- 1Department of Surgery, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH, United States
- 2Department of Urology, Shengjing Hospital, China Medical University, Shenyang, China
- 3Department of Biochemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
Aging and major chronic diseases are risk factors for lower urinary tract symptoms (LUTS). On the other hand, oxidative stress (OS) is one of the fundamental mechanisms of aging and the development of chronic diseases. Therefore, OS might be a candidate mechanism linking these two clinical entities. This article aims to summarize the studies on the prevalence of LUTS, the role of OS in aging and chronic diseases, and the potential mechanisms supporting the putative link. A comprehensive literature search was performed to identify recent reports investigating LUTS and OS in major chronic diseases. In addition, studies on the impact of OS on the lower urinary tract, including bladder, urethra, and prostate, were collected and summarized. Many studies showed LUTS are prevalent in aging and major chronic diseases, including obesity, metabolic syndrome, diabetes, cardiovascular disease, hypertension, obstructive sleep apnea, autoimmune diseases, Alzheimer’s disease, and Parkinson’s disease. At the same time, OS is a key component in the pathogenesis of those chronic diseases and conditions. Recent studies also provided evidence that exacerbated OS can cause functional and/or structural changes in the bladder, urethra, and prostate, leading to LUTS. The reviewed data support the concept that OS is involved in multiple risk factors-associated LUTS, although further studies are needed to confirm the causative relationship. The specific ROS/RNS and corresponding reactions/pathways involved in chronic diseases and associated LUTS should be identified in the future and could serve as therapeutic targets.
Introduction
Lower urinary tract symptoms (LUTS) are highly prevalent worldwide, affecting up to 70% of adult men and women, depending on the study population, age, and methodology (1–4). LUTS comprise (i) bladder storage symptoms including frequency, urgency, urge incontinence, and nocturia, (ii) voiding symptoms such as hesitancy, low and/or intermittent stream, straining, prolonged micturition, and (iii) postmicturition symptoms such as the feeling of incomplete emptying and postmicturition dribble (4, 5). These symptoms significantly affect the quality of life and impose a high social and economic burden. LUTS can be caused by a local disease or injury in the lower urinary tract, such as urinary tract infection, prostatitis, urethral stricture, and bladder stones. In addition, numerous clinical and epidemiological studies have demonstrated that LUTS are common in aging and aging-related major chronic diseases and conditions (4, 6–9).
There is a variation in the definition of chronic diseases regarding the included diseases and their duration (10). Generally, chronic diseases refer to a physical or mental health disorder that exists for 3 months (or 1 year by some definitions) or more, causes functional restrictions, or requires continuous monitoring or therapy (10). Chronic diseases are common in the United States. It is estimated that 133 million Americans (or 45% of the population) suffer from at least one chronic disease, and this number is continuously increasing (11). LUTS were found to be highly prevalent in the aging population (12–16), and patients with chronic diseases and conditions such as obesity (17–21), metabolic syndrome (MetS) (22–26), diabetes (27–31), cardiovascular disease (32–36), hypertension (37–39), obstructive sleep apnea (OSA) (40–46), autoimmune diseases (47–50), Alzheimer’s disease (51–53), and Parkinson’s disease (54–57). These diseases seem to adversely affect one or more organs in the lower urinary tract (bladder and urethra) and prostate, leading to LUTS. However, the mechanistic links between LUTS and the aforementioned chronic diseases and conditions are unclear. Enhanced oxidative stress (OS) is a possible candidate mechanism since OS has been shown to be potentially implicated in the pathogenesis of all the above diseases. Therefore, OS may represent a unifying common pathogenic pathway between LUTS and chronic diseases.
Oxidative stress is an overload of oxidants and free radicals, mainly reactive oxygen species (ROS) such as superoxide radical anion (O2–), hydrogen peroxide (H2O2), hydroxyl radical (HO–), and reactive nitrogen species (RNS) including nitric oxide (NO) and peroxynitrite (ONOO–), due to increased production and/or compromised antioxidant defenses. The endogenous ROS are mainly produced in mitochondria, endoplasmic reticulum, peroxisomes, and phagocytic cells through enzymatic or non-enzymatic reactions (58). ROS/RNS can be removed by free radical scavengers in physiological conditions. The antioxidant enzymes include superoxide dismutase (SOD), glutathione peroxidase (GPx), glutaredoxins, thioredoxins, and catalase. The non-enzymatic antioxidants include glutathione (GSH), thioredoxin, lipoic acid, melatonin, carotenoids, uric acid, bilirubin, polyamines, and Vitamin E and C, etc.
Oxidative stress has long been believed to cause lipid, protein, and DNA damage affecting cellular functions, ultimately contributing to multiple pathological conditions. The level of OS can be measured indirectly by detecting the oxidative or nitrosative products of lipids (malondialdehyde, 4-hydroxy-2-non-enal), proteins (protein carbonyls, 3-nitrotyrosine), or nucleic acids (8-Hydroxy-deoxyguanosine). On the other hand, recent evidence has shown that low or moderate levels of ROS/RNS can serve as second messengers in intracellular signaling cascades involved in various cellular pathways (59).
This review described the prevalence of LUTS in aging and major chronic diseases (Table 1), the role of OS in the pathogenesis of these diseases and conditions, and the impact of OS on the lower urinary tract. We explored that OS may be a mechanistic link between LUTS and chronic diseases and hopefully aid internal medicine specialists and urologists to realize the potential connections between two clinical entities when they manage such patients.
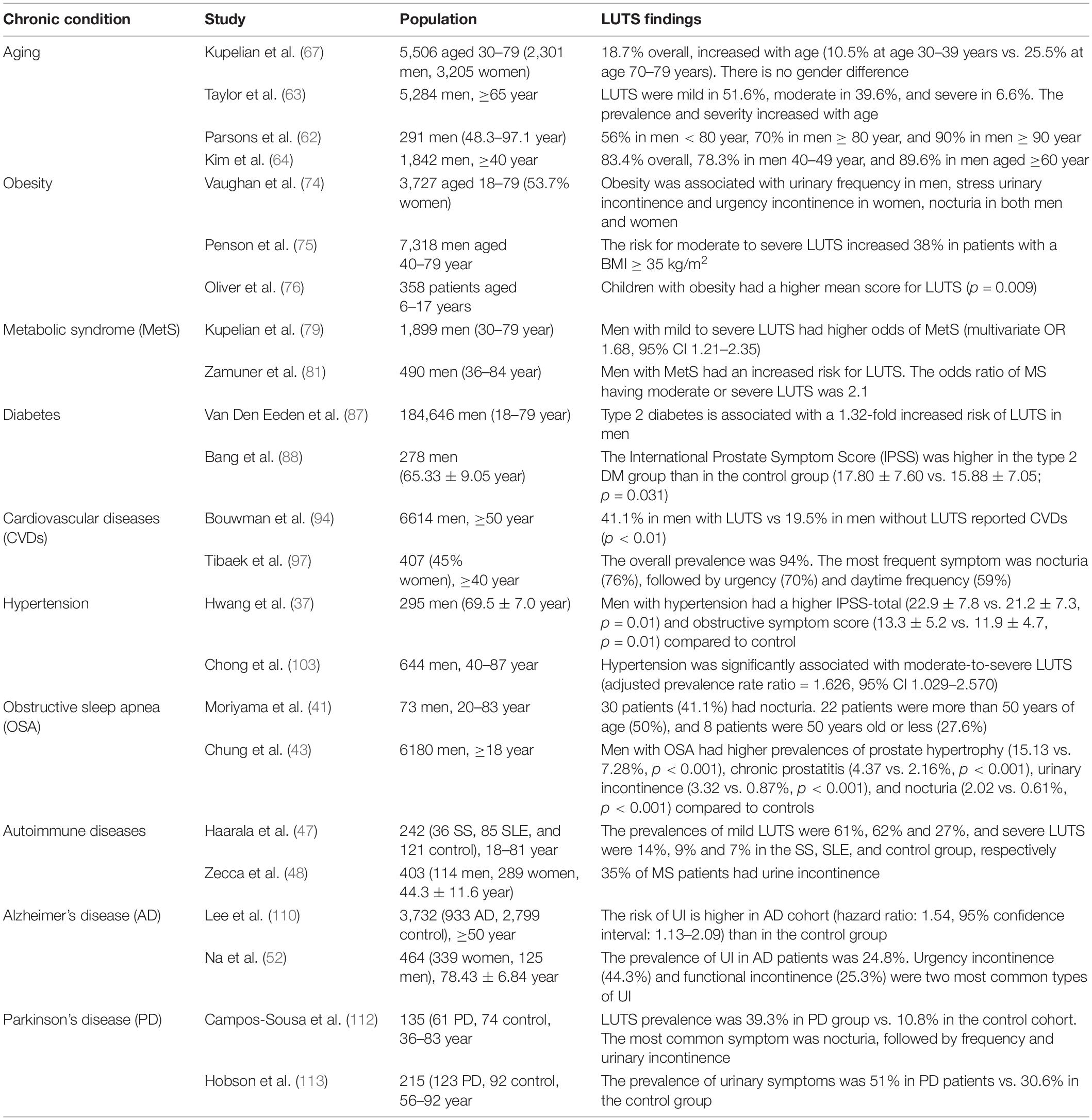
Table 1. Summary of the findings on lower urinary tract symptoms (LUTS) in aging and chronic diseases.
Lower Urinary Tract Symptoms are Prevalent in Aging and Major Chronic Diseases
Lower Urinary Tract Symptoms in Aging
According to the U.S. Census Bureau Data, 52 million (or 16% of the population) people are 65 or older in 2018; the number will reach 95 million by 2060 (60). Aging is the most significant risk factor for a wide range of chronic diseases. In the National Health Interview Survey performed by the Centers for Disease Control and Prevention (CDC) in 2008, 85.6% of the elderly population suffer from at least one chronic disease, and 56.0% experience at least two.
Urologic issues are ubiquitous in the elderly population and account for a greater number of clinic visits. It is estimated that about 50% of men at the age of 60 have histological benign prostatic hyperplasia (BPH)/LUTS, approximately 70–80% of men aged 60–89 years are affected, and by 90, the prevalence approaches 90% (61–63). Kim et al. (64) demonstrated that LUTS were typically seen in about 83.4% among 1,842 Korean men aged ≥40 years, and the prevalence and severity increased with age. In such a study, storage LUTS (70.1%) were more predominant than voiding LUTS (60.4%), while postmicturition LUTS occurred in 38.3% of the participants (64). Similar findings were obtained from another clinical study performed on 8,627 men aged 48–79 years old (65). Mild, moderate, and severe LUTS were reported in 75.3, 22.0, and 2.7% of patients, respectively, and the prevalence increased with age. Furthermore, age has been shown to be associated with the progression of LUTS in a population-based cohort of 5,502 participants ranging from 30 to 79 years old (66). A salient observation reported by Pöyhönen et al. (14) was that the most common LUTS among a population of men aged 30–80 years differed with increasing age, since men aged 30–40 years mainly complained of the dribble. While urgency and nocturia represented the most bothersome symptoms in men aged 70–80 years (14). In the Boston Area Community Health (BACH) Survey including both men and women, the prevalence of LUTS increased from 10.5% at age 30–39 years to 25.5% at age 70–79 years (67).
Although LUTS appears to be more prevalent in the elderly than in younger persons, they should not be considered a normal aspect of aging. Increased public awareness and appropriate therapeutic strategies should be adopted to manage these symptoms effectively.
