- 1Hebei Yanda Hospital, Langfang, China
- 2No. 988th Hospital of Joint Logistic Support Force of PLA, Zhengzhou, China
Background: The effect of N-acetylcysteine (NAC), an antioxidant, on preventing acute kidney injury (AKI) and major adverse cardiac events (MACE) remains controversial. Therefore, we conducted this meta-analysis and trial sequential analysis to evaluate its efficacy on cardiac surgery-related adverse events.
Methods: PubMed, Embase, and Cochrane Library were searched for relevant studies from inception to June 2021. We selected randomized controlled trials comparing NAC with controls in patients undergoing cardiac surgery.
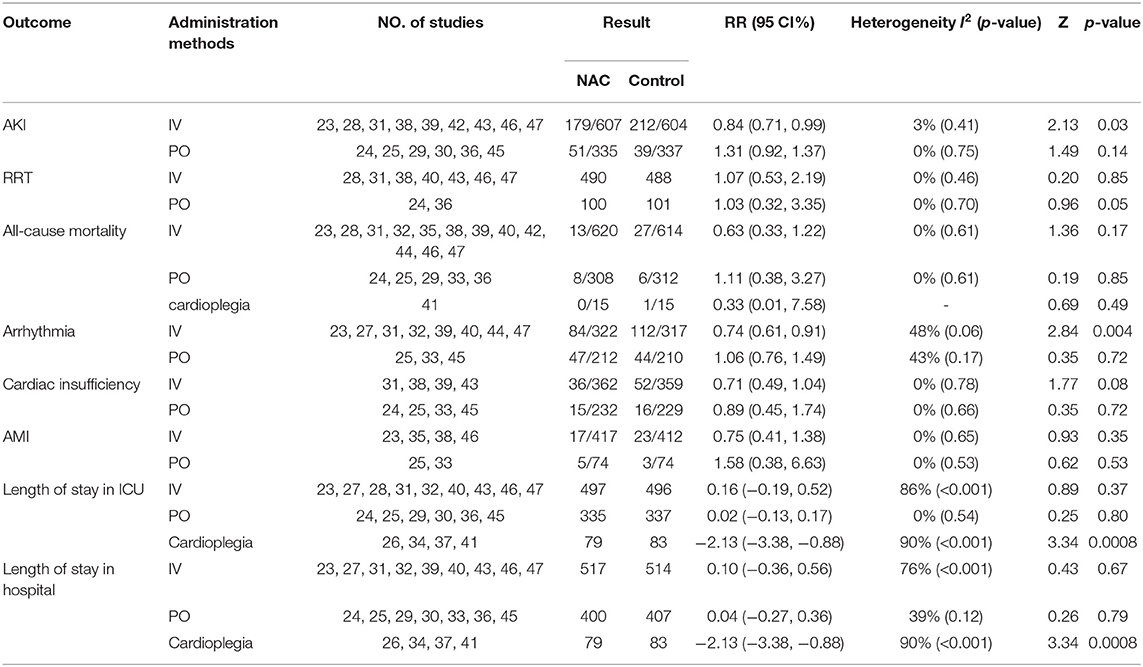
Results: Twenty-five studies including 2,444 patients met the inclusion criteria. The pooled results showed that there was no significant difference in the incidence of AKI between the NAC and control groups [relative risk (RR) = 0.91, 95% confidence interval (CI) = 0.77, 1.08, P = 0.28], but the trial sequential analysis (TSA) could not confirm this result. No difference was observed in the need for renal replacement therapy (RRT), all-cause mortality, MACE, length of stay in the intensive care unit (ICU), and length of stay in the hospital. Results of subgroup analysis results showed that intravenous infusion instead of oral NAC could significantly reduce the incidence of AKI and arrhythmia (RR = 0.84, 95% CI = 0.71, 0.99, P = 0.03, I2 = 3% and RR = 0.74, 95% CI = 0.61, 0.91, P = 0.004, I2 = 48%, respectively).
Conclusion: Intravenous administration of NAC can reduce the incidence of AKI and arrhythmia in patients after cardiac surgery, but cannot reduce all-cause mortality, AMI, cardiac insufficiency, and the number of patients using RRT. Oral NAC has no significant effect on the outcomes of patients after cardiac surgery.
Background
Coronary artery disease (CAD) is one of the major cardiovascular diseases worldwide and many CAD patients will be submitted for cardiac surgeries. The most common cardiac surgeries are coronary artery bypass graft (CABG) surgery and aortic or mitral valve repair or replacement (1, 2). They are commonly performed on-pump, which indicates that cardiopulmonary bypass (CPB) is used in the surgery. However, there is also a considerable number of cardiac surgeries that are performed off-pump without CPB assistance (3). Although the overall prognosis after cardiac surgery has been improved over the past decades, the occurrence of acute kidney injury (AKI) and major adverse cardiac events (MACEs) remains unsatisfactorily high (4, 5). AKI is the most common important complication in adult patients undergoing cardiac surgery and is associated with a prolonged hospital stay, use of dialysis, subsequent chronic kidney disease (CKD), and increased mortality (4). The incidence of AKI occurs in approximately 18% of patients undergoing cardiac surgery and approximately 2%-6% of them require renal replacement therapy (RRT). It is more likely for these kinds of patients to progress to CKD in the ensuing months and years than those who do not develop AKI and do not require RRT. A variety of risk factors, either renal or extrarenal, contribute to the development and progression of AKI after heart surgery, including renal ischemia, reperfusion, mechanical trauma, inflammation, hemolysis, oxidative stress, cholesterol emboli, and nephrotoxins (6). MACE is also an important composite primary endpoint assessed in most cardiovascular trials. Despite these unsatisfactory adverse events, cardiac surgery remains popular in CAD patients for its irreplaceable therapeutic effect. Thus, we must develop a more effective method to reduce the risk of postoperative complications of cardiac surgery.
N-acetylcysteine (NAC) is a cysteine prodrug and glutathione (GSH) precursor which has been used in clinical therapeutic practice as a mucolytic agent and for the treatment of numerous disorders including paracetamol intoxication, doxorubicin cardiotoxicity, ischemia-reperfusion cardiac injury and chemotherapy-induced toxicity associated with GSH deficiency for several decades (7). Also, NAC is a kind of free radical scavenger antioxidant agent and it is now well-known that it can reduce pro-inflammatory cytokines, oxygen free-radical production, and ameliorates ischemia-reperfusion injury which may consequently reduce postoperative complications in cardiac surgery (8). Researchers have investigated the efficacy and safety of NAC in several clinical trials in recent years. However, its effectiveness remains controversial. Although previous meta-analyses have investigated the role of NAC in preventing post-cardiac surgery complications, the results of them are conflicting (9–15). Therefore, we conducted this meta-analysis and trial sequential analysis (TSA) to further evaluate the efficacy of NAC in preventing AKI and MACEs after cardiac surgery.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines (16).
Data Sources and Search Strategy
Electronic databases including PubMed, Embase, and the Cochrane Library were systematically searched from inception to June 2021, using items related to “n-acetylcysteine,” and “cardiac surgery.” The search was limited to studies involving human subjects. No language restrictions or publication status were applied. The citations of included references were searched individually to identify potential additional relevant studies.
