- 1Nanshan School, Guangzhou Medical University, Guangzhou, China
- 2The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 3Department of Medical Oncology, School of Medicine, Tongji University, Shanghai, China
- 4Department of Medical Oncology, Shanghai Pulmonary Hospital, Tongji University Medical School Cancer Institute, Tongji University School of Medicine, Shanghai, China
Background: Observational studies indicated that circulating vitamin C (VitC) levels may be correlated with the risk of endometrial cancer (EC). However, the causal effects and direction between them were still unclear.
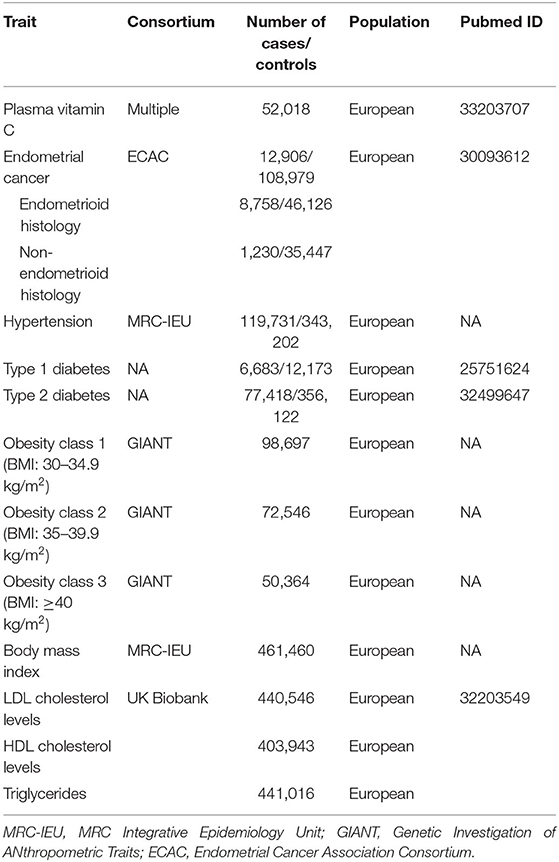
Methods: In this study, 11 single nucleotide polymorphisms (SNPs) robustly correlated with plasma VitC levels were extracted from the latest genome-wide association study (GWAS), containing 52,018 individuals. Genetic data of EC were obtained from the Endometrial Cancer Association Consortium (ECAC) (12,906 cases and 108,979 controls). An inverse-variance weighted method was utilized as the primary analysis of Mendelian randomization (MR), supplemented by the weighted median, MR Pleiotropy Residual Sum and Outlier test (MR-PRESSO), and MR-Egger methods. Additional sensitivity analyses excluding 3 SNPs with secondary phenotypes were conducted to rule out the possible pleiotropic effects. Potential impacts of several risk factors of EC, such as obesity, body mass index (BMI), hypertension, and diabetes on VitC levels, were assessed. We additionally evaluated the effects of VitC on LDL cholesterol levels, HDL cholesterol levels, and triglycerides levels to probe into the possible mediators in the VitC-EC pathway.
Results: Genetically predicted higher plasma VitC levels (per 1 SD increase, approximately 20 μmol/L) were causally associated with an increased risk of EC overall [odds ratio (OR) 1.374, 95% CI 1.128–1.674, p = 0.0016], supported by complementary sensitivity analyses. In the subgroup analyses, genetically predicted higher levels of VitC were associated with a tendency of increased risks of both endometrioid (ORSD 1.324, 95% CI 0.959–1.829, p = 0.0881) and non-endometrioid histology (ORSD 1.392, 95% CI 0.873–2.220, p = 0.1647) while without statistical significance. The association remained significant after the exclusion of the three pleiotropic SNPs (ORSD 1.394, 95% CI 1.090–1.784, p = 0.0082). The confounders and mediators were unlikely to affect the VitC-EC relationship. The causal effect of EC on VitC levels was not supported (OR 1.001, 95% CI 0.998–1.004, p = 0.4468).
Conclusions: This bi-directional MR study demonstrated a causal risk role of higher circulating VitC at physiological levels on an increased risk of EC, which was independent of confounders and mediators. Further studies are warranted to elucidate the possible mechanisms.
Introduction
Endometrial cancer (EC), which mainly affects postmenopausal women, is one of the commonest gynecological cancers worldwide (1). During the past few decades, the mortality rate of EC has been rising continuously in developed countries (2). Recently, vitamin C (VitC) has induced great attention as its potentially preventive effect on cancer. VitC is the most important water-soluble antioxidant in dietary fruit and vegetable sources, which may prevent cancer by reducing oxidative DNA damage, such as DNA mutations (3). Previous studies have indicated that VitC at pharmacological levels (i.e., intravenous medication) rather than physiological concentrations (i.e., oral administration) may manifest protective effects of cancers, while the findings were inconsistent. In general, there is relatively strong evidence supporting inverse associations between intake of VitC and breast cancer (4), lung cancer (5), and colon cancer (6). However, in the field of EC, the reports were relatively limited, and the results were inconsistent. The latest meta-analysis comprised of 9 case-control studies and 1 cohort study by Bandera et al. showed that intake of VitC from food resources rather than supplements decreased the risk of EC by 15% (7).
