- 1Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Beijing, China
- 3Zhanjiang Key Laboratory of Prevention and Management of Chronic Kidney Disease, Guangdong Medical University, Zhanjiang, China
Objective: To evaluate the effects of vitamin E, pioglitazone, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists in patients with non-alcoholic fatty liver disease (NAFLD).
Design: A network meta-analysis.
Data Sources: PubMed, Embase, Cochrane Library, and Web of Science databases from their inception until September 1, 2021.
Eligibility Criteria for Selecting Studies: Randomized controlled trials (RCTs) comparing the effects of four different drugs in patients with NAFLD were included. All superiority, non-inferiority, phase II and III, non-blinded, single-blinded, and double-blinded trials were included. Interventions of interest included vitamin E (α-tocopherol and δ-tocotrienol), pioglitazone, three kinds of GLP-1 receptor agonists (liraglutide, semaglutide, and dulaglutide), four SGLT2 inhibitors (dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin), and comparisons of these different drugs, and placebos.
Main Outcome Measures: The outcome measures included changes in non-invasive tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), controlled attenuation parameter (CAP), enhanced liver fibrosis (ELF) score, liver fat content (LFC), and keratin-18 (K-18)] and invasive tests [fibrosis score and resolution of non-alcoholic steatohepatitis (NASH)].
Results: Twenty-seven trials including 3,416 patients were eligible for inclusion in the study. Results refer to vitamin E, pioglitazone, GLP-1 receptor agonists, and SGLT2 inhibitors. First, placebos were used as a reference. δ-Tocotrienol was superior to placebo in decreasing the GGT level. Semaglutide, ipragliflozin, and pioglitazone induced a significantly higher decrease in the ALT level than a placebo. Semaglutide, pioglitazone, and dapagliflozin were superior to placebo in decreasing the AST level. Tofogliflozin and pioglitazone induced a significantly higher decrease in the K-18 level than a placebo. Liraglutide was superior to placebo in decreasing CAP. Liraglutide, pioglitazone, and vitamin E induced a significantly higher increase in resolution of NASH than a placebo. As for pairwise comparisons, semaglutide and pioglitazone were superior to liraglutide in decreasing the ALT level. Semaglutide induced a significantly higher decrease in the ALT level than dulaglutide. Semaglutide was obviously superior to empagliflozin, liraglutide, dulaglutide, and tofogliflozin in decreasing the AST level. Pioglitazone induced a significantly higher decrease in the GGT level than ipragliflozin. δ-Tocotrienol was superior to liraglutide in decreasing the GGT level. Tofogliflozin and pioglitazone induced a significantly higher decrease in the K-18 level than dulaglutide. Pioglitazone was superior to vitamin E in increasing the resolution of NASH. Furthermore, liraglutide treatment had the highest SUCRA ranking in decreasing CAP and ELF scores and increasing the resolution of NASH. Pioglitazone treatment had the highest SUCRA ranking in decreasing LFC and fibrosis scores. Tofogliflozin treatment had the highest SUCRA ranking in decreasing K-18, while dapagliflozin treatment had the highest SUCRA ranking in decreasing the GGT level. Semaglutide treatment had the highest SUCRA ranking in decreasing the levels of ALT and AST.
Conclusion: The network meta-analysis provided evidence for the efficacy of vitamin E, pioglitazone, SGLT2 inhibitors, and GLP-1 receptor agonists in treating patients with NAFLD. To find the best guide-level drugs, it is necessary to include more RCTs with these off-label drugs, so that patients and clinicians can make optimal decisions together.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier: CRD42021283129.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of steatosis in more than 5% of hepatocytes, in association with metabolic risk factors (in particular, obesity and type 2 diabetes) and in the absence of excessive alcohol consumption (≥30 g/day for men and ≥20 g/day for women) or other chronic liver diseases (1). According to epidemiological reports, NAFLD occurs in 47.3–63.7% of individuals with type 2 diabetes. As an established risk factor of NAFLD, diabetes contributes to the progression of non-alcoholic steatohepatitis (NASH) and the occurrence of hepatocellular carcinoma (HCC) (2–4). In patients with diabetes, NAFLD can accelerate the progression to cirrhosis, end-stage liver disease, and HCC (5). Therefore, it is extremely important to treat NAFLD with or without diabetes in a timely manner. At present, there are various unapproved drugs for NASH. However, it is still unclear whether these drugs can optimize the existing therapies [weight loss and exercise (6, 7)] to achieve improvement in two liver histological endpoints (NASH resolution without worsening of fibrosis; or an improvement in fibrosis of one stage or more without worsening of NASH) (8). Glucagon-like peptide-1 (GLP-1) receptor agonists and SGLT2 inhibitors are two kinds of common off-label hypoglycemic drugs. It has been shown that GLP-1 receptor agonists can control energy intake and body weight by lowering blood glucose or by prolonging gastric emptying and inducing satiety to reduce liver damage caused by any metabolic factor (9). SGLT2 inhibitors could reduce inflammatory markers, increase the oxidation of free fatty acids, and improve insulin resistance; they could also increase ketone body metabolism and improve fatty liver injury by upregulating ketogenic enzymes and transporters in the liver (10). In addition, vitamin E and pioglitazone have been endorsed by the current guidelines (Asia-Pacific Working Party on NAFLD) but not yet approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) guidelines in treating NAFLD linked with diabetes mellitus (recently renamed MAFLD) (11). Pioglitazone is a peroxisome proliferator-activated receptor γ (PPARγ) agonist, which can protect the liver by improving insulin resistance in patients with NAFLD and with type 2 diabetes mellitus. Although the mechanism is unclear, it has been shown that pioglitazone could improve biochemical and histological parameters (without diabetes) (12). In addition, one study had shown that the antioxidant effect of vitamin E (α-tocopherol) could significantly improve the disease progression of patients with NASH (13). GLP-1 receptor agonists and SGLT2 inhibitors are two kinds of hypoglycemic agents that are currently undergoing phase II and III trials. It has been shown that these drugs can protect the heart and kidneys of patients with NAFLD and with type 2 diabetes and induce weight loss (14–16). Several trials in patients with NAFLD have shown that pioglitazone, a drug from the class of thiazolidinediones, improve NASH activity and fibrosis (17). In addition, vitamin E has been found to be useful for patients with NAFLD and without type 2 diabetes (18).
The detection methods for NAFLD can be divided into non-invasive and invasive methods. The non-invasive methods lack specificity and sensitivity, and poorly correlate with histological severity [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT)], whereas the invasive method of biopsy is the golden standard for NAFLD diagnosis, but it has a high operating threshold and is prone to sampling errors. The majority of several previous network meta-analyses (7, 19, 20) have analyzed liver enzyme concentrations and a wide range of scores rather than invasive procedures such as liver biopsy. Here, we conducted a network meta-analysis of randomized controlled trials (RCTs) with the aim to directly and indirectly compare the effects of vitamin E, pioglitazone, GLP-1 receptor agonists, SGLT2 inhibitors, and a placebo for patients with NAFLD, thereby providing new potential clinical drugs for NAFLD. In addition to reporting the routine indicators as in the previous NMAs, we also added several non-invasive NAFLD detection methods closely related to the progression of liver histology, such as controlled attenuation parameter (CAP) and enhanced liver fibrosis (ELF) score. CAP (11) could be used to evaluate liver steatosis, while ELF score has been recognized by the United Kingdom's National Institute for Health and Clinical Excellence (NICE) and other associations in the UK for the evaluation and monitoring of patients suspected of NAFLD among metabolic risk factors (21).
Methods
Data Sources and Literature Search
We performed an extensive search of the PubMed, Embase, Cochrane Library, and Web of Science databases from their inception until September 1, 2021, for RCTs investigating different agents for treating patients with NAFLD. Additional studies were searched in the reference lists of all identified publications, including relevant meta-analyses. Search strategies are shown in Supplementary Material.
Study Selection
First, two reviewers (Luo and Cai) independently screened records according to the title/abstract and then screened the full text of the relevant records against the predefined selection criteria. Namely, eligible papers were RCTs that compared the effects of different treatments of patients with NAFLD. All superiority, non-inferiority, phase II and III, non-blinded, single-blinded, and double-blinded trials were included. Interventions of interest included vitamin E (α-tocopherol and δ-tocotrienol), pioglitazone, three kinds of GLP-1 receptor agonists (liraglutide, semaglutide, and dulaglutide), four types of SGLT2 inhibitors (dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin), and comparisons of these different drugs, and placebos. The outcome measures included changes in non-invasive tests [ALT, AST, GGT, CAP, liver fat content (LFC) on MRI–PDFF or H-MRS, ELF score, keratin-18 (K-18) M30 fragment] and invasive tests (fibrosis score and resolution of NASH). At the same time, we excluded clinical studies with the following features: (1) the presence of other conditions that may not be associated with NAFLD as identified by history, physical examination, and laboratory investigations; (2) the examination of a single drug for treating patients with NAFLD (e.g., comparison of different doses or frequencies of the same drug); and (3) the data of outcome indicators being unavailable, even after contacting the authors.