Eligibility Criteria
The inclusion criteria were as follows: (1) study design: randomized controlled trials (RCTs); (2) population: patients undergoing cardiac surgery (>18 years old); (3) intervention: n-acetylcysteine (NAC) compared with placebo or standard of care; and (4) outcome: assessed at least one of the following outcomes: the incidence of acute kidney injury (AKI), the need for renal replacement therapy (RRT), all-cause mortality, major adverse cardiac events (MACEs) including arrhythmia, cardiac insufficiency and acute myocardial infarction (AMI), length of stay in an intensive care unit (ICU) and hospital. The exclusion criteria were as follows: (1) studies that involved participants who are < 18 years old; (2) studies that evaluated different interventions or did not include a reference group; (3) studies that did not report predefined outcomes or the data could not be extracted.
Data Extraction and Risk of Bias Assessment
Two reviewers independently extracted the data using a predefined standardized form. The extracted data included first author, year of publication, sample size, patient characteristics, interventions, all clinical outcomes (the incidence of AKI, the need for RRT, all-cause mortality, MACEs including arrhythmia, cardiac insufficiency, and AMI, length of stay in ICU and length of stay in hospital). The risk of bias assessment was performed by two independent reviewers using the Cochrane risk of bias approach and a third reviewer was consulted if no consensus could be reached. The standard criteria included the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
Statistical Analysis
Outcomes were treated as dichotomous or continuous variables. We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous variables and mean differences (MDs) with 95% CIs for continuous variables. Heterogeneity among the studies was assessed by the I2 statistic, and when I2 was more than 50%, significant statistical heterogeneity was considered to be present (17). We used the fixed-effect model when the I2 values were < 25%. Otherwise, we used the random-effects model. Sensitivity analyses were conducted to test the robustness of the overall pooled effect. The presence of publication bias was evaluated by using a funnel plot. All comparisons were two-sided, and a P < 0.05 was considered statistically significant. If the mean or standard deviation of the outcomes could not be directly extracted from the studies, we estimated them from the sample size, median, range, and/or interquartile range (18, 19).
Review Manager (version 5.3, The Cochrane Collaboration, Oxford, United Kingdom) was used in all analyses.
Trial Sequential Analysis
We conducted trial sequential analysis (TSA) in this meta-analysis to control the risk of random errors and assess whether the results were conclusive (20). Firm evidence for accepting or rejecting the anticipated intervention effect is considered clear and no further studies are needed if the cumulative Z-curve crossed the trial sequential monitoring boundary or entered the futility area. No conclusion is made and more studies are required to confirm the results if the Z-curve did not cross any of the boundaries or the required information size (RIS) has not been reached (21). In our meta-analysis, we performed the TSA with an overall risk of 5% of the type I error and estimated the RIS based on a RR reduction of 20% with a power of 80%. The control event rate was calculated according to the comparator group (22).
Results
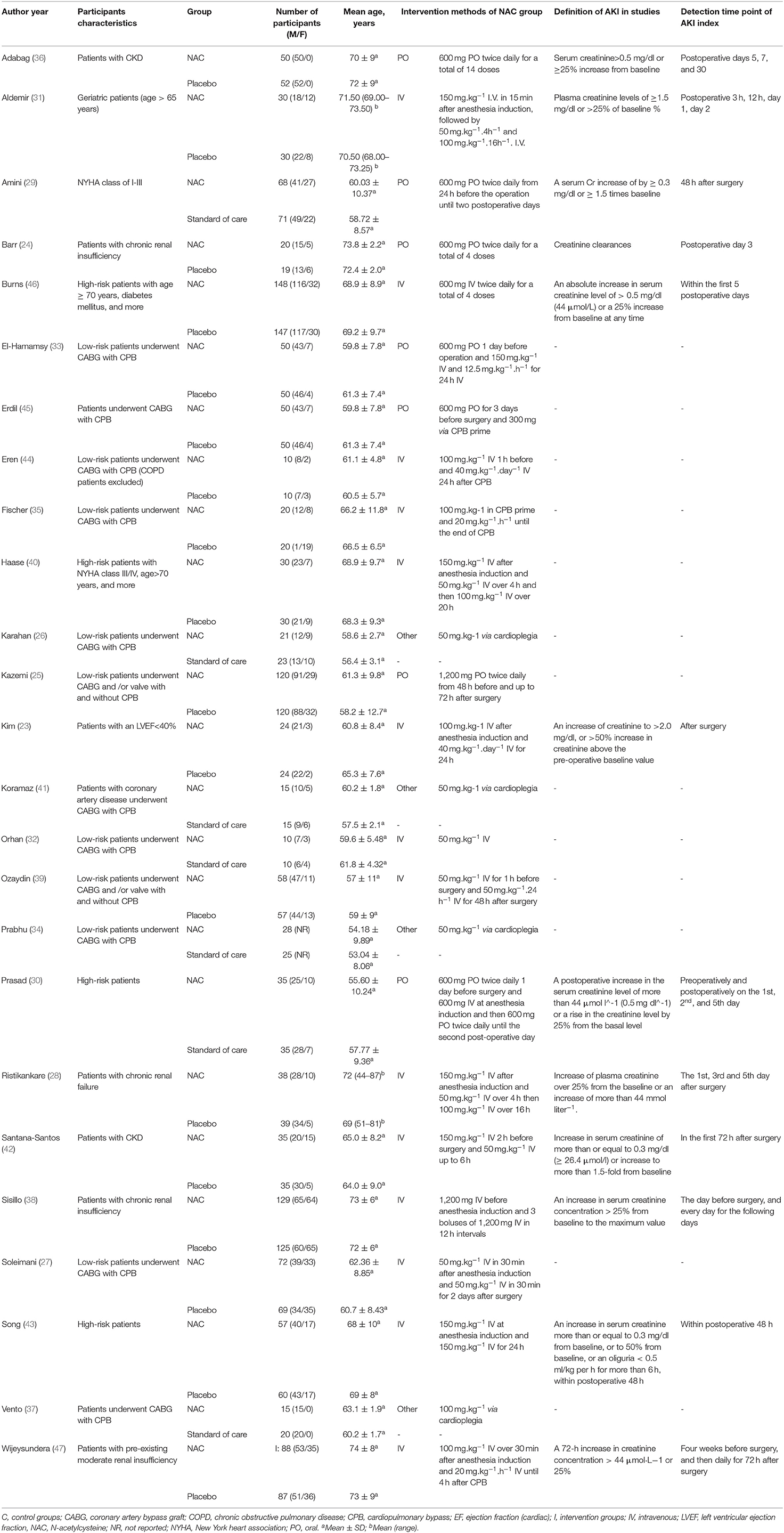
Search Results and Study Characteristics
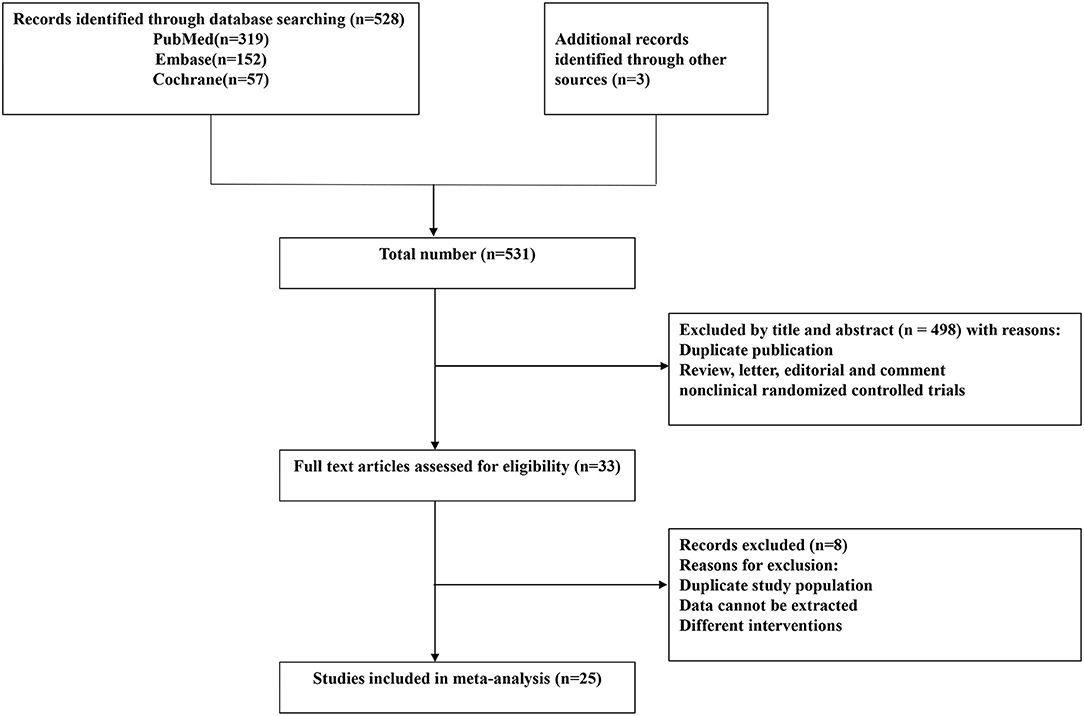
The flow chart describing the selection of the trials for this meta-analysis is presented in Figure 1. According to our search strategy, 531 potential studies were identified. After removing the duplicates and the studies that failed to meet the inclusion criteria, 33 studies were eligible for full-text reviews. Finally, only twenty-five (23–47) studies involving 2,444 patients were included in this meta-analysis. The characteristics of the included individual studies were summarized in Table 1. All included trials were reported between 2003 and 2018. The sample sizes of the included trials ranged from 20 to 295.