Lower Urinary Tract Symptoms in Obesity
Based on recent data released from the CDC: the prevalence of obesity increased from 30.5 to 42.4% from 1999 to 2000 through 2017–2018, and the prevalence of severe obesity increased from 4.7 to 9.2%. Increasing evidence suggests a link between obesity and LUTS. Obese patients are more likely to have LUTS, including voiding and storage symptoms as well as prostate enlargement in men than control subjects (20, 68). Increased waist circumference, as a manifestation of central obesity, is considered a predictive surrogate of the severity of LUTS in obese patients (69, 70) and animal models of obesity (71). Furthermore, central obesity has been recognized as a risk factor for storage problems and urinary incontinence after prostatectomy in patients with BPH (72). In contrast, surgical or behavioral weight loss results in modest improvements in urinary incontinence in obese women (73).
Vaughan et al. assessed the urinary storage symptoms in patients aged 18–79 years (74). They demonstrated that obesity was associated with nocturia in both men and women. Obesity was also associated with urinary frequency in men and stress urinary incontinence and urgency incontinence in women. Another study (75), including 7,318 men aged 40–79, found the risk for moderate to severe LUTS increased 38% in patients with a body mass index (BMI) of at least 35 kg/m2. Oliver et al. (76) prospectively evaluated 358 patients aged 6–17 years with LUTS using a 21-item questionnaire. They found that children with obesity had a higher mean score for LUTS than normal-weight children. The prevalence of overactive bladder increased as waist or BMI increased, although the relationship varies by gender (77).
Lower Urinary Tract Symptoms in Metabolic Syndrome
Metabolic syndrome (MetS) is a cluster of metabolic disorders that include central obesity, dyslipidemia, insulin resistance, and arterial hypertension. The prevalence of MetS in the United States increased from 32.9% in 2003–2004 to 34.7% in 2011–2012 (78). The association between LUTS and MetS is well established in many clinical and experimental studies. Patients with MetS have higher risks of LUTS, including incomplete emptying, intermittency, and nocturia (79). Multiple studies have reported associations between metabolic factors and higher American Urological Association Symptom Index scores in men (18, 69, 79, 80). In the Boston Area Community Health Survey, Kupelian et al. analyzed data on 1,899 men between the ages of 30–79 and found men with mild to severe LUTS had higher odds of MetS (79). The prevalence of MetS was increased from 20% with no symptoms or one symptom to about 40% with mild to severe LUTS (79). In another study, Zamuner et al. found a 2-fold increased risk for LUTS in male patients with MetS (81). MetS may predispose patients to BPH. A meta-analysis of eight studies, which enrolled 5,403 patients, suggests that BPH is highly linked to MetS and its individual component (82). Old and obese patients have a higher risk of developing an enlarged prostate (82). Similar findings were obtained from another meta-analysis including 52 studies, in which the authors demonstrated that MetS-induced inflammation is a potential contributor to BPH (83).
Lower Urinary Tract Symptoms in Diabetes
The CDC estimated that 34.2 million people, or 10.5% of the population in the United States, had diabetes in 2018, and approximately 90–95% of cases have type 2 diabetes. Multiple studies have shown a strong association between LUTS and diabetes. It has been estimated that between 25 and 87% of patients with diabetes experience some types of bladder dysfunction, including LUTS (84). The different etiology and pathophysiology of the two types of diabetes may give rise to different phenotypes of LUTS. In Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study including 652 women and 713 men with type 1 diabetes, the prevalence of LUTS is different in women (22%) and men (25%) (85). The risk of LUTS is increased in men with diabetes by 25% to threefold (86). Van Den Eeden et al. (87) analyzed the combined data from the California Men’s Health Study (CMHS) including 78,273 men, and the Research Program and Genes, Environment and Health (RPGEH) study including 106,373 men. They found that type 2 diabetes is associated with a 1.32-fold increased risk of LUTS in men (87). Another study has reported that men with type 2 diabetes had high storage and postmicturition symptom scores than age- and prostate volume-matched controls (88). In a survey of 2,115 white men aged 40–79 years, Burke et al. found the American Urological Association Symptom Index changed annually in patients with diabetes, indicating diabetes was associated with LUTS (89). It has been shown that women with type 2 diabetes are more likely to experience urinary incontinence compared with control subjects, although these studies fail to differentiate the different kinds of urinary incontinence (urge, stress, mixed, or overflow) (28, 90). Moreover, the prevalence of OAB in diabetic women is much higher than that of healthy controls (91, 92). LUTS in patients with diabetes are critically impacted by gender, possibly due to hormone variations and their effects on LUT organs, as well as the differences in physiology and anatomy between the male and female urethra (93).
Lower Urinary Tract Symptoms in Cardiovascular Diseases
Cardiovascular diseases (CVDs) refer to disorders of the heart or blood vessels and mainly include coronary heart disease, stroke, aneurysms, and other heart- and blood vessel-related diseases. CVDs are the leading cause of death worldwide. World Health Organization (WHO) estimated 17.9 million people died from CVDs globally in 2016, accounting for 31% of all deaths, and most cases (85%) died from a heart attack or stroke. There are many lines of evidence supporting the association between LUTS and cardiovascular events (94, 95). Kupelian et al. (32) reported a correlation between heart disease and the duration and severity of LUTS represented by nocturia and urinary frequency in men, while weak stream and strain in women. The correlation between urologic symptoms and heart disease has been shown to vary by gender, presumably due to the different responses of the male and female urinary tracts to the pathological condition (32). Whether LUTS severity can be a significant risk factor of cardiovascular disease has been investigated in some studies (35), and the findings were controversial. The meta-analysis results from pooled 15 studies revealed that moderate to severe LUTS could indicate the increased risk of cardiac disease in the male population (34). Obese men and women with LUTS are more likely to experience heart disease (33). On the contrary, another meta-analysis performed on five longitudinal studies does not confirm that LUTS can predict cardiac events in men without cardiovascular disease history (96).
Lower urinary tract symptoms are more common among stroke patients and have a significant impact on the quality of life. Nocturia (76%), urgency (70%), and daytime frequency (59%) are the most frequent symptoms in stroke patients (97). Tian et al. have shown that LUTS prevalence in patients with stroke history is about 62.6% (15). Storage symptoms in stroke patients have been found to be significantly correlated with increasing age, male gender, high education, living alone, snoring, and co-existing medical conditions such as hypertension, and coronary heart disease (15). While, voiding symptoms, including slow stream and straining, are markedly associated with age and physical activity following stroke (98).
Lower Urinary Tract Symptoms in Hypertension
The National Health and Nutrition Examination Survey in 2017–2018 revealed that the prevalence of hypertension was 51.0% in men and 39.7% in women. The prevalence of hypertension increased with age, from 22.4% of patients aged 18–39, 54.5% of patients aged 40–59, to 74.5% among those aged 60 and over (99).
Emerging evidence highlights hypertension as one of the risk factors that increase the frequency and severity of LUTS (100). Hypertension was highly associated with nocturnal polyuria in both genders (101, 102). A study by Hwang et al. has demonstrated that hypertensive patients have a higher International Prostate Symptom Score (IPSS) and larger prostate volume than normotensive men, indicating the association between hypertension and male LUTS (37). Chong et al. found hypertension was significantly associated with moderate-to-severe LUTS after adjusting for demographic and lifestyle factors (103). In a multicenter study in Japan including 10,744 men with LUTS, hypertension was the most common comorbidity (25.9%). The prevalence of hypertension was related to the degree of frequency and nocturia (38). Moreover, hypertension has been shown to worsen LUTS and may decrease the effectiveness of α1-blocker and terazosin therapy on BPH (39).
Lower Urinary Tract Symptoms in Obstructive Sleep Apnea
Obstructive Sleep Apnea (OSA) is a common respiratory disorder characterized by episodes of partial or complete obstruction of the upper airway during sleep. It was estimated that OSA may affect approximately 1 billion of the world’s adult population (7.3 billion) and is even continuing to rising (104, 105). Emerging evidence suggests a possible link between LUTS and OSA. The prevalence of nocturia in patients with OSA was from 52 to 70% depending on OSA severity (40–45), and the frequency of nocturia is directly proportional to the severity of OSA (46). The results from the Complementary and Alternative Medicine for Urological Symptoms (CAMUS) trial revealed that the severity of obstructive/voiding LUTS in men was significantly associated with sleep disturbance (106). Continuous Positive Airway Pressure therapy (CPAP) is an effective and widely used method for treating OSA. CPAP treatment can decrease the frequency of nocturia and therefore improve the quality of life (45, 107).
Lower Urinary Tract Symptoms in Autoimmune Diseases
Autoimmune diseases arise when the immune system cannot differentiate between self and foreign antigens, and both genetic and environmental factors are involved. More than 80 different autoimmune diseases affect nearly 4% of the world’s population. The prevalence in the United States is around 7%, or up to 23.5 million. Common autoimmune diseases include Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE), and multiple sclerosis (MS).
The prevalence of severe LUTS is higher in patients with autoimmune diseases than in healthy people. Haarala et al. (47) studied the prevalence of urinary symptoms in patients with SS (n = 36), SLE (n = 85), and 121 control subjects. Among the three groups, 61, 62, and 27% had mild symptoms, and 14, 9, and 7% had severe symptoms. Urinary frequency (27 and 62%, respectively) and suprapubic pain (36 and 34%, respectively) were mostly reported in SS and SLE patients (47). In addition, patients with SLE and SS have significantly worse overactive bladder symptoms compared to controls (50). LUTS are also commonly reported among MS patients and usually appear 6–8 years after the initial diagnosis. After 10 years of duration, more than 90% of MS patients report LUTS, and the symptom severity usually correlates with the disability status of patients (48, 49).
Lower Urinary Tract Symptoms in Alzheimer’s Disease
Alzheimer’s Disease (AD) is the most prevalent neurodegenerative disease accounting for dementia among older adults. It is characterized by the deposition of tau, neurofibrillary tangles, and beta-amyloid plaques in the brain, clinically showing a progressive loss of memory and higher executive functioning (108). Data from Alzheimer’s Association estimated that 5.8 million Americans are suffering from Alzheimer’s dementia in 2019. The prevalence of Alzheimer’s dementia increased with age: from 3% among adults aged 65–74, to 17% among aged 75–84, and 32% among those aged 85 and older (109).
The association between AD and LUTS was reported in several clinical studies. Takahashi et al. found AD was an independent risk factor of OAB and urinary incontinence in older people (51). The risk of urinary incontinence (UI) is higher in AD cohort (hazard ratio: 1.54, 95% confidence interval: 1.13–2.09) than in the control group (110). A clinical study conducted by Na et al. revealed that about 25% of patients with AD experienced UI (52). Recently, Jung et al. (53) have demonstrated that OAB is more common in patients with AD than control subjects, and the prevalence of OAB symptoms increases with age. In addition, the severity of OAB symptoms has been shown to be correlated with the severity of AD (53).
Lower Urinary Tract Symptoms in Parkinson’s Disease
Parkinson’s disease (PD) is the second most common degenerative neurological disease after AD, characterized by the progressive loss of dopamine (DA) neurons in the substantia nigra pars compacta, ultimately leading to debilitating symptoms like resting tremor, muscular rigidity, and postural imbalance. Parkinson’s Foundation estimated that 7–10 million people globally have PD, and nearly 1 million Americans suffer from PD.
Lower urinary tract symptoms are frequently observed in PD, affecting 27–85% of PD patients and significantly impacting their quality of life (111–113). Benli et al. reported significantly higher urologic complaints in PD patients than in age-matched controls (114). Patients with PD have storage problems or voiding problems, or both (54). Yeo et al. reported about 57–83% of PD patients had storage symptoms, whereas 17–27% of patients experienced voiding symptoms (115).