However, given the methodological limitations of observational reports, the effects of confounders, such as body mass index (BMI) and smoking, which were also common risk factors of cancers, cannot be thoroughly evaluated or eliminated. In addition, given that oxidative stress induced by cancer may enhance the consumption of VitC as well, findings were prone to reverse causality (8). Several randomized controlled trials (RCTs) focusing on this issue did not support the protective role of VitC in the development of cancers, whereas the sample size of incident cancer cases was limited (9, 10). Simultaneously, RCTs investigating the effect of VitC intake and EC risk are time-consuming and expensive, largely infeasible in a primary prevention condition. Hence, we may not establish the causal inference based on existing evidence with confidence. Considering EC has caused enormous health burdens worldwide, determining whether intake of VitC authentically plays a role in preventing EC development is important.
Mendelian randomization (MR), using genetic variations as instrumental variables (IVs), is a novel method for causal inference between exposures and outcomes. The genetic variants, utilized as proxies of exposures, are independent of environmental risk factors and determined prior to the diseases, avoiding the influences of confounders and reverse causation (11). It has been reported that single nucleotide polymorphisms (SNPs) can explain around 1.87% of the variance in plasma circulating VitC levels, suggesting that MR can provide a means for evaluating the causality between VitC and EC risk (12). In the present study, we performed a bi-directional MR method to probe into the putative effects of circulating VitC at physiological levels and the risk of EC. Previous MR studies have reported some of the risk factors of EC. For instance, Kho et al. found that higher LDL cholesterol levels were associated with a lower risk of EC overall, while higher HDL cholesterol levels raised the risk of non-endometrioid EC by 20% (13). Higher serum 17β-estradiol levels [odds ratio (OR) 1.09, 95% CI 1.06–1.11, p < 0.05] and fasting insulin levels (OR 2.34, 95% CI 1.06–5.14, p = 0.03) were also associated with a higher risk of EC (14, 15). Moreover, Nead et al. reported that increased age at menarche decreased the risk of EC by 22% (16). Therefore, we conducted additional MR analyses on these risk factors, which might play a role as a confounder and/or mediator on the VitC-EC pathway (17).
Materials and Methods
Genetic Variants Associated With Circulating VitC Levels
Initially, 11 SNPs robustly associated with plasma circulating VitC (i.e., at statistical significance threshold p < 5 × 10−8) were extracted from the latest study by Zheng et al. (Supplementary Table 1), containing 52,018 individuals of European origin (Table 1) (12). These SNPs aggregately accounted for 1.87% of the variance in VitC levels (12). Besides, all SNPs were kept for further analyses due to the absence of linkage disequilibrium (r2 < 0.01). Finally, we established the IVs based on these 11 VitC-related SNPs. Subsequently, we manually checked for the secondary phenotypes of each SNP in the PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk). Three potential pleiotropic SNPs, including rs56738967 with thyroid function, rs9895661 with serum urate, and rs174547 with fatty acids, were identified (Supplementary Table 1). The statistical power was generated using an online platform, “mRnd: Power calculations for Mendelian Randomization” (https://shiny.cnsgenomics.com/mRnd/), which was performed according to the formula derived by Brion et al. (18). On this basis, the effect size (OR = 0.85) of VitC on the risk of EC from the latest meta-analysis by Bandera et al. (7) was applied for power calculation at the threshold of the significance level of 0.05.
Study Participants of Endometrial Cancer
The Endometrial Cancer Association Consortium (ECAC) is a consortium formed to pool EC genetic studies to conduct large-scale genome-wide association study (GWAS) meta-analyses and identify genes associated with EC. Genetic data of EC that derived from European ancestry were obtained from the ECAC (12,906 EC cases and 108,979 controls) (https://ecac-studies.org/) (Table 1), which were publicly available on the MR-Base platform (https://www.mrbase.org/) (19). Subgroup analyses of different histological subtypes of EC, including endometrioid (8,758 cases and 46,126 controls) and non-endometrioid histology (1,230 cases and 35,447 controls) in ECAC, were also implemented.
Statistical Analysis
Mendelian randomization was applied as our statistical analysis tool, which is strictly subjected to three assumptions (20): (i) the IVs are robustly associated with increased VitC concentrations; (ii) the IVs affect EC only through their effects on increased VitC concentrations directly, and (iii) the IVs are independent of any confounders. Since the SNPs we chose were selected at the genome-wide significance threshold of p < 5 × 10−8 and the statistical power was 100% (>80%) as evaluated by an online tool (18), the first assumption was satisfied. Weighted median and MR-Egger methods were performed to test the second assumption indirectly. Regarding the sensitivity analysis, potential horizontal pleiotropic effects were obtained based on the intercept of the MR-Egger analyses. The MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) test was applied for identifying potential horizontal pleiotropy and removing outliner variants (21). The heterogeneity test was implemented as well, and I2 > 50.0% was considered significant. A leave-one-out analysis was conducted to appraise whether the estimation of MR was determined or biased by a single SNP. Single MR analysis was utilized to assess the effect size of individual SNP.
