- 1Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Yunnan Institute of Urology, Kunming, China
- 2Department of Anesthesiology, Peking University Third Hospital, Beijing, China
- 3Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
- 4Zhongke Jianlan Medical Research Institute, Beijing, China
According to the result released by the World Health Organization (WHO), non-communicable diseases have occupied four of the top 10 current causes for death in the world. Cancer is one of the significant factors that trigger complications and deaths; more than 80% cancer patients require surgical or palliative treatment. In this case, anesthetic treatment is indispensable. Since cancer is a heterogeneous disease, various types of interventions can activate oncogenes or mutate tumor suppressor genes. More and more researchers believe that anesthetics have a certain effect on the long-term recurrence and metastasis of tumors, but it is still controversial whether they promote or inhibit the progression of cancer. On this basis, a series of retrospective or prospective randomized clinical trials have been conducted, but it seems to be difficult to reach a conclusion within 5 years or longer. This article focuses on the effects of anesthetic drugs on immune function and cancer and reviews their latest targets on the tumor cells, in order to provide a theoretical basis for optimizing the selection of anesthetic drugs, exploring therapeutic targets, and improving the prognosis of cancer patients.
Introduction
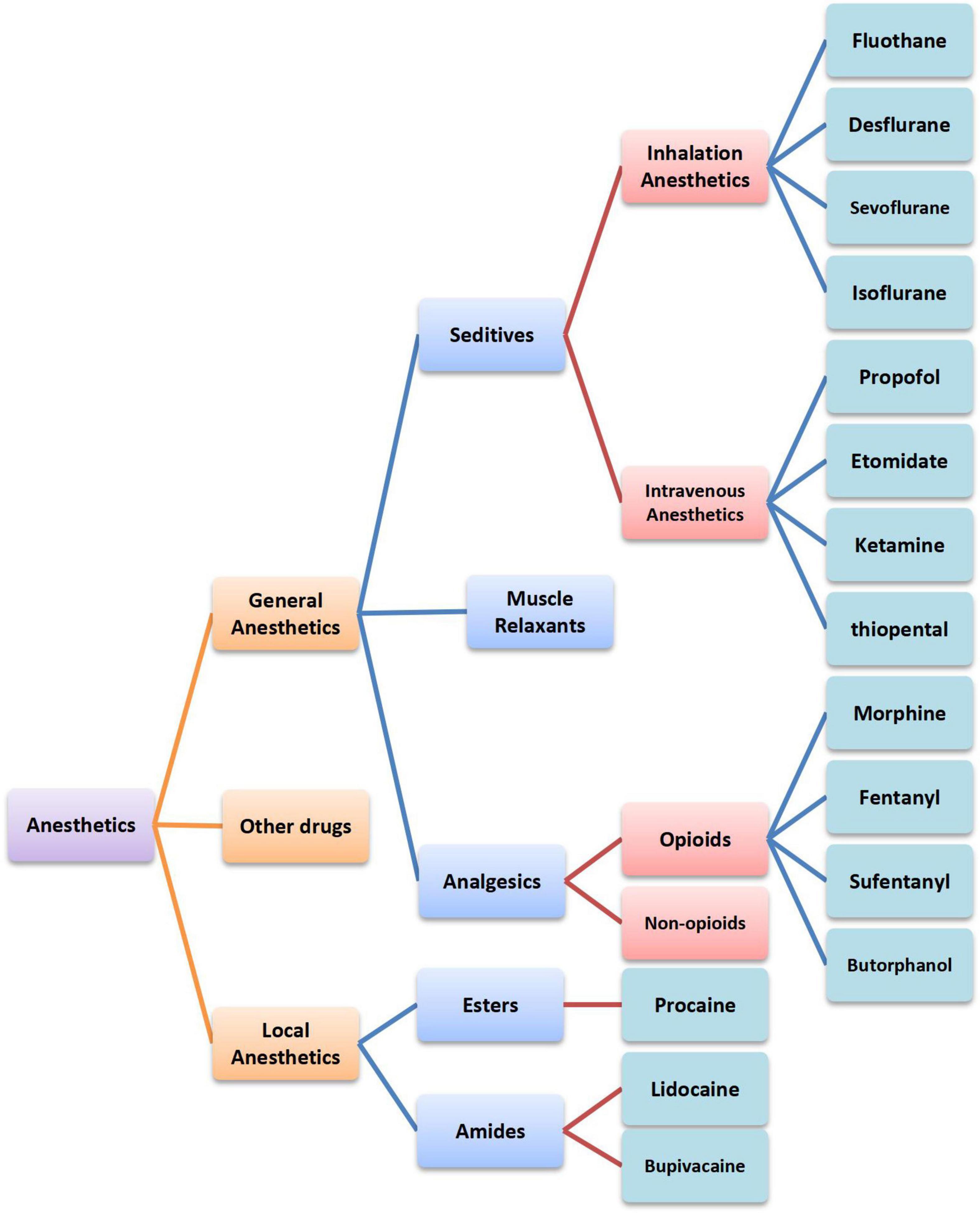
Anesthetics are a diverse group of drugs that are used in the management of pains and are generally categorized into two classes according to their functions, namely, general anesthetics and local anesthetics (1). General anesthetics are either volatile liquids or agents that are administered intravenously to produce a state of unconsciousness so that invasive and surgical procedures can be carried out. Local anesthetics act on any part of the nervous system, causing both sensory and motor paralysis, which can be further divided into esters and amides according to the chemical structure (2). Despite the widespread use of anesthetics, the precise mechanisms of general anesthesia remain poorly understood. In addition, its influencing mechanism on tumors is unclear as well. In this article, we summarized the immune function of anesthetics commonly used in clinical practice (Figure 1). Furthermore, the latest acting sites of anesthetics on tumors were discussed. This review will provide a theoretical basis for optimizing the selection of anesthetics, exploring therapeutic targets, and improving prognosis and survival qualities of patients.
Influence of anesthetics on immune function
Anti-tumor immunity includes innate and adaptive immunity. Anesthetic drugs can affect human body immune function. Normally, the immune system can recognize and remove mutated cells. However, some tumor cells have altered antigens, which help them escape from immune surveillance, resulting in their sustainable growth and metastases.
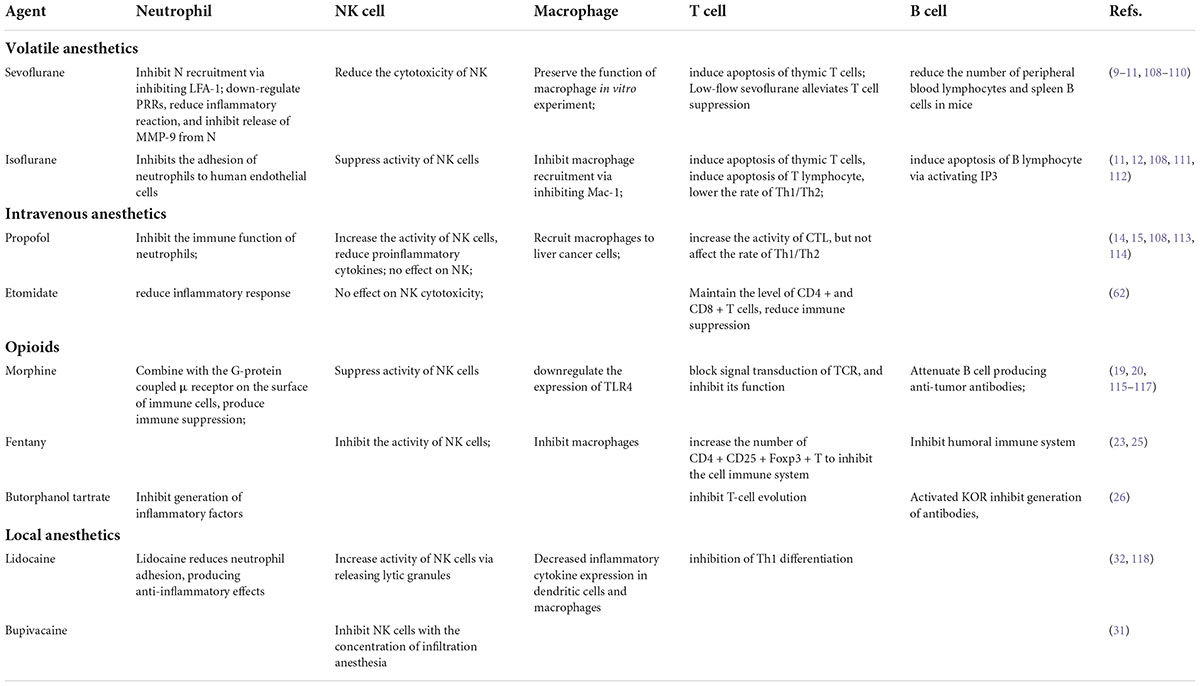
With the increasing attention to cancer–nerve crosstalk, cancer biologists realize that the nervous system has an impact on tumors. Neural activity of the brain or spinal cord can directly promote the growth of cancer in situ or infiltration (3). Surgical resection induces noxious stimulation, and anesthetic factors induce immunosuppression, which can active the hypothalamic–pituitary–adrenal axis (HPA) and the sympathetic nervous system (SNS). The activation of these two systems suppress cell-mediated immunity and release immunosuppressive cytokines (4–6). The immune suppression caused by anesthetics plays an important role in the progression and metastasis of tumors (Figure 2).