Data Extraction and Risk-of-Bias Assessment
Two reviewers (Luo and Wei) independently extracted data from the original trial reports using a standardized form (including author, sample size, sex, age, and outcome index), and then double-checked the extracted data. We assessed the risk of bias of the individual studies using the Cochrane Risk of Bias Tool (21), which is based on the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Items were scored as low, high, or unclear risk of bias. Two investigators (Luo and Wei) independently conducted study selection and data extraction. Two investigators (Luo and Cai) independently assessed the risk of bias of the individual studies. Any discrepancies were resolved by consensus and arbitration by a panel of adjudicators (Luo, Cai, Zheng, Zhao, and Guo) (18, 19).
Data Analyses
The statistical method for network meta-analysis was based on a frequency framework. All outcome measures were analyzed using a random-effects model for data analysis, except for two indicators (K-18 and CAP, for which we used the fixed-effects model). If the evaluated indicators were continuous variables, standardized mean difference (SMD) or mean difference (MD) was used for the determination of effect size; if they were binary variables, odds ratio (OR) was used as the effect size, and the corresponding 95% CI was calculated. Multiple independent groups in a study (e.g., different semaglutide doses) were considered separate datasets. More than one record of treatment duration in one study (23) was divided into independent datasets. Stata16.0 software (StataCorp, Texas, USA) was used to select the frequentist framework random-effects model for network meta-analysis; the ranking group command was used for data preprocessing; the network relationship diagram was drawn for the comparison between the interventions of each outcome indicator; the efficacy ranking was performed; and the cumulative probability ranking diagram was drawn to obtain the area under the curve (SUCRA). In the reticulation diagram, dot size represents the number of patients with relevant interventions, and the thickness of the line between points represents the number of included studies (22). SUCRA was expressed as a percentage. When SUCRA was 100%, it indicated that the intervention was absolutely effective, and when it was 0, it indicated that the intervention was absolutely ineffective. The inconsistency test was mainly used to assess the degree of consistency between the direct comparison results and the indirect comparison results. Finally, the presence of small sample effects in the network was identified by drawing a funnel plot (publication bias was only examined if ≥10 study comparisons were included in the analysis). Review Manager 5.4 software (Nordic Cochrane Centre) was used for literature quality evaluation in this study.
Results
Study Characteristics
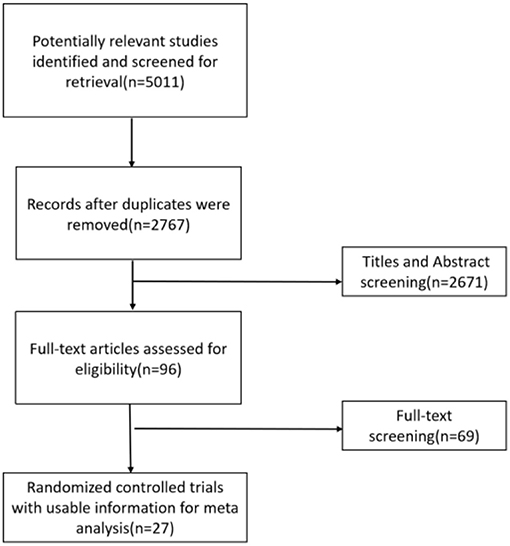
A total of 27 eligible studies, published between 1989 and 2021, corresponding to 3,416 adults, were selected for pooled analyses (7, 17–20, 23–44). Five (23, 29, 30, 32, 37) of them were ≥3-arm trials, and the remaining 22 were double-arm trials. The baseline characteristics of the included RCTs are provided in Supplementary Table 1. The literature search process is shown in Figure 1.
Quality of the Included Studies
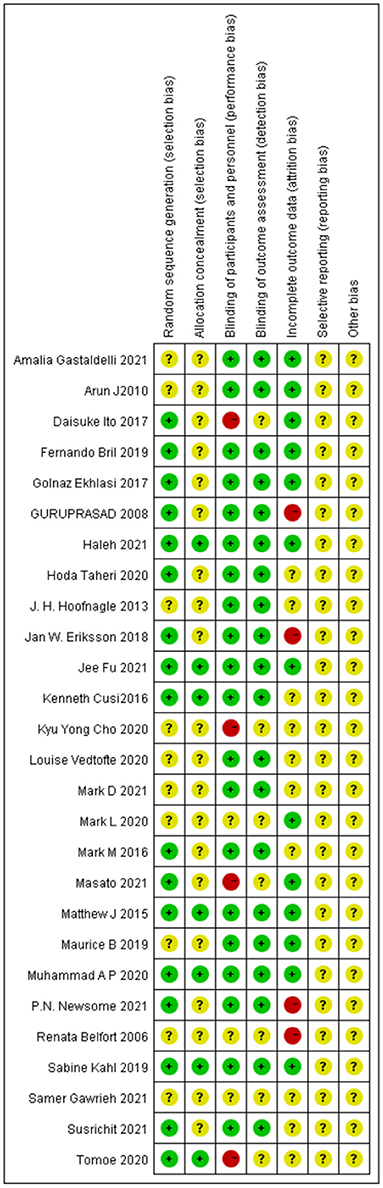
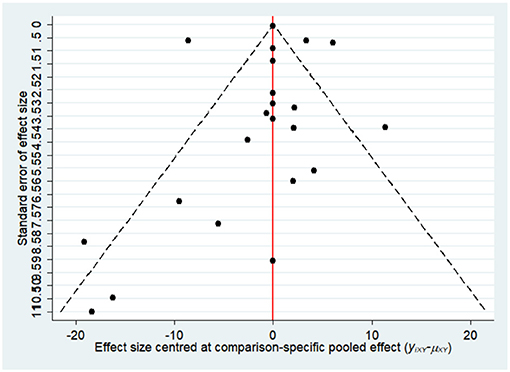
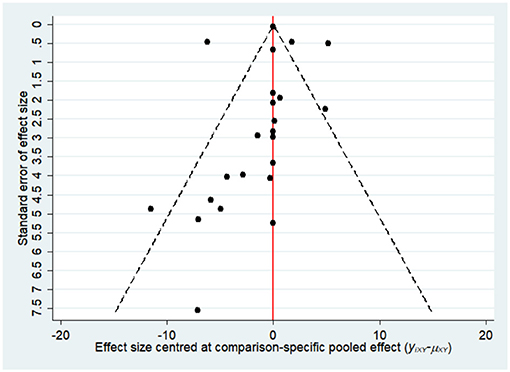
Most of the studies were judged to be at low or unclear risk of bias for the six domains according to the Cochrane Collaboration's tool. Four studies (28, 33, 36, 44) were judged to be at a high risk of bias for blinding participants and personnel due to performing single-blinding or open-label trials. Four studies (17, 26, 30, 31) were judged to be at a high risk of bias for incomplete outcome data due to the high rate of dropout. The risk-of-bias assessment of the trials included in this study is presented in Figure 2. In addition, the funnel plot results suggested there may be some publication bias in ALT, AST, and GGT, as shown in Figures 3–5.
Network Meta-Analysis Results
We assessed the efficacy of four different off-label agents, including three different GLP-1 receptor agonists, four kinds of SGLT2 inhibitors, vitamin E, and pioglitazone for patients with NAFLD. Figures 6A–14A show network graphs of all the drugs included in the study. The width of the lines is proportional to the number of trials comparing each pair of treatments. The sizes of the circles are proportional to the number of patients.