Risk of Bias Assessment and Publication Bias of the Included Studies
Review Manager 5.3 was used to assess the study quality in this study. A summary of the risk of bias in the included studies is presented in Figure 2. Random sequence generation was judged to be at a low risk of bias in all included studies. There is no significant publication bias of mortality (P = 0.652 for the Begg's test, P = 0.475 for the Egger's test), length of stay in ICU (P = 0.086 for the Begg's test, P = 0.163 for the Egger's test) and length of stay in hospital (P = 0.475 for the Begg's test, P = 0.181 for the Egger's test).
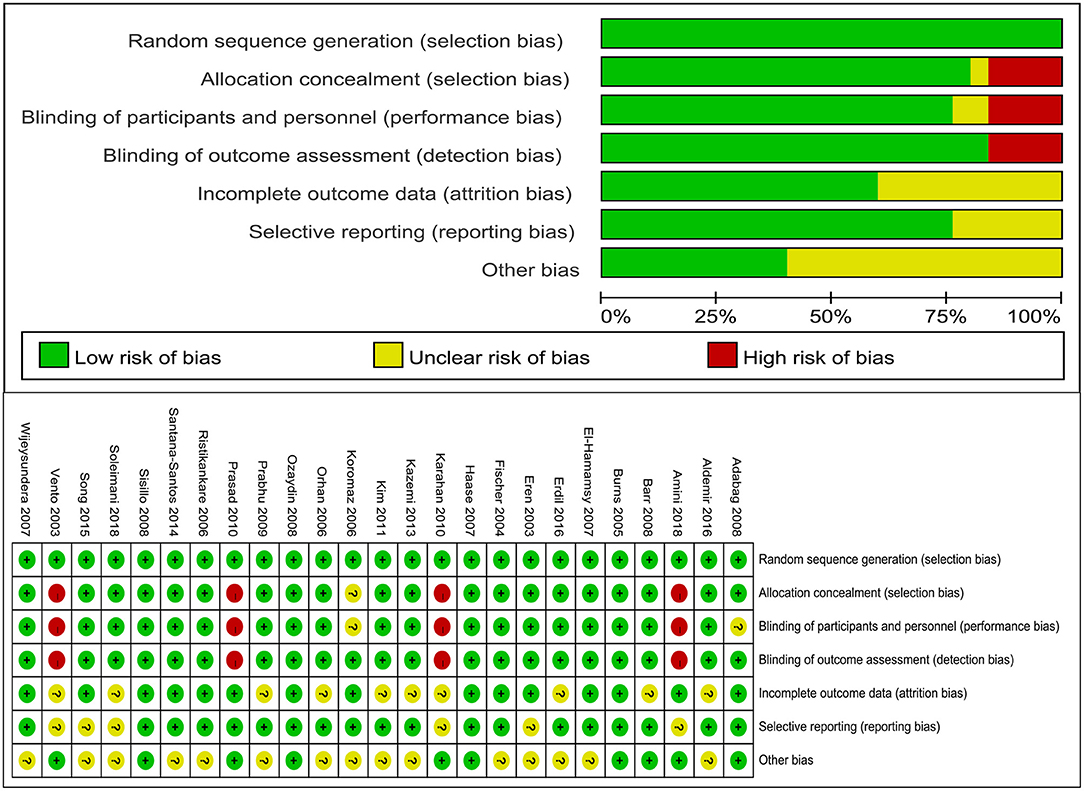
Figure 2. Risk of bias assessment. “+” indicates low risk of bias, “−” indicates high risk of bias, “?” indicates unclear risk of bias.
The Incidence of AKI
Fifteen studies (23–25, 28–31, 36, 38, 39, 42, 43, 45–47) reported the incidence rate of AKI. The data from the trials showed that there was no significant difference in the incidence of AKI between the NAC groups and the controlled groups (RR = 0.91, 95%CI = 0.77, 1.08, P = 0.28, I2 = 15%) as shown in Figure 3A. However, the TSA could not confirm this result because the cumulative Z-curve did not cross the conventional boundary or the trial sequential monitoring boundary and did not cross the futility boundary (Figure 3B).