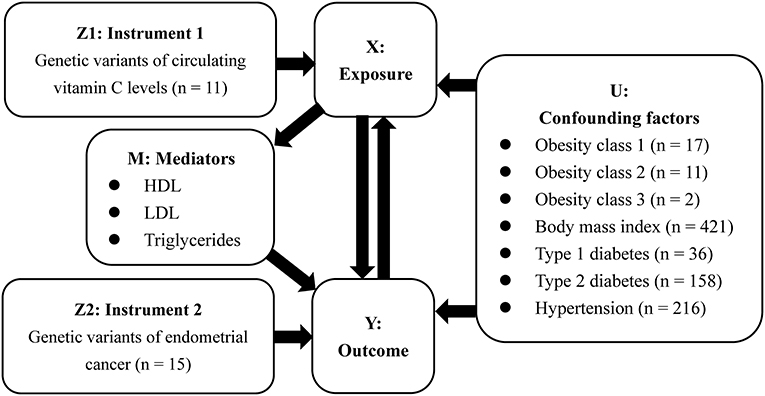
In this study, we used the random-effects inverse-variance weighted (IVW) to obtain the MR estimate based on multiple IVs. As described in Figure 1, the results were presented as OR and 95% CI, providing an estimate of relative risk on EC (Y) caused by a per 1 standard deviation (SD) increase in VitC levels (X). Additional sensitivity analysis excluding 3 SNPs with secondary phenotypes was also performed to eliminate the possible pleiotropic effects. In addition, a bi-directional MR analysis was further performed to investigate whether EC (Y) would reversely affect VitC concentrations (X).
Aiming to assess the potential confounders intervening the mechanisms on the VitC-EC relationship, additional MR analyses were performed to investigate whether genetic predisposition toward the common risk factors of EC (U) could be associated with circulating VitC levels. In 2019, Raglan et al. proposed an umbrella review to summarize the risk factors of EC based on literature, in which obesity (strong evidence), diabetes (highly suggestively evidence), and hypertension (suggestively evidence) were stated (17). Additionally, there is evidence that a higher BMI can increase the risk of EC (22). Therefore, further investigation was needed to appraise whether these factors would bias the MR results, leading to the hypothesis of the above-mentioned third assumption. Genetic summary data on obesity were extracted from the GIANT, BMI, and hypertension from the MRC-IEU, and diabetes (both type 1 and type 2) from published GWASs (Table 1). Given that several RCT showed VitC could affect LDL cholesterol levels, HDL cholesterol levels, and triglycerides levels and MR study suggested these factors were associated with the risk of EC, they were considered mediators (M) in the VitC-EC pathway (13, 23). For exposure (i.e., VitC)-mediator analysis, genetic data of these mediators were from the UK Biobank. MR analyses were performed in R (version 4.0.0) using the package TwoSampleMR (version 0.5.1) (24).
Results
Based on the previously reported effect size (OR = 0.85) to evaluate the causal effect of VitC on EC (7), our MR analyses with large-scale consortium had sufficient power (100%). The primary MR results indicated that genetically predisposed higher plasma VitC levels (per 1 SD increase, ~20 μmol/L) were causally associated with an increased EC risk (OR 1.374; 95% CI, 1.128–1.674; p = 0.0016), supported by complementary sensitivity analyses (ORSD 1.363, 95% CI 1.098–1.692, p = 0.0050 for weighted median; ORSD 1.318, 95% CI 1.120–1.479, p = 0.0103 for MR-PRESSO; ORSD 1.363, 95% CI 0.978–1.900, p = 0.1010 for MR-Egger) (Figures 2, 3, Supplementary Table 2). In the subgroup analyses, genetically predicted higher levels of VitC were associated with a tendency of increased risks of both endometrioid (ORSD 1.324, 95% CI 0.959–1.829, p = 0.0881 for IVW; ORSD 1.270, 95% CI 0.974–1.656, p = 0.0775 for weighted median; ORSD 1.239, 95% CI 0.722–2.216, p = 0.4563 for MR-Egger; ORSD 1.281, 95% CI 0.958–1.604, p = 0.1189 for MR-PRESSO) and non-endometrioid histology (ORSD 1.392, 95% CI 0.873–2.220, p = 0.1647 for IVW; ORSD 1.278, 95% CI 0.710–2.298, p = 0.6053 for weighted median; ORSD 1.227, 95% CI 0.580–2.594, p = 0.6053 for MR-Egger; ORSD 1.331, 95% CI 1.007–1.655, p = 0.0731 for MR-PRESSO) while without statistical significance (Figure 3).