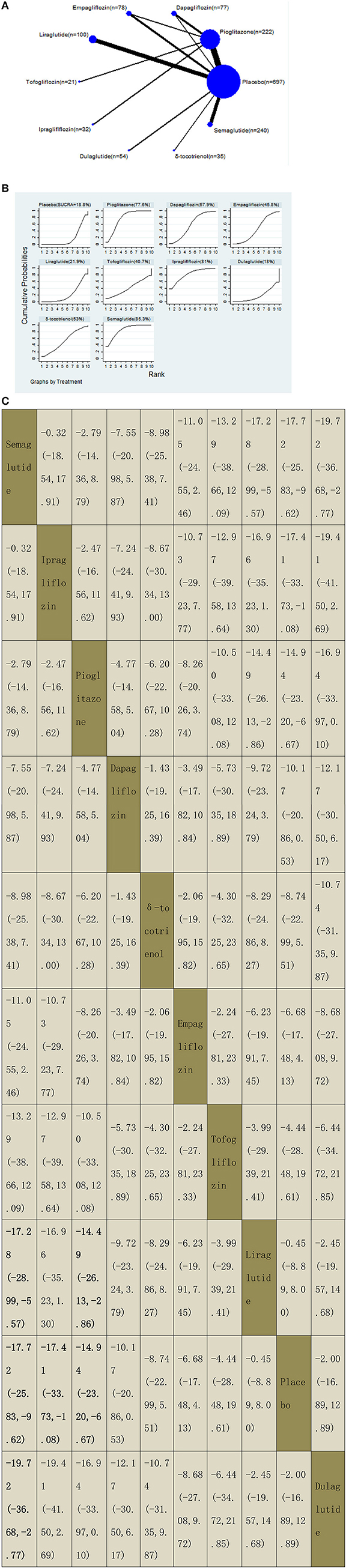
Figure 6. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for alanine aminotransferase (ALT).
Non-invasive Tests
Alanine Aminotransferase
Our NMA included 20 RCTs reporting the administration of vitamin E, pioglitazone, three GLP-1 receptor agonists, and four SGLT2 inhibitors among 1,506 patients. A placebo was used as a reference. We found that three drugs significantly decreased the ALT levels, with MDs of −17.72 (95% CI: −25.83, −9.62) for semaglutide, −17.41 (−33.73, −1.08) for ipragliflozin, and −14.94 (−23.20, −6.67) for pioglitazone. Compared with liraglutide, semaglutide and pioglitazone significantly decreased the ALT levels, with MDs of −17.28 (−28.99, −5.57) for semaglutide and −14.49 (−26.13, −2.86) for pioglitazone. In addition, semaglutide decreased the ALT levels compared with dulaglutide, with MDs of −19.72 (−36.68, −2.77). The results of pairwise comparisons are indicated by the MDs and 95% CI (Figure 6B). According to the results of SUCRA, semaglutide may be the most effective intervention to reduce the level of ALT in patients with NAFLD, and the results of the SUCRA probability ranking are shown in Figure 6C.
Aspartate Aminotransferase
Our NMA included 21 RCTs reporting the administration of vitamin E, pioglitazone, three GLP-1 receptor agonists, and four SGLT2 inhibitors among 1,597 patients. We found that semaglutide could significantly decrease the levels of AST than five drugs, with MDs of −10.06 (95% CI: −18.50, −1.62) than empagliflozin, −10.51 (−18.41, −2.61) than liraglutide, −15.58 (−27.24, −3.91) than dulaglutide, −14.88 (−19.95, −9.80) than placebo, and −19.02 (−34.23, −3.81) than tofogliflozin. Compared with a placebo, three included agents significantly decreased the levels of AST, with MDs of −14.88 (−19.95, −9.80) for semaglutide, −7.96 (−12.78, −3.13) for pioglitazone, and −7.49 (−14.63, −0.34) for dapagliflozin. The results of the pairwise comparison are indicated by the MDs and 95% CI (Figure 7B). According to the SUCRA results, semaglutide may be the most effective intervention to reduce the level of AST in patients with NAFLD, and the results of the SUCRA probability ranking are shown in Figure 7C.
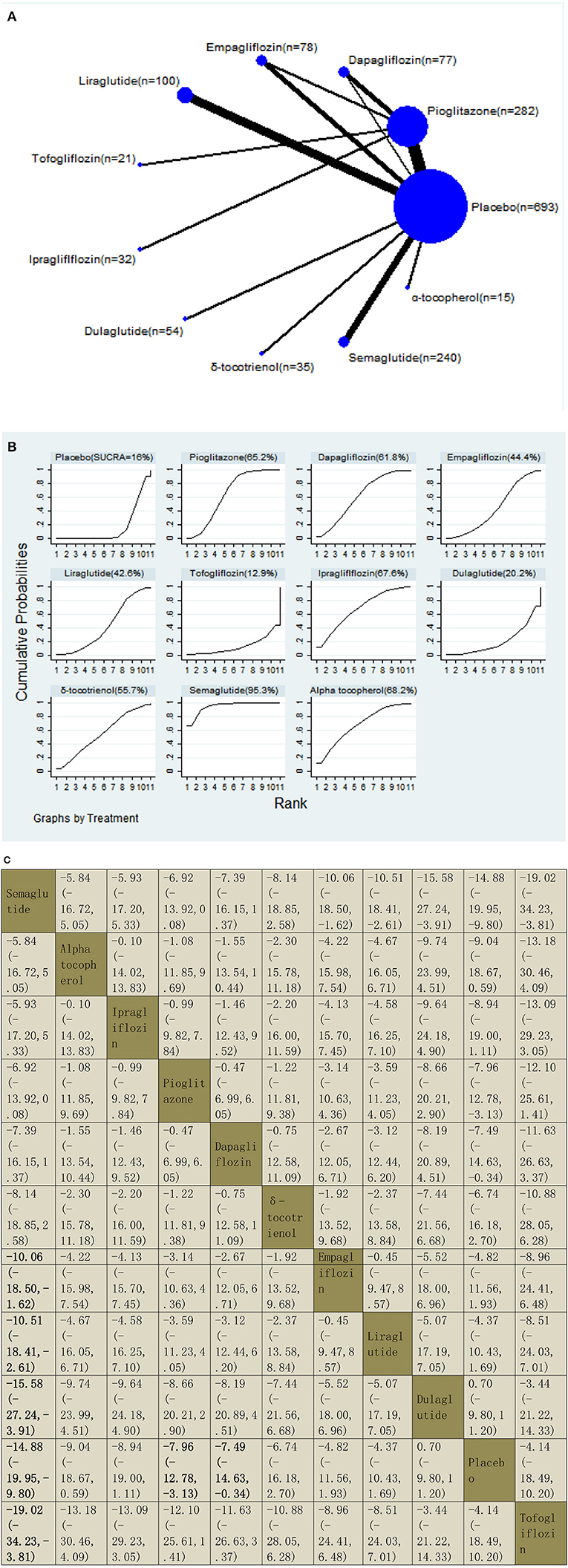
Figure 7. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for aspartate aminotransferase (AST).
Gamma-Glutamyl Transferase
Our NMA included 10 RCTs reporting the administration of vitamin E, pioglitazone, one GLP-1 receptor agonist, and three SGLT2 inhibitors among 579 patients. Tofogliflozin was used as a reference. We found that three drugs could significantly decrease the levels of GGT, with MDs of −30.28 (95% CI: −50.99, −9.58) for dapagliflozin, −29.40 (−49.11, −9.70) for pioglitazone, and −24.30 (−44.19, −4.41) for ipragliflozin. δ-Tocotrienol decreased the levels of GGT significantly compared with placebo and liraglutide, with MDs of −5.33 (−7.47, −3.19) compared with placebo and −5.61 (−8.50, −2.72) compared with liraglutide. We also found that pioglitazone decreased the levels of GGT significantly compared with ipragliflozin, with MDs of −5.10 (−7.80, −2.40). The results of the pairwise comparison are indicated by the MDs and 95% CI (Figure 8B). According to the SUCRA results, dapagliflozin may be the most effective intervention to reduce the level of GGT in patients with NAFLD, and the results of the SUCRA probability ranking are shown in Figure 8C.