Influence of inhalation anesthetics on the immune system
Inhalation anesthetics with fluorinated ethers can act on the receptor of the central nervous system and generate anesthesia and sedation, as well as have direction actions on receptors located on the surface of immune cells, such as Ca/Mg ion channel protein, TLRs, integrin β2, and Ras1 protein (Rap 1), to promote degranulation of immune cells like NK cells and macrophages and consequently decrease their killing abilities and inhibit anti-tumor effects (5, 7). It has been revealed in a study that fluothane can affect the cytostatic activity of NK cells and increase the expression of hypoxia-inducible factor 1-alpha (HIF-1α) (8), decrease their secretion of IFN (interferon), and lower the killing abilities. Isoflurane can also decrease the cytostatic activity of NK cells, induce apoptosis of T and B lymphocytes, and reduce the Th1/Th2 ratio. Sevoflurane will contribute to the decrease in NK cells and increase the number of leukocytes and neutrophils at the same time (8, 9). Both sevoflurane and desflurane can have pre-treatment on neutrophils to inhibit the release of matrix metalloproteinase-9 (MMP-9) and then restrain the metastasis of colon cancer cells (10). In vitro experiments have shown that inhalation anesthetics can regulate immune cells to recognize antigens, recruit proinflammatory cells, and affect immune reactions mediated by cells (9). By dose-dependent effects, both sevoflurane and desflurane induce apoptosis of thymus T cells, and desflurane induces apoptosis of B lymphocytes by activating IP3 (inositol triphosphate) (11, 12), which might be the cause for immune suppression after operations.
Influence of intravenous anesthetics on immune system function
Ketamine belongs to non-barbiturate intravenous anesthetics. Research studies suggested that ketamine and thiopental would have several implications on the immune system. Ketamine inhibits the cytostatic activity of NK cells, induces lymphocytic apoptosis via the mitochondrial pathway, and inhibits the maturation of dendritic cells. However, thiopental inhibits T-lymphocyte apoptosis by inducing heat shock proteins (HSPs) (4, 13). Propofol, which is different from other intravenous anesthetics, can increase the activity of NK cells, significantly inhibit the generation of COX-2 and PGE2, enhance the body protection against tumor immune reaction, and affect the tumor directly (14). Regarding adaptive immunity, disoprofol can increase the activity of cytotoxic T lymphocytes (CTL) and decrease proinflammatory cytokines, but it do not influence the Th1/Th2 ratio and can relieve the immune suppression caused by surgical traumas (15).
Influence of opioid analgesics on immune system function
Immunocytes, such as neutrophils, macrophages, and T cells, can secrete endogenous opioid peptides (EOP), combine with the peripheric opioid receptor, and relieve inflammation and neuropathic pains (16). Meanwhile, immunologically competent cells also express opioid receptors and degrade the function of NK cells, macrophages, and subpopulation of B/T cells. For the mouse with the μ-opioid receptor (MOR) knocked out, using morphine does not affect the killing of NK cells (17, 18). Except for inhibiting the activity of NK cells, morphine can also decrease the expression of the toll-like receptor 4 (TLR4) of macrophages, inhibit the differentiation of T cells, and promote lymphocytic apoptosis (19, 20). It has been verified that opioid analgesics inhibit the proliferation of T lymphocytes, damage the killing function of T cells, and influence the production of antibodies (21, 22). Agonists of the MOR, such as fentanyl and sufentanyl, can inhibit cellular immunity of the body and the humoral immune system, as well as the activity of NK cells and macrophages and the production of antibodies (23). It was revealed in a colorectal surgery that sufentanyl lowers the rate of T-cell subsets, but remifentanil improved the IL-6 level and the secretion of hydrocortisone (24). Both fentanyl and sufentanyl with the clinical concentration can reinforce the suppression of the immune system by increasing the number of CD4, CD25, Foxp3, and T cells. In addition, fentanyl has a stronger inhibiting effect (25). Some research suggests that the expression of the κ-opioid receptor (KOR) exists in multiple types of blood cells, and the MOR activates the production of inhibiting antibodies and inhibits the evolution of T cells and the generation of inflammatory factors (26).
Influence of local anesthetics on immune system function
Surgical traumas can activate the hypothalamic–pituitary–adrenal axis (HPA) of patients, which contributes to the change in neuroendocrine function and then inhibits immune functions. The use of local anesthetics can alleviate the stress reaction after operation and lower the inhibiting effect of stress on the immune system by blocking the signaling to nerves. Blocking peripheral nerves or intraspinal anesthesia can lower the conduction of stimulation caused by surgical injuries to the central nervous system and then reduce the immune suppression resulting from the activated HPA (27). Apart from the aforementioned indirect influences, local anesthetics can also directly have an effect on NK cells and T cells. Stress, pains, and opioids can inhibit the activity of NK cells (28–30), and the inhibiting or activating effect on NK cells brought by local anesthetics is related to their concentrations. Lidocaine, ropivacaine, and bupivacaine will inhibit NK cells when their concentrations are equivalent to infiltration anesthesia (31). However, with the intravenous concentration, lidocaine can enhance the activity of NK cells to resist tumor cancers by releasing lytic particles (32). Studies have shown that local anesthetics have analgesic and anti-inflammatory effects, as well as monitoring and protection of the immune system, which can improve the prognosis of cancer patients and improve disease-free survival (DFS) and overall survival (OS).
Based on the previous research, different anesthetics and methods can affect the immune function positively or negatively, and immune balance plays a role in tumor dissemination and recurrence. Depending on existing evidence, sevoflurane and isoflurane mostly play a negative role in anti-tumor immunity, but it is relatively vague for propofol. Local anesthetics can block neurotransmission from afferent nerves to the central nervous system, thus preventing surgical pains and reducing surgically induced neuroendocrine stress so that the HPA axis and SNS reactions are avoided. Toxicity that local anesthetics have on the cardiovascular system and the central nervous system, particular types of local anesthetics function, and their safe concentrations that effectively regulate the function of immune cells all need to be further studied.
Study on influencing mechanism and acting site of inhalation anesthetics on tumors
The major review is that the influence of inhalation anesthetics on the OS of patients with cancers is stronger than that of intravenous anesthetics.
Sevoflurane
Sevoflurane, a kind of inhalation anesthetics which is widely used in clinical practices, has many advantages, such as rapid recovery, strong controllability, and organ protection. The influence of evoflurane on tumors is currently being disputed.
Ru Li et al. made a comparative analysis between the respective use of sevoflurane and propofol in the mouse model of breast cancer and found that the mice with the adoption of sevoflurane had increased proinflammatory cytokines, which were related to tumor metastasis. On the first day after the operation, the volume of IL-6 and VEGF in the mouse serum with sevoflurane was higher than that in the group with propofol. Particularly, by increasing the expression of IL-6, sevoflurane activated the signaling pathway of IL-6/JAK/STAT3, induced the aggregation of CD11b bone marrow stromal cells in lung tissues, and promoted the metastasis of tumor cells to the lung. AZD1480, a JAK inhibitor, can be used to significantly reduce the number of metastasized tumors in the lung of the mice narcotized with sevoflurane and to reduce the level of p-STAT3 (33).
More studies put forward that sevoflurane promotes tumor, but some researchers hold the opposite view. Liang et al. revealed that sevoflurane could downregulate HIF-1α via the p38/MAPK signal channel and inhibit hypoxia-induced growth and metastasis of lung cancer cells (34). Similar research verified that sevoflurane inhibited the invasion and metastasis of colorectal cancer cells by regulating the ERK/MMP-9 pathway via miR-203 (35). Liang H et al. discovered that sevoflurane could lower the platelet aggregation rate (PAR) and inhibit the platelet activity by downregulating GPIIb/IIIa and CD62P, and then inhibit the invasion of lung cancer cells induced by platelet activity, which was not tested in the isoflurane group (36). The study in relation to breast cancer indicated that sevoflurane with low concentration significantly promoted the proliferation of primary cancer cells but remarkably inhibited metastatic cells, which indicated that sevoflurane had different effects on the proliferation of cancer cells at different stages (37).
Multiple reports show that sevoflurane affects the proliferation, apoptosis, migration, and invasion of cancer cells in relation to cervical cancer, optic glioma, stomach cancer, etc., which is currently not finally concluded and needs to particularly analyze its influence on the biological behavior of cancer cells of different types and statuses.
Isoflurane
Isoflurane, a kind of inhalation anesthetics widely used in clinical practices, is thought to promote cancer progression by most of the research. However, it is believed from some of the results that isoflurane has inhibiting or no effects.
Research revealed that isoflurane could increase the expression of insulin-like growth factor (IGF), which stimulates the development of tumors. With isoflurane use, an increased expression of IGF-1 and IGF-1 RSKOV3 was found in ovarian tumor cells, which accelerated the cell cycle and proliferation (38, 39). Through the dependency mechanism of caveolin-1 (Cav-1), isoflurane resisted the apoptosis of colorectal cancer cells (40), increased the proliferation and invasion of squamous cell cancer of head and neck (SCCHN), inhibited apoptosis, and promoted cancer progression and metastasis (41). A report indicated that isoflurane upregulated the expression of HIF-1α in prostate cancer cells via the PI3K/AKT/mTOR signal pathway and contributed to the increased cell invasion and migration (42), but the use of propofol could reverse the activation of the signaling pathway involved, which indicated its application value in the operation of patients with tumors. The genotoxicity of inhalation anesthetics was closely related to the recurrence of tumor patients after operation (43).
