate analysis (LDA), and the other four articles utilized Principal-component linear discriminant analysis (PC-LDA). In term of spectra, two studies applied 785 nm, other two studies applied 784.12 nm, and the other two studies applied 430 and 514.5 nm, respectively. All of six studies utilized tissue to research, two studies were in vivo, therefor their samples were cervix in vivo, and the other four studies were in vitro, so their samples were ex vivo tissues. Three of four studies in vitro obtained fresh tissue slices, the rest of one study obtained Formalin-fixed paraffin preserved tissue.
The sensitivity and specificity were calculated to assess diagnostic accuracy of all the six studies. And the forest plot of pooled sensitivity and specificity was shown in Figure 3. The sensitivity which meant the detection of uterine cervical tumors by RS, ranged from 0.91 (95% CI, 0.82–0.97) to 1.00 (95% Cl, 0.95–1.00) and the pooled sensitivity was 0.98 (95% Cl, 0.93–0.99). The sensitivity of all the six studies was more than 0.90, which was mean that the missed diagnosis rate of RS for uterine cervical tumors is very low. The specificity ranged from 0.75 (95% CI, 0.58–0.88) to 0.99 (95% Cl, 0.96–1.00), and the pooled specificity was 0.95 (95% Cl, 0.89–0.98). It should be noted that except for one study with sensitivity of 0.75, specificity of the other five studies were more than 0.90. In a word, the ability of RS to distinguish cancer from normal people was worthy of recognition.
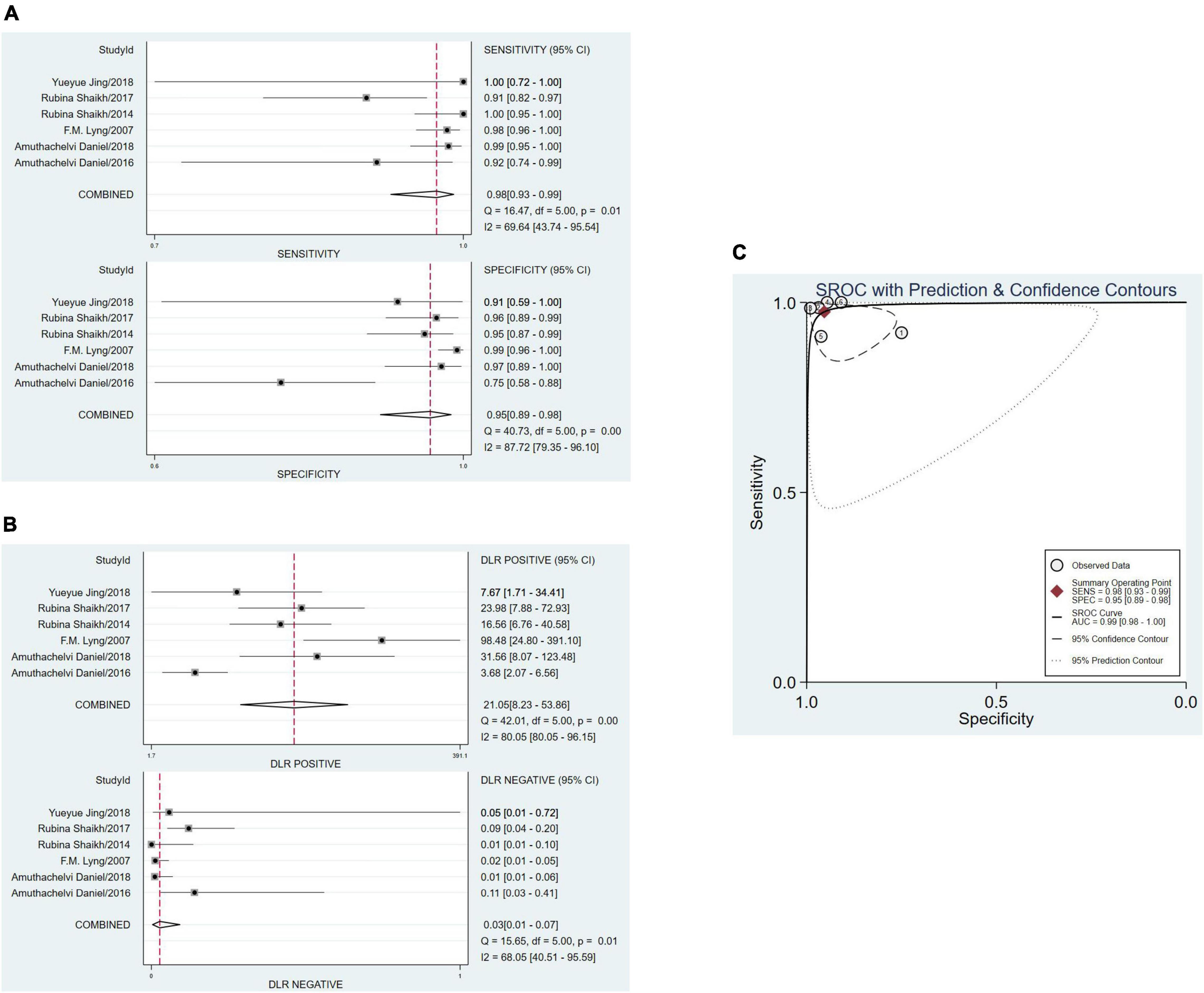