Systemic Oxidative Stress is Implicated in Aging and Major Chronic Diseases
Oxidative Stress in Aging
Aging is a process characterized by a progressive functional decline of all body organs. Among the proposed aging theories, the most plausible one is the free radical theory or the oxidative stress theory. In a study of 100 healthy men and women in five age groups, Kasapoglu et al. found protein carbonyl content increased in subjects aged 60–69 years compared to those aged 20–29 and 30–39 years old (116). The activities of the main antioxidative enzymes in blood including Cu, Zn-SOD, GPx, catalase decreased significantly in older people (65–90+ years) compared to the younger group (aged 55–59 years) (117). Another study showed that the activation response of Nrf2 signaling to OS and the expression of its target antioxidant genes decreased in an age-dependent manner (118). The decrease of mitochondrial oxidative phosphorylation in aging may contribute to the increase in the production of ROS. The precise mechanisms of OS-induced aging are still not completely understood. The progressive aging-related functional loss may be associated with accumulated oxidative damage of all types of biological molecules, including lipids, DNA, and protein (119). Increased free radicals may cause cellular senescence, therefore preventing cellular proliferation processes in response to injury. In addition, senescent cells gain an irreversible senescence-associated secretory phenotype (SASP), secreting soluble factors (such as chemokines and interleukins) and matrix-degrading enzymes, altering the microenvironment and affecting the behavior of the surrounding cells (120, 121).
Oxidative Stress in Obesity
Both experimental and clinical studies have shown that obesity and OS are strongly interconnected, despite being difficult to define the temporal sequence of their association (122). Gutierrez-Lopez et al. (123) found plasma OS markers including malondialdehyde (MDA), carbonyl group, and dityrosine increased in obese patients (BMI, 30–34.9 kg/m2) compared to control subjects (BMI, 18.5–24.9 kg/m2). These changes could be reversed by treatment with a hypocaloric diet for 90 days (123). Several mechanisms have recently been proposed to explain the augmented OS during obesity, including altered glucose and lipid homeostasis, systemic inflammation, hyperleptinemia, and abnormal postprandial ROS production (122). On the other hand, the systemic OS can induce obesity by stimulating pre-adipocyte proliferation and adipocyte differentiation and increasing the size of mature adipocytes (124). Furthermore, OS has been postulated to be an important mechanism underlying obesity-related MetS (125).
Oxidative Stress in Metabolic Syndrome
Metabolic syndrome is strongly associated with chronic systemic OS (126). Van Guilder et al. examined the plasma biomarkers of OS in 48 normal-weight, 20 obese, and 20 obese with MetS adults (127). They found that the plasma concentrations of oxidized low-density lipoprotein inhere groups are 45.1 ± 1.8, 54.0 ± 4.0, and 62.3 ± 3.2 U/L, respectively, indicating increased OS levels in obesity and further in MetS. In another study, Jialal et al. found increased levels of oxidized low-density lipoprotein (Ox-LDL) and nitrotyrosine in plasma, and enhanced superoxide anion release from monocytes in MetS patients compared with controls (128).
Increased ROS production and decreased potency of the free radical scavenging system contribute to the increased OS level in MetS. High energy diet increases the mitochondria’s metabolic load, resulting in an overactive electron transport chain that generates excessive ROS. The activity of NADPH oxidase (Nicotinamide adenine dinucleotide phosphate oxidase, NOX) is upregulated in the aorta, kidney, and adipose tissues in high fat diet-fed rats, leading to excessive production of superoxide ions – a major oxygen free radical (129). On the other hand, MetS-induced OS may be related to impaired antioxidative activity (130). Lucie et al. examined the activities of antioxidant enzymes in hemolysed erythrocytes. They found increased activities of Cu, Zn-SOD, and glutathione reductase, while decreased activities of catalase and paraoxonase 1 in the MetS group compared to healthy controls, indicating a disorder in antioxidant defense mechanism (131).
Oxidative Stress in Diabetes
The redox imbalance between the production of ROS and antioxidant defense systems is also associated with the development of diabetes and its complications. Metabolic abnormalities in diabetes cause excessive production of ROS and RNS, which leads to OS and cellular damage (132, 133). MDA, protein carbonyl (134), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) (135) increased consistently in patients with type 2 diabetes compared to healthy subjects. In addition, reduced total antioxidant status was also observed in type 2 diabetic patients compared to controls and prediabetic patients (136). Several sources of ROS have been identified during diabetes, including enhanced blood glucose flux through the polyol and hexosamine pathway, increased formation of advanced glycation end products, and activation of protein kinase C pathway (137, 138).
Oxidative Stress in Cardiovascular Diseases
Oxidative stress is one of the important mechanisms for the development and progression of CVDs (139, 140). The major contributors of ROS production during CVDs are mitochondrial NOX (141), lypoxidases (142), xanthine oxidase (XO) (143), and myeloperoxidases (144). In a study including 50 coronary heart disease (CHD) patients and 50 healthy volunteers, Mostafa et al. found serum GSH level, and GPx and catalase activities were significantly lower, whereas MDA level was higher in CHD patients than in healthy individuals (145). Patel et al. (146) measured the plasma levels of oxidized and reduced cystine and GSH in 1,411 patients undergoing coronary angiography. They found a high extracellular oxidant burden or reduced intracellular antioxidant capacity is associated with higher mortality in patients with coronary artery disease (146).
Oxidative stress has emerged as a key detrimental player in ischemic stroke. Impaired antioxidant defense system has also been observed in ischemic stroke patients (147). During brain ischemia, oxygen depletion stimulates the glucolytic pathway as an anaerobic source of ATP production, resulting in lactic acid accumulation and acidosis. The latter promotes pro-oxidant production and oxidative injury to neurons (148). Milanlioglu et al. reported higher levels of serum MDA and lower levels of antioxidant enzyme activity in acute ischemic stroke patients than in healthy subjects (149). Laura et al. measured the OS markers in 50 patients with acute ischemic stroke (150). They found the levels of lipoperoxide, hydroperoxide, and conjugated diene in platelets were significantly higher in the early stage and positively correlated with the NIH Stroke Scale/Score (NIHSS).
Oxidative Stress in Hypertension
Oxidative stress plays an important role in the pathogenesis of hypertension. Lip et al. have shown that patients with malignant hypertension exhibited increased OS damage, manifested by higher levels of serum lipid hydroproxides, compared with normotensive subjects (151). Furthermore, the activities of endogenous antioxidant enzymes including SOD and GPx in erythrocytes and whole blood were markedly suppressed in untreated mild hypertensive patients than in age-matched healthy controls (152). Vascular ROS can be produced in both endothelium and smooth muscle. The major enzymatic systems involved in ROS production during hypertension include NOX, XO, endothelial nitric oxide synthase (eNOS) uncoupling, cytochrome P450 epoxygenase, and cyclooxygenase (153). Non-phagocytic NOX is potentially activated by angiotensin II during hypertension, causing the excessive production of hydrogen peroxide, superoxide, and peroxynitrite (154). eNOS uncoupling is caused by deficiency or oxidation of tetrahydrobiopterin, an important cofactor for eNOS action, leading to increased generation of superoxide and decreased NO bioavailability (155). XO has been shown to underlie the development of hypertension-induced end-organ damage (156). In addition, ROS generated in mitochondria and the endoplasmic reticulum also contribute to the development of hypertension (157, 158).
Oxidative Stress in Obstructive Sleep Apnea
A number of studies have shown that OS is present in patients with OSA. The circulating polymorphonuclear neutrophils from patients with OSA released more superoxide radical anions, possibly due to the increased activation of NOX (159). 8-isoprostane increased significantly in exhaled breath condensate of OSA patients (160). Serkan et al. (161) measured plasma total antioxidative capacity and total oxidative status in 25 healthy volunteers and 59 obstructive sleep apnea patients. They found the total antioxidative capacity was significantly lower, but the total oxidative status was significantly higher in OSA patients than the healthy controls (161). Ekin et al. found the serum levels of NOX4, ischemia-modified albumin (IMA), MDA, and 8-OHdG in OSA patients were significantly higher than those in healthy subjects (162), which might be due to cyclic hypoxia and reoxygenation in OSA. The salivary levels of OS markers, including thiobarbituric acid reacting substances, advanced oxidation protein products, and advanced glycation end products (AGEs), in patients with severe OSA were higher in the morning compared to the past evening. The change can be partially reversed by short-term continuous positive airway pressure (CPAP) (163).
Oxidative Stress in Autoimmune Diseases
Excessive OS and/or defective antioxidant potential were also reported in patients with autoimmune diseases. In a cross-sectional study of 15 healthy subjects and 26 SS patients, Norheim et al. found plasma levels of protein carbonyl and advanced oxidation protein products were higher in the SS patients than in healthy subjects, indicating increased levels of OS (164). A strong association between the development of SLE and the polymorphisms of genes encoding NADPH and several antioxidant enzymes including SOD, GPx, and catalase, has been reported (165). In a study of nine controls and 21 SLE patients for up to 38 months, Morgan et al. found the serum levels of protein carbonyls were correlated with disease activity (166). Mitochondrial dysfunction, which can cause an overproduction of ROS and RNS, was observed in the early stage of MS patients (167). The urinary levels of 8-iso-PGF2α (a marker of lipid peroxidation) were higher in MS patients than in healthy controls (168). OS is involved in MS through both central and peripheral mechanisms (169). ROS-induced damage contributes to the demyelination in MS. At the same time, ROS-mediated pathways regulate immune cell priming in peripheral lymphoid organs. T cells traveled to the brain parenchyma can be activated by microglia or macrophages, promoting inflammatory cascade (169).
Oxidative Stress in Alzheimer’s Disease
The brain requires an abundant supply of both oxygen and glucose since the oxidation of glucose under aerobic conditions is the primary energy source for the brain. On the other hand, the brain is more vulnerable to OS because it has abundant, easily oxidizable lipid (170). Previous studies found reduced glucose metabolism and impaired oxygen consumption in the brains of AD patients, indicating mitochondrial dysfunction (171). Mitochondrial dysfunction and associated accumulation of ROS may participate in AD development. The levels of OS markers, including protein carbonyls, 4-hydroxy-2-non-enal (HNE), 3-nitrotyrosine (3-NT), and 8-OHdG, are increased in the blood and cerebrospinal fluid of patients with AD (172, 173). OS can cause neuron injury and degeneration partially through the release of excitatory amino acids and an increase in intracellular free Ca2+ (174). Therefore, OS has been considered one of the causative factors for developing AD (175).
Oxidative Stress in Parkinson’s Disease
Oxidative stress has been demonstrated to play a role in dopaminergic cell death in PD. Markers of lipid peroxidation, nucleic acid, and protein oxidation, along with reduced levels of GSH have been found in dopaminergic neurons of PD patient (176). A recent meta-analysis, including 7,212 PD patients and 6,037 healthy subjects from 80 studies, found the levels of blood MDA and 8-OhdG were increased, but catalase and GSH were decreased in PD patients compared with controls (177). The primary sources of ROS in PD include dopamine neurotransmitter metabolism, endoplasmic reticulum stress, mitochondrial dysfunction, and neuroinflammation (178). The nigral dopaminergic neurons contain iron which catalyzes the Fenton reaction where ferrous iron (Fe2+) reduces H2O2 to yield hydroxyl radical (HO⋅), a highly reactive ROS. Besides, dopaminergic neurons are susceptible to OS and have ROS-producing enzymes, such as, tyrosine hydroxylase and monoamine oxidase. For these reasons, even moderate OS can trigger a series of events that ultimately lead to cellular death (179).
Impact of Oxidative Stress on the Lower Urinary Tract
Impact of Oxidative Stress on the Urinary Bladder
Several lines of evidence support that OS is a major pathogenic factor underlying the development and progression of bladder dysfunction in certain pathologic conditions, including diabetic cystopathy (180), bladder outlet obstruction (181), and chronic bladder ischemia (182). ROS can damage detrusor muscle mitochondria, resulting in decreased efficacy of energy production and impaired bladder contraction (183).