Combined with statistical results of clinical samples, it is found that compared to total intravenous anesthesia, the use of isoflurane increases the risk of death in tumor patients, which indicates that propofol is more applicable to anesthesia in tumor excision surgeries. The anti-immune effect of isoflurane may not be applied to the population with low immunity but can be potentially used in the organ transplantation or patients with host immunity response stimulated by infection.
Influence and acting sites of intravenous anesthetics on tumors
Propofol
Propofol, an alkyl acid intravenous anesthetic with short-term effects which is widely used for intravenous injection in clinical practices, realizes sedative effects by enhancing the neurotransmission of central inhibition (the GABA pathway) and decreasing that of central excitation (the NMDA pathway) and is commonly used as an anesthetic in tumor resection operations. It is worthy of exploring whether propofol affects tumors when generating central sedation.
N-methyl-D-aspartate receptor (NMDAR), a subset of ionic glutamic acid receptor which is controlled by the membrane potential as well as by other neurotransmitters, plays an important role in the synaptic transmission and plasticity regulation in the central nervous system (44). By inhibiting glutamic acid release in the presynaptic element, propofol with clinical concentration induces the allosteric regulation of various sub-units of NMDAR, conducts negative controls, and produces sedative effects (45). After NMDAR is activated, the ion permeation of Ca2 + and K + is increased, which causes a large number of Ca2 + to flood into the cell; thus, the associated transcription, translation, and post-translational modification are increased as well. At present, the related research is concentrated in the cognitive deletion caused by abnormal activities of NMDAR, such as research in relation to epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), and anti-NMDAR encephalitis (46); however, rare studies involve the relation between NMDAR and tumors as well as the mechanism of action. Based on the testing and screening on lung cancer, colon cancer, breast cancer, prostate cancer, stomach cancer, liver cancer, esophageal cancer, and cervical cancer, some researchers found that NMDAR was positively expressed in tumor tissues, which was significantly different from normal paracancer tissues (P < 0.05) (47), but the research did not further clarify the effect that NMDAR had on the biological behavior of tumors. North et al. reported the positive expression of NMDAR in neuroblastoma and found that the use of glutamic acid and NMDAR agonist could trigger the change in the ion channel which had toxic injuries on neuroblastoma cells (48). Sub-types of NMDAR were also tested in the stomach cancer cell line, and with the use of AP-5, a retardant of NMDAR, proliferation of stomach cancer cells was effectively inhibited (49). Similar reports of research related to breast cancer, particularly, NMDAR, was expressed negatively in normal breast tissues but positively in the breast cancer cell line and patients’ breast cancer tissues. In addition, MK-801, another NMDAR retardant, could significantly inhibit the proliferation of breast cancer cells (50). A recent study revealed that the expression of NMDAR in colorectal cancer was significantly different from that in normal tissues, and as an important marker of endothelial cells, NMDAR promoted tumor angiogenesis and then facilitated tumor progression (51).
GABAR, the receptor of γ-aminobutyric acid, is the another crucial acting site of propofol. As the most significant inhibitory neurotransmitter in the central nervous system of mammals, by combining with GABAR, the receptor can lead to changes in ion permeation in the cell membrane. There are three pharmacological subtypes of GABAR, particularly, GABAR(A), GABAR(B), and GABAR(C). By combining with receptors of different subtypes, GABA will produce different regulatory effects via the specific signal transduction pathway. Recent research indicates that except for the existence of GABA and GABAR (the receptor of GABA) in the central nervous system, with their expressions in some tumor tissues, they regulate tumor proliferation, invasion, and metastasis and engage in the development and progression of tumors via specific signal transduction pathways. The research suggests GABA is significantly expressed in tumor tissues such as neuroglioma, prostate cancer, and colorectal cancer, but the expression of GABAR is significantly increased in liver cancer and breast cancer (52–54), which indicates that the high expression of GABA and its receptor in tumor tissues is potentially related to tumor development. The expression of GABAR is increased in the mouse with liver cancer which is induced by N-nitrosodiethylamine. Mitosis and DNA synthesis of liver cancer cells can be promoted with the adoption of the GABAR agonist. Inamoto et al. (55) studied the regulatory effect that GABA had on activating the MAPK signal pathway in Caki-2 (human renal clear cell carcinoma cell line) and found that GABA activated receptor B to promote phosphorylation of the MAPK family, which included ERKl/2, cJNK, and P38, and then improved the invasion ability of renal cell carcinoma. However, another research results showed that (56–58) the adoption of the GABAR agonist can inhibit the proliferation of tumor cells. By increasing the activity of GABAR, muscimol effectively lowers the expression of alpha fetoprotein (AFP) in HepG2 (human liver cancer cell line), as well as cell proliferation, and a similar result is also obtained in research on colorectal cancer and stomach cancer, which reveals that the intervention of the GABAR pathway is potentially an effective therapeutic method for gastroenteric tumors.
Due to differences of the structure and function between tumor cells and neuronal cells, the GABAR pathway in neuronal cells is different from that in tumor cells. It needs to be further explored by researchers with regard to whether the expression of GABA and GABAR in tumor tissues is a tumor promoter or a protective response of the body, what kind of receptor pathway is adopted to play the regulatory role, and what is the signal transduction pathway.
Etomidate
Etomidate, a kind of non-barbiturate intravenous anesthetics and a derivant of imidazole, produces analgesic effects by activating GABAR (59, 60). It is especially suitable for anesthesia induction on critical patients and can also be applied to undergoing little operations due to its slight influences on hemodynamics, short action time, and rapid onset (61). Currently, there is few research on the influence of etomidate on tumors.
A research indicated that with the adoption of etomidate in resection operation of lung adenocarcinoma, the quantity of CD8 + T cells in patients’ blood after the operation was lower than in that in the propofol group but higher than that in the propofol group 24 hours after the operation, which illustrated that etomidate had smaller effects on the immune system of patients with lung adenocarcinoma (62), and its adoption could improve immune suppression of the body in the perioperative period. In another research on lung cancer, after A549 (human lung adenocarcinoma cell line) treated with etomidate, the activation of MMP2 was inhibited, and expressions of PKC, MMP7, MMP1, MMP9, and p-p-38 were significantly downregulated, but those of RAS, PI3K, and P-ERK (phosphorylation extracellular signal-regulated kinase) were upregulated; therefore, the migration and invasion of A549 were inhibited (63). Etomidate contributed to the loss of mitochondrial membrane potential (MMP) in N2a cells; produced reactive oxygen species (ROS); promoted the generation of apoptin such as PARP, caspase-9, and procaspase3; and facilitated the apoptosis of neuroblastoma Neuro-2a cells (N2a) (64). Limited research reveals the inhibiting effect that etomidate has on tumors, which still needs to be verified by basic experiments and clinical trials with large-scale samples.
As one of the most commonly used intravenous anesthetics, only a few studies have shown that propofol can promote the proliferation of some cancer cells, and clinical data show that propofol has no great influence on long-term prognosis. But most cytological and animal studies suggest that propofol seems to have a tumor-suppressive effect by decreasing cancer cell migration, invasion, proliferation, and angiogenesis and by inducing apoptosis, as demonstrated in many experimental studies. At present, few research studies are found on the effects of etomidate on tumors. It is still necessary to further reveal the regulatory effect and molecular mechanism of propofol and etomidate in different types of cancers. Clinical studies are also needed to confirm their effects and provide a reference for the rational selection of propofol or etomidate.
Influence and acting sites of opioid analgesics on tumors
The opioid receptor, a kind of G-protein-coupled receptors (GPCRs) with seven transmembrane domains (TMDs), mainly exists in the central nervous system with four subtypes (μ, κ, δ, and ORL1), namely, MOR, KOR, DOR, and ORL1 receptors. With approximately 60% of amino acids are structurally identical, these receptors of different types could have analgesic, sedative, and other effects after being activated (65, 66). Emerging research has suggested that the expression of opioid receptors is detected in tumor cells such as lung cancer, prostate cancer, breast cancer, and liver cancer (67–69). The opioid drug indicates a kind of drug that is naturally created or partially synthesized and produces reactions by combining with opioid receptors and is the most effective analgesics available. Due to its strong analgesic effects, opioid drugs are widely applied to the abirritation in the perioperative period and treatments of postoperaticve pains and chronic cancer pains. The result of recent research indicates that the effect that opioid drugs have on patients with tumors is greatly disputed.
Morphine
Morphine is the agonist of MOR, KOR, and DOR, which has gradually decreased effects on subtypes of the aforementioned three receptors. It is currently believed that the effect that morphine has on tumor development is contradictory, and both its promoting and inhibiting effects are reported. Based on a cytological study, it was found that morphine could activate the MOR in lung cancer cells, induce phosphorylation of the epidermal growth factor receptor (EGFR), lead to activation of the lower MAPK/ERK/Akt pathway, and promote cell proliferation and invasion. Increasing expressions of the EGFR and MOR in lung cancer could promote growth of tumor cells, trigger angiogenesis mediated by the vascular endothelial growth factor (VEGF), increase vascular permeability, accelerate tumor progression, and increase the risk of micrometastasis (70).
