- 1Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China
- 2School of Basic Medical Sciences, Guangxi Medical University, Nanning, China
Background: For patients with obscure gastrointestinal bleeding (OGIB), finding the bleeding site is challenging. Balloon-assisted enteroscopy (BAE) has become the preferred diagnostic modality for OGIB. The long-term outcome of patients with negative BAE remains undefined. The present study aimed to evaluate the long-term outcomes of patients with negative BAE results for OGIB and to clarify the effect of further investigations at the time of rebleeding with a systematic review and meta-analysis of the available cohort studies.
Methods: Studies were searched through the PubMed, EMBASE, and Cochrane library databases. The following indexes were analyzed: rebleeding rate after negative BAE, rebleeding rate after different follow-up periods, the proportion of patients who underwent further evaluation after rebleeding, the percentage of patients with identified rebleeding sources, and the percentage of patients with rebleeding sources in the small intestine. Heterogeneity was assessed using the I2 test.
Results: Twelve studies that involved a total of 407 patients were included in the analysis. The pooled rebleeding rate after negative BAE for OGIB was 29.1% (95% CI: 17.2–42.6%). Heterogeneity was significant among the studies (I2 = 88%; p < 0.0001). The Chi-squared test did not show a difference in rebleeding rates between the short and long follow-up period groups (p = 0.142). The pooled proportion of patients who underwent further evaluation after rebleeding was 86.1%. Among the patients who underwent further evaluation, rebleeding sources were identified in 73.6% of patients, and 68.8% of the identified rebleeding lesions were in the small intestine.
Conclusion: A negative result of BAE in patients with OGIB indicates a subsequently low risk of rebleeding. Further evaluation should be considered after rebleeding.
Introduction
The small intestine has always been difficult to evaluate thoroughly because of its long length and variable looped configuration. Before balloon-assisted enteroscopy (BAE) was introduced in clinical practice in 2001, endoscopic examination of the small intestine was unsatisfactory, and treatment of small intestinal disease often required surgical laparotomy with intraoperative enteroscopy. The development of BAE had made the entire small intestine accessible to endoscopic observation. BAE can achieve a complete small intestinal examination by using one (single balloon enteroscopy, SBE) or two balloons (double balloon enteroscopy, DBE) to fix the intestinal wall and to facilitate endoscopic intubation in the small intestine (1). The most common indication of BAE is obscure gastrointestinal bleeding (OGIB), which is defined as bleeding of an unknown origin despite traditional endoscopy (2). OGIB accounts for ~1.2–5% of all GI bleeding events (3, 4) and can be further classified as overt or occult OGIB. Obscure-overt GI bleeding in patients presents as clinically visible bleeding, such as hematemesis, melena, or hematochezia (5, 6). In contrast, the occult type presents as iron deficiency anemia or a positive occult blood test in the stool (7, 8). Most OGIB events are attributable to small intestinal diseases. BAE has become the preferred method for examination of the small intestine in OGIB. BAE can examine much more of the small intestine compared with push enteroscopy and can achieve a much higher diagnostic yield (9, 10). The diagnostic yields of BAE for patients with OGIB have been reported as 43–81% (11–20), but the long-term outcome of patients with OGIB after BAE has been indeterminate. A prospective study of patients with OGIB who underwent BAE showed reduced bleeding and blood transfusion (21). However, some follow-up studies found a rebleeding rate ranging from 40 to 46% in patients with OGIB who are treated with BAE (22–24).
For some patients with OGIB, the bleeding source may not be found with BAE. It is believed that the prognosis of patients with identified bleeding sites responsible for OGIB was better than that of patients with a negative result. Studies about capsule endoscopy (CE) in OGIB reported rebleeding rates ranging from 5 to 53% after negative CE (25–30). The long-term outcomes of positive BAE results for small intestinal bleeding have been investigated in some studies and the rebleeding rates are between 10 and 50% (31–37). There are a few reports about the outcomes of patients after negative BAE. A few small studies have evaluated the rebleeding rate after negative BAE, and the reported rebleeding rates range from 33.3 to 55.9% (38–41). The long-term outcome of patients with negative BAE is not clear until now, and the role of a further evaluation at the time of rebleeding needs to be investigated. The optimal management strategy for patients with negative findings of initial BAE remains elusive. The present study attempts to investigate the long-term outcomes of OGIB patients with negative BAE and to evaluate the role of further examinations at the time of rebleeding with a systematic review and meta-analysis of available cohort studies.