In previous studies, we found increased nitrotyrosine levels in the bladders of 9-week diabetic mice (184), and 20-week (185) and 44-week (186) diabetic rats. To investigate the role of OS on bladder dysfunction, we generated a mouse model with inducible smooth muscle (SM) - specific deletion of manganese superoxide dismutase (MnSOD), encoded by the superoxide dismutase-2 gene (Sod2). The depletion of MnSOD caused increased nitrotyrosine expression in the bladder, indicating OS. Meanwhile, the SM-specific Sod2 knockout mice had overactive bladder symptoms (187). One possible explanation for OS-induced bladder hyperactivity may be related to the activation of C fiber afferent pathway (188). OS has also been shown to mediate bladder hyperactivity in a chronic bladder ischemia rat model induced by atherosclerosis (189–191), possibly through affecting the release of nerve growth factor and the expression of its receptor p75, leading to neurodegeneration and neuropathy in the bladder (192). On the other hand, the severe and prolonged OS has been shown to impair detrusor contractility, leading to bladder underactivity (193, 194). Therefore, it is conceivable that the effects of ROS/RNS on bladder function are dose and/or duration-dependent. Induction of mild OS in the bladder of rats by intravesical administration of a low dose of H2O2 (0.003%) has been found to cause higher peak voiding pressure and detrusor hyperactivity. In contrast, a high dose of H2O2 (3%) induced a decline of the peak voiding pressure and impaired detrusor contractility (188). The mild OS may activate the bladder afferent nerves (188) and stimulate the detrusor muscle cells directly (195), leading to bladder hyperactivity, while severe or prolonged OS can induce detrimental alterations in nerves and the detrusor muscle ultimately resulting in detrusor underactivity (196).
Impact of Oxidative Stress on the Urethra
Oxidative stress has emerged as a potential pathogenic factor for urethral dysfunction in diabetes (197, 198) and aging (199). Urethral dysfunction can manifest as the impairment of contraction or relaxation (200, 201). The former is related to urinary incontinence, while the latter leads to urethral tightening and functional outlet obstruction, lowering the voiding efficiency. OS significantly attenuates the generation and bioavailability of NO, a major transmitter that induces urethral relaxation during bladder voiding (202). In addition, overproduction of ROS has been shown to induce oxidative injury and cause atrophy of the urethral sphincter muscles, leading to impaired urethral closure (198). Enhanced OS coupled with increased collagen deposition has been observed in rats with castration, and the changes can be attenuated by alpha-tocopherol supplementation (203). Alexandre et al. (204) have reported higher levels of ROS, along with decreased levels of NO and SOD in the urethral smooth muscle of obese mice. All these abnormalities are reversed by treatment with resveratrol (204). In a streptozotocin-induced diabetic rat model, urethral dysfunction was associated with increased OS markers, decreased activity of antioxidant enzymes, along with the downregulation of nNOS, NO, and cGMP (197).
Impact of Oxidative Stress on the Prostate
There is increasing evidence highlighting OS as an important mechanism that triggers a series of events involved in the development and progression of BPH. Patients with BPH have higher plasma and urine levels of OS markers than age-matched healthy subjects (205, 206). Resveratrol is a nutraceutical belonging to the polyphenols’ stilbenoids group, mostly found in grapes’ skin and seeds, some berries, and peanuts. Numerous studies have demonstrated that resveratrol possesses anti-inflammatory and antioxidant properties (207). After 2-months of treatment with resveratrol, patients with prostate fibrosis and LUTS showed significant improvement of NIH-Chronic Prostatic Symptom Index (NIH-CPSI) and IPSS scores (208). In another clinical study, Matsumoto et al. (209) used Eviprostat, a phytotherapeutic agent composed of several plant extracts, to treat BPH-associated LUTS. They found the total IPSS decreased from 16.56 ± 2.74 to 13.67 ± 2.30 after Eviprostat treatment. This effect is accompanied by a 2.5-fold decrease of urinary 8-OHdG. The authors concluded that the antioxidant activity of Eviprostat may be responsible for its treatment effects (209).
The causal role of OS in BPH has been supported by Vital et al.’s (210) study, in which they generated a transgenic mouse model with overexpression of Nox4 under the control of the prostate-specific promoter ARR2PB. The transgenic ARR2PB-Nox4 mice were found to display oxidative injury in prostate tissue, prostate hyperplasia along with histological remodeling (epithelial proliferation and fibrosis) (210). Several plausible mechanisms have been proposed to understand the role of OS in causing BPH. OS induces oxidative DNA damage, adversely affecting the balance between cell proliferation and programmed cell death leading to hyperplasia. In addition, NF-κB is the master inflammatory transcriptional regulator of genes involved in inflammation, cell proliferation, cell migration, and apoptosis (211). ROS can activate the NF-κB pathway, contributing to chronic prostate inflammation and BPH (212). Furthermore, OS is associated with increased plasma activity of prolidase, a key enzyme in collagen turnover and matrix remodeling, promoting the development of BPH (213).
Conclusion
Many studies showed that LUTS are highly prevalent in the aging population and patients with chronic diseases, supporting the speculation that those disease-related pathophysiological changes also contribute to the development of LUTS. There may be one or more common underlying factors for the aforementioned chronic diseases and LUTS. Studies also showed that OS is a mutual pathogenic factor of aging and major chronic diseases. Meanwhile, recent studies showed that exacerbated OS could cause functional and/or structural changes in the bladder, urethra, and prostate, leading to LUTS. Taken together, these data suggest that OS seems to be a potential link between LUTS and aging and major chronic diseases (Figure 1), although further studies are needed to confirm the causative relationship. Urologists should consider factors outside the lower urinary tract that may be contributing to LUTS and the higher likelihood of LUTS during aging and these major chronic diseases. It remains to be determined whether treatment of these diseases can significantly improve urologic symptoms. On the other hand, general antioxidant treatment might not be the best choice since ROS/RNS also serve as second messengers in the physiological regulation of intracellular signaling cascades involved in various cellular functions. Future investigation is required to identify the specific ROS/RNS and corresponding reactions/pathways involved in aging and chronic diseases and associated LUTS, which would be a reasonable basis for developing particular antioxidants for clinical application.
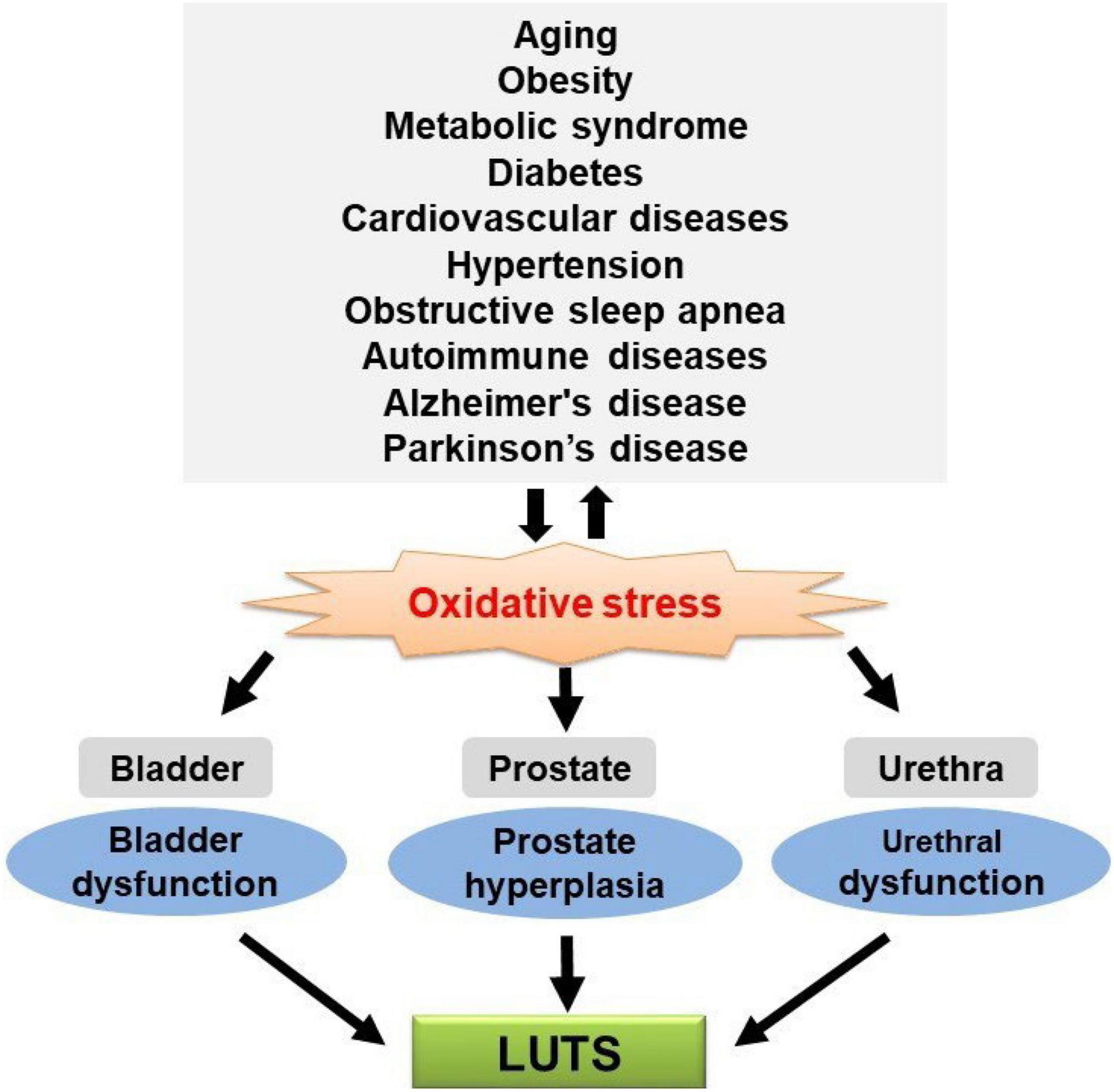
Figure 1. Oxidative stress (OS) is a putative mechanistic link between lower urinary tract symptoms (LUTS) and aging and chronic diseases.
Author Contributions
GL: conceptualization and writing—review and editing. ZX, RE, BL, and GL: writing—original draft preparation, read, and agreed to the published version of the manuscript.