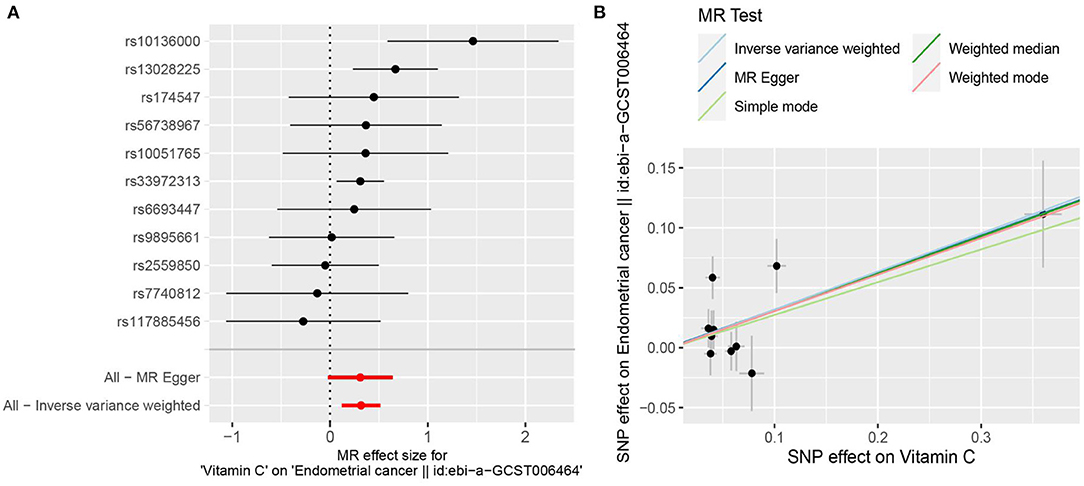
Figure 2. Mendelian randomization estimating the effects of genetically predicted higher plasma circulating vitamin C (VitC) levels (per 1 SD increase, ~20 μmol/L) on the risk of endometrial cancer (EC). (A) Forest plot of single nucleotide polymorphisms (SNPs) associated with higher circulating VitC levels and their risk of EC. (B) Scatter plots of SNPs correlated with higher circulating VitC concentrations and their risk of EC.
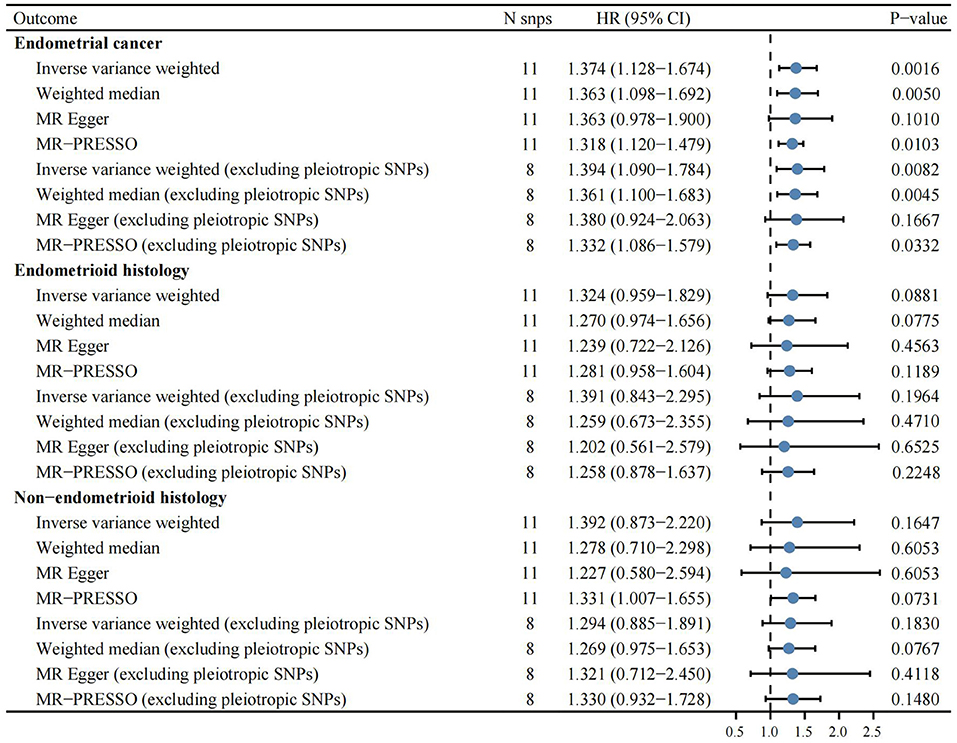
Figure 3. Complementary Mendelian randomization analyses estimating the effects of genetically predicted higher circulating vitamin C levels (per 1 SD increase, ~20 μmol/L) on the risk of endometrial cancer overall and different histological subtypes.