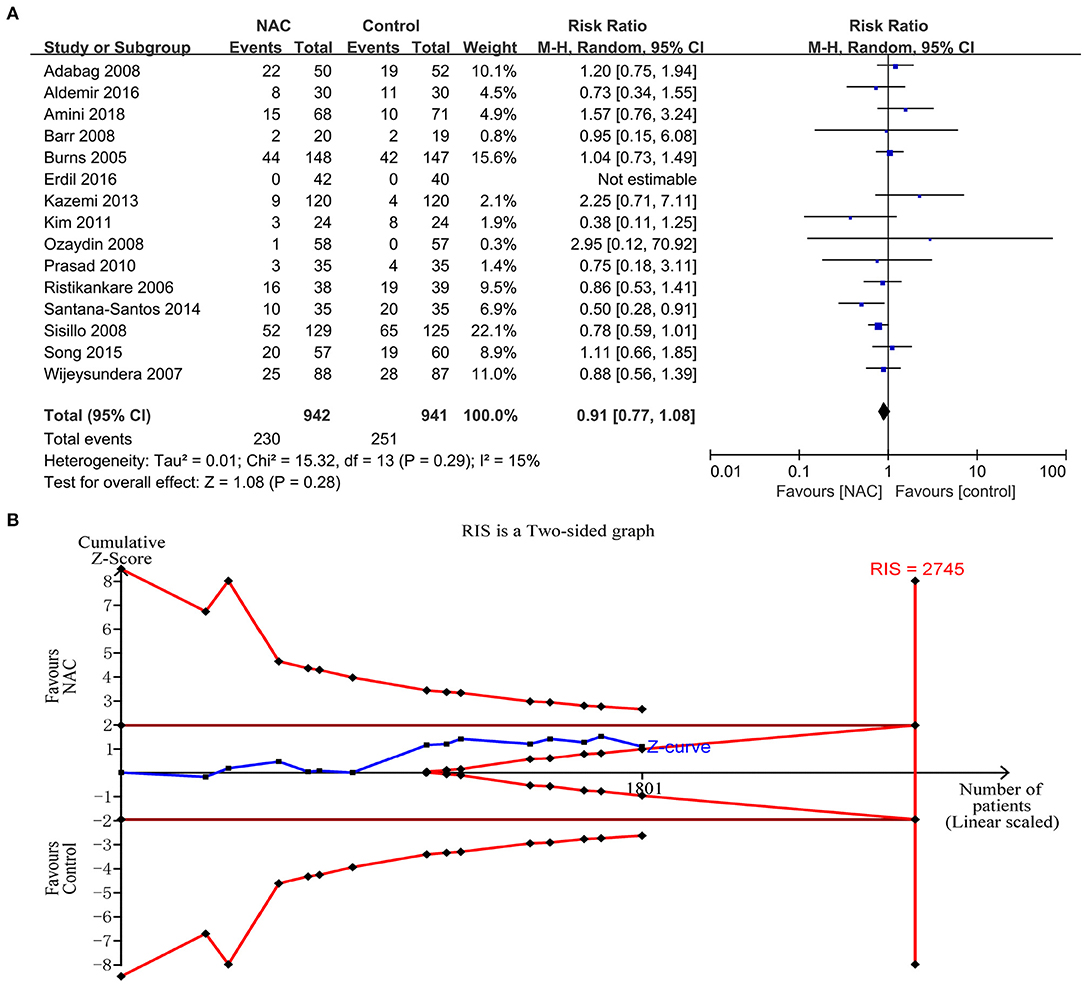
Figure 3. The effects of NAC on the incidence of AKI. (A) Forest plot of the incidence of AKI between NAC and controlled groups. (B) Fixed effects model of the TSA of the incidence of AKI between NAC and controlled groups. A diversity-adjusted information size of 2,745 participants was calculated based on a control event rate of 26.7% and a RR reduction of 20%, with α = 5% (two-sided), β = 20%, and I2 = 15%. The solid blue line represents the cumulative Z-curve, which did not cross the conventional boundary (solid dark red line), the trial sequential monitoring boundary (solid red line), and the futility boundary (solid red line).
The Need for RRT Among the Patients
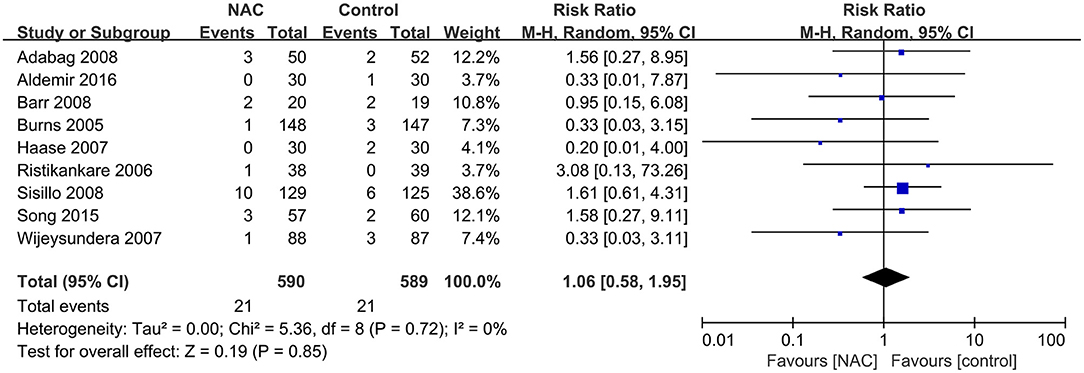
Nine studies (24, 28, 31, 36, 38, 40, 43, 46, 47) with 1,179 patients reported the number of patients who required RRT among all included patients. No significant difference was found between the NAC and the controlled groups (RR = 1.06, 95% CI = 0.58, 1.95, P = 0.85, I2 = 0%) as depicted in Figure 4.
All-Cause Mortality
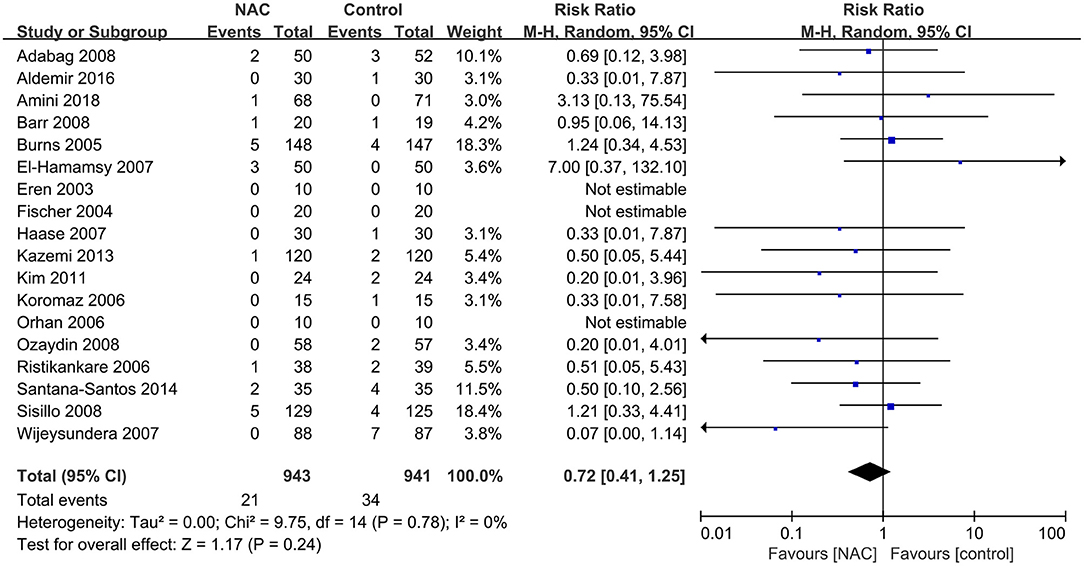
Eighteen (23–25, 28, 29, 31–33, 35, 36, 38–42, 44, 46, 47) of the included studies reported all-cause mortality. The mortality in the NAC group and the controlled group was 2.2 and 3.6%, respectively. The pooled results showed that the use of NAC could not reduce the risk of all-cause mortality compared with the use of placebo or just standard of care (RR = 0.72, 95% CI = 0.41, 1.25, P = 0.24, I2 = 0%) as presented in Figure 5.
The Incidence of MACEs
The outcomes of MACEs analyzed in this study included arrhythmia, cardiac insufficiency and AMI. Eleven studies (23, 25, 27, 31–33, 39, 40, 44, 45, 47) reported the outcome of arrhythmia and the meta-analysis of these results shows that NAC treatment did not decrease the incidence of arrhythmia (RR = 0.84, 95% CI = 0.62, 1.13, P = 0.24, I2 = 43%) as illustrated in Figure 6A. Nine studies (24, 25, 31, 33, 38, 39, 43, 45, 47) and six studies (23, 25, 33, 35, 38, 46) reported cardiac insufficiency and AMI, respectively. The overall pooled analysis found no significant difference in the incidence of cardiac insufficiency and AMI between the NAC treatment and the controlled groups (RR = 0.75, 95% CI = 0.54, 1.04, P = 0.09, I2 = 0% and RR = 0.84, 95% CI = 0.48, 1.47, P = 0.54, I2 = 0%, respectively) as revealed in Figures 6B,C.