Funding
GL was supported by the National Institutes of Health (NIH) NIDDK grant R01-DK110567.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Przydacz M, Golabek T, Dudek P, Lipinski M, Chlosta P. Prevalence and bother of lower urinary tract symptoms and overactive bladder in Poland, an Eastern European Study. Sci Rep. (2020) 10:19819. doi: 10.1038/s41598-020-76846-0
2. Harlow BL, Bavendam TG, Palmer MH, Brubaker L, Burgio KL, Lukacz ES, et al. The prevention of lower urinary tract symptoms (PLUS) research consortium: a transdisciplinary approach toward promoting bladder health and preventing lower urinary tract symptoms in women across the life course. J Womens Health (Larchmt). (2018) 27:283–9. doi: 10.1089/jwh.2017.6566
3. Plata M, Bravo-Balado A, Robledo D, Trujillo CG, Caicedo JI, Catano JG, et al. Prevalence of lower urinary tract symptoms and overactive bladder in men and women over 18 years old: the Colombian overactive bladder and lower urinary tract symptoms (COBaLT) study. Neurourol Urodyn. (2019) 38:200–7. doi: 10.1002/nau.23828
4. Irwin DE, Milsom I, Kopp Z, Abrams P, Artibani W, Herschorn S. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol. (2009) 56:14–20. doi: 10.1016/j.eururo.2009.02.026
5. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the international continence society. Urology. (2003) 61:37–49. doi: 10.1016/s0090-4295(02)02243-4
6. Cox L, Rovner ES. Lower urinary tract symptoms in women: epidemiology, diagnosis, and management. Curr Opin Urol. (2016) 26:328–33. doi: 10.1097/MOU.0000000000000283
7. Milsom I. Lower urinary tract symptoms in women. Curr Opin Urol. (2009) 19:337–41. doi: 10.1097/MOU.0b013e32832b659d
8. Mariappan P, Chong WL. Prevalence and correlations of lower urinary tract symptoms, erectile dysfunction and incontinence in men from a multiethnic Asian population: results of a regional population-based survey and comparison with industrialized nations. BJU Int. (2006) 98:1264–8. doi: 10.1111/j.1464-410X.2006.06525.x
9. Vedanayagam M, Kumar A, Madaan S. Lower urinary tract symptoms in an older man. BMJ. (2017) 357:j1493. doi: 10.1136/bmj.j1493
10. Bernell S, Howard SW. Use your words carefully: what is a chronic disease? Front Public Health. (2016) 4:159. doi: 10.3389/fpubh.2016.00159
11. Raghupathi W, Raghupathi V. An empirical study of chronic diseases in the United States: a visual analytics approach. Int J Environ Res Public Health. (2018) 15:431. doi: 10.3390/ijerph15030431
12. Osuga Y, Okamura K, Ando F, Shimokata H. Prevalence of lower urinary tract symptoms in middle-aged and elderly Japanese. Geriatr Gerontol Int. (2013) 13:1010–7. doi: 10.1111/ggi.12048
13. Camoes J, Coelho A, Castro-Diaz D, Cruz F. Lower urinary tract symptoms and aging: the impact of chronic bladder ischemia on overactive bladder syndrome. Urol Int. (2015) 95:373–9. doi: 10.1159/000437336
14. Poyhonen A, Auvinen A, Hakkinen JT, Koskimaki J, Tammela TL. Population-level and individual-level bother of lower urinary tract symptoms among 30- to 80-year-old men. Urology. (2016) 95:164–70. doi: 10.1016/j.urology.2016.06.023
15. Tian Y, Guan Y, Wen J, Shang X, Li J, Wang Y. Survey and risk factors for lower urinary tract storage symptoms in middle-aged and older stroke patients in urban China. Lower Urinary Tract Symp. (2016) 8:91–9. doi: 10.1111/luts.12078
16. Vahabi B, Wagg AS, Rosier P, Rademakers KLJ, Denys MA, Pontari M, et al. Can we define and characterize the aging lower urinary tract?-ICI-RS 2015. Neurourol Urodyn. (2017) 36:854–8. doi: 10.1002/nau.23035
17. Coban S, Cander S, Altuner MS, Keles I, Gul OO. Does metabolic syndrome increase erectile dysfunction and lower urinary tract symptoms. Urol J. (2014) 11:1820–4. doi: 10.1111/bju.13038
18. Mondul AM, Giovannucci E, Platz EA. A prospective study of obesity, and the incidence and progression of lower urinary tract symptoms. J Urol. (2014) 191:715–21. doi: 10.1016/j.juro.2013.08.110
19. Kim JH, Sun HY, Lee HY, Soh MJ, Park S, Kim YJ, et al. Improvement of voiding characteristics in morbidly obese women after bariatric surgery: a single-center study with a 1-year follow-up. Surg Obes Relat Dis. (2017) 13:836–41. doi: 10.1016/j.soard.2017.01.047
20. Kim JH, Sun HY, Park SY, Soh MJ, Kim YJ, Song YS. Association between obesity and lower urinary tract symptoms: propensity score matching study between healthy controls and obese patients seeking bariatric surgery. Surg Obes Relat Dis. (2016) 12:1585–93. doi: 10.1016/j.soard.2016.04.027
21. Groutz A, Gordon D, Schachter P, Amir H, Shimonov M. Effects of bariatric surgery on male lower urinary tract symptoms and sexual function. Neurourol Urodyn. (2017) 36:636–9. doi: 10.1002/nau.22980
22. Russo GI, Regis F, Spatafora P, Frizzi J, Urzi D, Cimino S, et al. Association between metabolic syndrome and intravesical prostatic protrusion in patients with benign prostatic enlargement and lower urinary tract symptoms (MIPS Study). BJU Int. (2018) 121:799–804. doi: 10.1111/bju.14007
23. Saratlija Novakovic Z, Tesija RA, Puljak L. Association between metabolic syndrome and overactive bladder: a case-control study. Scand J Urol. (2017) 51:470–3. doi: 10.1080/21681805.2017.1354912
24. Ngai HY, Yuen KS, Ng CM, Cheng CH, Chu SP. Metabolic syndrome and benign prostatic hyperplasia: an update. Asian J Urol. (2017) 4:164–73. doi: 10.1016/j.ajur.2017.05.001
25. Denys MA, Anding R, Tubaro A, Abrams P, Everaert K. Lower urinary tract symptoms and metabolic disorders: ICI-RS 2014. Neurourol Urodyn. (2016) 35:278–82. doi: 10.1002/nau.22765
26. Plata M, Caicedo JI, Trujillo CG, Marino-Alvarez AM, Fernandez N, Gutierrez A, et al. Prevalence of metabolic syndrome and its association with lower urinary tract symptoms and sexual function. Actas Urol Esp. (2017) 41:522–8. doi: 10.1016/j.acuro.2016.12.009
27. Tam CA, Helfand BT, Erickson BA. The relationship between diabetes, diabetes severity, diabetes biomarkers, and the presence of lower urinary tract symptoms: findings from the national health and nutrition examination survey. Urology. (2017) 105:141–8. doi: 10.1016/j.urology.2017.03.040
28. Tai HC, Tai TY, Yang WS, Wang SW, Yu HJ. Associations between lower urinary tract dysfunction and glycemic control in women with type 2 diabetes: a cross-sectional study. J Diabetes Complications. (2016) 30:415–9. doi: 10.1016/j.jdiacomp.2016.01.002
29. Dereli Yilmaz S, Demirgoz Bal M, Celik S, Rathfisch G, Kizilkaya Beji N, Dinccag N, et al. Lower urinary tract symptoms in women with type 2 Diabetes mellitus. J Wound Ostomy Continence Nurs. (2016) 43:523–8. doi: 10.1097/WON.0000000000000259
30. Vignozzi L, Gacci M, Maggi M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat Rev Urol. (2016) 13:108–19. doi: 10.1038/nrurol.2015.301
31. Gali A, Mucciardi G, Buttice S, Subba E, D’Amico C, Lembo F, et al. Correlation between advanced glycation end-products, lower urinary tract symptoms and bladder dysfunctions in patients with type 2 diabetes mellitus. Lower Urinary Tract Symptoms. (2017) 9:15–20. doi: 10.1111/luts.12102
32. Kupelian V, Rosen RC, Link CL, McVary KT, Aiyer LP, Mollon P, et al. Association of urological symptoms and chronic illness in men and women: contributions of symptom severity and duration–results from the BACH Survey. J Urol. (2009) 181:694–700. doi: 10.1016/j.juro.2008.10.039
33. Kupelian V, Araujo AB, Wittert GA, McKinlay JB. Association of moderate to severe lower urinary tract symptoms with incident type 2 diabetes and heart disease. J Urol. (2015) 193:581–6. doi: 10.1016/j.juro.2014.08.097
34. Gacci M, Corona G, Sebastianelli A, Serni S, De Nunzio C, Maggi M, et al. Male lower urinary tract symptoms and cardiovascular events: a systematic review and meta-analysis. Eur Urol. (2016) 70:788–96. doi: 10.1016/j.eururo.2016.07.007
35. Wehrberger C, Temml C, Gutjahr G, Berger I, Rauchenwald M, Ponholzer A, et al. Is there an association between lower urinary tract symptoms and cardiovascular risk in men? A cross sectional and longitudinal analysis. Urology. (2011) 78:1063–7. doi: 10.1016/j.urology.2011.05.065
36. Fode M, Gratzke C, Sonksen J. Male lower urinary tract symptoms reveal and predict important cardiovascular disease. Eur Urol. (2016) 70:797–8. doi: 10.1016/j.eururo.2016.07.030
37. Hwang EC, Kim SO, Nam DH, Yu HS, Hwang I, Jung SI, et al. Men with hypertension are more likely to have severe lower urinary tract symptoms and large prostate volume. Lower Urinary Tract Symptoms. (2015) 7:32–6. doi: 10.1111/luts.12046
38. Ito H, Yoshiyasu T, Yamaguchi O, Yokoyama O. Male lower urinary tract symptoms: hypertension as a risk factor for storage symptoms, but not voiding symptoms. Lower Urinary Tract Symptoms. (2012) 4:68–72. doi: 10.1111/j.1757-5672.2011.00115.x
39. Sugaya K, Kadekawa K, Ikehara A, Nakayama T, Gakiya M, Nashiro F, et al. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. Int J Urol. (2003) 10:569–74; discussion 75. doi: 10.1046/j.1442-2042.2003.00707.x
40. Oztura I, Kaynak D, Kaynak HC. Nocturia in sleep-disordered breathing. Sleep Med. (2006) 7:362–7. doi: 10.1016/j.sleep.2005.10.004
41. Moriyama Y, Miwa K, Tanaka H, Fujihiro S, Nishino Y, Deguchi T. Nocturia in men less than 50 years of age may be associated with obstructive sleep apnea syndrome. Urology. (2008) 71:1096–8. doi: 10.1016/j.urology.2008.02.038
42. Raheem OA, Orosco RK, Davidson TM, Lakin C. Clinical predictors of nocturia in the sleep apnea population. Urol Ann. (2014) 6:31–5. doi: 10.4103/0974-7796.127019
43. Chung SD, Hung SH, Lin HC, Tsai MC, Kao LT. Obstructive sleep apnea and urological comorbidities in males: a population-based study. Sleep Breath. (2016) 20:1203–8. doi: 10.1007/s11325-016-1336-x
44. Miyazaki T, Kojima S, Yamamuro M, Sakamoto K, Izumiya Y, Tsujita K, et al. Nocturia in patients with sleep-disordered breathing and cardiovascular disease. Circ J. (2015) 79:2632–40. doi: 10.1253/circj.CJ-15-0654
45. Irer B, Celikhisar A, Celikhisar H, Bozkurt O, Demir O. Evaluation of sexual dysfunction, lower urinary tract symptoms and quality of life in men with obstructive sleep Apnea syndrome and the efficacy of continuous positive airway pressure therapy. Urology. (2018) 121:86–92. doi: 10.1016/j.urology.2018.08.001
46. Ayik S, Bal K, Akhan G. The association of nocturia with sleep disorders and metabolic and chronic pulmonary conditions: data derived from the polysomnographic evaluations of 730 patients. Turk J Med Sci. (2014) 44:249–54. doi: 10.3906/sag-1210-20
47. Haarala M, Alanen A, Hietarinta M, Kiilholma P. Lower urinary tract symptoms in patients with Sjogren’s syndrome and systemic lupus erythematosus. Int Urogynecol J Pelvic Floor Dysfunct. (2000) 11:84–6. doi: 10.1007/s001920050075