Figure 3. The pooled date analysis of Raman spectroscopy (RS) in uterine cervical tumors. (A) The forest plot of pooled sensitivity and specificity of Raman spectroscopy to diagnose uterine cervical tumors of all the six studies. (B) The pooled PLR and NLR of Raman spectroscopy in diagnosis of uterine cervical tumors. PLR, positive likelihood ratios; NLR, negative likelihood ratios. (C) The SROC curve of Raman spectroscopy in diagnosis of uterine cervical tumors. SROC, summary receiver operator characteristics.
The total PLR and NLR were 21.05 (95% CI, 8.23–53.86) and 0.03 (95% CI, 0.01–0.07), respectively. And the AUC of the SROC curve which show the overall diagnostic accuracy was 0.99 (0.98–1.00). The plots were shown in Figure 3C.
Two studies (15, 16) showed the research of RS to uterine cervical tumors in vivo which had a total of 87 samples and 300 tested spectra. The sensitivity of two studies was 1.00 (95% Cl, 0.95–1.00) and 0.91 (95% Cl, 0.82–0.97), respectively, and the specificity was 0.95 (95% Cl, 0.87–0.99) and 0.96 (95% Cl, 0.89–0.99), respectively. Since the number of study included in this group is less than 4, data analysis cannot be done in STATA. All of the data and grouping situation were shown in Figure 4.
Four studies (12–14, 17) showed the research of RS to uterine cervical tumors in vitro which had a total of 322 samples and 420 tested spectra (two articles didn’t provide the number of spectra). The sensitivity of four studies ranged from 0.92 (95% Cl, 0.74–0.99) to 1.00 (95% Cl, 0.72–1.00), and the pooled sensitivity was 0.98 (95% Cl, 0.89–1.00). The specificity ranged from 0.75 (95% Cl, 0.58–0.88) to 0.99 (95% Cl, 0.96–1.00), and the pooled specificity was 0.97 (95% Cl, 0.94–0.99). Total PLR and NLR were 33.38 (95% Cl, 15.00–74.28) and 0.02 (95% Cl, 0.00–0.12), respectively. The SROC curve was described and the AUC was 0.99 (0.98–1.00). All of the plots of vitro group were shown in Figure 5.