However, there is also evidence that morphine can inhibit tumor progression. It was revealed in an in vivo and in vitro research on nude mice that a 24-hour incubation of 10 μM morphine could significantly increase the apoptosis rate of Hep3B/HepG2 (71), while 5 μM or 10 μM morphine significantly decreased proliferation and invasion of liver cancer cells and lowered the occurrence rate of pulmonary metastasis. By upregulating OGFR and downregulating MOR, uPA, and MMP-9, morphine inhibited the biological characteristics of tumors. In addition, morphine with the clinical concentration could significantly inhibit tumor progression, which is thought to be a safe and effective pain treatment for patients with liver cancer. Another data also supported the fact that morphine could affect the opioid growth factor receptor (OGFR) and finally inhibit proliferation of lung cancer cells (72). OGFR, the ζ-opioid receptor, does not relieve pain, which differs from the common μ-opioid receptor, κ-opioid receptor, and δ-opioid receptor. The expression of the OGFR in lung cancer tissues is significantly higher than in para-carcinoma tissues, and morphine plays a role in the OGFR and inhibits cell proliferation. Zagon et al. tested 31 cases of human tumor cell lines and found that 90% of the tumor cells had a high expression of the OGFR, and 42% of cell proliferation was decreased after OGF was added into the culture dish, but 44% was accelerated after naltrexone (opioid receptor antagonist) was added (73), which indicates that application of morphine in the treatment of pains for patients with tumors is safe. Based on research on colorectal tumors, it was found that morphine could significantly inhibit lipopolysaccharide (LPS) and decrease the expression of endothelial cell adhesion molecules and therefore inhibit the tumor progression induced by LPS and prevent tumor growth and metastasis (74).
Fentanyl
Fentanyl, which belongs to short-acting opioid analgesics, is a strong MOR agonist with powerful analgesic effects, with no influences on the respiratory system within the scope of safe dose, fairly stable hemodynamics, short acting time, and fast metabolism. Increasing research has testified that the expression of the MOR exists not only in the central nervous system but also in many tumor cells.
Compared to normal lung tissues, the expression of the MOR in tumor tissues of patients with non-small-cell lung cancer (NSCLC) was 5∼10 times increased, which was consistent with that in the NSCLC cell line, which indicates that high expression of the MOR may have regulated a series of biological characteristics including cell proliferation and differentiation (75, 76). A retrospective analysis of about 113 cases of patients with prostate cancer shown that the increased expression of the MOR predicted a poor survival rate of patients (77). It was speculated by some researchers that the MOR could affect various aspects such as angiogenesis and immune regulation by activating signal pathways of PI3K, Akt, and mTOR, and promote tumor recurrence and metastasis (74, 77–79). Except for the central nervous system, opioid receptors also exist in multiple kinds of stem cells of the body, such as neural stem cell, embryonic stem cell (ES cell), bone marrow mesenchymal stem cell (BMMSC), and epidermal stem cell, which play an important role in regulating proliferation and differentiation of nerve cells, improving ventricular remodeling (VR) and healing wounds. Our previous research indicated that acting as one of the biomarkers of liver cancer stem cells (LCSCs), the high expression of MOR promoted the self-renewal of LCSCs (69).
Although most of the research results revealed that the activated MOR could promote tumor progression, for patients with advanced tumors, the effect of the MOR agonist on the opioid receptor in the central nervous system could effectively relieve their pains, reduce inflammatory reactions caused by tumors, inhibit angiogenesis, and alleviate tumor recurrence and metastasis (78). Research suggested that fentanyl could directly work on human pancreatic cancer cells to inhibit their activity and lower the speed of tumor growth (79). It was also revealed in similar research results that fentanyl could decrease miR-182 and MMP-9 generated by β-catenin to inhibit the growth and invasion of tumor cells for colorectal cancer (80). The direct biological effects that opioid drugs have on tumors are closely related to the drug type and administration concentration and method.
Butorphanol tartrate
The major metabolite of butorphanol tartrate activates KOR and has double actions on the MOR particularly, as the activator and the inhibitor. It majorly plays its pharmacological role indirectly by combining with the receptor in the central nervous system.
Currently, there are few research studies on KOR and tumors, which is available in relation to studies on liver cancer, bone cancer, prostate cancer, and melanoma. Most of the research studies were inclined to support that the KOR had anti-tumor effects and patients with low expression of KOR had poor survival rates (81). A study on bone cancer believed that the KOR agonist could effectively relieve pains but would not promote tumor progression synchronously (82). The application of U50 and 488H (KOR agonists) combined with the targeted therapeutic drug gefitinib could significantly inhibit the growth of NSCLC cells by activating the phosphorylated glycogen synthase kinase 3β pathway (83). KOR had the effect of inhibiting angiogenesis, and it could relieve pains and inhibit tumor progression by promoting apoptosis (84–86), which indicates that KOP is potentially a new target to treat tumors. A research on human epithelial cancer cells thought that the KOR promoted apoptosis of tumor cells via the PKC or Bcl-2 pathway (85, 86). However, there were also opposite views which believed that the expression of the KOR was in positive correlation to lymph node metastasis of esophageal cancer, and the KOR was an independent prognostic factor, which indicated poor prognosis (87). Currently, there is no convincing evidence to testify that through kinds of the pathway the KOR affects in tumors and influences patients’ survival; therefore, more experimental data associated are wanted to support the view.
Evidence on the association of opioids with tumor recurrence and prognosis in cancer patients is still lacking. Current evidence mainly comes from cell and animal experiments, and some retrospective studies and small randomized controlled studies have emerged. However, there is still a lack of evidence from large-scale randomized controlled clinical studies. Due to mixed factors which were related to the type, administration method, and use time of opioid drugs clinically, it was really difficult to determine the “direct effects” that opioid receptors had on tumor development and progression by clinical studies alone, and research on the associated mechanism is an urgent need (88, 89). Overemphasizing the potential role of opioids in promoting tumor recurrence and metastasis and limiting their application in the perioperative period may harm the interests of patients. Opioids remain an important component of perioperative analgesia.
Influence and acting sites of local anesthetics on tumors
Local anesthetics are a kind of drugs applied to block the occurrence and conduction of nerve impulses to make short-term and reversible analgesia occurred in innervation areas. Based on different chemical structures, local anesthetics can be divided into esters and amides. Common esters include procaine, tetracaine, and cocaine, and amides often include lidocaine, bupivacaine, and ropivacaine.
In recent years, it was found in the research that local anesthetics could have effects on proliferation, invasion, and metastasis of multiple types of tumor cells with different mechanisms of action and, in addition, their different administration methods would also influence recurrence, metastasis, and prognosis after the tumor operation (90). Through different administration methods, such as local infiltration and intravenous, intraspinal, and regional block, local anesthetics provide the personalized treatment plan for tumor patients in the perioperative period. The discussion on the mechanism of action in tumor metastasis and recurrence can provide new strategies and basis for the appropriate use of local anesthetics in tumor patients in the perioperative period.
Esters
It was found in the research that as a kind of ester local anesthetics, procaine with 10 nM concentration could inhibit A549 proliferation. In an animal experiment, 3-week administration of procaine on the mice with lung cancer (50 mg/kg/day) could significantly reduce the expression of the EGFR and the tumor volume (91). By increasing demethylation, procaine inhibited the growth of breast cancer cells, made 5-methylated DNA 40% be downregulated in MCF-7 (breast cancer cell line), and made high-methylated tumor suppressor genes be demethylated, which indicated that procaine had high application value in patients with targeted drug resistance (92). The in vitro experiment illustrated that procaine could effectively inhibit the growth of HLE, HuH7 and HuH6 (human liver cancer cells) and had demethylation effects, which was potentially a candidate drug for liver cell cancer (93). The associated research also revealed that procaine promoted proliferation arrest and apoptosis via the regulation of DNA methylation in stomach cancer, which indicated that procaine had specific anti-tumor potentials (94). The application of procaine in research on nasopharyngeal cancer could significantly inhibit the proliferation of CNE-2Z and make cells be arrested before the G1 phase, which was presented in relations of time dependency and dose effect (95).
Amides
Lidocaine
The effects that different types of amide local anesthetics had on tumors differed from others, particularly lidocaine and ropivacaine increased demethylation of breast cancer cells, but bupivacaine did not (96). Lidocaine, which was presented in time and dose dependency, could increase the demethylation of breast cancer cells, and the methylation-inhibiting effect of DAC (5-azacytidine), which is a kind of DNMTs (DNA methyltransferases), could decrease the CpG (CpG island) methylation of tumor suppressor genes—RARβ and RASSF1A, and increase the sensitivity of cisplatin to breast cancer cells (97, 98). The infiltration anesthesia concentration of lidocaine commonly used in clinical practices is 0.5%∼2% (19mM∼74mM), and the regional concentration after being spread in the tissue is about 0.4mM. The research suggested that lidocaine with 0.4mM concentration could inhibit the EGFR by combining with EGF to generate phosphorylation and lower proliferation of corneal epithelial cells (99), as well as inhibit the growth of CAL27 (tongue cancer cell line) by decreasing EGFR phosphorylation (100).
miRNA can be involved in regulating the proliferation, differentiation, migration, angiogenesis, and apoptosis of tumor cells. As an important member of the miR-520 family, the decreased expression of miR-520a-3p is in connection with the development and progression of multiple tumors such as colorectal cancer and NSCLC. Lidocaine with 0.5 mM concentration could decrease the expression of EGFR by upregulating the level of colorectal cancer cells and miR-520a-3p (retinoblastoma cell line) and inhibit the proliferation of tumor cells (101, 102). However, in lung cancer, lidocaine with a higher concentration (8 mM) was required to promote miR-539, inhibit the expression of EGFR as well as its ERK (extracellular regulated protein kinases), and the PI3K/Akt signal pathway to play its role in inhibiting the proliferation, migration, and invasion of tumor cells (103, 104).