Methods
Literature Search
Relevant studies were identified by searching in the PubMed, EMBASE, and Cochrane Library databases from January 2001 to December 2020. Search items were listed as follows: “obscure gastrointestinal bleeding” or “OGIB” or “small intestinal bleeding” or “small bowel bleeding” and “double balloon enteroscopy” or “single balloon enteroscopy” or “balloon-assisted enteroscopy” and “negative” or “normal”, and “follow-up”. The search was limited to studies in humans published in English. References of eligible articles and review articles were manually searched.
Selection of Articles
The selection criteria were studies in (1) patients who underwent DBE or SBE due to OGIB; (2) initial BAE results were negative; (3) the patients were followed up; and (4) the rebleeding rates after negative BAE were presented. The exclusion criteria were abstracts, reviews and meta-analyses, editorials, case reports, and studies that did not report the rebleeding rate. Each eligible article was reviewed in full text. Two reviewers (SHT and ZYG) independently performed literature search and then cross-checked the search results. Two authors (SHT and ZYG) fulfilled study selection and data extraction and a third reviewer (SXD) was involved if there was any conflict.
Data Extraction
The following data were extracted from all eligible studies: author, country, publication year, publication type, study design, type of BAE used, the number of patients with negative BAE, the number of patients with rebleeding, the number of patients who underwent further evaluation, the number of patients whose rebleeding source was identified in the further evaluation, the number of patients whose identified lesion was in the small intestine, and the length of follow-up.
Definitions
Negative BAE: No obvious cause of blood loss was identified during BAE.
Rebleeding: Evidence of GI bleeding was found at least 30 days after an initial BAE.
Long-term follow-up: A follow-up continued for ≥2 years after BAE.
Short-term follow-up: A follow-up continued for <2 years after BAE.
Risk of Bias and Publication Bias Analysis
The risk of bias was assessed by the Newcastle-Ottawa Scale (NOS) criteria for cohort studies. There are three major parts assessed: (1) selection (score 0–4); (2) comparability (score 0–2); and (3) outcome (score 0–3). The maximum score is 9. A score of 0–3, 4–6, and 7–9 represents a low, moderate, and high quality, respectively. The publication bias was assessed by the Egger test.
Statistical Analysis
A random-effects model with StatsDirect statistical software Version 2.7.8 (StatsDirect Ltd., Sale, Cheshire, UK) was performed in all analyses to generate a more conservative estimate. We pooled proportions with 95% CIs, which are presented as forest plots. The heterogeneity between studies was estimated by the Cochran Q test and the I2 statistics. p < 0.1 and I2 > 50% were considered to be significantly heterogeneous. Categorical variables are presented as absolute numbers and percentages. Statistically significant differences were evaluated using the Chi-squared test for categorical variables. Results were considered as significant at p < 0.05.