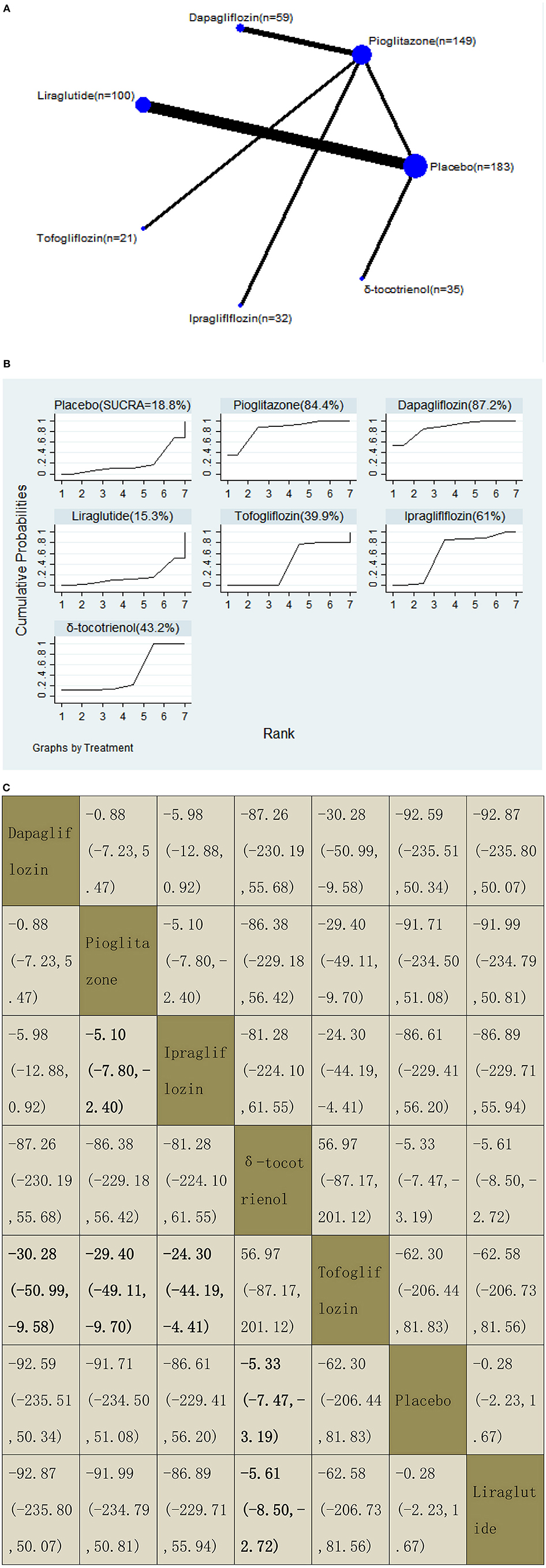
Figure 8. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for gamma-glutamyl transferase (GGT).
Controlled Attenuation Parameter
With regard to CAP decrease, our NMA included three RCTs, reporting the administration of pioglitazone, one GLP-1 receptor agonist, and one SGLT2 inhibitor among 278 patients. Liraglutide decreased the CAP significantly compared with a placebo, with MDs of −30.30 (95% CI: −52.17, −8.43). The results of the pairwise comparison are indicated by the MDs and 95% CI (Figure 9B). According to the SUCRA results, liraglutide may be the most effective intervention to reduce the CAP of patients with NAFLD, and the results of the SUCRA probability ranking are shown in Figure 9C.
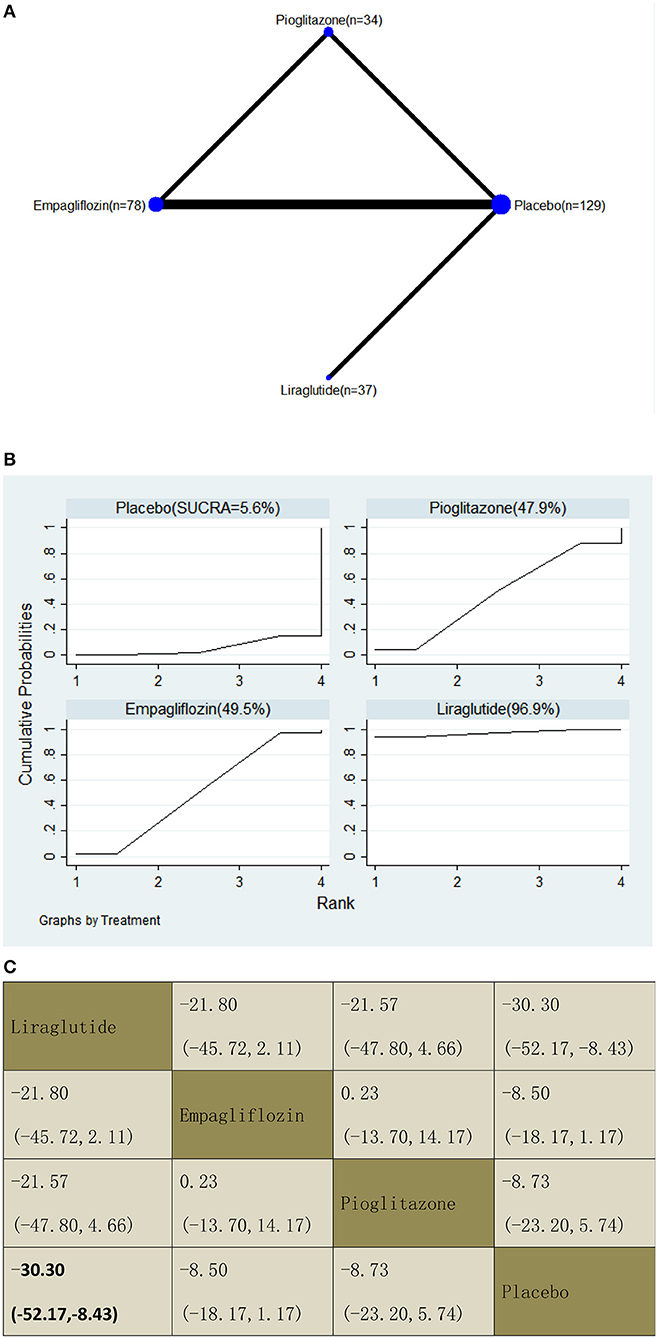
Figure 9. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for controlled attenuation parameter (CAP).
Liver Fat Content on MRI–PDFF or H-MRS
With regard to LFC decrease, our NMA included seven RCTs, reporting the administration of pioglitazone, one GLP-1 receptor agonist, and three SGLT2 inhibitors among 366 patients. There was no statistically significant difference between the five drugs and a placebo, i.e., for pioglitazone −21.24 (95% CI: −50.86, 8.38), tofogliflozin −17.67 (−74.47, 39.13), empagliflozin −1.27 (−49.95, 47.41), dapagliflozin −1.64 (−50.24, 46.96), and liraglutide −0.30 (−49.59, 48.99). The results of the pairwise comparison are indicated by the MDs and 95% CI (Figure 10B). According to the SUCRA results, pioglitazone may be the most effective intervention to reduce the LFC of patients, and the results of the SUCRA probability ranking are shown in Figure 10C.
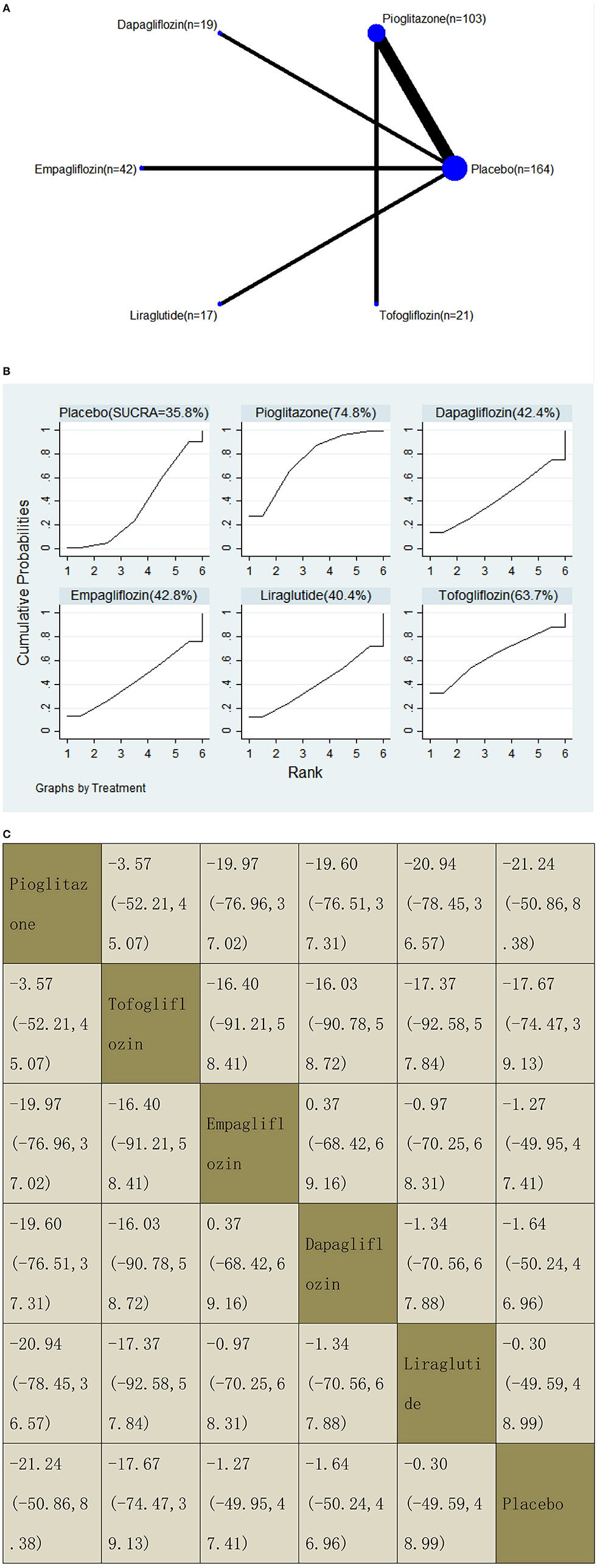
Figure 10. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for liver fat content (LFC).