48. Zecca C, Riccitelli GC, Disanto G, Singh A, Digesu GA, Panicari L, et al. Urinary incontinence in multiple sclerosis: prevalence, severity and impact on patients’ quality of life. Eur J Neurol. (2016) 23:1228–34. doi: 10.1111/ene.13010
49. Aharony SM, Lam O, Corcos J. Evaluation of lower urinary tract symptoms in multiple sclerosis patients: review of the literature and current guidelines. Can Urol Assoc J. (2017) 11:61–4. doi: 10.5489/cuaj.4058
50. Pereira ESR, Romao VC, Neves M, Garcia R, Oliveira S, Brites J, et al. Overactive bladder symptom bother and health-related quality of life in patients with systemic lupus erythematosus and primary Sjogren syndrome. Lupus. (2019) 28:27–33. doi: 10.1177/0961203318811605
51. Takahashi O, Sakakibara R, Panicker J, Fowler CJ, Tateno F, Kishi M, et al. White matter lesions or Alzheimer’s disease: which contributes more to overactive bladder and incontinence in elderly adults with dementia? J Am Geriatr Soc. (2012) 60:2370–1. doi: 10.1111/jgs.12004
52. Na HR, Park MH, Cho ST, Lee BC, Park S, Kim KH, et al. Urinary incontinence in Alzheimer’s disease is associated with clinical dementia rating-sum of boxes and barthel activities of daily living. Asia Pac Psychiatry. (2015) 7:113–20. doi: 10.1111/appy.12007
53. Jung HB, Choi DK, Lee SH, Cho ST, Na HR, Park MH. Correlation between overactive bladder symptom score and neuropsychological parameters in Alzheimer’s disease patients with lower urinary tract symptom. Int Braz J Urol. (2017) 43:256–63. doi: 10.1590/s1677-5538.ibju.2015.0664
54. Robinson JP, Bradway CW, Bunting-Perry L, Avi-Itzhak T, Mangino M, Chittams J, et al. Lower urinary tract symptoms in men with Parkinson disease. J Neurosci Nurs. (2013) 45:382–92; quiz E1-2. doi: 10.1097/JNN.0b013e3182a3cf67
55. Madan A, Ray S, Burdick D, Agarwal P. Management of lower urinary tract symptoms in Parkinson’s disease in the neurology clinic. Int J Neurosci. (2017) 127:1136–49. doi: 10.1080/00207454.2017.1327857
56. Kapoor S, Bourdoumis A, Mambu L, Barua J. Effective management of lower urinary tract dysfunction in idiopathic Parkinson’s disease. Int J Urol. (2013) 20:79–84. doi: 10.1111/j.1442-2042.2012.03220.x
57. Witte LP, Odekerken VJJ, Boel JA, Schuurman PR, Gerbrandy-Schreuders LC, de Bie RMA, et al. Does deep brain stimulation improve lower urinary tract symptoms in Parkinson’s disease? Neurourol Urodyn. (2018) 37:354–9. doi: 10.1002/nau.23301
58. Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. (2017) 38:592–607. doi: 10.1016/j.tips.2017.04.005
59. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
60. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. (2014) 159:709–13. doi: 10.1016/j.cell.2014.10.039
61. Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. (2005) 173:1256–61. doi: 10.1097/01.ju.0000155709.37840.fe
62. Parsons JK, Bergstrom J, Silberstein J, Barrett-Connor E. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology. (2008) 72:318–21. doi: 10.1016/j.urology.2008.03.057
63. Taylor BC, Wilt TJ, Fink HA, Lambert LC, Marshall LM, Hoffman AR, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. (2006) 68:804–9. doi: 10.1016/j.urology.2006.04.019
64. Kim TH, Han DH, Lee KS. The prevalence of lower urinary tract symptoms in korean men aged 40 years or older: a population-based survey. Int Neurourol J. (2014) 18:126–32. doi: 10.5213/inj.2014.18.3.126
65. Rohrmann S, Katzke V, Kaaks R. Prevalence and progression of lower urinary tract symptoms in an aging population. Urology. (2016) 95:158–63. doi: 10.1016/j.urology.2016.06.021
66. Maserejian NN, Chen S, Chiu GR, Araujo AB, Kupelian V, Hall SA, et al. Treatment status and progression or regression of lower urinary tract symptoms in a general adult population sample. J Urol. (2014) 191:107–13. doi: 10.1016/j.juro.2013.07.005
67. Kupelian V, Wei JT, O’Leary MP, Kusek JW, Litman HJ, Link CL, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston area community health (BACH) survey. Arch Intern Med. (2006) 166:2381–7. doi: 10.1001/archinte.166.21.2381
68. Mongiu AK, McVary KT. Lower urinary tract symptoms, benign prostatic hyperplasia, and obesity. Curr Urol Rep. (2009) 10:247–53.
69. Lee RK, Chung D, Chughtai B, Te AE, Kaplan SA. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. (2012) 110:540–5. doi: 10.1111/j.1464-410X.2011.10819.x
70. He Q, Wang H, Yue Z, Yang L, Tian J, Liu G, et al. Waist circumference and risk of lower urinary tract symptoms: a meta-analysis. Aging Male. (2014) 17:223–9. doi: 10.3109/13685538.2014.967671
71. He Q, Babcook MA, Shukla S, Shankar E, Wang Z, Liu G, et al. Obesity-initiated metabolic syndrome promotes urinary voiding dysfunction in a mouse model. Prostate. (2016) 76:964–76. doi: 10.1002/pros.23185
72. Gacci M, Sebastianelli A, Salvi M, De Nunzio C, Tubaro A, Gravas S, et al. The impact of central obesity on storage luts and urinary incontinence after prostatic surgery. Curr Urol Rep. (2016) 17:61. doi: 10.1007/s11934-016-0620-4
73. Yazdany T, Jakus-Waldman S, Jeppson PC, Schimpf MO, Yurteri-Kaplan LA, Ferzandi TR, et al. American urogynecologic society systematic review: the impact of weight loss intervention on lower urinary tract symptoms and urinary incontinence in overweight and obese women. Female Pelvic Med Reconstr Surg. (2020) 26:16–29. doi: 10.1097/SPV.0000000000000802
74. Vaughan CP, Auvinen A, Cartwright R, Johnson TM II, Tahtinen RM, Ala-Lipasti MA, et al. Impact of obesity on urinary storage symptoms: results from the FINNO study. J Urol. (2013) 189:1377–82. doi: 10.1016/j.juro.2012.10.058
75. Penson DF, Munro HM, Signorello LB, Blot WJ, Fowke JH. Urologic diseases in America P. Obesity, physical activity and lower urinary tract symptoms: results from the southern community Cohort study. J Urol. (2011) 186:2316–22. doi: 10.1016/j.juro.2011.07.067
76. Oliver JL, Campigotto MJ, Coplen DE, Traxel EJ, Austin PF. Psychosocial comorbidities and obesity are associated with lower urinary tract symptoms in children with voiding dysfunction. J Urol. (2013) 190(4 Suppl.):1511–5. doi: 10.1016/j.juro.2013.02.025
77. Link CL, Steers WD, Kusek JW, McKinlay JB. The association of adiposity and overactive bladder appears to differ by gender: results from the Boston area community health survey. J Urol. (2011) 185:955–63. doi: 10.1016/j.juro.2010.10.048
78. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. (2015) 313:1973–4. doi: 10.1001/jama.2015.4260
79. Kupelian V, McVary KT, Kaplan SA, Hall SA, Link CL, Aiyer LP, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J Urol. (2013) 189(1 Suppl.):S107–14; discussion S15–6. doi: 10.1016/j.juro.2012.11.026
80. Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the third national health and nutrition examination survey (NHANES III). Int J Obes. (2005) 29:310–6. doi: 10.1038/sj.ijo.0802881
81. Zamuner M, Laranja WW, Alonso JC, Simoes FA, Rejowski RF, Reis LO. Is metabolic syndrome truly a risk factor for male lower urinary tract symptoms or just an epiphenomenon? Adv Urol. (2014) 2014:203854. doi: 10.1155/2014/203854
82. Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, De Nunzio C, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int. (2015) 115:24–31. doi: 10.1111/bju.12728
83. He Q, Wang Z, Liu G, Daneshgari F, MacLennan GT, Gupta S. Metabolic syndrome, inflammation and lower urinary tract symptoms: possible translational links. Prostate Cancer Prostatic Dis. (2016) 19:7–13. doi: 10.1038/pcan.2015.43
85. Wessells H, Braffett BH, Holt SK, Jacobson AM, Kusek JW, Cowie C, et al. Burden of Urological complications in men and women with long-standing type 1 diabetes in the diabetes control and complications trial/epidemiology of diabetes interventions and complications Cohort. Diabetes Care. (2018) 41:2170–7. doi: 10.2337/dc18-0255
86. Sarma AV, Kellogg Parsons J. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Curr Urol Rep. (2009) 10:267–75. doi: 10.1007/s11934-009-0044-5
87. Van Den Eeden SK, Ferrara A, Shan J, Jacobsen SJ, Quinn VP, Haque R, et al. Impact of type 2 diabetes on lower urinary tract symptoms in men: a cohort study. BMC Urol. (2013) 13:12. doi: 10.1186/1471-2490-13-12
88. Bang WJ, Lee JY, Koo KC, Hah YS, Lee DH, Cho KS. Is type-2 diabetes mellitus associated with overactive bladder symptoms in men with lower urinary tract symptoms? Urology. (2014) 84:670–4. doi: 10.1016/j.urology.2014.05.017
89. Burke JP, Jacobson DJ, McGree ME, Roberts RO, Girman CJ, Lieber MM, et al. Diabetes and benign prostatic hyperplasia progression in Olmsted County, Minnesota. Urology. (2006) 67:22–5. doi: 10.1016/j.urology.2005.08.010
90. Phelan S, Kanaya AM, Subak LL, Hogan PE, Espeland MA, Wing RR, et al. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the look AHEAD trial. J Urol. (2012) 187:939–44. doi: 10.1016/j.juro.2011.10.139
91. Lawrence JM, Lukacz ES, Liu IL, Nager CW, Luber KM. Pelvic floor disorders, diabetes, and obesity in women: findings from the Kaiser permanente continence associated risk epidemiology study. Diabetes Care. (2007) 30:2536–41. doi: 10.2337/dc07-0262
92. Ho CH, Tai HC, Yu HJ. Urodynamic findings in female diabetic patients with and without overactive bladder symptoms. Neurourol Urodyn. (2010) 29:424–7. doi: 10.1002/nau.20727
93. Daneshgari F, Liu G, Hanna-Mitchell AT. Path of translational discovery of urological complications of obesity and diabetes. Am J Physiol Renal Physiol. (2017) 312:F887–96. doi: 10.1152/ajprenal.00489.2016
94. Bouwman II, Kollen BJ, van der Meer K, Nijman RJ, van der Heide WK. Are lower urinary tract symptoms in men associated with cardiovascular diseases in a primary care population: a registry study. BMC Fam Pract. (2014) 15:9. doi: 10.1186/1471-2296-15-9
95. Fitzgerald MP, Link CL, Litman HJ, Travison TG, McKinlay JB. Beyond the lower urinary tract: the association of urologic and sexual symptoms with common illnesses. Eur Urol. (2007) 52:407–15. doi: 10.1016/j.eururo.2007.03.014
96. Bouwman II, Voskamp MJ, Kollen BJ, Nijman RJ, van der Heide WK, Blanker MH. Do lower urinary tract symptoms predict cardiovascular diseases in older men? A systematic review and meta-analysis. World J Urol. (2015) 33:1911–20. doi: 10.1007/s00345-015-1560-1
97. Tibaek S, Gard G, Klarskov P, Iversen HK, Dehlendorff C, Jensen R. Prevalence of lower urinary tract symptoms (LUTS) in stroke patients: a cross-sectional, clinical survey. Neurourol Urodyn. (2008) 27:763–71. doi: 10.1002/nau.20605
98. Miyazato M, Tana T, Higa A, Wakugami K, Tokashiki T, Sakima H, et al. A questionnaire survey to assess lower urinary tract symptoms in patients with chronic stroke. Neurourol Urodyn. (2017) 36:1890–5. doi: 10.1002/nau.23206
99. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief. (2020) 1–8.