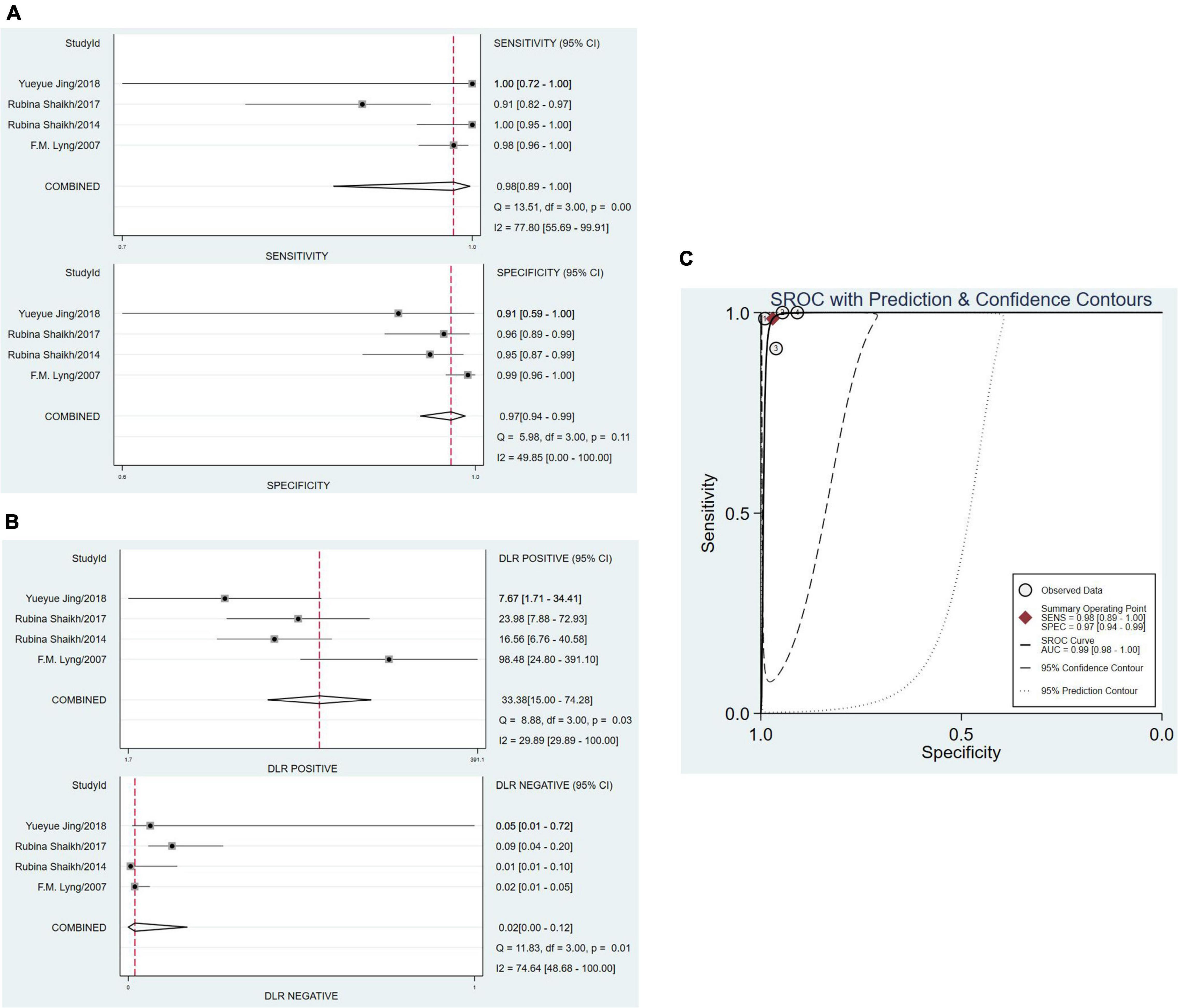
Figure 5. The pooled date analysis of Raman spectroscopy (RS) in uterine cervical tumors in vitro group. (A) The forest plot of pooled sensitivity and specificity of Raman spectroscopy to diagnose uterine cervical tumors of four studies. (B) The pooled PLR and NLR of Raman spectroscopy in diagnosis of uterine cervical tumors. PLR, positive likelihood ratios; NLR, negative likelihood ratios. (C) The SROC curve of Raman spectroscopy in diagnosis of uterine cervical tumors. SROC, summary receiver operator characteristics.