Enhanced Liver Fibrosis Score
With regard to a decrease in the ELF score, our NMA included two RCTs, reporting the administration of vitamin E, pioglitazone, and one GLP-1 receptor agonist among 696 patients. There was no statistically significant difference between the three drugs and placebo, i.e., for liraglutide −0.40 (95% CI: −0.90, 0.10), vitamin E −0.08 (−0.24, 0.07), and pioglitazone −0.05 (−0.21, 0.11). The results of the pairwise comparison are indicated by the MDs and 95% CI (Figure 11B). According to the results of SUCRA, liraglutide may be the most effective intervention to reduce the ELF score of patients, and the results of the SUCRA probability ranking are shown in Figure 11C.
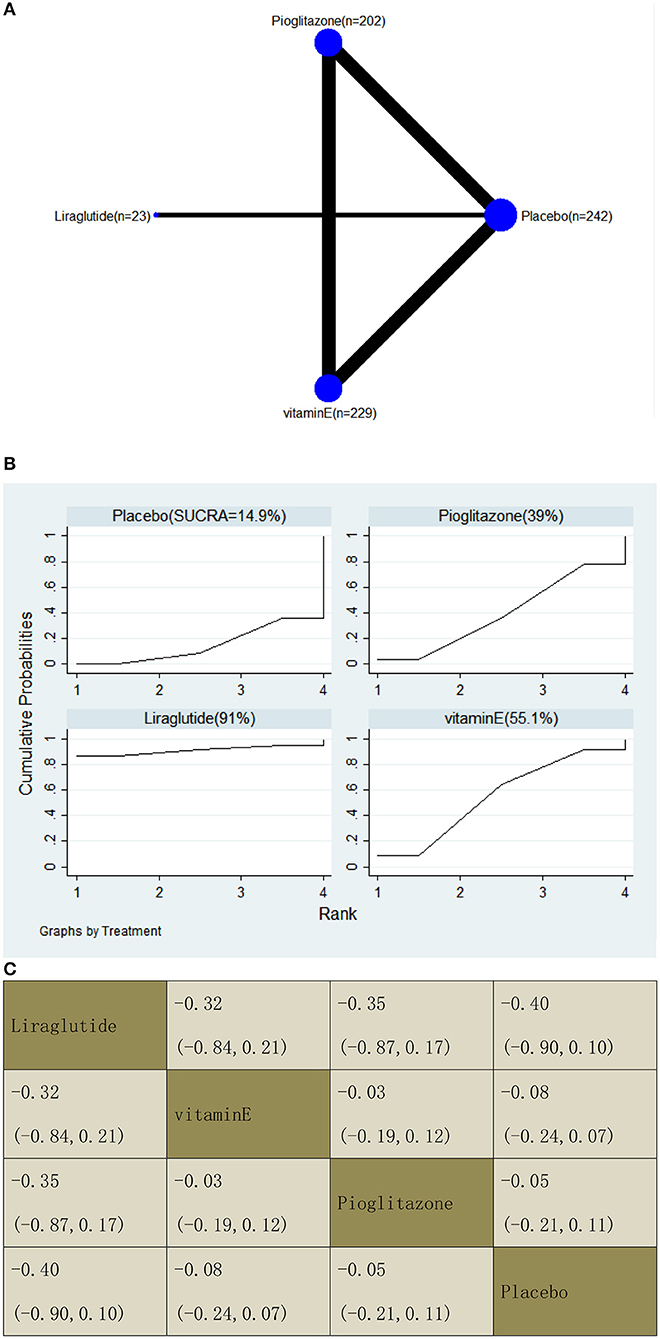
Figure 11. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for enhanced liver fibrosis (ELF) score.
Keratin-18 (K-18) M30 Fragment
With regard to a decrease in K-18, our NMA included four RCTs, reporting the administration of pioglitazone, two GLP-1 receptor agonists, and one SGLT2 inhibitor among 289 patients. Compared with dulaglutide, two included agents significantly decreased the K-18 levels, with MDs of −282.30 (95% CI: −549.35, −15.26) for tofogliflozin and −141.42 (−246.65, −36.18) for pioglitazone. Also, compared with placebo, two included agents significantly decreased the K-18 levels, with MDs of −326.60 (−574.06, −79.15) for tofogliflozin and −185.72 (−217.29, −154.14) for pioglitazone. The results of the pairwise comparison are indicated by the MDs and 95% CI (Figure 12B). According to the results of SUCRA, tofogliflozin may be the most effective intervention to reduce the K-18 of patients, and the results of the SUCRA probability ranking are shown in Figure 12C.
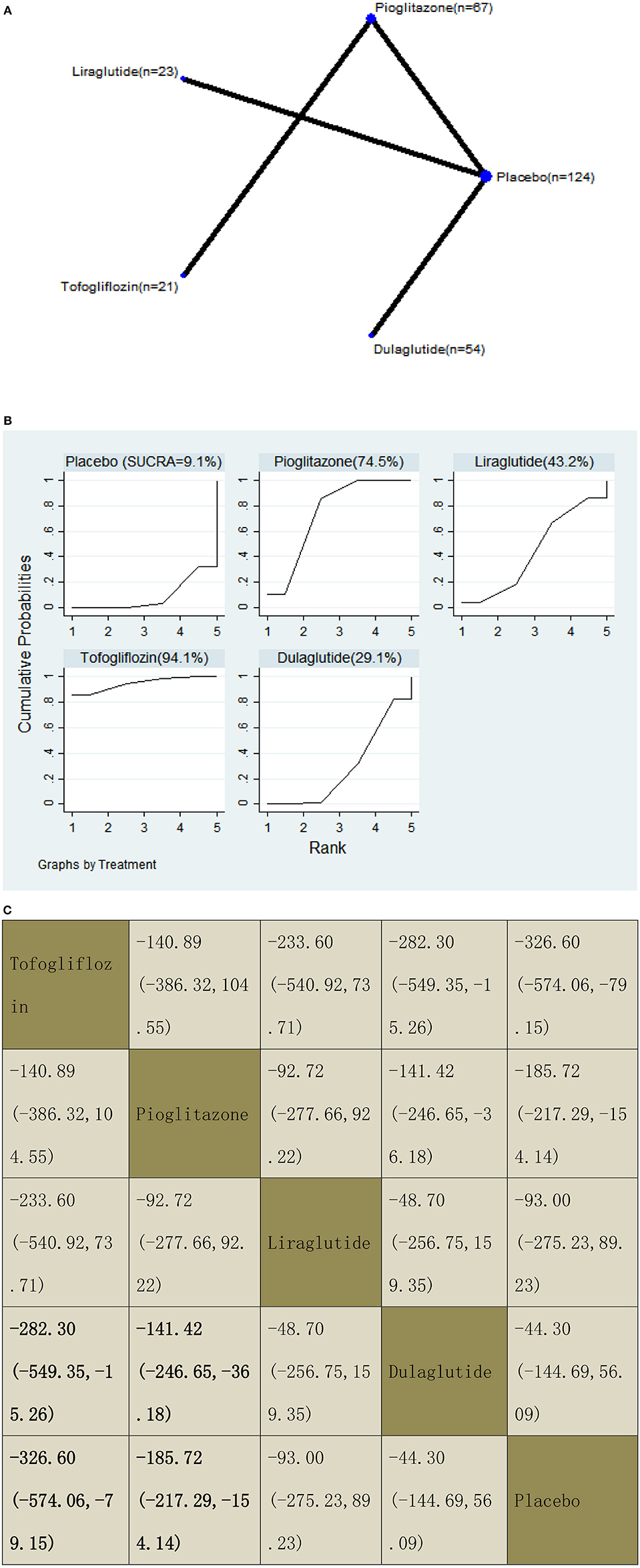
Figure 12. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for keratin-18 (K-18).
Invasive Tests
Fibrosis Score
With regard to a decrease in the fibrosis score, our NMA included six RCTs, reporting the administration of vitamin E, pioglitazone, and one SGLT2 inhibitor among 407 patients. There was no statistically significant association between the three drugs and a placebo, i.e., for pioglitazone −0.91 (95% CI: −1.82, 0.01), vitamin E −0.28 (−2.07, 1.51), and empagliflozin 0.02 (−1.76, 1.79). The results of the pairwise comparison are indicated by the SMDs and 95% CI (Figure 13B). According to the SUCRA results, pioglitazone may be the most effective intervention to reduce the fibrosis score of patients, and the results of the SUCRA probability ranking are shown in Figure 13C.