Bupivacaine
Bupivacaine is a kind of long-term amide local anesthetic, which has strong combination with plasma proteins. The toxicity of bupivacaine is four times greater than lidocaine, its cardiotoxicity should be noticed especially, and the rate between circulatory collapse and convulsions (CC/CNS) induced by is low.
The degree of apoptosis induced by lidocaine and bupivacaine in MCF-7 (breast cancer cell line) was significantly higher than that in MCF-10A (mammary epithelial cell line), both of which could activate caspase 7, caspase 8, and caspase 9 in MCF-7 and promote the lysed nucleosome and thus induced the apoptosis of MCF-7 via intrinsic and extrinsic pathways of mitochondria (105). Chang et al. (106) proved in their studies that with the concentration-dependent method, lidocaine and bupivacaine affected the cytotoxicity of thyroid cancer cells and inhibited the MAPK pathway to lower the survival and colony formation of 8505C and K1 (thyroid cancer cell lines) and induce the apoptosis of cancer cells (106). Lidocaine and bupivacaine could lead to the division of mitochondrial membrane protein and release of cytochrome C, activate caspase 3 and caspase 7 synchronously, and contribute to the break of poly ADP-ribose polymerase (PARP) and Bax/Bcl-2 with a higher rate. They could weaken the activity of ERK1/2 (extracellular signal-regulated protein kinases) and promote the activation of caspase 3 and break of PARP through inhibiting mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK).
Current literature has pointed out that the intravenous infusion of lidocaine provides an antiproliferative tumor suppressive effect. Many molecular pathways/mechanisms are described. Local anesthetics could directly act on tumor cells and induce apoptosis of tumor cells through intrinsic and extrinsic pathways (107). Lidocaine used in the perioperative period benefited postoperative analgesia and inflammatory reaction control, had protective effects on immune surveillance of the innate immune system, improved the disease-free survival rate and the overall survival rate via migration of anti-tumor cells, and improved the prognosis of patients with tumors, which was possibly an ideal adjuvant drug in the treatment of cancers. However, it should be further verified by clinical research on how it can be clinically used.
Conclusion
Cancer is a disease with high heterogeneity. Various factors contribute to the activation of oncogenes or mutation of tumor suppressor genes, which results in great differences between the therapeutic effectiveness of cancers.
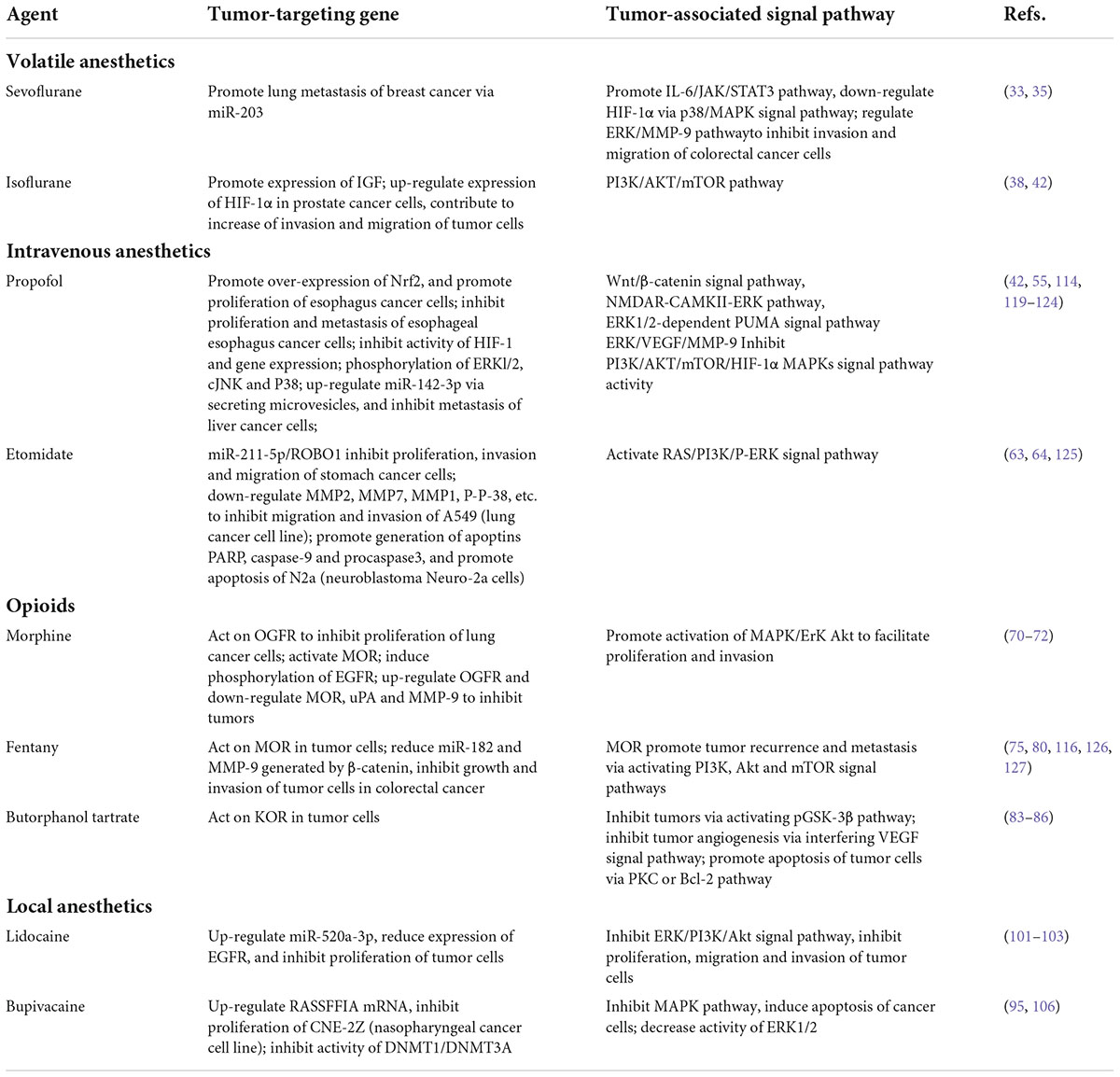
Surgical stress and immunosuppression are unevadable topics. With the activation of the HPA axis and SNS, neuroendocrine regulators are increased, which promotes the metastasis of residual or circulating tumor cells to regional lymph nodes and distant sites. We can reduce the noxious stimulus as much as possible by prioritizing the selection of local anesthetics. In the perioperative period, anesthetics and analgesics are inevitable environmental exposure factors for patients with tumors. As shown in Tables 1, 2, increasing evidence has indicated that anesthetics can directly act on the immune function and relevant sites in tumor cells to activate a series of signal transduction pathways and affect tumor proliferation, invasion, migration, etc. The aforementioned findings suggest that different anesthetics have different or even opposite effects on anti-tumor results. The mechanisms are mainly related to cell cycle regulatory pathways and immune pathways. Meanwhile, animal models greatly benefit research on the mechanism of action that anesthetics have in tumor metastasis, which will provide reference for clinical medication as well as a basis for overcoming side effects of anesthetics. These findings will provide potential research and application directions for rational drug use in different surgeries and patients.
Furthermore, it is necessary for scientists of clinical medicine to conduct a series of retrospective or perspective and stochastic clinical trials to explore the effect that anesthetics have on long-term recurrence and metastasis of tumors, such as to provide a theoretical basis for optimizing the selection of anesthetics, exploring therapeutic targets, and improving the life quality and prognosis of tumor patients.
Author contributions
HW, JW, and CL designed and supervised the review. TL and YL searched the literature and wrote the manuscript. LS and SX searched the literature and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82060464, 81860452, 81972390, and 82002566); Joint Project of Science and Technology, Department of Yunnan and Kunming Medical University (No. 202001AY070001-148); Key Clinical Projects of Peking University Third Hospital (No. BYSY2017001); Beijing-Tianjin-Hebei Basic Research Cooperation Special Project (19JCZDJC65800Z); and Zhongke Jianlan Science Trust Fund (20220717).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aminoff MJ, Daroff RB. Encyclopedia of the Neurological Sciences. Waltham, MA: Academic Press (2014).
2. Schüttler J, Schwilden H. Modern Anesthetics. Berlin: Springer (2008). doi: 10.1007/978-3-540-74806-9
3. Shi DD, Guo JA, Hoffman HI, Su J, Mino-Kenudson M, Barth JL, et al. Therapeutic avenues for cancer neuroscience: translational frontiers and clinical opportunities. Lancet Oncol. (2022) 23:e62–74. doi: 10.1016/S1470-2045(21)00596-9
4. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. (2018) 16:8.