Results
Literature Search Results
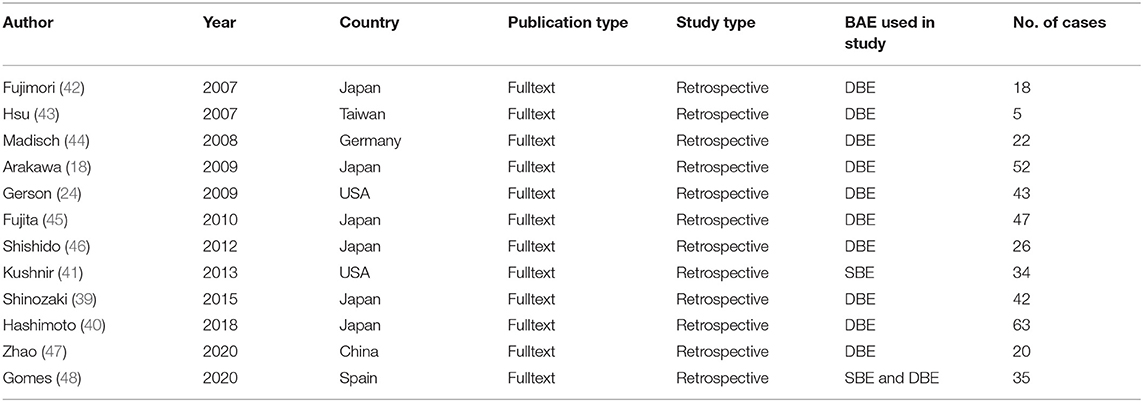
Twelve studies that involved a total of 407 patients were included in the analysis. All studies were retrospective and published between 2007 and 2020. The results of the literature search are summarized in Figure 1. The characteristics of the 12 eligible studies are summarized in Table 1.
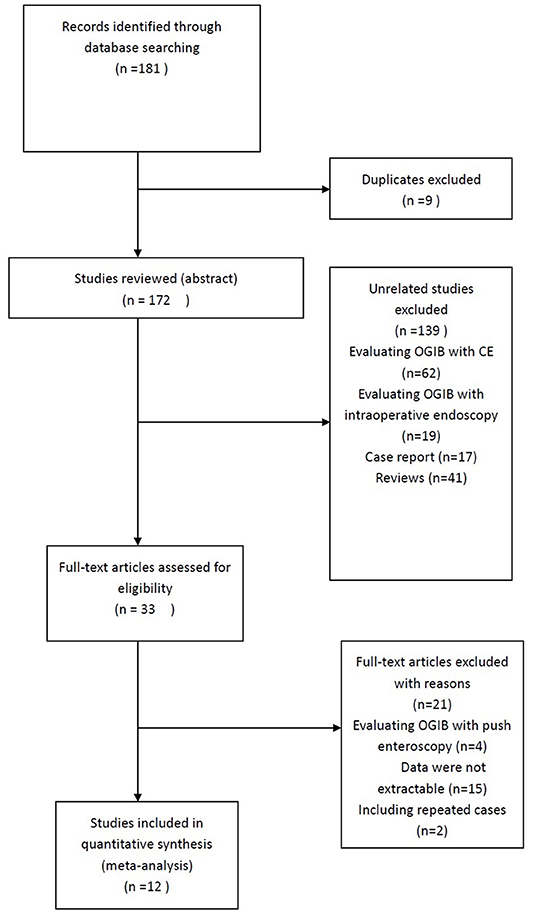
Figure 1. Study selection flow chart. Of a total of 181 studies, only 12 studies met selection criteria.
Characteristics of Study
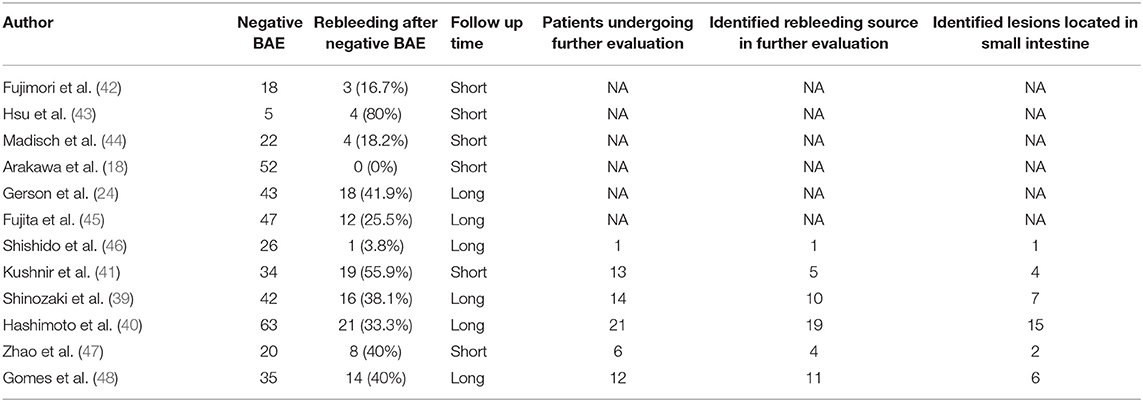
In the 12 studies, a total of 407 patients underwent BAE procedures for OGIB with negative results. All studies were conducted between 2007 and 2020. The included 12 studies were retrospective, six of which were performed in Japan, followed by the United States (2/12), China (1/12), Germany (1/12), Spain (1/12), and Taiwan (1/12). The number of patients in each eligible study was more than 5 and the largest one included 63 patients. In one study, only SBE was used (41) and both SBE and DBE were adopted in another study (48). In the other 10 studies, only DBE was performed. Six studies had a long duration of follow-up (2 years or more). The results of the various outcomes of the individual studies are shown in Table 2.