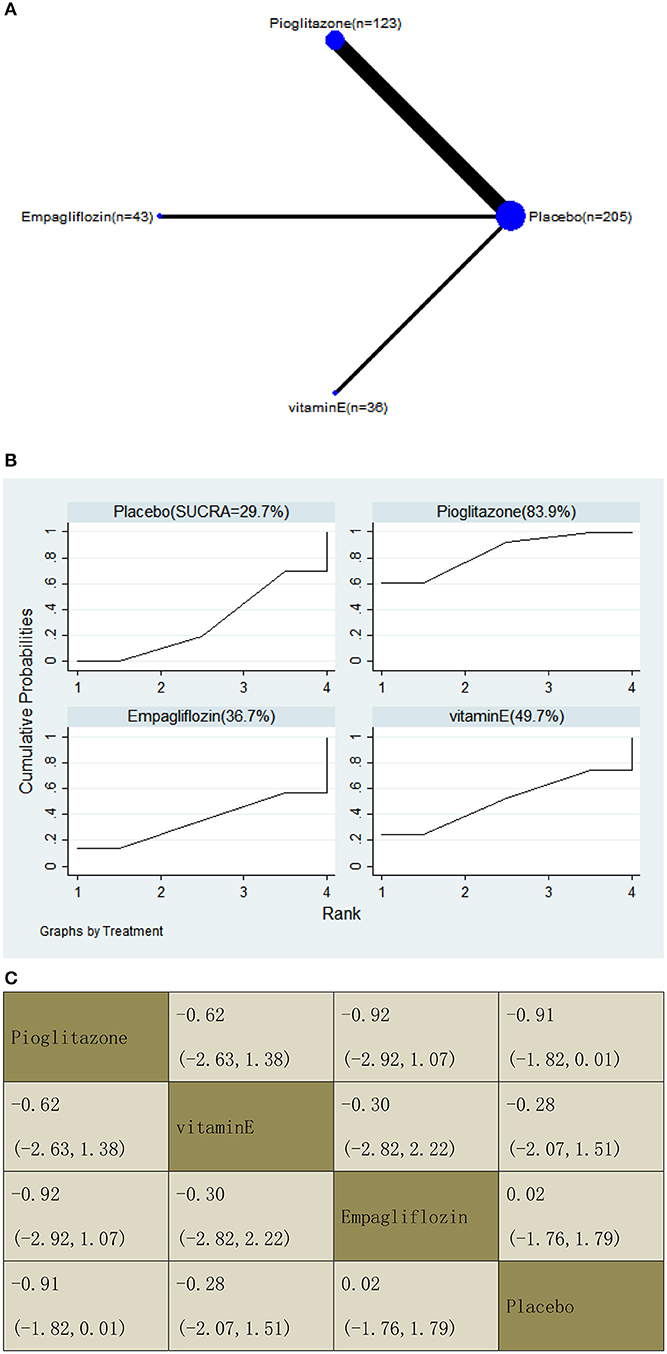
Figure 13. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for fibrosis score.
Resolution of NASH
With regard to an increase in the resolution of NASH, our NMA included seven RCTs, reporting the administration of vitamin E, pioglitazone, and one GLP-1 receptor agonist among 891 patients. Compared with a placebo, three included agents significantly increased the resolution, with OR of 6.43 (95% CI: 1.20, 34.41) for liraglutide, 3.55 (2.40, 5.28) for pioglitazone, and 2.27 (1.54, 3.35) for vitamin E. In addition, we found that pioglitazone increased the resolution compared with vitamin E, with an OR of 1.56 (1.03, 2.37). The results of the pairwise comparison are indicated by the OR and 95% CI (Figure 14B). According to the SUCRA results, liraglutide may be the most effective intervention to increase the NASH resolution of patients, and the results of the SUCRA probability ranking are shown in Figure 14C.
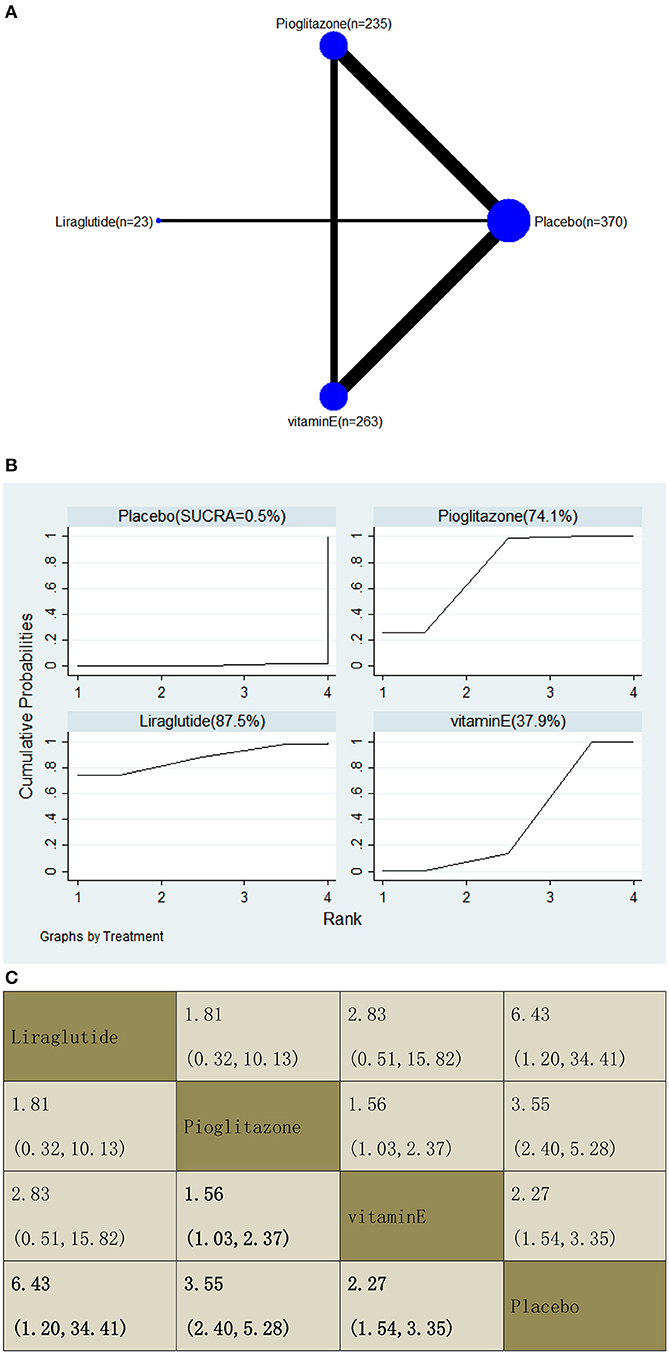
Figure 14. Network graphs (A), pairwise comparisons (B), and SUCRA probability ranking (C) for resolution of non-alcoholic steatohepatitis (NASH).
Heterogeneity and Inconsistency Assessment
We tested the inconsistency of all comparisons. The results are presented in Supplementary Table 2. A p > 0.05 indicated no inconsistency among the direct and indirect comparisons, whereas a p < 0.05 indicated high inconsistency among the direct and indirect comparisons. The results showed that the direct and indirect inconsistencies of the included drugs were not high, except for one group in the analysis of ALT level (placebo vs. empagliflozin).
Discussion
This study is a network meta-analysis designed to specifically evaluate the efficacy of vitamin E, pioglitazone, GLP-1 receptor agonists, and SGLT2 inhibitors for treating patients with NAFLD. The direct and indirect comparison results showed the evidence from recent RCTs. First, a placebo was used as a reference. δ-Tocotrienol was superior to placebo in decreasing the GGT level. Semaglutide, ipragliflozin, and pioglitazone induced a significantly higher decrease in the ALT level than a placebo. Semaglutide, pioglitazone, and dapagliflozin were superior to placebo in decreasing the AST level. Tofogliflozin and pioglitazone induced a significantly higher decrease in the K-18 level than a placebo. Liraglutide was superior to placebo in decreasing the CAP. Liraglutide, pioglitazone, and vitamin E induced a significantly higher increase in resolution of NASH compared with a placebo. As for pairwise comparisons, semaglutide and pioglitazone were superior to liraglutide in decreasing the ALT level. Semaglutide induced a significantly higher decrease in the ALT level than dulaglutide. Semaglutide was superior to empagliflozin, liraglutide, dulaglutide, and tofogliflozin in decreasing the AST level. Pioglitazone induced a significantly higher decrease in GGT level than ipragliflozin. δ-Tocotrienol was superior to liraglutide in decreasing the GGT level. Tofogliflozin and pioglitazone induced a significantly higher decrease in the K-18 level than dulaglutide. Pioglitazone was obviously superior to vitamin E in increasing the resolution of NASH. Furthermore, liraglutide treatment had the highest SUCRA ranking in decreasing the CAP and ELF score, and in increasing the resolution of NASH. Pioglitazone treatment had the highest SUCRA ranking in decreasing the LFC and fibrosis score. Tofogliflozin treatment had the highest SUCRA ranking in decreasing the K-18. Dapagliflozin treatment had the highest SUCRA ranking in decreasing the GGT level. Semaglutide treatment had the highest SUCRA ranking in decreasing the levels of ALT and AST. These findings indicate that more studies should be carried out to explore the potential of the above drugs in treating patients with NAFLD. Liraglutide, dulaglutide, and tofogliflozin were ranked last in the SUCRA ranking in reducing GGT, ALT, and AST, respectively. Additional large, high-quality studies are needed to confirm the efficacy of these drugs.