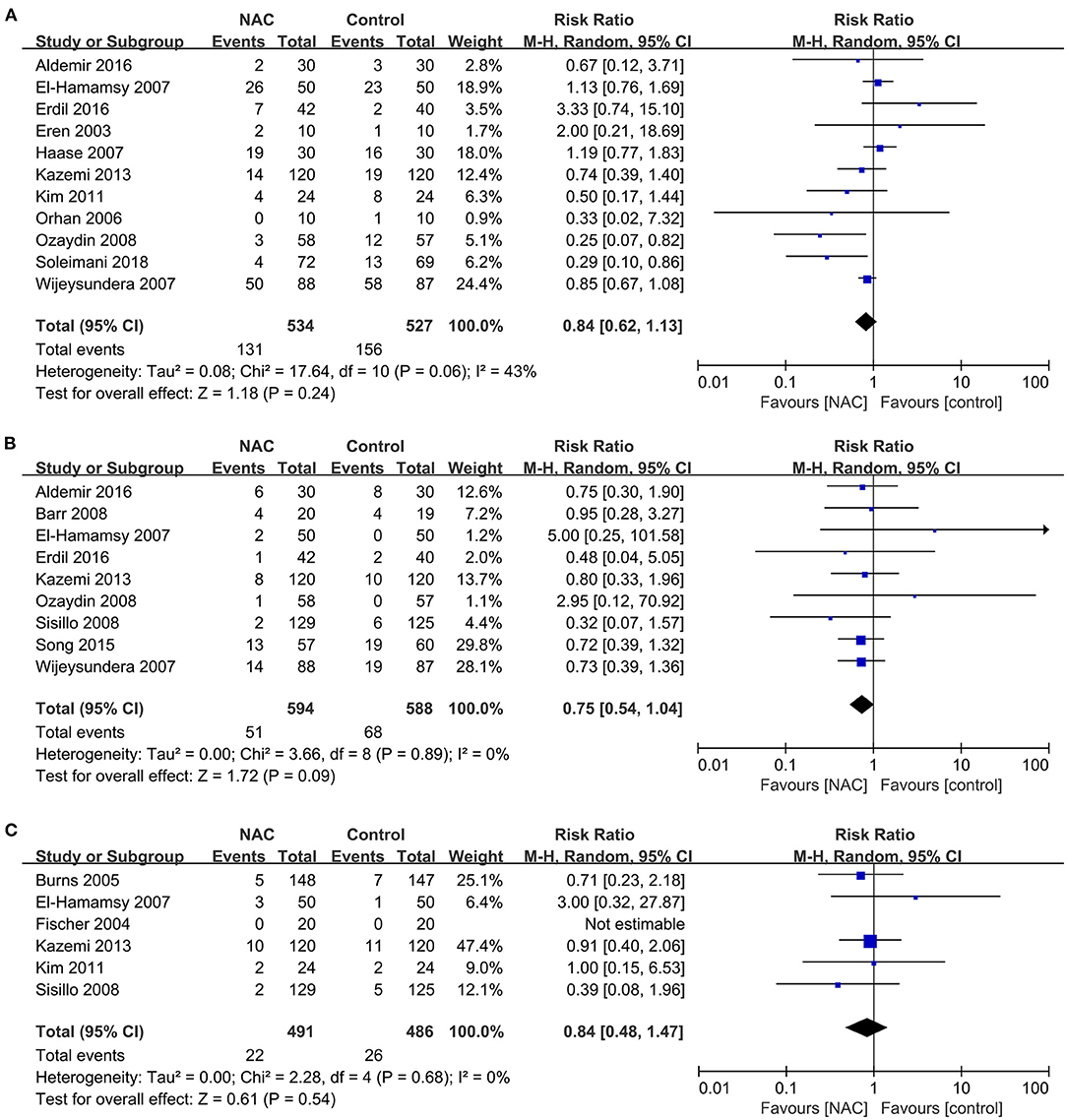
Figure 6. Forest plot of the effects of NAC on the incidence of MACEs. (A) Forest plot of arrhythmia. (B) Forest plot of cardiac insufficiency. (C) Forest plot of AMI.
Length of Stay in ICU and Hospital
Nineteen studies (23–32, 34, 36, 37, 40, 41, 43, 45–47) reported the length of stay in ICU in patients submitted to cardiac surgery. And twenty studies (23–27, 29–34, 36, 37, 39–41, 43, 45–47) reported the length of stay in hospital in patients. Results of these studies showed a non-statistically significant difference in the length of ICU and hospital stay between the NAC and controlled groups (MD = −0.07, 95% CI = −0.28, 0.14, P = 0.54, I2 = 95% and RR = −0.16, 95% CI = −0.59, 0.27, P = 0.45, I2 = 91%, respectively) as exhibited in Figures 7A,B.
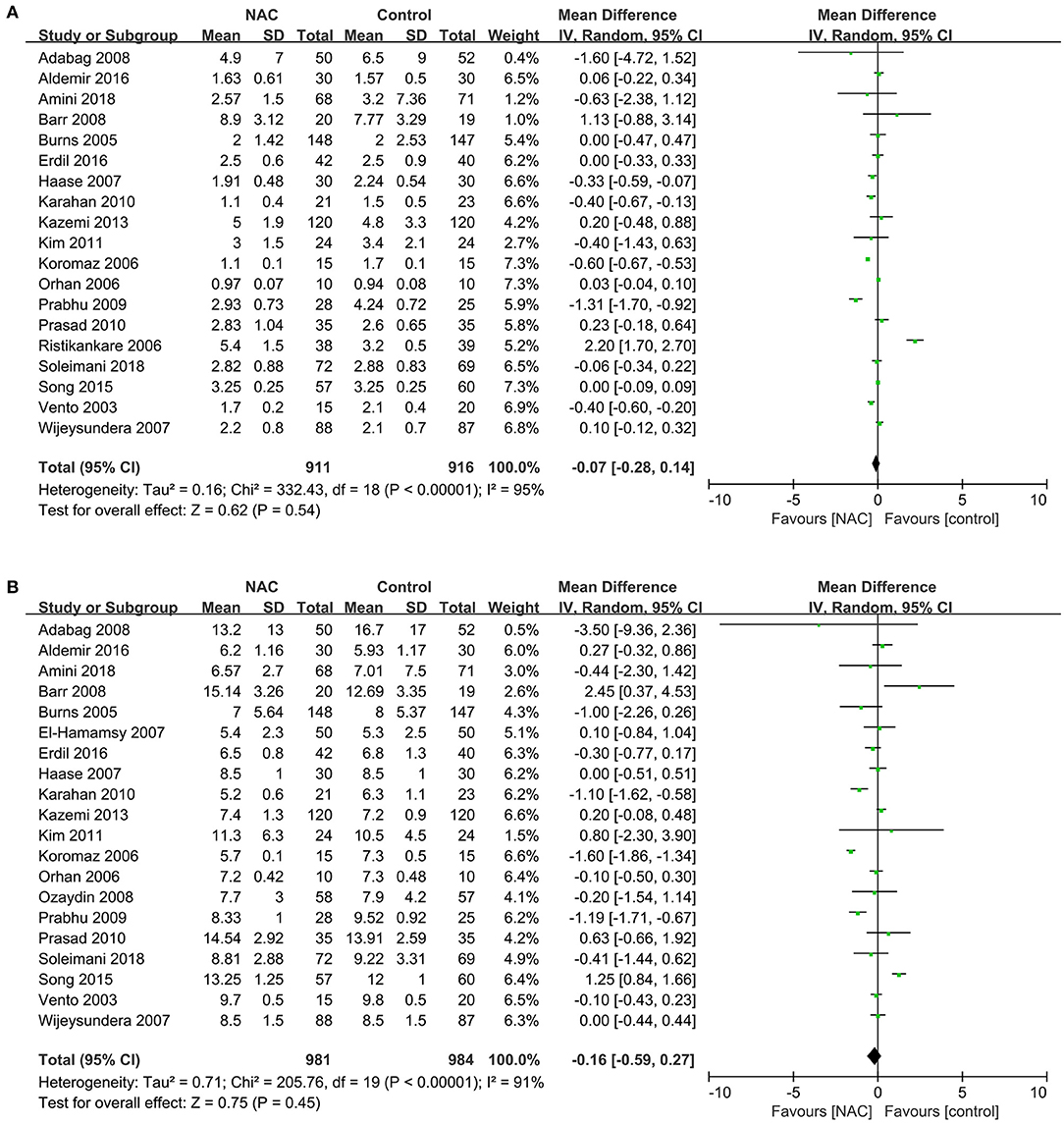
Figure 7. Forest plot of the effects of NAC on the length of stay in ICU and hospital. (A) Forest plot of the length of stay in ICU. (B) Forest plot of the length of stay in hospital.