Risk of Bias and Publication Bias Analysis
The NOS score ranged from 6 to 8 points. Six studies were considered to be of moderate quality, and 6 were of high quality (41–43, 45–47) (Supplementary Table S1). Publication bias (Egger test): 4.967 (95% CI = 3.259–6.674); p < 0.0001.
Rebleeding Rate After Negative BAE
The rebleeding rates of all included studies ranged from 0 to 80%. The overall, pooled rebleeding rate after negative BAE for OGIB was 29.1% (95% CI: 17.2–42.6%; Figure 2). There was significant heterogeneity among the studies (I2 = 88%; p < 0.0001). One study where only SBE was used in all patients had a higher rebleeding rate (55.9%) (41).

Figure 2. Rebleeding rate after negative balloon-assisted enteroscopy (BAE) is ~ 29%. Rebleeding in patients after negative BAE for obscure gastrointestinal bleeding (OGIB). Rebleeding episodes during the follow-up period were reported in 29.1% (95% CI: 17.2–42.6%) of the 407 patients in the 12 studies. There was significant heterogeneity among the studies (p < 0.0001).
Rebleeding Rate After a Different Follow-Up Period
As shown in Table 2, the rebleeding rates in the short follow-up period group were ranged from 0 to 80%. The pooled rebleeding rate was 29.6% (95% CI: 7.5–58.6%) in this group (Figure 3). Significant heterogeneity was found among the studies (I2 = 92.4%; p < 0.0001). The rebleeding rates in the long follow-up period group ranged from 3.8 to 41.9%, and the pooled rebleeding rate was 30.2% (95% CI: 19.9–41.7%; Figure 4). Heterogeneity was significant among the studies (I2 = 73.9%; p = 0.0018). The Chi-squared test did not show a difference in rebleeding rates between the short and long follow-up period groups (p = 0.142).

Figure 3. Rebleeding rate of patients in the short follow-up group is ~30%. Forest plot shows that 29.6% (95% CI: 7.5–58.6%) of the patients who had been followed up less than 2 years experienced rebleeding. There was evidence of heterogeneity among the studies (p < 0.0001).

Figure 4. Rebleeding rate of patients in the long follow-up group is ~30%. Forest plot shows that 30.2% (95% CI: 19.9–41.7%) of the patients who had been followed up more than 2 years experienced rebleeding. There was evidence of heterogeneity among the studies (p = 0.0018).
Further Evaluation After Rebleeding
Six studies provided the data of patients who underwent further evaluation after rebleeding (Table 2). The pooled proportion of patients who underwent further evaluation after rebleeding was 86.1% (95% CI: 74.4–97.9%). Among the patients who underwent further evaluation, the rebleeding sources were identified in 73.6% (95% CI: 54.9–88.7%) of patients, and 68.8% (95% CI: 56.1–80.1%) of the identified rebleeding lesions were in the small intestine.