100. Haidinger G, Temml C, Schatzl G, Brossner C, Roehlich M, Schmidbauer CP, et al. Risk factors for lower urinary tract symptoms in elderly men. For the prostate study group of the Austrian society of urology. Eur Urol. (2000) 37:413–20. doi: 10.1159/000020162
101. Yoshimura K, Terada N, Matsui Y, Terai A, Kinukawa N, Arai Y. Prevalence of and risk factors for nocturia: analysis of a health screening program. Int J Urol. (2004) 11:282–7. doi: 10.1111/j.1442-2042.2004.00791.x
102. Yokoyama O, Nishizawa O, Homma Y, Takeda M, Gotoh M, Kakizaki H, et al. Nocturnal polyuria and hypertension in patients with lifestyle related diseases and overactive bladder. J Urol. (2017) 197:423–31. doi: 10.1016/j.juro.2016.08.087
103. Chong C, Fong L, Lai R, Koh YT, Lau WK, Hartman M, et al. The prevalence of lower urinary tract symptoms and treatment-seeking behaviour in males over 40 years in Singapore: a community-based study. Prostate Cancer Prostatic Dis. (2012) 15:273–7. doi: 10.1038/pcan.2011.69
104. Lyons MM, Bhatt NY, Pack AI, Magalang UJ. Global burden of sleep-disordered breathing and its implications. Respirology. (2020) 25:690–702. doi: 10.1111/resp.13838
105. Dragonieri S, Bikov A. Obstructive sleep Apnea: a view from the back door. Medicina (Kaunas). (2020) 56:208. doi: 10.3390/medicina56050208
106. Cakir OO, McVary KT. LUTS and sleep disorders: emerging risk factor. Curr Urol Rep. (2012) 13:407–12. doi: 10.1007/s11934-012-0281-x
107. Miyazato M, Tohyama K, Touyama M, Nakamura H, Oshiro T, Ueda S, et al. Effect of continuous positive airway pressure on nocturnal urine production in patients with obstructive sleep apnea syndrome. Neurourol Urodyn. (2017) 36:376–9. doi: 10.1002/nau.22936
108. Reddy PH, Oliver DM. Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer’s disease. Cells. (2019) 8:488. doi: 10.3390/cells8050488
109. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. (2013) 80:1778–83. doi: 10.1212/WNL.0b013e31828726f5
110. Lee HY, Li CC, Juan YS, Chang YH, Yeh HC, Tsai CC, et al. Urinary incontinence in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. (2017) 32:51–5. doi: 10.1177/1533317516680900
111. McDonald C, Winge K, Burn DJ. Lower urinary tract symptoms in Parkinson’s disease: prevalence, aetiology and management. Parkinsonism Relat Disord. (2017) 35:8–16. doi: 10.1016/j.parkreldis.2016.10.024
112. Campos-Sousa RN, Quagliato E, da Silva BB, de Carvalho RM Jr, Ribeiro SC, de Carvalho DF. Urinary symptoms in Parkinson’s disease: prevalence and associated factors. Arq Neuropsiquiatr. (2003) 61:359–63. doi: 10.1590/s0004-282x2003000300007
113. Hobson P, Islam W, Roberts S, Adhiyman V, Meara J. The risk of bladder and autonomic dysfunction in a community cohort of Parkinson’s disease patients and normal controls. Parkinsonism Relat Disord. (2003) 10:67–71. doi: 10.1016/j.parkreldis.2003.07.001
114. Benli E, Ozer FF, Kaya Y, Ozcan TS, Ayyildiz A. Is there a difference between Parkinson disease patients and a control group in terms of urinary symptoms and quality of life? Turk J Med Sci. (2016) 46:1665–71. doi: 10.3906/sag-1507-148
115. Yeo L, Singh R, Gundeti M, Barua JM, Masood J. Urinary tract dysfunction in Parkinson’s disease: a review. Int Urol Nephrol. (2012) 44:415–24. doi: 10.1007/s11255-011-9969-y
116. Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. (2001) 36:209–20. doi: 10.1016/s0531-5565(00)00198-4
117. Kozakiewicz M, Kornatowski M, Krzywinska O, Kedziora-Kornatowska K. Changes in the blood antioxidant defense of advanced age people. Clin Interv Aging. (2019) 14:763–71. doi: 10.2147/CIA.S201250
118. Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. (2015) 88(Pt B):314–36. doi: 10.1016/j.freeradbiomed.2015.05.036
119. Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. (2012) 2012:646354. doi: 10.1155/2012/646354
120. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. (2018) 13:757–72. doi: 10.2147/CIA.S158513
121. Strzyz P. Cell signalling: signalling to cell cycle arrest. Nat Rev Mol Cell Biol. (2016) 17:536. doi: 10.1038/nrm.2016.108
122. Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. (2013) 14:10497–538. doi: 10.3390/ijms140510497
123. Gutierrez-Lopez L, Garcia-Sanchez JR, Rincon-Viquez Mde J, Lara-Padilla E, Sierra-Vargas MP, Olivares-Corichi IM. Hypocaloric diet and regular moderate aerobic exercise is an effective strategy to reduce anthropometric parameters and oxidative stress in obese patients. Obes Facts. (2012) 5:12–22. doi: 10.1159/000336526
124. Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. (2015) 13:423–44. doi: 10.1089/met.2015.0095
125. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig. (2004) 114:1752–61. doi: 10.1172/JCI21625
126. Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. (2009) 84:705–12. doi: 10.1016/j.lfs.2009.02.026
127. Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring). (2006) 14:2127–31. doi: 10.1038/oby.2006.248
128. Jialal I, Devaraj S, Adams-Huet B, Chen X, Kaur H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J Clin Endocrinol Metab. (2012) 97:E1844–50. doi: 10.1210/jc.2012-2498
129. Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, et al. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. (2011) 16:223–9. doi: 10.1179/174329211X13049558293713
130. Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. (2004) 89:4963–71. doi: 10.1210/jc.2004-0305
131. Vavrova L, Kodydkova J, Zeman M, Dusejovska M, Macasek J, Stankova B, et al. Altered activities of antioxidant enzymes in patients with metabolic syndrome. Obes Facts. (2013) 6:39–47. doi: 10.1159/000348569
132. Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. (2014) 1840:2709–29. doi: 10.1016/j.bbagen.2014.05.017
133. Newsholme P, Rebelato E, Abdulkader F, Krause M, Carpinelli A, Curi R. Reactive oxygen and nitrogen species generation, antioxidant defenses, and beta-cell function: a critical role for amino acids. J Endocrinol. (2012) 214:11–20. doi: 10.1530/JOE-12-0072
134. Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem. (2010) 43:508–11. doi: 10.1016/j.clinbiochem.2009.11.011
135. Liu X, Gan W, Zou Y, Yang B, Su Z, Deng J, et al. Elevated levels of urinary markers of oxidative DNA and RNA damage in type 2 diabetes with complications. Oxid Med Cell Longev. (2016) 2016:4323198. doi: 10.1155/2016/4323198
136. Jimenez-Osorio AS, Picazo A, Gonzalez-Reyes S, Barrera-Oviedo D, Rodriguez-Arellano ME, Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci. (2014) 15:20290–305. doi: 10.3390/ijms151120290
137. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. (2001) 414:813–20. doi: 10.1038/414813a
138. Yang H, Jin X, Kei Lam CW, Yan SK. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. (2011) 49:1773–82. doi: 10.1515/CCLM.2011.250
139. Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. (2009) 2:259–69. doi: 10.4161/oxim.2.5.9441
140. Cervantes Gracia K, Llanas-Cornejo D, Husi H. CVD and oxidative stress. J Clin Med. (2017) 6:22. doi: 10.3390/jcm6020022
141. Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. (2014) 20:2794–814. doi: 10.1089/ars.2013.5607
142. Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. (2010) 86:243–53. doi: 10.1093/cvr/cvq016
143. Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther. (2012) 30:217–26. doi: 10.1111/j.1755-5922.2011.00277.x
144. Anatoliotakis N, Deftereos S, Bouras G, Giannopoulos G, Tsounis D, Angelidis C, et al. Myeloperoxidase: expressing inflammation and oxidative stress in cardiovascular disease. Curr Top Med Chem. (2013) 13:115–38. doi: 10.2174/1568026611313020004
145. Cheraghi M, Ahmadvand H, Maleki A, Babaeenezhad E, Shakiba S, Hassanzadeh F. Oxidative stress status and liver markers in coronary heart disease. Rep Biochem Mol Biol. (2019) 8:49–55.
146. Patel RS, Ghasemzadeh N, Eapen DJ, Sher S, Arshad S, Ko YA, et al. Novel biomarker of oxidative stress is associated with risk of death in patients with coronary artery disease. Circulation. (2016) 133:361–9. doi: 10.1161/CIRCULATIONAHA.115.019790
147. Kotur-Stevuljevic J, Bogavac-Stanojevic N, Jelic-Ivanovic Z, Stefanovic A, Gojkovic T, Joksic J, et al. Oxidative stress and paraoxonase 1 status in acute ischemic stroke patients. Atherosclerosis. (2015) 241:192–8. doi: 10.1016/j.atherosclerosis.2015.05.016
148. Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. (2013) 62:712–8. doi: 10.1016/j.neuint.2012.11.009
149. Milanlioglu A, Aslan M, Ozkol H, Cilingir V, Nuri Aydin M, Karadas S. Serum antioxidant enzymes activities and oxidative stress levels in patients with acute ischemic stroke: influence on neurological status and outcome. Wien Klin Wochenschr. (2016) 128:169–74. doi: 10.1007/s00508-015-0742-6
150. Nanetti L, Raffaelli F, Vignini A, Perozzi C, Silvestrini M, Bartolini M, et al. Oxidative stress in ischaemic stroke. Eur J Clin Invest. (2011) 41:1318–22. doi: 10.1111/j.1365-2362.2011.02546.x
151. Lip GY, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG. Oxidative stress in malignant and non-malignant phase hypertension. J Hum Hypertens. (2002) 16:333–6. doi: 10.1038/sj.jhh.1001386
152. Pedro-Botet J, Covas MI, Martin S, Rubies-Prat J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens. (2000) 14:343–5. doi: 10.1038/sj.jhh.1001034
153. Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. J Res Med Sci. (2014) 19:358–67.
154. Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med. (2012) 44(Suppl. 1):S2–16. doi: 10.3109/07853890.2011.653393
155. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. (2001) 103:1282–8. doi: 10.1161/01.cir.103.9.1282
156. Laakso JT, Teravainen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. (2004) 22:1333–40. doi: 10.1097/01.hjh.0000125441.28861.9f
157. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. (2010) 107:106–16. doi: 10.1161/CIRCRESAHA.109.214601
158. Carlisle RE, Werner KE, Yum V, Lu C, Tat V, Memon M, et al. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J Hypertens. (2016) 34:1556–69. doi: 10.1097/HJH.0000000000000943
159. Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. (2000) 162(2 Pt 1):566–70. doi: 10.1164/ajrccm.162.2.9908091
160. Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. (2003) 124:1386–92. doi: 10.1378/chest.124.4.1386
161. Ozben S, Huseyinoglu N, Hanikoglu F, Guvenc TS, Yildirim BZ, Cort A, et al. Advanced oxidation protein products and ischaemia-modified albumin in obstructive sleep apnea. Eur J Clin Invest. (2014) 44:1045–52. doi: 10.1111/eci.12338
162. Ekin S, Yildiz H, Alp HH. NOX4, MDA, IMA and oxidative DNA damage: can these parameters be used to estimate the presence and severity of OSA? Sleep Breath. (2021) 25:529–36. doi: 10.1007/s11325-020-02093-2
163. Tothova L, Celec P, Mucska I, Hodosy J. Short-term effects of continuous positive airway pressure on oxidative stress in severe sleep apnea. Sleep Breath. (2019) 23:857–63. doi: 10.1007/s11325-018-01777-0
164. Norheim KB, Jonsson G, Harboe E, Hanasand M, Goransson L, Omdal R. Oxidative stress, as measured by protein oxidation, is increased in primary Sjogren’s syndrome. Free Radic Res. (2012) 46:141–6. doi: 10.3109/10715762.2011.645206
165. Kim-Howard X, Sun C, Molineros JE, Maiti AK, Chandru H, Adler A, et al. Allelic heterogeneity in NCF2 associated with systemic lupus erythematosus (SLE) susceptibility across four ethnic populations. Hum Mol Genet. (2014) 23:1656–68. doi: 10.1093/hmg/ddt532
166. Morgan PE, Sturgess AD, Davies MJ. Evidence for chronically elevated serum protein oxidation in systemic lupus erythematosus patients. Free Radic Res. (2009) 43:117–27. doi: 10.1080/10715760802623896
167. Rajda C, Pukoli D, Bende Z, Majlath Z, Vecsei L. Excitotoxins, mitochondrial and redox disturbances in multiple sclerosis. Int J Mol Sci. (2017) 18:353. doi: 10.3390/ijms18020353
168. Guan JZ, Guan WP, Maeda T, Guoqing X, GuangZhi W, Makino N. Patients with multiple sclerosis show increased oxidative stress markers and somatic telomere length shortening. Mol Cell Biochem. (2015) 400:183–7. doi: 10.1007/s11010-014-2274-1
169. Ohl K, Tenbrock K, Kipp M. Oxidative stress in multiple sclerosis: central and peripheral mode of action. Exp Neurol. (2016) 277:58–67. doi: 10.1016/j.expneurol.2015.11.010
171. Weidling IW, Swerdlow RH. Mitochondria in Alzheimer’s disease and their potential role in Alzheimer’s proteostasis. Exp Neurol. (2020) 330:113321. doi: 10.1016/j.expneurol.2020.113321
172. Abolhassani N, Leon J, Sheng Z, Oka S, Hamasaki H, Iwaki T, et al. Molecular pathophysiology of impaired glucose metabolism, mitochondrial dysfunction, and oxidative DNA damage in Alzheimer’s disease brain. Mech Ageing Dev. (2017) 161(Pt A):95–104. doi: 10.1016/j.mad.2016.05.005
173. Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. (2019) 20:148–60. doi: 10.1038/s41583-019-0132-6
174. Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, et al. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol. (2016) 53:648–61. doi: 10.1007/s12035-014-9053-6
175. Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. (2016) 4:519–22. doi: 10.3892/br.2016.630
176. Garcia-Garcia A, Zavala-Flores L, Rodriguez-Rocha H, Franco R. Thiol-redox signaling, dopaminergic cell death, and Parkinson’s disease. Antioxid Redox Signal. (2012) 17:1764–84. doi: 10.1089/ars.2011.4501
177. Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative stress in Parkinson’s disease: a systematic review and meta-analysis. Front Mol Neurosci. (2018) 11:236. doi: 10.3389/fnmol.2018.00236
178. Hwang O. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. (2013) 22:11–7. doi: 10.5607/en.2013.22.1.11
179. Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. (2013) 3:461–91. doi: 10.3233/JPD-130230
180. Beshay E, Carrier S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology. (2004) 64:1062–7. doi: 10.1016/j.urology.2004.06.021
181. Lin WY, Wu SB, Lin YP, Chang PJ, Levin RM, Wei YH. Reversing bladder outlet obstruction attenuates systemic and tissue oxidative stress. BJU Int. (2012) 110:1208–13. doi: 10.1111/j.1464-410X.2012.11185.x
182. Nomiya M, Burmeister DM, Sawada N, Campeau L, Zarifpour M, Yamaguchi O, et al. Effect of melatonin on chronic bladder-ischaemia-associated changes in rat bladder function. BJU Int. (2013) 112:E221–30. doi: 10.1111/j.1464-410X.2012.11746.x
183. Lin AT, Yang CH, Chen KK, Chang LS. Detrusor mitochondrial lipid peroxidation and superoxide dismutase activity in partial bladder outlet obstruction of rabbits. Neurourol Urodyn. (2005) 24:282–7. doi: 10.1002/nau.20109
184. Elrashidy RA, Kavran M, Asker ME, Mohamed HE, Daneshgari F, Liu G. Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am J Physiol Renal Physiol. (2019) 317:F906–12. doi: 10.1152/ajprenal.00221.2019
185. Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G. Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J Urol. (2013) 189:1130–6. doi: 10.1016/j.juro.2012.08.222
186. Elrashidy RA, Liu G. Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp Mol Pathol. (2019) 111:104304. doi: 10.1016/j.yexmp.2019.104304
187. Liu G, Elrashidy RA, Xiao N, Kavran M, Huang Y, Tao M, et al. Bladder function in mice with inducible smooth muscle-specific deletion of the manganese superoxide dismutase gene. Am J Physiol Cell Physiol. (2015) 309:C169–78. doi: 10.1152/ajpcell.00046.2015
188. Masuda H, Kihara K, Saito K, Matsuoka Y, Yoshida S, Chancellor MB, et al. Reactive oxygen species mediate detrusor overactivity via sensitization of afferent pathway in the bladder of anaesthetized rats. BJU Int. (2008) 101:775–80. doi: 10.1111/j.1464-410X.2007.07310.x
189. Nomiya M, Sagawa K, Yazaki J, Takahashi N, Kushida N, Haga N, et al. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol Urodyn. (2012) 31:185–9. doi: 10.1002/nau.21191
190. Matsui T, Oka M, Fukui T, Tanaka M, Oyama T, Sagawa K, et al. Suppression of bladder overactivity and oxidative stress by the phytotherapeutic agent, eviprostat, in a rat model of atherosclerosis-induced chronic bladder ischemia. Int J Urol. (2012) 19:669–75. doi: 10.1111/j.1442-2042.2012.03000.x
191. Kim JW, Jang HA, Bae JH, Lee JG. Effects of coenzyme Q10 on bladder dysfunction induced by chronic bladder ischemia in a rat model. J Urol. (2013) 189:2371–6. doi: 10.1016/j.juro.2012.12.101
192. Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J Urol. (2007) 178:710–5. doi: 10.1016/j.juro.2007.03.096
193. Changolkar AK, Hypolite JA, Disanto M, Oates PJ, Wein AJ, Chacko S. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol. (2005) 173:309–13. doi: 10.1097/01.ju.0000141583.31183.7a
194. Mannikarottu AS, Changolkar AK, Disanto ME, Wein AJ, Chacko S. Over expression of smooth muscle thin filament associated proteins in the bladder wall of diabetics. J Urol. (2005) 174:360–4. doi: 10.1097/01.ju.0000161602.18671.c7
195. Wang M, Xing N, Wu L, Huang WC, Xu Z, Liu G. Regulation of spontaneous contractions in intact rat bladder strips and the effects of hydrogen peroxide. Biomed Res Int. (2018) 2018:2925985. doi: 10.1155/2018/2925985
196. Kanika ND, Chang J, Tong Y, Tiplitsky S, Lin J, Yohannes E, et al. Oxidative stress status accompanying diabetic bladder cystopathy results in the activation of protein degradation pathways. BJU Int. (2011) 107:1676–84. doi: 10.1111/j.1464-410X.2010.09655.x
197. Zhang B, Zhang Z, Ji H, Shi H, Chen S, Yan D, et al. Grape seed proanthocyanidin extract alleviates urethral dysfunction in diabetic rats through modulating the NO-cGMP pathway. Exp Ther Med. (2018) 15:1053–61. doi: 10.3892/etm.2017.5499
198. Liu G, Lin YH, Yamada Y, Daneshgari F. External urethral sphincter activity in diabetic rats. Neurourol Urodyn. (2008) 27:429–34. doi: 10.1002/nau.20543
199. Birder LA, Wolf-Johnston A, Wein AJ, Grove-Sullivan M, Stoltz D, Watkins S, et al. A uro-protective agent with restorative actions on urethral and striated muscle morphology. World J Urol. (2021) 39:2685–90. doi: 10.1007/s00345-020-03492-6
200. Lee YC, Lin G, Wang G, Reed-Maldonado A, Lu Z, Wang L, et al. Impaired contractility of the circular striated urethral sphincter muscle may contribute to stress urinary incontinence in female zucker fatty rats. Neurourol Urodyn. (2017) 36:1503–10. doi: 10.1002/nau.23165
201. Cao N, Gu B, Gotoh D, Yoshimura N. Time-dependent changes of urethral function in diabetes mellitus: a review. Int Neurourol J. (2019) 23:91–9. doi: 10.5213/inj.1938050.025
202. Yang Z, Dolber PC, Fraser MO. Diabetic urethropathy compounds the effects of diabetic cystopathy. J Urol. (2007) 178:2213–9. doi: 10.1016/j.juro.2007.06.042
203. Kracochansky M, Reis LO, Lorenzetti F, Ortiz V, Dambros M. Impact of castration with or without alpha-tocopherol supplementation on the urethral sphincter of rats. Int Braz J Urol. (2012) 38:277–83. doi: 10.1590/s1677-55382012000200017
204. Alexandre EC, Calmasini FB, Sponton A, de Oliveira MG, Andre DM, Silva FH, et al. Influence of the periprostatic adipose tissue in obesity-associated mouse urethral dysfunction and oxidative stress: effect of resveratrol treatment. Eur J Pharmacol. (2018) 836:25–33. doi: 10.1016/j.ejphar.2018.08.010
205. Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. (2006) 39:176–9. doi: 10.1016/j.clinbiochem.2005.11.018
206. Kullisaar T, Turk S, Punab M, Mandar R. Oxidative stress–cause or consequence of male genital tract disorders? Prostate. (2012) 72:977–83. doi: 10.1002/pros.21502
207. Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, et al. Resveratrol: a double-edged sword in health benefits. Biomedicines. (2018) 6:91. doi: 10.3390/biomedicines6030091
208. Vicari E, Arancio A, Catania VE, Vicari BO, Sidoti G, Castiglione R, et al. Resveratrol reduces inflammation-related prostate fibrosis. Int J Med Sci. (2020) 17:1864–70. doi: 10.7150/ijms.44443
209. Matsumoto S, Hanai T, Matsui T, Oka M, Tanaka M, Uemura H. Eviprostat suppresses urinary oxidative stress in a rabbit model of partial bladder outlet obstruction and in patients with benign prostatic hyperplasia. Phytother Res. (2010) 24:301–3. doi: 10.1002/ptr.2909
210. Vital P, Castro P, Ittmann M. Oxidative stress promotes benign prostatic hyperplasia. Prostate. (2016) 76:58–67. doi: 10.1002/pros.23100
211. Minciullo PL, Inferrera A, Navarra M, Calapai G, Magno C, Gangemi S. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urol Int. (2015) 94:249–54. doi: 10.1159/000366210
212. Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, Salonia A, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. (2013) 112:432–41. doi: 10.1111/bju.12118
Keywords: lower urinary tract symptoms (LUTS), oxidative stress, aging, chronic diseases, bladder
Citation: Xu Z, Elrashidy RA, Li B and Liu G (2022) Oxidative Stress: A Putative Link Between Lower Urinary Tract Symptoms and Aging and Major Chronic Diseases. Front. Med. 9:812967. doi: 10.3389/fmed.2022.812967
Received: 11 November 2021; Accepted: 14 February 2022;
Published: 10 March 2022.
Edited by:
Tzvi Dwolatzky, Technion Israel Institute of Technology, IsraelReviewed by:
Zongwei Wang, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesGuiting Lin, University of California, San Francisco, United States
Copyright © 2022 Xu, Elrashidy, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiming Liu, Z3VpbWluZy5saXVAY2FzZS5lZHU=
†These authors have contributed equally to this work
 Zhenqun Xu
Zhenqun Xu Rania A. Elrashidy3†
Rania A. Elrashidy3† Guiming Liu
Guiming Liu