After the exclusion of the three pleiotropic SNPs, the association remained significant (ORSD 1.394, 95% CI 1.090–1.784, p = 0.0082 for IVW; ORSD 1.361, 95% CI 1.100–1.683, p = 0.0045 for weighted median; ORSD 1.332, 95% CI 1.086–1.579, p = 0.0322 for MR-PRESSO; ORSD 1.380, 95% CI 0.924–2.063, p = 0.1667 for MR-Egger) (Supplementary Table 3). Despite without statistical significance, the effects of VitC on EC remained directionally consistent in the subgroup analysis (ORSD 1.391, 95% CI 0.843–2.295, p = 0.1964 for endometrioid histology; ORSD 1.294, 95% CI 0.885–1.891, p = 0.1830 for non-endometrioid histology) (Figure 3). Single MR analysis demonstrated that rs10136000 (β = 1.46, p = 0.0010, nearest gene-serine-threonine protein kinase), rs13028225 (β = 0.67, p = 0.0026, nearest gene-sodium-dependent vitamin C transporter (SVCT) 3), and rs33972313 (β = 0.31, p = 0.0124, nearest gene-SVCT 1) were independently associated with an increased EC risk while other SNPs showed insignificant associations with EC (Supplementary Table 4).
Sensitivity Analysis
Using MR-Egger regression, we further tested for the global pleiotropic effect, wherein no global violation of pleiotropic assumptions existed (intercept = 0.0007, p = 0.9516 for EC overall; intercept = 0.0058, p = 0.7636 for endometrioid histology; intercept = 0.0110, p = 0.6822 for non-endometrioid histology) (Supplementary Table 5). Besides, heterogeneity was not observed (I2 = 32.65% for EC overall; I2 = 3.51% for endometrioid histology; I2 = 20.78% for non-endometrioid histology) in the study (Supplementary Table 6). Leave-one-out studies supported no evidence that a single SNP had an impact on the gross effect of genetically predisposed VitC on EC (Supplementary Table 7). We further evaluated whether the association between genetically predisposed one-SD increase in the VitC concentrations and EC was influenced by potential confounders (i.e., obesity, BMI, diabetes, and hypertension). The IVW results demonstrated that genetically predisposed obesity class 3 (BMI: ≥40 kg/m2) (OR 0.997, 95% CI 0.994–1.000, p = 0.0302) and BMI (OR 0.991, 95% CI 0.987–0.996, p < 0.001) but not obesity class 1 (BMI: 30–34.9 kg/m2) (OR 0.996, 95% CI 0.992–1.000, p = 0.0789) or obesity class 2 (OR 0.998, 95% CI 0.995–1.002, p = 0.3811) were inversely associated with VitC levels (Table 2, Supplementary Table 8). Genetically predicted hypertension was negatively correlated with VitC levels (OR 0.985, 95% CI 0.972–0.999, p = 0.0306) (Table 2). No causality between diabetes (both type 1 and type 2) and VitC levels was observed (Table 2, Supplementary Table 8). Since the effect size was marginal, the causal direction and association were unlikely to be affected by these confounders.
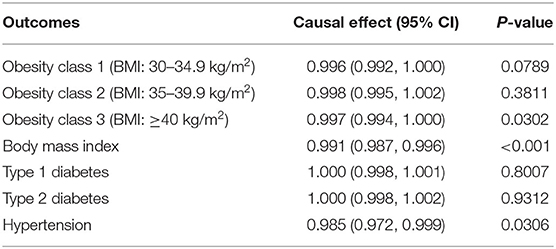
Table 2. Causal effects between genetic predisposition toward common risk factors of endometrial cancer and circulating plasma vitamin C.
For exposure (i.e., VitC)-mediator effects, consistent with the meta-analysis by McRae et al. that VitC supplementation can decrease LDL cholesterol levels (23), we found that genetic predisposition towards higher circulating VitC levels were associated with a tendency of lower LDL cholesterol levels (OR 0.968, 95% CI 0.873–1.073, p = 0.5385) while without statistical significance. On the contrary, since VitC supplementation was reported to raise HDL cholesterol levels and decrease triglycerides levels (23), we found that genetically predicted higher circulating VitC levels were correlated with lower HDL cholesterol levels (OR 0.943, 95% CI 0.792–1.122, p = 0.5081) and higher triglycerides levels (OR 1.062, 95% CI 0.908–1.242, p = 0.4546) while without statistical significance (Table 3, Supplementary Table 9). Consequently, these mediators appeared to have no bearing on the VitC-EC relation. The causal effect of EC on VitC levels was not supported (OR 1.001, 95% CI 0.998–1.004, p = 0.4468) (Supplementary Table 10).