Mahadevan-Jansen et al. first researched uterine cervical tumors in vivo and in vitro by RS in 1998 (18). That means the research of uterine cervical tumors by RS has had more than 20 years history. Related articles research different substances, such as fresh cervical tissues, cervical cells, blood serum and so on. According to searching, this study is the first meta-analysis attempt to analyze the meaning of RS for uterine cervical tumors by researching fresh cervical tissues, and we intend to confirm its diagnostic accuracy by means of this study.
Meta-analysis showed that RS had high diagnostic accuracy for uterine cervical tumors. The sensitivity of all included articles was more than 90%, and the specificity of most included articles (except for one 75%) were also more than 90%. In the subgroup analysis, the sensitivity and specificity also achieved high standard, that meant whether RS analyze uterine cervical tumors tissues in vivo or in vitro both showed high diagnostic accuracy. This is strong evidence to explain the diagnostic effect of RS in uterine cervical tumors. Although there are only two literatures in vivo subgroup analysis, but for new technologies, such high sensitivity and specificity deserve our attention, and we look forward to seeing more research. And from the perspective of the combination of engineering with medicine, such new technologies and new ideas really deserve our attention.
RS also was used in researching uterine cervical tumors by cervical cells and blood serum except fresh cervical tissue. Sitarz et al. (19) studied the cervical cells of 96 women after TCT and HPV testing. They evaluated Glycogen levels in cells of all study groups to prove that RS can also diagnose HPV infected cells. Karunakaran et al. (20) found that the accuracy of RS in diagnosing uterine cervical tumors and normal people using single cells, cell clusters and DNA were 93.84, 74.26, and 92.21%, respectively. Lu et al. (21) studied the serum of 150 women and detected the levels of SCCA and OPN in the serum by RS. This is a convenient and efficient method which maybe a new screening measure for uterine cervical tumors.
With the prevalence of TCT and HPV examination, pathological biopsy is widely used in clinic and is considered as the gold standard of cervical cancer, what are the outstanding advantages of Raman technology? In other words, how should Raman technology position itself in clinical application?
After reading a lot of literature, people generally believe that the outstanding advantage of Raman microscope lies in its timeliness, such as real-time images, convenience and rapidity, reducing the demand and burden of pathologists and so on. According to the current research progress, Raman technology does not seem to be enough to make us think that it can replace postoperative pathology. However, with the rapid development of modern science and technology, there is an emerging technology called handheld Raman spectrometer, which can quickly and quantitatively detect the anti-cancer drug 5-fluorouracil (5-FU) in serum (22). We have every reason to expect that this technology can be innovated and applied to clinic as soon as possible, such as handheld portable Raman device. This device is smaller, imaging is faster, it is more convenient to determine the scope of lesions, reduce the burden of pathologists, and shorten the time waiting for intraoperative freezing during surgery, so as to realize efficient diagnosis in cost and time.
There are some limitations in this article. First and foremost, the heterogeneity was high. In order to explore the reasons for this result, we conducted a sensitivity analysis, and the results have been analyzed in Figure 1E. Excluding the included literature one by one did not have a great impact on heterogeneity. And meta regression, grouped by year, country, analysis tool, and Raman wave number, respectively, P-values are greater than 0.05, it means no great significance (Figure 6). We believe that the most likely reason is that there is too few research included due to the lack of current research. Second, because the vast majority of studies do not strictly abide by the double-blind test rules when conducting Raman test, there are some errors in the screening of patients, which may affect the analysis results. Third, one of the documents was published in 2007, and the rest were studied in recent 8 years. We don’t know whether microscope technology has developed greatly during this period. However, because there are few articles in conformity, we did not rule it out, and we think this meta can better explain the diagnostic effect of Raman technology in cervical cancer in the past 15 years. If someone continues to choose research in the follow-up, they can directly choose the literature from this time. Fourth, there are only two literatures in vivo subgroup analysis. Too few may not directly indicate the effectiveness of Raman technology, which needs more sample size and literature research.