Serum biomarkers (such as ALT, AST, GGT) are the most common non-invasive tests for evaluating liver diseases and are usually used as clinical indicators of liver cell injury. However, they are often used as auxiliary indicators of the improvement of NASH, owing to their lack of specificity and sensitivity (45). According to mouse models of NASH, GLP-1 analogs reduces liver enzymes and oxidative stress, and also improve liver histology (46). We found that semaglutide (a GLP-1 receptor agonist) is superior to other drugs (including placebo) in reducing the ALT and AST levels. The same was reported by Cusi (47). K-18, a biomarker of hepatocyte apoptosis, was also analyzed in this NMA. The results showed that tofogliflozin and pioglitazone could obviously reduce the level of K-18.
In view of the fact that liver biopsy is related to patients' severe advents, some non-invasive alternative indicators have been suggested in different countries and regions, such as tests used to quantify the degree of liver steatosis (e.g., MRI–PDFF, L/S ratio, and CAP score) and tests used to judge the severity of liver fibrosis (e.g., FIB-4 index, Pro-3, LSM, APRI, and ELF score) (48). This NMA reported on CAP, ELF score, and LFC. CAP evaluates steatosis based on shear-wave propagation, and its performance has been supported by several biopsy-controlled clinical studies (27, 32, 43). Liver fat measured by MRI–PDFF or H-MRS quantitatively explores LFC through free radiation mode (31, 34, 40). The ELF score has mostly been determined in cohort studies with a high prevalence of advanced fibrosis and has been tested in various cross-sectional studies and clinical trials (11). The results of this NMA showed that the liraglutide treatment had the highest ranking in decreasing CAP and ELF scores, while the pioglitazone treatment had the highest ranking in decreasing the LFC and fibrosis score. Previous studies have reported that GLP-1 analogs can act directly on human hepatocytes in vitro, reducing steatosis by decreasing de novo lipogenesis and increasing fatty acid oxidation (49–51). Pioglitazone can improve insulin sensitivity and transfer fat accumulation from central to subcutaneous regions (37). These results may explain the mechanism of clinical efficacy of the above drugs. FIB-4 index, Pro-3, L/S ratio, APRI, and LSM were not analyzed in this study because large, high-quality studies are still needed.
The gold standard method for diagnosing NAFLD is liver biopsy, which reveals the main histopathological features of NAFLD, such as steatosis, hepatocellular ballooning, lobular inflammation, and fibrosis (45). These findings can be used to score histology and judge the activity of NAFLD. Although there are some objective problems, such as difficult puncture techniques and errors in sample acquisition, liver biopsy is still the primary method of diagnosis, as there are no accurate non-invasive tests. Due to the limitations of the included literature, this NMA only analyzed the fibrosis score and the resolution of histological features that met the diagnostic criteria of NASH. According to the results of SUCRA, we found that liraglutide could be the most effective intervention to achieve resolution of NASH, followed by pioglitazone and vitamin E. Vitamin E is thought to improve NASH by reducing oxidative stress of hepatocytes and then reduce liver injury and inflammation, while pioglitazone can improve insulin sensitivity (52–54). Previous studies have found that liraglutide can improve body weight and blood glucose control, which may have a beneficial effect on the risk of cardiovascular disease and premature death in patients with NASH in the future. However, evidence for directly improving the hepatic histology of NASH remains to be explored (42).
There are some other off-label drugs used in the treatment of NAFLD worldwide, such as omega-3 fatty acids, metformin, and statins. However, these drugs have shown no discernible histological benefit on NASH yet (11). Furthermore, obeticholic acid, a potent farnesoid × receptor agonist, has shown a side effect (pruritus) that has reduced the possibility of its conditional approval (11). In addition, silybin or its complexes might play a potential role in the treatment of NAFLD patients. A preliminary observational study in 2006 (55) showed that treatment with the silybin–vitamin E–phospholipid complex for 6 months was able to improve the ultrasound appearance of the “bright” liver, the levels of liver enzymes, homeostatic model assessment for insulin resistance (HOMA-IR), and serum indices of hepatic fibrosis; these preliminary results were then also confirmed in a phase III study on 180 patients (55). In other words, silybin and its complexes are areas worthy of further study in the future. Our study did not consider some current ideas based on specific genetic background (including polymorphisms in key genes in the pathogenesis of NAFLD) that could potentially negatively influence or decrease responses to certain therapeutic regimens (including silybin-based regimens) (56), so these ideas still need to be explored.
Our study has several limitations. First, there was a small number of open-label studies we could include (17, 26, 30, 31). Second, the basic characteristics of some studies mentioned that several patients had temporarily used other drugs (e.g., to avoid serious endocrine imbalance). Third, the drugs mentioned in this review were in phase II or III clinical trials at that time, so the accuracy of the results of indirect comparison sounded bad with lacking direct comparison. Therefore, our results need to be verified in multicenter and large-scale studies; especially those with long-term and complete follow-up.
Conclusion
The network meta-analysis provided evidence for the efficacy of vitamin E, pioglitazone, SGLT2 inhibitors, and GLP-1 receptor agonists in NAFLD patients. In order to find the best guide-level drugs, it is necessary to include more RCT experiments with these off-label drugs, so that patients and clinicians can make optimal decisions together.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
QL, RW, and YC: research idea, study design, and data analyses/interpretation. YC and QZ: data acquisition and statistical analyses. QL, RW, YC, and QZ: supervision or mentorship. All author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions on the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
This study was supported by the National Major Scientific and Technological Special Project for ‘‘Significant New Drugs Development'' (grant no. 2017ZX09304019), National Natural Science Foundation of China (no. 81774278).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank LetPub (https://www.letpub.com) for its linguistic assistance during the preparation of this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.793203/full#supplementary-material
Abbreviations
NAFLD, non-alcoholic fatty liver disease; CAP, controlled attenuation parameter; LFC, liver fat content; ELF, enhanced liver fibrosis; K-18, keratin-18 (K-18) M30 fragment; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FDA, Food and Drug Administration; EMA, European Medicines Agency; GGT, gamma-glutamyl transferase; GLP-1, glucagon-like peptide-1; HCC, hepatocellular carcinoma; HOMA-IR, homeostatic model assessment for insulin resistance; PPARγ, peroxisome proliferator-activated receptor γ; SGLT2, sodium-glucose cotransporter-2; RCT, Randomized controlled trial; NICE, the United Kingdom's National Institute for Health and Clinical Excellence; NASH, non-alcoholic steatohepatitis.