5. Yuki K, Eckenhoff RG. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg. (2016) 123:326–35. doi: 10.1213/ANE.0000000000001403
6. Niwa H, Rowbotham DJ, Lambert DG, Buggy DJ. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? J Anesth. (2013) 27:731–41. doi: 10.1007/s00540-013-1615-7
7. Yuki K, Hou L, Shibamura-Fujiogi M, Koutsogiannaki S, Soriano SG. Mechanistic consideration of the effect of perioperative volatile anesthetics on phagocytes. Clin Immunol. (2021) 222:108635. doi: 10.1016/j.clim.2020.108635
8. Das J, Kumar S, Khanna S, Mehta Y. Are we causing the recurrence-impact of perioperative period on long-term cancer prognosis: review of current evidence and practice. J Anaesthesiol Clin Pharmacol. (2014) 30:153–9. doi: 10.4103/0970-9185.129996
9. Sedghi S, Kutscher HL, Davidson BA, Knight PR. Volatile anesthetics and immunity. Immunol Investig. (2017) 46:793–804. doi: 10.1080/08820139.2017.1373905
10. Muller-Edenborn B, Roth-Z’graggen B, Bartnicka K, Borgeat A, Hoos A, Borsig L, et al. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology. (2012) 117:293–301. doi: 10.1097/ALN.0b013e3182605df1
11. Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. (2005) 102:1147–57. doi: 10.1097/00000542-200506000-00014
12. Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. (2008) 108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e
13. Roesslein M, Schibilsky D, Muller L, Goebel U, Schwer C, Humar M, et al. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J Pharmacol Exp Ther. (2008) 325:217–25. doi: 10.1124/jpet.107.133108
14. Li R, Liu H, Dilger JP, Lin J. Effect of propofol on breast cancer cell, the immune system, and patient outcome. BMC Anesthesiol. (2018) 18:77. doi: 10.1186/s12871-018-0543-3
15. Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. (2004) 59:954–9. doi: 10.1111/j.1365-2044.2004.03837.x
16. Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. (2018) 175:2717–25. doi: 10.1111/bph.13750
17. Gaveriaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc Natl Acad Sci USA. (1998) 95:6326–30. doi: 10.1073/pnas.95.11.6326
18. Brejchova J, Holan V, Svoboda P. Expression of opioid receptors in cells of the immune system. Int J Mol Sci. (2020) 22:315. doi: 10.3390/ijms22010315
19. Franchi S, Moretti S, Castelli M, Lattuada D, Scavullo C, Panerai AE, et al. Mu opioid receptor activation modulates toll like receptor 4 in murine macrophages. Brain Behav Immun. (2012) 26:480–8. doi: 10.1016/j.bbi.2011.12.010
20. Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. (2000) 90:1411–4. doi: 10.1097/00000539-200006000-00028
21. Eisenstein TK, Meissler JJ Jr, Rogers TJ, Geller EB, Adler MW. Mouse strain differences in immunosuppression by opioids in vitro. J Pharmacol Exp Ther. (1995) 275:1484–9.
22. Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. (1993) 265: 1071–8.
23. Connolly C, Buggy DJ. Opioids and tumour metastasis: does the choice of the anesthetic-analgesic technique influence outcome after cancer surgery? Curr Opin Anaesthesiol. (2016) 29:468–74. doi: 10.1097/ACO.0000000000000360
24. Qi Y, Yao X, Zhang B, Du X. Comparison of recovery effect for sufentanil and remifentanil anesthesia with TCI in laparoscopic radical resection during colorectal cancer. Oncol Lett. (2016) 11:3361–5. doi: 10.3892/ol.2016.4394
25. Gong L, Qin Q, Zhou L, Ouyang W, Li Y, Wu Y, et al. Effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol. (2014) 7:7708–16.
26. Rogers TJ. Kappa opioid receptor expression and function in cells of the immune system. Handb Exp Pharmacol. (2022) 271:419–33. doi: 10.1007/164_2021_441
27. Xuan W, Hankin J, Zhao H, Yao S, Ma D. The potential benefits of the use of regional anesthesia in cancer patients. Int J Cancer. (2015) 137:2774–84. doi: 10.1002/ijc.29306
28. Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. (1999) 80:9. doi: 10.1002/(SICI)1097-0215(19990315)80:6<880::AID-IJC14>3.0.CO;2-Y
29. Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. (2003) 97:1331–9. doi: 10.1213/01.ANE.0000082995.44040.07
30. Provinciali M, Di Stefano G, Stronati S, Raffaeli W, Pari G, Fabris N. Role of prolactin in the modulation of NK and LAK cell activity after shor-or long term morphin administration in neolastic tatients. Int J Immunopharmacol. (1996) 18:10. doi: 10.1016/S0192-0561(96)00059-8
31. Krog J, Hokland M, Ahlburg P, Parner E, Tønnesen E. Lipid solubility- and concentration-dependent attenuation of in vitro natural killer cell cytotoxicity by local anesthetics. Acta Anaesthesiol Scand. (2002) 7:875–81. doi: 10.1034/j.1399-6576.2002.460719.x
32. Ramirez MF, Tran P, Cata JP. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity. Reg Anesth Pain Med. (2015) 40:43–8. doi: 10.1097/AAP.0000000000000191
33. Li R, Huang Y, Lin J. Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models. Nat Commun. (2020) 11:642. doi: 10.1038/s41467-019-14065-6
34. Liang H, Yang CX, Zhang B, Wang HB, Liu HZ, Lai XH, et al. Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1alpha. J Anesth. (2015) 29:821–30. doi: 10.1007/s00540-015-2035-7
35. Fan L, Wu Y, Wang J, He J, Han X. Sevoflurane inhibits the migration and invasion of colorectal cancer cells through regulating ERK/MMP-9 pathway by up-regulating miR-203. Eur J Pharmacol. (2019) 850:43–52. doi: 10.1016/j.ejphar.2019.01.025
36. Liang H, Yang CX, Zhang B, Zhao ZL, Zhong JY, Wen XJ. Sevoflurane attenuates platelets activation of patients undergoing lung cancer surgery and suppresses platelets-induced invasion of lung cancer cells. J Clin Anesth. (2016) 35:304–12. doi: 10.1016/j.jclinane.2016.08.008
37. Argano M, De Maria R, Vogl C, Rodlsberger K, Buracco P, Larenza Menzies MP. Canine mammary tumour cells exposure to sevoflurane: effects on cell proliferation and neuroepithelial transforming gene 1 expression. Vet Anaesth Analg. (2019) 46:369–74. doi: 10.1016/j.vaa.2018.12.006
38. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. (2016) 124:69–79. doi: 10.1097/ALN.0000000000000936
39. Luo X, Zhao H, Hennah L, Ning J, Liu J, Tu H, et al. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. (2015) 114:831–9. doi: 10.1093/bja/aeu408
40. Kawaraguchi Y, Horikawa YT, Murphy AN, Murray F, Miyanohara A, Ali SS, et al. Volatile anesthetics protect cancer cells against tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via caveolins. Anesthesiology. (2011) 115:499–508. doi: 10.1097/ALN.0b013e3182276d42
41. Jun R, Gui-he Z, Xing-xing S, Hui Z, Li-xian X. Isoflurane enhances malignancy of head and neck squamous cell carcinoma cell lines: a preliminary study in vitro. Oral Oncol. (2011) 47:329–33. doi: 10.1016/j.oraloncology.2011.03.002
42. Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, et al. Prostate cancer cell malignancy via modulation of HIF-1alpha pathway with isoflurane and propofol alone and in combination. Br J Cancer. (2014) 111:1338–49. doi: 10.1038/bjc.2014.426
43. Schifilliti D, Mondello S, D’Arrigo MG, Chille G, Fodale V. Genotoxic effects of anesthetic agents: an update. Expert Opin Drug Saf. (2011) 10:891–9. doi: 10.1517/14740338.2011.586627
44. Jin WZ, Liu H, Wan P, Chu CP, Qiu DL. Propofol enhances facial stimulation-evoked responses in the cerebellar granule cell layer via NMDA receptor activation in mice in vivo. Eur J Pharmacol. (2016) 788:37–44. doi: 10.1016/j.ejphar.2016.05.027
45. Ghasemi M, Phillips C, Fahimi A, McNerney MW, Salehi A. Mechanisms of action and clinical efficacy of NMDA receptor modulators in mood disorders. Neurosci Biobehav Rev. (2017) 80:555–72. doi: 10.1016/j.neubiorev.2017.07.002
46. Gonzalez J, Jurado-Coronel JC, Avila MF, Sabogal A, Capani F, Barreto GE. NMDARs in neurological diseases: a potential therapeutic target. Int J Neurosci. (2015) 125:315–27. doi: 10.3109/00207454.2014.940941
47. Zhu BZ, Hu HQ, Liu YY, Wang SC, Cao BZ. Expression and distribution of NMDA receptors in common tumor tissues. In: Proceedings of the 18th National Conference of Neurology of Chinese Medical Association. Chengdu (2015).