Due to the high cost and expense of RS, there are not many related studies at present. But in the existing research, it is believed that RS does play an important role in the diagnosis of uterine cervical tumors. This is a satisfactory result which predicts the emergence of a new and efficient diagnostic technology.
Through this meta-analysis, we can confidently believe that Raman spectroscopy has high specificity and sensitivity in the diagnosis of uterine cervical tumors, and we have reason to believe that Raman spectroscopy will become an efficient diagnostic method of uterine cervical tumors in the future. However, more research and evidence are needed to fully demonstrate the role of Raman spectroscopy in the diagnosis of uterine cervical tumors before it is used in clinic. We are also looking forward to more samples and more researches.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Z-WS, L-JZ, Z-YS, XZ, Z-FZ, and FX wrote the manuscript together and made great contributions to the article. This article has taken place under the guidance of two experienced tutors, ZX and RL. All authors agreed to be responsible for the content of this article and the submitted version.
This work was supported by the National Natural Science Foundation of China-Liaoning Joint Fund 2019-BS-073 and the Scientific Research Fund of Liaoning Provincial Education Department LZ2019044.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
2. de Kok IM, van der Aa MA, van Ballegooijen M, Siesling S, Karim-Kos HE, van Kemenade FJ, et al. Trends in cervical cancer in the Netherlands until 2007: has the bottom been reached? Int J Cancer. (2011) 128:2174–81. doi: 10.1002/ijc.25553
3. Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, et al. European guidelines for quality assurance in cervical cancer screening. Second edition–summary document. Ann Oncol. (2010) 21:448–58. doi: 10.1093/annonc/mdp471
4. Hu L, Bell D, Antani S, Xue Z, Yu K, Horning MP, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. (2019) 111:923–32. doi: 10.1093/jnci/djy225
5. Shu C, Yan H, Zheng W, Lin K, James A, Selvarajan S, et al. Deep learning-guided fiberoptic Raman spectroscopy enables real-time in vivo diagnosis and assessment of nasopharyngeal carcinoma and post-treatment efficacy during endoscopy. Anal Chem. (2021) 93:10898–906. doi: 10.1021/acs.analchem.1c01559
6. Harmsen S, Rogalla S, Huang R, Spaliviero M, Neuschmelting V, Hayakawa Y, et al. Detection of premalignant gastrointestinal lesions using surface-enhanced resonance Raman scattering-nanoparticle endoscopy. ACS Nano. (2019) 13:1354–64. doi: 10.1021/acsnano.8b06808
7. Shin H, Oh S, Hong S, Kang M, Kang D, Ji YG, et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. (2020) 14:5435–44. doi: 10.1021/acsnano.9b09119
8. Almond LM, Hutchings J, Lloyd G, Barr H, Shepherd N, Day J, et al. Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett’s esophagus. Gastrointest Endosc. (2014) 79:37–45. doi: 10.1016/j.gie.2013.05.028
9. He C, Wu X, Zhou J, Chen Y, Ye J. Raman optical identification of renal cell carcinoma via machine learning. Spectrochim Acta A Mol Biomol Spectrosc. (2021) 252:119520. doi: 10.1016/j.saa.2021.119520
10. Jermyn M, Mok K, Mercier J, Desroches J, Pichette J, Saint-Arnaud K, et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med. (2015) 7:274ra19. doi: 10.1126/scitranslmed.aaa2384