References
1. European European Association for the Study of the Liver (EASL) European European Association for the Study of Diabetes (EASD) European European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
2. DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. (2017) 13:11–26. doi: 10.1038/nrneph.2016.170
3. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. (2019) 92:82–97. doi: 10.1016/j.metabol.2018.11.014
4. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
5. Ray K. NAFLD-the next global epidemic. Nat Rev Gastroenterol Hepatol. (2013) 10:621. doi: 10.1038/nrgastro.2013.197
6. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
7. Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. (2013) 38:134–43. doi: 10.1111/apt.12352
8. Rinella ME, Tacke F, Sanyal AJ, Anstee QM Participants Participants of the AASLD/EASL Workshop. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology. (2019) 70:1424–36. doi: 10.1002/hep.30782
9. Rezaei S, Tabrizi R, Nowrouzi-Sohrabi P, Jalali M, Atkin SL, Al-Rasadi K, et al. GLP-1 Receptor agonist effects on lipid and liver profiles in patients with nonalcoholic fatty liver disease: systematic review and meta-analysis. Can J Gastroenterol Hepatol. (2021) 2021:8936865. doi: 10.1155/2021/8936865
10. Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W. Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. J Diabetes Investig. (2020) 11:1238–47. doi: 10.1111/jdi.13237
11. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. (2021) 397:2212–24. doi: 10.1016/S0140-6736(20)32511-3
12. Lian J, Fu J. Pioglitazone for NAFLD patients with prediabetes or type 2 diabetes mellitus: a meta-analysis. Front Endocrinol. (2021) 12:615409. doi: 10.3389/fendo.2021.615409
13. Vadarlis A, Antza C, Bakaloudi DR, Doundoulakis I, Kalopitas G, Samara M, et al. Systematic review with meta-analysis: the effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2021) 36:311–9. doi: 10.1111/jgh.15221
14. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
15. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
16. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
17. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. (2006) 355:2297–307. doi: 10.1056/NEJMoa060326
18. Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin e for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. (2019) 42:1481–8. doi: 10.2337/dc19-0167
19. Ekhlasi G, Zarrati M, Agah S, Hosseini AF, Hosseini S, Shidfar S, et al. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. (2017) 16:278–90. doi: 10.17179/excli2016-846
20. Gastaldelli A, Sabatini S, Carli F, Gaggini M, Bril F, Belfort-DeAguiar R, et al. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int. (2021) 41:2659–70. doi: 10.1111/liv.15005
21. Glen J, Floros L, Day C, Pryke R, Guideline Development Group. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. (2016) 354:i4428. doi: 10.1136/bmj.i4428
22. Zheng Q, Yang H, Sun L, Wei R, Fu X, Wang Y, et al. Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: a network meta-analysis. Pharmacol Res. (2020) 159:105020. doi: 10.1016/j.phrs.2020.105020
23. Gawrieh S, Wilson LA, Yates KP, Cummings OW, Vilar-Gomez E, Ajmera V, et al. Relationship of ELF and PIIINP with liver histology and response to vitamin e or pioglitazone in the PIVENS trial. Hepatol Commun. (2021) 5:786–97. doi: 10.1002/hep4.1680
24. Huang JF, Dai CY, Huang CF, Tsai PC, Yeh ML, Hsu PY, et al. First-in-Asian double-blind randomized trial to assess the efficacy and safety of insulin sensitizer in nonalcoholic steatohepatitis patients. Hepatol Int. (2021) 15:1136–47. doi: 10.21203/rs.3.rs-596467/v1
25. Pervez MA, Khan DA, Slehria AUR, Ijaz A. Delta-tocotrienol supplementation improves biochemical markers of hepatocellular injury and steatosis in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Complement Ther Med. (2020) 52:102494. doi: 10.1016/j.ctim.2020.102494
26. Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. (2008) 135:1176–84. doi: 10.1053/j.gastro.2008.06.047
27. Vedtofte L, Bahne E, Foghsgaard S, Bagger JI, Andreasen C, Strandberg C, et al. One year's treatment with the glucagon-like peptide 1 receptor agonist liraglutide decreases hepatic fat content in women with nonalcoholic fatty liver disease and prior gestational diabetes mellitus in a randomized, placebo-controlled trial. J Clin Med. (2020) 9:3213. doi: 10.3390/jcm9103213
28. Cho KY, Nakamura A, Omori K, Takase T, Miya A, Yamamoto K, et al. Favorable effect of sodium-glucose cotransporter 2 inhibitor, dapagliflozin, on non-alcoholic fatty liver disease compared with pioglitazone. J Diabetes Investig. (2021) 12:1272–7. doi: 10.1111/jdi.13457
29. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. (2010) 362:1675–85. doi: 10.1056/NEJMoa0907929
30. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A Placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. (2021) 384:1113–24. doi: 10.1056/NEJMoa2028395
31. Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. (2018) 61:1923–34. doi: 10.1007/s00125-018-4675-2
32. Chehrehgosha H, Sohrabi MR, Ismail-Beigi F, Malek M, Reza Babaei M, Zamani F, et al. Empagliflozin improves liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease and type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Ther. (2021) 12:843–61. doi: 10.1007/s13300-021-01011-3
33. Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, et al. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care. (2017) 40:1364–72. doi: 10.2337/dc17-0518
34. Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care. (2020) 43:298–305. doi: 10.2337/dc19-0641
35. Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. (2020) 43:1352–5. doi: 10.2337/dc19-1892
36. Kinoshita T, Shimoda M, Nakashima K, Fushimi Y, Hirata Y, Tanabe A, et al. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, open-label, three-arm, active control study. J Diabetes Investig. (2020) 11:1612–22. doi: 10.1111/jdi.13279
37. Gurka MJ, Mack JA, Chi X, DeBoer MD. Use of metabolic syndrome severity to assess treatment with vitamin E and pioglitazone for non-alcoholic steatohepatitis. J Gastroenterol Hepatol. (2021) 36:249–56. doi: 10.1111/jgh.15131
38. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. (2016) 165:305–15. doi: 10.7326/M15-1774
39. Phrueksotsai S, Pinyopornpanish K, Euathrongchit J, Leerapun A, Phrommintikul A, Buranapin S, et al. The effects of dapagliflozin on hepatic and visceral fat in type 2 diabetes patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2021) 36:2952–9. doi: 10.1111/jgh.15580
40. Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den Bos IC, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. (2016) 59:2588–93. doi: 10.1007/s00125-016-4100-7
41. Bizino MB, Jazet IM, de Heer P, van Eyk HJ, Dekkers IA, Rensen PCN, et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia. (2020) 63:65–74. doi: 10.1007/s00125-019-05021-6
42. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. (2016) 387:679–90. doi: 10.1016/S0140-6736(15)00803-X
43. Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, et al. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv Ther. (2020) 37:4697–708. doi: 10.1007/s12325-020-01498-5
44. Yoneda M, Honda Y, Ogawa Y, Kessoku T, Kobayashi T, Imajo K, et al. Comparing the effects of tofogliflozin and pioglitazone in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus (ToPiND study): a randomized prospective open-label controlled trial. BMJ Open Diabetes Res Care. (2021) 9:e001990. doi: 10.1136/bmjdrc-2020-001990
45. de Brito E, Silva MB, Tustumi F, de Miranda Neto AA, Dantas ACB, Santo MA, Cecconello I. Gastric bypass compared with sleeve gastrectomy for nonalcoholic fatty liver disease: a systematic review and meta-analysis. Obes Surg. (2021) 31:2762–72. doi: 10.1007/s11695-021-05412-y
46. Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, et al. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS ONE. (2012) 7:e31394. doi: 10.1371/journal.pone.0031394
47. Cusi K. Incretin-based therapies for the management of nonalcoholic fatty liver disease in patients with type 2 diabetes. Hepatology. (2019) 69:2318–22. doi: 10.1002/hep.30670
48. Wong C, Yaow CYL, Ng CH, Chin YH, Low YF, Lim AYL, et al. Sodium-glucose co-transporter 2 inhibitors for non-alcoholic fatty liver disease in asian patients with type 2 diabetes: a meta-analysis. Front Endocrinol. (2021) 11:609135. doi: 10.3389/fendo.2020.609135
49. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. (2006) 43:173–81. doi: 10.1002/hep.21006
50. Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. (2011) 54:1214–23. doi: 10.1016/j.jhep.2010.09.032
51. Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. (2010) 51:1584–92. doi: 10.1002/hep.23569
52. Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. (2016) 13:196–205. doi: 10.1038/nrgastro.2016.3
53. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. (2014) 371:2237–8. doi: 10.1056/NEJMc1412427
54. DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. (2013) 29:379–86. doi: 10.1016/j.nut.2012.07.003
55. Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Del Vecchio Blanco C, et al. new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut. (2006) 55:901–2. doi: 10.1136/gut.2006.091967
Keywords: NAFLD, diabetes mellitus, vitamin E, pioglitazone, GLP-1 receptor agonists, SGLT2 inhibitors
Citation: Luo Q, Wei R, Cai Y, Zhao Q, Liu Y and Liu WJ (2022) Efficacy of Off-Label Therapy for Non-alcoholic Fatty Liver Disease in Improving Non-invasive and Invasive Biomarkers: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Med. 9:793203. doi: 10.3389/fmed.2022.793203
Received: 12 October 2021; Accepted: 10 January 2022;
Published: 25 February 2022.
Edited by:
Marcello Dallio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Mario Romeo, University of Campania Luigi Vanvitelli, ItalyGiulia Besutti, IRCCS Local Health Authority of Reggio Emilia, Italy
Copyright © 2022 Luo, Wei, Cai, Zhao, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jing Liu, bGl1d2VpamluZy0xOTc3JiN4MDAwNDA7aG90bWFpbC5jb20=; Yuning Liu, eXVuaW4xOTY0JiN4MDAwNDA7c2luYS5jb20=
†These authors share first authorship
 Qian Luo
Qian Luo Ruojun Wei1,2†
Ruojun Wei1,2† Yuzi Cai
Yuzi Cai Wei Jing Liu
Wei Jing Liu