Effect of NAC Administration Methods on Outcomes
All the studies were divided according to NAC administration methods, and the effects on the outcome were analyzed respectively. The results showed that intravenous infusion instead of oral NAC could significantly reduce the incidence of AKI and arrhythmia (RR = 0.84, 95% CI = 0.71, 0.99, P = 0.03, I2 = 3% and RR = 0.74, 95% CI = 0.61, 0.91, P = 0.004, I2 = 48% respectively) as shown in Figures 8A,B. The addition of NAC to cardioplegia may reduce ICU and hospital stay, but significant statistical heterogeneity was observed. NAC has no significant effect on RRT, all-cause mortality, AMI, and cardiac insufficiency. Results of subgroup analysis showed administration methods of NAC had no significant effect on the need for RRT, all-cause mortality, AMI, and cardiac insufficiency among patients (Table 2).
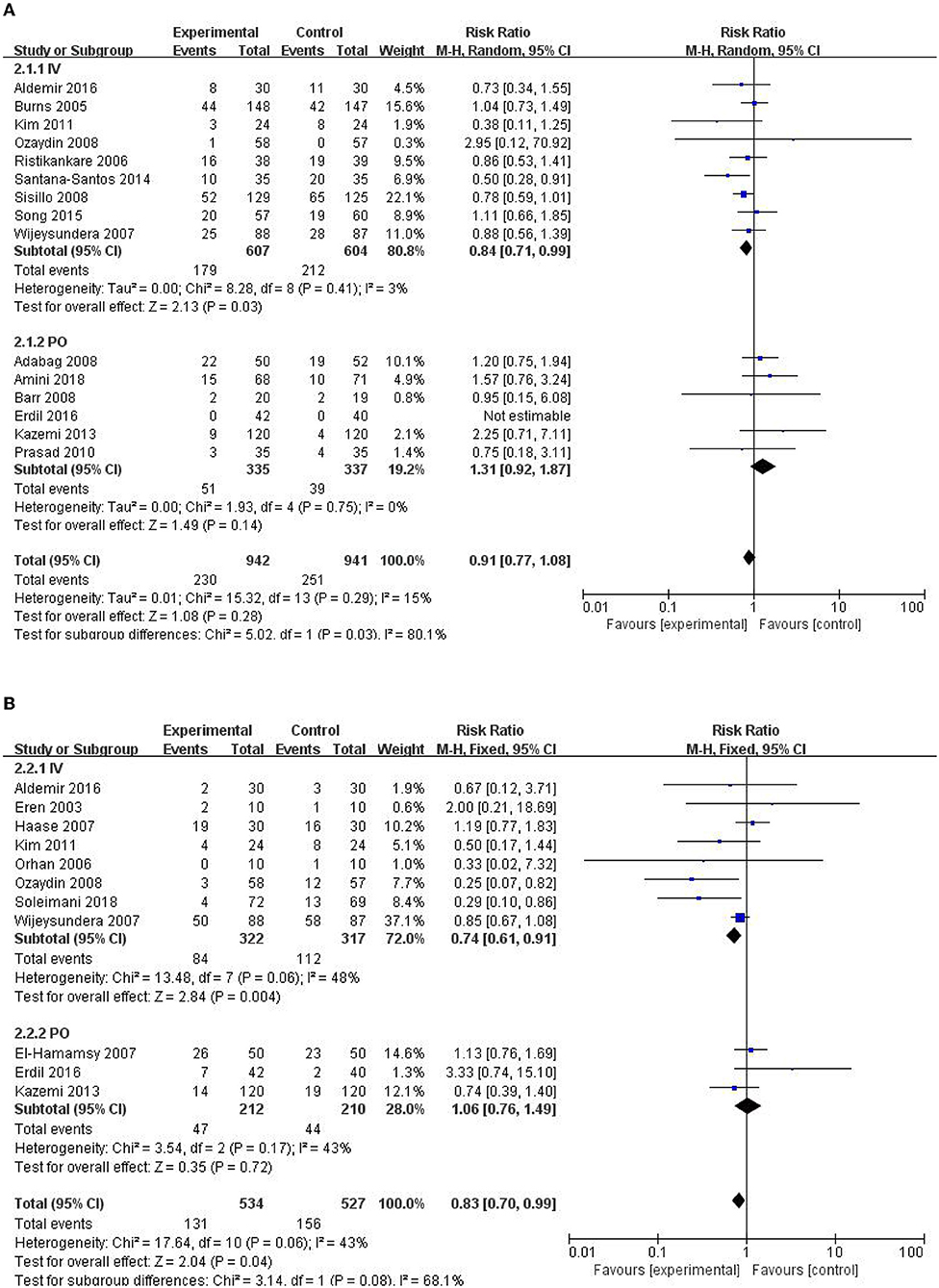
Figure 8. Forest plot of the effects of NAC administration methods on the incidence of AKI and arrhythmia. (A) Forest plot of the incidence of AKI. (B) Forest plot of the incidence of arrhythmia.
Discussion
Our meta-analysis showed some novel findings. The incidence of AKI in postoperative cardiac patients who received intravenous rather than oral NAC treatment was significantly lower than that in the control group. Intravenous injection of NAC can reduce the incidence of arrhythmia in patients after cardiac surgery while no difference was found in the incidence between intravenous injection and oral subgroups. Conversely, NAC has no significant effect on RRT, all-cause mortality, AMI, and cardiac insufficiency.