11. Ramos IR, Malkin A, Lyng FM. Current advances in the application of Raman spectroscopy for molecular diagnosis of cervical cancer. Biomed Res Int. (2015) 2015:561242. doi: 10.1155/2015/561242
12. Daniel A, Prakasarao A, Dornadula K, Ganesan S. Polarized Raman spectroscopy unravels the biomolecular structural changes in cervical cancer. Spectrochim Acta A Mol Biomol Spectrosc. (2016) 152:58–63. doi: 10.1016/j.saa.2015.06.053
13. Daniel A, Prakasarao A, Ganesan S. Near-infrared Raman spectroscopy for estimating biochemical changes associated with different pathological conditions of cervix. Spectrochim Acta A Mol Biomol Spectrosc. (2018) 190:409–16. doi: 10.1016/j.saa.2017.09.014
14. Lyng FM, Faoláin EO, Conroy J, Meade AD, Knief P, Duffy B, et al. Vibrational spectroscopy for cervical cancer pathology, from biochemical analysis to diagnostic tool. Exp Mol Pathol. (2007) 82:121–9. doi: 10.1016/j.yexmp.2007.01.001 x
15. Shaikh R, Dora TK, Chopra S, Maheshwari A, Kedar KD, Bharat R, et al. In vivo Raman spectroscopy of human uterine cervix: exploring the utility of vagina as an internal control. J Biomed Opt. (2014) 19:087001. doi: 10.1117/1.JBO.19.8.087001
16. Shaikh R, Prabitha VG, Dora TK, Chopra S, Maheshwari A, Deodhar K, et al. A comparative evaluation of diffuse reflectance and Raman spectroscopy in the detection of cervical cancer. J Biophotonics. (2017) 10:242–52. doi: 10.1002/jbio.201500248
17. Jing Y, Wang Y, Wang X, Song C, Ma J, Xie Y, et al. Label-free imaging and spectroscopy for early detection of cervical cancer. J Biophotonics. (2018) 11:e201700245. doi: 10.1002/jbio.201700245
18. Mahadevan-Jansen A, Mitchell MF, Ramanujam N, Malpica A, Thomsen S, Utzinger U, et al. Near-infrared Raman spectroscopy for in vitro detection of cervical precancers. Photochem Photobiol. (1998) 68:123–32. doi: 10.1562/0031-865519980682.3.co;2
19. Sitarz K, Czamara K, Bialecka J, Klimek M, Zawilinska B, Szostek S, et al. HPV infection significantly accelerates glycogen metabolism in cervical cells with large nuclei: Raman microscopic study with subcellular resolution. Int J Mol Sci. (2020) 21:2667. doi: 10.3390/ijms21082667
20. Karunakaran V, Saritha VN, Joseph MM, Nair JB, Saranya G, Raghu KG, et al. Diagnostic spectro-cytology revealing differential recognition of cervical cancer lesions by label-free surface enhanced Raman fingerprints and chemometrics. Nanomedicine. (2020) 29:102276. doi: 10.1016/j.nano.2020.102276
21. Lu D, Ran M, Liu Y, Xia J, Bi L, Cao X. SERS spectroscopy using Au-Ag nanoshuttles and hydrophobic paper-based Au nanoflower substrate for simultaneous detection of dual cervical cancer-associated serum biomarkers. Anal Bioanal Chem. (2020) 412:7099–112. doi: 10.1007/s00216-020-02843-x
Keywords: Raman spectroscopy, uterine cervical tumors, diagnostic efficacy, meta-analysis, translational medicine
Citation: Shen ZW, Zhang LJ, Shen ZY, Zhang ZF, Xu F, Zhang X, Li R and Xiao Z (2022) Efficacy of Raman Spectroscopy in the Diagnosis of Uterine Cervical Neoplasms: A Meta-Analysis. Front. Med. 9:828346. doi: 10.3389/fmed.2022.828346
Received: 03 December 2021; Accepted: 13 April 2022;
Published: 06 May 2022.
Edited by:
Pietro Lio’, University of Cambridge, United KingdomReviewed by:
Jinwei Qiang, Jinshan Hospital, ChinaCopyright © 2022 Shen, Zhang, Shen, Zhang, Xu, Zhang, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Li, cmxpQGRsdXQuZWR1LmNu; Zhen Xiao, c2VyaW91c2RvY0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.