48. North WG, Fay MJ, Du J, Cleary M, Gallagher JD, McCann FV. Presence of functional NMDA receptors in a human neuroblastoma cell line. Mol Chem Neuropathol. (1997) 30:77–94. doi: 10.1007/BF02815151
49. Watanabe K, Kanno T, Oshima T, Miwa H, Tashiro C, Nishizaki T. The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem Biophys Res Commun. (2008) 367:487–90. doi: 10.1016/j.bbrc.2007.12.167
50. North WG, Gao G, Memoli VA, Pang RH, Lynch L. Breast cancer expresses functional NMDA receptors. Breast Cancer Res Treat. (2010) 122:307–14. doi: 10.1007/s10549-009-0556-1
51. Ferguson HJ, Wragg JW, Ward S, Heath VL, Ismail T, Bicknell R. Glutamate dependent NMDA receptor 2D is a novel angiogenic tumour endothelial marker in colorectal cancer. Oncotarget. (2016) 7:20440–54. doi: 10.18632/oncotarget.7812
52. Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, et al. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. (2003) 63:8090–6.
53. Zafrakas M, Chorovicer M, Klaman I, Kristiansen G, Wild PJ, Heindrichs U, et al. Systematic characterisation of GABRP expression in sporadic breast cancer and normal breast tissue. Int J Cancer. (2006) 118:1453–9. doi: 10.1002/ijc.21517
54. Biju MP, Pyroja S, Rajeshkumar NV, Paulose CS. Enhanced GABA(B) receptor in neoplastic rat liver: induction of DNA synthesis by baclofen in hepatocyte cultures. J Biochem Mol Biol Biophys. (2002) 6:209–14. doi: 10.1080/10258140290018667
55. Inamoto T, Azuma H, Sakamoto T, Kiyama S, Ubai T, Kotake Y, et al. Invasive ability of human renal cell carcinoma cell line Caki-2 is accelerated by gamma-aminobutyric acid, via sustained activation of ERK1/2 inducible matrix metalloproteinases. Cancer Invest. (2007) 25:574–83. doi: 10.1080/07357900701522471
56. Tatsuta M, Iishi H, Baba M, Taniguchi H. Attenuation by the GABA receptor agonist baclofen of experimental carcinogenesis in rat colon by azoxymethane. Oncology. (1992) 49:241–5. doi: 10.1159/000227048
57. Tatsuta M, Iishi H, Baba M, Uehara H, Nakaizumi A, Taniguchi H. Inhibition by amiloride of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Br J Cancer. (1993) 67:1011–4. doi: 10.1038/bjc.1993.185
58. Wang T, Huang W, Chen F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. (2008) 82:536–41. doi: 10.1016/j.lfs.2007.12.014
59. Moody EJ, Knauer CS, Granja R, Strakhovaua M, Skolnick P. Distinct structural requirements for the direct and indirect actions of the anaesthetic etomidate at GABA(A) receptors. Toxicol Lett. (1998) 101:209–15. doi: 10.1016/S0378-4274(98)00187-8
60. Kim JJ, Gharpure A, Teng J, Zhuang Y, Howard RJ, Zhu S, et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. (2020) 585:303–8. doi: 10.1038/s41586-020-2654-5
61. Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. (2009) 374:293–300. doi: 10.1016/S0140-6736(09)60949-1
62. Liu J, Dong W, Wang T, Liu L, Zhan L, Shi Y, et al. Effects of etomidate and propofol on immune function in patients with lung adenocarcinoma. Am J Transl Res. (2016) 8:5748–55.
63. Chu CN, Wu KC, Chung WS, Zheng LC, Juan TK, Hsiao YT, et al. Etomidate suppresses invasion and migration of human A549 lung adenocarcinoma cells. Anticancer Res. (2019) 39:215–23. doi: 10.21873/anticanres.13100
64. Chen HT, Zhou J, Fan YL, Lei CL, Li BJ, Fan LX, et al. Anesthetic agent etiomidate induces apoptosis in N2a brain tumor cell line. Mol Med Rep. (2018) 18:3137–42. doi: 10.3892/mmr.2018.9298
65. Anand JP, Montgomery D. Multifunctional opioid ligands. Handb Exp Pharmacol. (2018) 247:21–51. doi: 10.1007/164_2018_104
66. Azzam A, McDonald J, Lambert DG. Hot topics in opioid pharmacology: mixed and biased opioids. Br J Anaesth. (2019) 122:e136–45. doi: 10.1016/j.bja.2019.03.006
67. Bimonte S, Barbieri A, Palma G, Arra C. The role of morphine in animal models of human cancer: does morphine promote or inhibit the tumor growth? Biomed Res Int. (2013) 2013:258141. doi: 10.1155/2013/258141
68. Singleton PA, Moss J. Effect of perioperative opioids on cancer recurrence: a hypothesis. Future Oncol. (2010) 6:1237–42. doi: 10.2217/fon.10.99
69. Li Y, Li G, Tao T, Kang X, Liu C, Zhang X, et al. The mu-opioid receptor (MOR) promotes tumor initiation in hepatocellular carcinoma. Cancer Lett. (2019) 453:1–9. doi: 10.1016/j.canlet.2019.03.038
70. Fujioka N, Nguyen J, Chen CS, Li YF, Pasrija T, Niehans G, et al. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg. (2011) 113:1353–64. doi: 10.1213/ANE.0b013e318232b35a
71. Zhang HW, Wang F, Zhou YQ, Xu SP, Yu SY, Zhang ZG. Morphine suppresses liver cancer cell tumor properties in vitro and in vivo. Front Oncol. (2021) 11:666446. doi: 10.3389/fonc.2021.666446
72. Kim JY, Ahn HJ, Kim JK, Kim J, Lee SH, Chae HB. Morphine suppresses lung cancer cell proliferation through the interaction with opioid growth factor receptor: an in vitro and human lung tissue study. Anesth Analg. (2016) 123:1429–36. doi: 10.1213/ANE.0000000000001293
73. Zagon IS, Donahue RN, McLaughlin PJ. Opioid growth factor-opioid growth factor receptor axis is a physiological determinant of cell proliferation in diverse human cancers. Am J Physiol Regul Integr Comp Physiol. (2009) 297:R1154–61. doi: 10.1152/ajpregu.00414.2009
74. Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, Ramkrishnan S, et al. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol. (2014) 184:1073–84. doi: 10.1016/j.ajpath.2013.12.019
75. Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. (2011) 112:558–67. doi: 10.1213/ANE.0b013e31820568af
76. Singleton PA, Mirzapoiazova T, Hasina R, Salgia R, Moss J. Increased mu-opioid receptor expression in metastatic lung cancer. Br J Anaesth. (2014) 113(Suppl. 1):i103–8. doi: 10.1093/bja/aeu165
77. Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. (2013) 119:4103–10. doi: 10.1002/cncr.28345
78. Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anesth. (2016) 63:184–92. doi: 10.1007/s12630-015-0523-8
79. Miao J, Wang L, Chen L, Yang T, Jin L, Lin L. Fentanyl inhibits cell viability in human pancreatic cancer cell line and tumor growth in pancreatic cancer cell-transplanted mice. Int J Clin Exp Med. (2015) 8:17684–93.
80. Zhang XL, Chen ML, Zhou SL. Fentanyl inhibits proliferation and invasion of colorectal cancer via beta-catenin. Int J Clin Exp Pathol. (2015) 8:227–35.
81. Chen D, Chen Y, Yan Y, Pan J, Xing W, Li Q, et al. Down-regulation of the tumour suppressor kappa-opioid receptor predicts poor prognosis in hepatocellular carcinoma patients. BMC Cancer. (2017) 17:553. doi: 10.1186/s12885-017-3541-9
82. Edwards KA, Havelin JJ, McIntosh MI, Ciccone HA, Pangilinan K, Imbert I, et al. A kappa opioid receptor agonist blocks bone cancer pain without altering bone loss, tumor size, or cancer cell proliferation in a mouse model of cancer-induced bone pain. J Pain. (2018) 19:612–25. doi: 10.1016/j.jpain.2018.01.002
83. Kuzumaki N, Suzuki A, Narita M, Hosoya T, Nagasawa A, Imai S, et al. Effect of kappa-opioid receptor agonist on the growth of non-small cell lung cancer (NSCLC) cells. Br J Cancer. (2012) 106:1148–52. doi: 10.1038/bjc.2011.574
84. Yamamizu K, Hamada Y, Narita M. kappa opioid receptor ligands regulate angiogenesis in development and in tumours. Br J Pharmacol. (2015) 172:268–76. doi: 10.1111/bph.12573
85. Diao CT, Li L, Lau SY, Wong TM, Wong NS. kappa-Opioid receptor potentiates apoptosis via a phospholipase C pathway in the CNE2 human epithelial tumor cell line. Biochim Biophys Acta. (2000) 1499:49–62. doi: 10.1016/S0167-4889(00)00107-5
86. Wong N, Diao CT, Wong T. The overexpression of Bcl-2 antagonizes the proapoptotic function of the kappa-opioid receptor. Ann N Y Acad Sci. (2003) 1010:358–60. doi: 10.1196/annals.1299.066
87. Zhang YF, Xu QX, Liao LD, Xu XE, Wu JY, Shen J, et al. kappa-Opioid receptor in the nucleus is a novel prognostic factor of esophageal squamous cell carcinoma. Hum Pathol. (2013) 44:1756–65. doi: 10.1016/j.humpath.2012.11.025
88. Colvin LA, Fallon MT, Buggy DJ. Cancer biology, analgesics, and anaesthetics: is there a link. Br J Anaesth. (2012) 109:140–3. doi: 10.1093/bja/aes255
89. Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. (2011) 30:225–38. doi: 10.1007/s10555-011-9285-0
90. Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. (2019) 123:135–50. doi: 10.1016/j.bja.2019.04.062
91. Ma XW, Li Y, Han XC, Xin QZ. The effect of low dosage of procaine on lung cancer cell proliferation. Eur Rev Med Pharmacol Sci. (2016) 20:4791–5.