The intravenous NAC to patients with cardiac surgery is associated with a lower incidence of AKI, and the efficacy of oral NAC in preventing CI-AKI is inconclusive. Cardiac surgery-related acute renal injury (CSA-AKI) is the most common major complication of cardiac surgery (48). Although there is no consensus on the definition of post-cardiac surgery ARI, most of the fifteen studies stated that an increase in serum creatinine concentration > 25% from baseline is one of the conditions for the diagnosis of AKI. NAC has been shown to reduce the level of oxidative stress and reduce acute renal failure induced by ischemia-reperfusion. It can also improve renal aging and renal interstitial fibrosis through Sirtuin 1 activation and p53 deacetylation (49). There is no conclusive evidence supporting the benefit of the administration of NAC to prevent CSA-AKI in previous meta-analyses but our subgroup analysis reveals different results (12, 13). Of all the 15 studies that reported the incidence of AKI, 9 were administered intravenously with NAC, 6 were done orally. Results of a subgroup analysis according to the methods of administration show that the effect of NAC is related to the administration methods. Several possible mechanisms caused these differences such as dosage and duration. We listed the treatment protocol of NAC in each study in Table 1. The dosage of NAC by intravenous injection is often much larger than that by oral administration. For example, Aldemir gave patients 150 mg.kg−1 I.V. in 15 min after anesthesia induction, followed by 50 mg.kg−1.4h−1 and 100 mg.kg−1.16h−1. While the drug was administered at 600 mg PO twice daily for a total of 14 doses in Adabag's study. Furthermore, oral NAC was first absorbed through the digestive system, which may have a first-pass elimination effect, resulting in a decrease in blood concentration. It is worth mentioning that high-dose NAC is effective, but it has been also demonstrated to frequently cause adverse effects (50, 51). Before a large-scale application of NAC after cardiac surgery is recommended, further research is needed to explore the optimal dosage and method.
This meta-analysis shows that intravenous administration of NAC after cardiac surgery may reduce the incidence of arrhythmias. Baker and other studies found that the incidence of postoperative atrial fibrillation in the NAC group was 36% lower than that in the control group. But they did not explore the effect of drug use on the outcome (52). On the one hand, this confirms that NAC can significantly reduce the incidence of arrhythmias after cardiac surgery. On the other hand, there is a significant correlation between the occurrence of postoperative atrial fibrillation and the level of postoperative inflammation. NAC may reduce the occurrence of arrhythmias by inhibiting inflammatory storms.
In addition, we found that there was no significant difference in all-cause mortality, risk of AMI and cardiac insufficiency, rate of renal replacement therapy, and length of stay between the NAC group and the control group. This is consistent with previous studies (53). Given that we have included more RCT trials, the results may apply to a larger population.
Previous meta-analyses evaluating this topic have been published. However, there are several differences between the present study and the previous works. The present analysis includes the most RCT experiments representing the latest and most comprehensive study and we conducted a subgroup analysis of NAC for the first time based on the administration methods of NAC. Our meta-analysis also has some limitations. First, we only assessed the impact of NAC on the incidence of AKI but failed to assess the impact of the use of NAC on AKI to varying degrees because the vast majority of studies did not classify AKI or used different grading criteria. Second, a limitation is imposed on this meta-analysis because the relevant literatures included different degrees of differences such as the inclusion criteria of the study population and the dose and time of the use of NAC. These shortcomings may affect the results. Third, there is a lack of long-term follow-up results for patients, and a shortage of further subdivided MACEs, such as subgroup analysis of different types of arrhythmias.
Conclusion
To sum up, this meta-analysis suggests that intravenous administration of NAC can reduce the incidence of AKI and arrhythmia in patients after cardiac surgery, but cannot reduce all-cause mortality, AMI, cardiac insufficiency, and the number of patients using RRT. Oral NAC has no significant effect on the outcomes of patients after cardiac surgery.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. (2019) 234:16812–23. doi: 10.1002/jcp.28350
2. Myles PS. Meaningful outcome measures in cardiac surgery. J Extra Corpor Technol. (2014) 46:23–7.
3. Taggart DP and Altman DG. Off-pump vs. on-pump CABG: are we any closer to a resolution? Eur Heart J. (2012) 33:1181–3. doi: 10.1093/eurheartj/ehr374
4. O'Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. (2016) 20:187. doi: 10.1186/s13054-016-1352-z
5. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. (2009) 360:961–72. doi: 10.1056/NEJMoa0804626
6. Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. (2015) 10:500–14. doi: 10.2215/CJN.07830814
7. Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. (2013) 1830:4117–29. doi: 10.1016/j.bbagen.2013.04.016
8. Wang G, Bainbridge D, Martin J, Cheng D. N-acetylcysteine in cardiac surgery: do the benefits outweigh the risks? A meta-analytic reappraisal. J Cardiothorac Vasc Anesth. (2011) 25:268–75. doi: 10.1053/j.jvca.2010.04.022
9. Liu XH, Xu CY, Fan GH. Efficacy of N-acetylcysteine in preventing atrial fibrillation after cardiac surgery: a meta-analysis of published randomized controlled trials. BMC Cardiovasc Disord. (2014) 14:52. doi: 10.1186/1471-2261-14-52
10. He G, Li Q, Li W, Wang L, Yang J, Zeng F. N-Acetylcysteine for preventing of acute kidney injury in chronic kidney disease patients undergoing cardiac surgery: a Metaanalysis. Heart Surg Forum. (2018) 21:E513–E21. doi: 10.1532/hsf.2193
11. Pereira JEG, El Dib R, Braz LG, Escudero J, Hayes J, Johnston BC. N-acetylcysteine use among patients undergoing cardiac surgery: a systematic review and meta-analysis of randomized trials. PLoS ONE. (2019) 14:e0213862. doi: 10.1371/journal.pone.0213862
12. Adabag AS, Ishani A, Bloomfield HE, Ngo AK, Wilt TJ. Efficacy of N-acetylcysteine in preventing renal injury after heart surgery: a systematic review of randomized trials. Eur Heart J. (2009) 30:1910–7. doi: 10.1093/eurheartj/ehp053
13. Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-Acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg. (2018) 31:14–23. doi: 10.1080/08941939.2016.1269853
14. Naughton F, Wijeysundera D, Karkouti K, Tait G, Beattie WS. N-acetylcysteine to reduce renal failure after cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. (2008) 55:827–35. doi: 10.1007/BF03034054
15. Baker WL, Anglade MW, Baker EL, White CM, Kluger J, Coleman CI. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: a meta-analysis. Eur J Cardiothorac Surg. (2009) 35:521–7. doi: 10.1016/j.ejcts.2008.11.027
16. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
17. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
20. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. (2008) 61:763–9. doi: 10.1016/j.jclinepi.2007.10.007
21. Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. (2016) 20:144. doi: 10.1186/s13054-016-1299-0