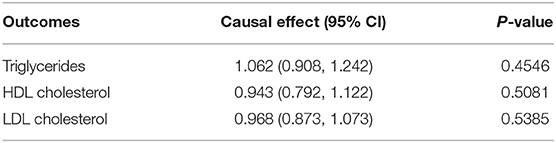
Table 3. Causal effects between genetic predisposition toward circulating plasma vitamin C and mediators.
Discussion
Involving 11 VitC-related SNPs as IVs and genetic statistics from the ECAC (12,906 EC cases and 108,979 controls), we used a bi-directional MR method to investigate the putative causality between increased VitC concentrations and EC risk for the first time. MR results showed that genetically predicted per SD increase (~20 μmol/L) in VitC at physiological levels was causally associated with a 37% higher EC risk, independent of confounders and mediators.
Our MR finding was consistent with the only cohort study focusing on this issue, which included 221 EC cases, that intake of VitC was correlated with a slightly increased risk of EC (10%) while without statistical significance (25). Similarly, a case-control study that included 42 EC cases and 68 controls indicated a 13% higher EC risk with the increment of VitC intake (26). However, a study proposed by Negri et al., which contained 368 cases and 713 controls, demonstrated that the EC risk decreased by 40% in the highest quintile than the lowest quintile of VitC intake (27). In addition, the other two studies showed negative association (28, 29) and most other case-control studies indicated null (8, 25, 30–33) association. The latest dose-response meta-analysis enrolling one cohort study and nine case-control studies showed that per 50 mg increase in VitC intake decreased the risk of EC by 15% (7).
Nevertheless, since traditional observational studies are susceptible to potential confounders or reverse causality, limitations of previous studies existed. First, the number of EC cases enrolled in previous studies was rather small, with the maximum sample size up to 368, which may lack substantial statistical performance to assess the causal effect of increased VitC concentrations on EC. Second, given EC is comprised of a distinct set of histological subtypes and differences in histology related to differences in molecular features and clinical behaviors, no studies have managed to investigate the correlations between VitC concentrations and different histotypes of EC. Third, none of the previous studies managed to control BMI, while high BMI is regarded as a pivotal risk factor for EC. Similarly, the use of medication (e.g., oral contraceptive) or hormonal therapy can impact the incidence of EC, while no studies have taken these confounders into consideration. More importantly, no prospective large-scale cohort studies have been implemented currently. Thereby, it is insufficient to completely address the causal direction between increased VitC concentrations and EC.
Previous MR studies have investigated the effects of VitC levels with several health issues (e.g., Alzheimer's disease and hyperuricemia) with only one SNP as IV (34, 35). In the field of cancer, Fu et al. implemented the MR analysis to probe into the causal direction between VitC concentrations and lung, colon, rectal, prostate, and breast cancer with 11 SNPs as IVs while they found null associations (36). Our study first investigated the causal relationships between VitC and EC risk with bi-directional MR design, of which several advantages were listed as follows. First, we used the most comprehensive and proved GWASs-identified SNPs as IV sets. With large sample size (n = 52,018 for circulating VitC levels, n = 121,885 for EC) and strongly associated IVs (power = 100.0%), our MR study with substantial statistical performance may estimate the causal effect more precisely. Second, we attempted to address the three key assumptions underlying the MR design with robust methods (e.g., MR-PRSSO and excluding pleiotropic SNPs), resulting in mostly unbiased findings. Third, we evaluated the influence of potential confounders and mediators, including obesity, BMI, hypertension, LDL cholesterol levels, HDL cholesterol levels, triglycerides levels, and diabetes, on the VitC-EC pathway. Considering the gross effect that genetically predisposed increased VitC concentrations were causally correlated with an increased EC risk, these confounders and mediators were unlikely to influence the VitC-EC relation, implying a comparatively independent relationship between them.
In addition, several limitations should be noted. First, despite using the most comprehensive set of SNPs currently, it solely explained a small part of the variance of increased VitC concentrations in the population. It is probable that several unknown VitC-related SNPs may also influence the progression of EC. Second, the observed causal effect between genetically predisposed elevated concentrations of VitC and EC was modest, with a 37% higher risk of EC. Therefore, the clinical significance of increased concentrations of VitC in the progression of EC is relatively limited and whether people with increased VitC levels should be monitored continuously remains uncertain. Third, we cannot evaluate the potential non-linear relationships between VitC levels and EC risk. Additionally, given that the IVs of both the exposure and outcome phenotypes were of European descent, whether our findings can be extended to other ethnicities was uncertain. Consequently, our results should not be considered definitive.