92. Grandhi RK, Perona B. Mechanisms of action by which local anesthetics reduce cancer recurrence: a systematic review. Pain Med. (2020) 21:401–14. doi: 10.1093/pm/pnz139
93. Tada M, Imazeki F, Fukai K, Sakamoto A, Arai M, Mikata R, et al. Procaine inhibits the proliferation and DNA methylation in human hepatoma cells. Hepatol Int. (2007) 1:355–64. doi: 10.1007/s12072-007-9014-5
94. Li YC, Wang Y, Li DD, Zhang Y, Zhao TC, Li CF. Procaine is a specific DNA methylation inhibitor with anti-tumor effect for human gastric cancer. J Cell Biochem. (2018) 119:2440–9. doi: 10.1002/jcb.26407
95. Zhou H, Xu M, Luo G, Zhang Y. Effect of procaine on proliferation of human nasopharyngeal carcinoma cells CNE-2Z. J Clin Otorhinolaryngol Head Neck Surg. (2007) 21:1118–21.
96. Lirk P, Hollmann MW, Fleischer M, Weber NC, Fiegl H. Lidocaine and ropivacaine, but not bupivacaine, demethylate deoxyribonucleic acid in breast cancer cells in vitro. Br J Anaesth. (2014) 113:i32–8. doi: 10.1093/bja/aeu201
97. Lirk P, Berger R, Hollmann MW, Fiegl H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro †. Br J Anaesth. (2012) 109:200–7. doi: 10.1093/bja/aes128
98. Li K, Yang J, Han X. Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARbeta2 and RASSF1A demethylation. Int J Mol Sci. (2014) 15:23519–36. doi: 10.3390/ijms151223519
99. Hirata M, Sakaguchi M, Mochida C, Sotozono C, Kageyama K, Kuroda Y, et al. Lidocaine inhibits tyrosine kinase activity of the epidermal growth factor receptor and suppresses proliferation of corneal epithelial cells. Anesthesiology. (2004) 100:5. doi: 10.1097/00000542-200405000-00024
100. Sakaguchi M, Kuroda Y, Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth Analg. (2006) 102:1103–7. doi: 10.1213/01.ane.0000198330.84341.35
101. Zhang R, Liu R, Liu C, Niu Y, Zhang J, Guo B, et al. A novel role for MiR-520a-3p in regulating EGFR expression in colorectal cancer. Cell Physiol Biochem. (2017) 42:1559–74. doi: 10.1159/000479397
102. Xia W, Wang L, Yu D, Mu X, Zhou X. Lidocaine inhibits the progression of retinoblastoma in vitro and in vivo by modulating the miR520a3p/EGFR axis. Mol Med Rep. (2019) 20:1333–42. doi: 10.3892/mmr.2019.10363
103. Sun H, Sun Y. Lidocaine inhibits proliferation and metastasis of lung cancer cell via regulation of miR-539/EGFR axis. Artif Cells Nanomed Biotechnol. (2019) 47:2866–74. doi: 10.1080/21691401.2019.1636807
104. Chamaraux-Tran TN, Piegeler T. The amide local anesthetic lidocaine in cancer surgery-potential antimetastatic effects and preservation of immune cell function? A narrative review. Front Med (Lausanne). (2017) 4:235. doi: 10.3389/fmed.2017.00235
105. Chang YC, Liu CL, Chen MJ, Hsu YW, Chen SN, Lin CH, et al. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. (2014) 118:116–24. doi: 10.1213/ANE.0b013e3182a94479
106. Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. (2014) 9:e89563. doi: 10.1371/journal.pone.0089563
107. Liu H, Dilger JP, Lin J. Effects of local anesthetics on cancer cells. Pharmacol Ther. (2020) 212:107558. doi: 10.1016/j.pharmthera.2020.107558
108. Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. (2014) 119:251–61. doi: 10.3109/03009734.2014.922649
109. Puig NR, Ferrero P, Bay ML, Hidalgo G, Valenti J, Amerio N, et al. Effects of sevoflurane general anesthesia: immunological studies in mice. Int Immunopharmacol. (2002) 2:95–104. doi: 10.1016/S1567-5769(01)00151-5
110. Pirbudak Cocelli L, Ugur MG, Karadasli H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr Ther Res Clin Exp. (2012) 73:41–51. doi: 10.1016/j.curtheres.2012.02.005
111. Saad MM, Eom W, Hu G, Kim SJ, Crystal GJ. Persistency and pathway of isoflurane-induced inhibition of superoxide production by neutrophils. Can J Anaesth. (2010) 57:50–7. doi: 10.1007/s12630-009-9205-8
112. Wada H, Seki S, Takahashi T, Kawarabayashi N, Higuchi H, Habu Y, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. (2007) 106:499–506. doi: 10.1097/00000542-200703000-00014
113. Chen RM, Wu CH, Chang HC, Wu GJ, Lin YL, Sheu JR, et al. Propofol suppresses macrophage functions and modulates mitochondrial membrane potential and cellular adenosine triphosphate synthesis. Anesthesiology. (2003) 98:1178–85. doi: 10.1097/00000542-200305000-00021
114. Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX, Jin HY, et al. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J Transl Med. (2014) 12:279. doi: 10.1186/s12967-014-0279-x
115. Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. (2011) 115:1363–81. doi: 10.1097/ALN.0b013e318238bba6
116. Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth. (2012) 109:i17–28. doi: 10.1093/bja/aes421
118. Piegeler T, Votta-Velis EG, Bakhshi FR, Mao M, Carnegie G, Bonini MG, et al. Endothelial barrier protection by local anesthetics: ropivacaine and lidocaine block tumor necrosis factor-alpha-induced endothelial cell Src activation. Anesthesiology. (2014) 120:1414–28. doi: 10.1097/ALN.0000000000000174
119. Zhang L, Wang N, Zhou S, Ye W, Jing G, Zhang M. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. J Exp Clin Cancer Res. (2012) 31:66. doi: 10.1186/1756-9966-31-66
120. Xu YB, Du QH, Zhang MY, Yun P, He CY. Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci. (2013) 17:2486–94.
121. Yang N, Liang Y, Yang P, Ji F. Propofol suppresses LPS-induced nuclear accumulation of HIF-1alpha and tumor aggressiveness in non-small cell lung cancer. Oncol Rep. (2017) 37:2611–9. doi: 10.3892/or.2017.5514
122. Chen L, Wan Y, Liu Y, Li T. Propofol inhibits biological functions of leukaemia stem and differentiated cells through suppressing Wnt/beta-catenin and Akt/mTOR. Clin Exp Pharmacol Physiol. (2020) 47:127–34. doi: 10.1111/1440-1681.13167
123. Chen X, Wu Q, Sun P, Zhao Y, Zhu M, Miao C. Propofol disrupts aerobic glycolysis in colorectal cancer cells via inactivation of the NMDAR-CAMKII-ERK pathway. Cell Physiol Biochem. (2018) 46:492–504. doi: 10.1159/000488617
124. Xing SG, Zhang KJ, Qu JH, Ren YD, Luan Q. Propofol induces apoptosis of non-small cell lung cancer cells via ERK1/2-dependent upregulation of PUMA. Eur Rev Med Pharmacol Sci. (2018) 22:4341–9.
125. Deng F, Ouyang M, Wang X, Yao X, Chen Y, Tao T, et al. Differential role of intravenous anesthetics in colorectal cancer progression: implications for clinical application. Oncotarget. (2016) 7:77087–95. doi: 10.18632/oncotarget.12800
126. Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, Moss J, et al. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS One. (2014) 9:e91577. doi: 10.1371/journal.pone.0091577
Keywords: volatile anesthetics, intravenous, opioids, local anesthetics, immune effect, tumor-targeting gene, tumor-associated signal pathway
Citation: Luan T, Li Y, Sun L, Xu S, Wang H, Wang J and Li C (2022) Systemic immune effects of anesthetics and their intracellular targets in tumors. Front. Med. 9:810189. doi: 10.3389/fmed.2022.810189
Received: 09 November 2021; Accepted: 06 July 2022;
Published: 28 July 2022.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Anca Irina Ristescu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaXiaozhen Zhang, Zhejiang University, China
Copyright © 2022 Luan, Li, Sun, Xu, Wang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifeng Wang, aGlnaHBob25lQDEyNi5jb20=; Jiansong Wang, amlhbnNvbmd3YW5na21AMTI2LmNvbQ==; Chong Li, bGljaG9uZ0Btb29uLmlicC5hYy5jbg==
†These authors have contributed equally to this work
 Ting Luan
Ting Luan Yi Li
Yi Li Lihui Sun
Lihui Sun Siqi Xu
Siqi Xu Haifeng Wang
Haifeng Wang Jiansong Wang
Jiansong Wang Chong Li
Chong Li