22. Liu C, Lu G, Wang D, Lei Y, Mao Z, Hu P, et al. Balanced crystalloids versus normal saline for fluid resuscitation in critically ill patients: a systematic review and meta-analysis with trial sequential analysis. Am J Emerg Med. (2019) doi: 10.1016/j.ajem.2019.02.045
23. Kim JC, Hong SW, Shim JK, Yoo KJ, Chun DH, Kwak YL. Effect of N-acetylcysteine on pulmonary function in patients undergoing off-pump coronary artery bypass surgery. Acta Anaesthesiol Scand. (2011) 55:452–9. doi: 10.1111/j.1399-6576.2011.02407.x
24. Barr LF, Kolodner K. N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit Care Med. (2008) 36:1427–35. doi: 10.1097/CCM.0b013e31816f48ba
25. Kazemi B, Akbarzadeh F, Safaei N, Yaghoubi A, Shadvar K, Ghasemi K. Prophylactic high-dose oral-N-acetylcysteine does not prevent atrial fibrillation after heart surgery: a prospective double blind placebo-controlled randomized clinical trial. Pacing Clin Electrophysiol. (2013) 36:1211–9. doi: 10.1111/pace.12190
26. Karahan SC, Koramaz I, Altun G, Ucar U, Topbas M, Mentese A, et al. Ischemia-modified albumin reduction after coronary bypass surgery is associated with the cardioprotective efficacy of cold-blood cardioplegia enriched with N-acetylcysteine: a preliminary study. Eur Surg Res. (2010) 44:30–6. doi: 10.1159/000262324
27. Soleimani A, Habibi MR, Hasanzadeh Kiabi F, Alipour A, Habibi V, Azizi S, et al. The effect of intravenous N-acetylcysteine on prevention of atrial fibrillation after coronary artery bypass graft surgery: a double-blind, randomised, placebo-controlled trial. Kardiol Pol. (2018) 76:99–106. doi: 10.5603/KP.a2017.0183
28. Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta-Ylinen R, et al. Lack of renoprotective effect of i. v N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. (2006) 97:611–6. doi: 10.1093/bja/ael224
29. Amini S, Robabi HN, Tashnizi MA, Vakili V. Selenium, vitamin C and N-Acetylcysteine do not reduce the risk of acute kidney injury after off-pump CABG: a randomized clinical trial. Braz J Cardiovasc Surg. (2018) 33:129–34. doi: 10.21470/1678-9741-2017-0071
30. Prasad A, Banakal S, Muralidhar K. N-acetylcysteine does not prevent renal dysfunction after off-pump coronary artery bypass surgery. Eur J Anaesthesiol. (2010) 27:973–7. doi: 10.1097/EJA.0b013e3283383506
31. Aldemir M, Koca HB, Dogan Baki E, Carsanba G, Ozturk Kavrut N, Kavakli AS, et al. Effects of N-acetyl cysteine on renal functions evaluated by blood neutrophil gelatinase-associated lipocalin levels in geriatric patients undergoing coronary artery bypass grafting. Anatol J Cardiol. (2016) 16:504–11. doi: 10.5152/AnatolJCardiol.2015.6287
32. Orhan G, Yapici N, Yuksel M, Sargin M, Senay S, Yalcin AS, et al. Effects of N-acetylcysteine on myocardial ischemia-reperfusion injury in bypass surgery. Heart Vessels. (2006) 21:42–7. doi: 10.1007/s00380-005-0873-1
33. El-Hamamsy I, Stevens LM, Carrier M, Pellerin M, Bouchard D, Demers P, et al. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. (2007) 133:7–12. doi: 10.1016/j.jtcvs.2006.05.070
34. Prabhu A, Sujatha DI, Kanagarajan N, Vijayalakshmi MA, Ninan B. Effect of N-acetylcysteine in attenuating ischemic reperfusion injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg. (2009) 23:645–51. doi: 10.1016/j.avsg.2008.12.005
35. Fischer UM, Tossios P, Huebner A, Geissler HJ, Bloch W, Mehlhorn U. Myocardial apoptosis prevention by radical scavenging in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2004) 128:103–8. doi: 10.1016/j.jtcvs.2003.11.034
36. Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. (2008) 155:1143–9. doi: 10.1016/j.ahj.2008.01.013
37. Vento AE, Nemlander A, Aittomaki J, Salo J, Karhunen J, Ramo OJ. N-acetylcysteine as an additive to crystalloid cardioplegia increased oxidative stress capacity in CABG patients. Scand Cardiovasc J. (2003) 37:349–55. doi: 10.1080/14017430310015406
38. Sisillo E, Ceriani R, Bortone F, Juliano G, Salvi L, Veglia F, et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med. (2008) 36:81–6. doi: 10.1097/01.CCM.0000295305.22281.1D
39. Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, et al. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J. (2008) 29:625–31. doi: 10.1093/eurheartj/ehn011
40. Haase M, Haase-Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, et al. Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med. (2007) 35:1324–31. doi: 10.1097/01.CCM.0000261887.69976.12
41. Koramaz I, Pulathan Z, Usta S, Karahan SC, Alver A, Yaris E, et al. Cardioprotective effect of cold-blood cardioplegia enriched with N-acetylcysteine during coronary artery bypass grafting. Ann Thorac Surg. (2006) 81:613–8. doi: 10.1016/j.athoracsur.2005.08.013
42. Santana-Santos E, Gowdak LH, Gaiotto FA, Puig LB, Hajjar LA, Zeferino SP, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg. (2014) 97:1617–23. doi: 10.1016/j.athoracsur.2014.01.056
43. Song JW, Shim JK, Soh S, Jang J, Kwak YL. Double-blinded, randomized controlled trial of N-acetylcysteine for prevention of acute kidney injury in high risk patients undergoing off-pump coronary artery bypass. Nephrology. (2015) 20:96–102. doi: 10.1111/nep.12361
44. Eren N, Cakir O, Oruc A, Kaya Z, Erdinc L. Effects of N-acetylcysteine on pulmonary function in patients undergoing coronary artery bypass surgery with cardiopulmonary bypass. Perfusion. (2003) 18:345–50. doi: 10.1191/0267659103pf696oa
45. Erdil N, Eroglu T, Akca B, Disli OM, Yetkin O, Colak MC, et al. The effects of N-acetylcysteine on pulmonary functions in patients undergoing on-pump coronary artery surgery: a double blind placebo controlled study. Eur Rev Med Pharmacol Sci. (2016) 20:180–7.
46. Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, et al. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing cabg surgery: a randomized controlled trial. JAMA. (2005) 294:342–50. doi: 10.1001/jama.294.3.342
47. Wijeysundera DN, Beattie WS, Rao V, Granton JT, Chan CT. N-acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anaesth. (2007) 54:872–81. doi: 10.1007/BF03026790
48. Bove T, Monaco F, Covello RD, Zangrillo A. Acute renal failure and cardiac surgery. HSR Proc Intensive Care Cardiovasc Anesth. (2009) 1:13–21.
49. Livingston MJ, Wang J, Zhou J, Wu G, Ganley IG, Hill JA, et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy. (2019) 15:2142–62. doi: 10.1080/15548627.2019.1615822
50. Li M, Gao W, Ma J, Zhu Y, Li X. Early-stage lupus nephritis treated with N-acetylcysteine: A report of two cases. Exp Ther Med. (2015) 10:689–92. doi: 10.3892/etm.2015.2510
51. Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol. (2009) 47:81–8. doi: 10.1080/15563650802665587
52. Djordjević A, Šušak S, Velicki L, Antonič M. Acute kidney injury after open-heart surgery procedures. Acta Clin Croat. (2021) 60:120–6. doi: 10.20471/acc.2021.60.01.17
Keywords: N-acetylcysteine, acute kidney injury, major adverse cardiac events (MACEs), cardiac surgery, trial sequential analysis
Citation: Zhao J, Li M and Tan C (2022) Efficacy of N-acetylcysteine in Preventing Acute Kidney Injury and Major Adverse Cardiac Events After Cardiac Surgery: A Meta-Analysis and Trial Sequential Analysis. Front. Med. 9:795839. doi: 10.3389/fmed.2022.795839
Received: 15 October 2021; Accepted: 12 May 2022;
Published: 22 June 2022.
Edited by:
Michele Provenzano, Università di Bologna, ItalyReviewed by:
Feilong Wang, Shanghai East Hospital, ChinaYu-Chang Yeh, National Taiwan University Hospital, Taiwan
Copyright © 2022 Zhao, Li and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Tan, aGFwcHl0YW5jaGVuQDEyNi5jb20=
 Jingtao Zhao
Jingtao Zhao Maowei Li
Maowei Li Chen Tan
Chen Tan