Since EC comprises a genetically and histologically broad range of tumors, the different signaling pathways underlying increased VitC concentrations to different subtypes of EC risk could be complicated and affected by various factors, whereas related research was quite limited. Since the circulating VitC levels were seldomly measured in most observational studies, the preventative effects of VitC against cancers were based on dietary intake or supplementation. As is well known, VitC cannot be composited by the human body and has to be gained from the diet (e.g., fruit and vegetable). Hence, the VitC maybe just a biomarker of vegetables and fruits consumption, and the reported protective effects of VitC were likely to be biased by other factors, such as fibers and polyphenols in vegetables and fruits. In this case, it is difficult to disentangle the unconfounded VitC-EC relation in traditional studies. Herein, we used the MR design analysis to dissect the relationship between genetically predicted VitC at physiological levels and EC risk. Under the circumstance that high pharmacological levels of VitC through intravenous injection alone or combined with other drugs manifested promising outcomes on treating cancers, it is essential for public health to determine whether maintaining the high physiological levels of VitC contributes to cancer prevention. Consequently, combined previous reports with our findings, universal screening of the general population for hypovitaminosis and maintaining high physiological VitC levels should not be supposed to be a tactic for primary EC prevention at present. As we preliminarily identified VitC as a risk factor of EC overall, future experimental and longitudinal studies are warranted to verify our findings and elucidate the possible mechanisms. Subsequently, people with high circulating VitC levels may need to be screened routinely (e.g., hysteroscopy and transvaginal ultrasound) (37), which may lower the mortality rates of EC in the future. Moreover, with the emerging data from GWAS and epidemiological studies, precisely defining the high-risk populations of EC and refining risk classification may lower the disease burden of EC. Meanwhile, it is pivotal for future studies to recognize the changeable risk factors (e.g., diets and lifestyles) of EC and take preventative measures subsequently to lower the incidence of EC.
Conclusions
The present bi-directional MR study indicated a causal risk role of higher circulating VitC at physiological levels on an increased risk of EC in European descent, which was independent of confounders and mediators. Future large-scale GWASs with individual-level data and more SNPs of VitC, which can be used to build genetic scores of VitC metabolic pathway, and experimental studies, are warranted to better understand the mechanisms from VitC concentrations to EC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The study protocol complied with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the National Clinical Research Center for Respiratory Disease of the First Affiliated Hospital of Guangzhou Medical University.
Author Contributions
HP and XW: conception, design, collection, assembly of data, data analysis, and interpretation. All authors provided study materials or patients, contributed to writing the manuscript, and approved the final version of the manuscript.
Funding
This work was supported by Cultivation of Guangdong College Students' Scientific and Technological Innovation (Climbing Program Special Funds) (Grant Numbers: pdjh2020a0480 and pdjh2021a0407).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledged the efforts of the MRC Integrative Epidemiology Unit (MRC-IEU), the Genetic Investigation of ANthropometric Traits (GIANT), the UK Biobank, and the Endometrial Cancer Association Consortium (ECAC) in providing the high quality GWAS data for researchers. The authors acknowledged the efforts of the GWAS consortia in providing high-quality resources in the MR-Base platform (https://www.mrbase.org/) for researchers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.792008/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. CA: cancer statistics, 2021. Cancer J Clin. (2021) 71:e34408. doi: 10.3322/caac.21654
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Lee KW, Lee HJ, Surh Y-J, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. (2003) 78:1074–8. doi: 10.1093/ajcn/78.6.1074
4. Zhang D, Xu P, Li Y, Wei B, Yang S, Zheng Y, et al. Association of vitamin C intake with breast cancer risk and mortality: a meta-analysis of observational studies. Aging. (2020) 12:18415–35. doi: 10.18632/aging.103769
5. Luo J, Shen L, Zheng D. Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Sci Rep. (2014) 4:6161. doi: 10.1038/srep06161
6. Heine-Bröring RC, Winkels RM, Renkema JMS, Kragt L, van Orten-Luiten A-CB, Tigchelaar EF, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer. (2015) 136:2388–401. doi: 10.1002/ijc.29277
7. Bandera EV, Gifkins DM, Moore DF, McCullough ML, Kushi LH. Antioxidant vitamins and the risk of endometrial cancer: a dose-response meta-analysis. Cancer Causes Control. (2009) 20:699–711. doi: 10.1007/s10552-008-9283-x
8. Pawlowska E, Szczepanska J, Blasiak J. Pro- and antioxidant effects of vitamin C in cancer in correspondence to its dietary and pharmacological concentrations. Oxid Med Cell Longev. (2019) 2019:7286737. doi: 10.1155/2019/7286737
9. Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. (2009) 101:14–23. doi: 10.1093/jnci/djn438
10. Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the physicians' health study II randomized controlled trial. JAMA. (2009) 301:52–62. doi: 10.1001/jama.2008.862
11. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
12. Zheng J-S, Luan Ja, Sofianopoulou E, Imamura F, Stewart ID, Day FR, et al. Plasma vitamin C and type 2 diabetes: genome-wide association study and mendelian randomization analysis in European populations. Diabetes Care. (2021) 44:98–106. doi: 10.2337/dc20-1328
13. Kho P-F, Amant F, Annibali D, Ashton K, Attia J, Auer PL, et al. Mendelian randomization analyses suggest a role for cholesterol in the development of endometrial cancer. Int J Cancer. (2021) 148:307–19. doi: 10.1002/ijc.33206
14. Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, et al. Evidence of a causal association between insulinemia and endometrial cancer: a mendelian randomization analysis. J Natl Cancer Inst. (2015) 107:djv178. doi: 10.1093/jnci/djv178
15. Larsson SC, Kar S, Perry JRB, Carter P, Vithayathil M, Mason AM, et al. Serum estradiol and 20 site-specific cancers in women: mendelian randomization study. J Clin Endocrinol Metab. (2021) 107:e467–74. doi: 10.1210/clinem/dgab713
16. Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. (2017) 49:834–41. doi: 10.1038/ng.3841
17. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. (2019) 145:1719–30. doi: 10.1002/ijc.31961
18. Brion M-JA, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
19. O'Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. (2018) 9:3166. doi: 10.1038/s41467-018-05427-7
20. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology. (2014) 25:427–35. doi: 10.1097/EDE.0000000000000081
21. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
22. Masuda T, Ogawa K, Kamatani Y, Murakami Y, Kimura T, Okada Y, et al. Mendelian randomization study identified obesity as a causal risk factor of uterine endometrial cancer in Japanese. Cancer Sci. (2020) 111:4646–51. doi: 10.1111/cas.14667
23. McRae MP. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J Chiropr Med. (2008) 7:48–58. doi: 10.1016/j.jcme.2008.01.002
24. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
25. Jain MG, Rohan TE, Howe GR, Miller AB. A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol. (2000) 16:899–905. doi: 10.1023/A:1011012621990
26. Tzonou A, Lipworth L, Kalandidi A, Trichopoulou A, Gamatsi I, Hsieh CC, et al. Dietary factors and the risk of endometrial cancer: a case–control study in Greece. Br J Cancer. (1996) 73:1284–90. doi: 10.1038/bjc.1996.246
27. Negri E, La Vecchia C, Franceschi S, Levi F, Parazzini F. Intake of selected micronutrients and the risk of endometrial carcinoma. Cancer. (1996) 77:917–23.
28. McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States). Cancer Causes Control. (2000) 11:965–74. doi: 10.1023/A:1026551309873
29. Xu W-H, Dai Q, Xiang Y-B, Zhao G-M, Ruan Z-X, Cheng J-R, et al. Nutritional factors in relation to endometrial cancer: a report from a population-based case-control study in Shanghai, China. Int J Cancer. (2007) 120:1776–81. doi: 10.1002/ijc.22456
30. Barbone F, Austin H, Partridge EE. Diet and endometrial cancer: a case-control study. Am J Epidemiol. (1993) 137:393–403. doi: 10.1093/oxfordjournals.aje.a116687
31. Goodman MT, Hankin JH, Wilkens LR Lyu LC, McDuffie K, Liu LQ, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. (1997) 57:5077–85.
32. Potischman N, Swanson CA, Brinton LA, McAdams M, Barrett RJ, Berman ML, et al. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. (1993) 4:239–50.
33. Shu XO, Zheng W, Potischman N, Brinton LA, Hatch MC, Gao YT, et al. A population-based case-control study of dietary factors and endometrial cancer in Shanghai, People's Republic of China. Am J Epidemiol. (1993) 137:155–65. doi: 10.1093/oxfordjournals.aje.a116655
34. Kobylecki CJ, Afzal S, Nordestgaard BG. Genetically high plasma vitamin C and urate: a Mendelian randomization study in 106 147 individuals from the general population. Rheumatology. (2018) 57:1769–76. doi: 10.1093/rheumatology/key171
35. Williams DM, Hägg S, Pedersen NL. Circulating antioxidants and Alzheimer disease prevention: a Mendelian randomization study. Am J Clin Nutr. (2019) 109:90–8. doi: 10.1093/ajcn/nqy225
36. Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang J, et al. Circulating vitamin C concentration and risk of cancers: a Mendelian randomization study. BMC Med. (2021) 19:171. doi: 10.1186/s12916-021-02041-1
Keywords: vitamin C, endometrial cancer, Mendelian randomization, single nucleotide polymorphism, causality
Citation: Peng H, Wu X and Wen Y (2022) Plasma Circulating Vitamin C Levels and Risk of Endometrial Cancer: A Bi-Directional Mendelian Randomization Analysis. Front. Med. 9:792008. doi: 10.3389/fmed.2022.792008
Received: 30 October 2021; Accepted: 31 January 2022;
Published: 23 March 2022.
Edited by:
Luca Ermini, Luxembourg Institute of Health, LuxembourgReviewed by:
Dylan Glubb, The University of Queensland, AustraliaPrasenjit Mitra, Post Graduate Institute of Medical Education & Research (PGIMER), India
Copyright © 2022 Peng, Wu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haoxin Peng, MTU3Njc3NzE2MzZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Haoxin Peng
Haoxin Peng Xiangrong Wu
Xiangrong Wu Yaokai Wen
Yaokai Wen