Discussion
The introduction of BAE has improved the diagnostic yield for patients with OGIB. However, some small intestinal lesions can be missed during BAE and false-negative BAE results for OGIB are not rare. The delayed BAE may be performed when the original bleeding site had healed. Some lesions were self-limiting and experienced a rapid resolution. When the etiologic factors were addressed, a prompt stop of bleeding could be expected in some cases. The timing of BAE could contribute to improving the diagnostic yield of patients with OGIB. Some studies have shown that emergency DBE is associated with a lower rebleeding rate compared with non-emergency DBE (49, 50). A study demonstrated that emergency DBE is helpful for the diagnosis and management of patients with small intestinal bleeding (51). Therefore, some experts suggested that BAE should be performed within the first few days after OGIB. Some small intestinal lesions found with additional diagnostic modalities after initial negative BAE were not within the reach of the first BAE. This was due to an insufficient insertion depth of the first procedure. In comparison with CE and DBE, CT has a lower diagnostic yield for OGIB (52). However, a study showed that CT enterography found the lesion in 50% of OGIB cases with negative CE results (53). A meta-analysis indicated that urgent CT angiography could localize the bleeding lesion of OGIB patients with high sensitivity and specificity (54). Based on these reports, the concomitant use of CT examination could also improve the detection of bleeding sources missed during BAE.
Rebleeding episodes can occur many years after the initial negative BAE results. In the present study, we found that the pooled rebleeding rate after an initial negative BAE was 29.1%. This is similar to the rebleeding rate after positive DBE (31–33). The rebleeding rates after negative BAE varied from 0 to 80% among included studies. The severity of OGIB in these reports was different. A study had found that the severity of OGIB is a key factor of the long-term outcomes after a DBE with negative results (39). The degree of OGIB may explain the differences in the reported rebleeding rates after a negative BAE. The follow-up period in these reports is also variable and may have an effect on the clinical outcomes. However, there was no difference in rebleeding rate between the long and short follow-up period groups in our study (30.2 vs. 29.6%, p = 0.142). Some reports estimated that, in 5–53% of patients with negative CE, rebleeding is expected (25, 26, 29). Other studies suggested that a negative CE could reliably predict a low rebleeding rate in the future (28, 55). More than 70% of OGIB patients with negative BAE had no rebleeding during the follow-up in the present study. This means that a close follow-up for patients with negative BAE may be appropriate. Our results support a watch-and-wait policy that does not recommend a second-look BAE unless there is strong evidence of rebleeding.
In the present study, 86.1% of patients with rebleeding underwent further evaluation, such as BAE repetition, CE, and CT, and the identification of the bleeding source was achieved in 73.6% of these patients. Currently, there are no guidelines to clarify the preferred diagnostic modality after a rebleeding event in a patient with a previously negative BAE. When an episode of rebleeding occurs, prompt endoscopy examination may find the bleeding site and allow therapeutic interventions in some cases. For patients who have rebleeding after OGIB episode, BAE is safe and effective to detect the origin of bleeding. Repeated BAE should be considered when a patient has rebleeding with negative BAE results during previous bleeding. In fact, in our study, 68.8% of the identified rebleeding lesions were in the small intestine. A thorough endoscopic examination could improve clinical outcomes in these patients. Apparently, in the presence of a rebleeding episode, the use of alternative non-invasive methods, such as CE and CT, may also contribute to lesion detection. Although CT usually has a lower diagnostic yield for OGIB compared to CE or BAE, it has already been shown that a repeat CT at the time of rebleeding may help to identify the source of rebleeding.
There are some limitations in the present study. The heterogeneity of the studies was significant. Much of the literature mixes patients with overt and occult obscure GI bleeding. These patients should be analyzed separately because they may have different prognoses. Many of the included studies did not intentionally evaluate outcomes after negative BAE. A study showed that enteroscopy with DBE had a higher complete enteroscopy rate and a higher diagnostic yield compared with SBE (56). Another report demonstrated that the complete enteroscopy rate is higher for DBE than for SBE (11). Most of the included studies adopted DBE to investigate OGIB, but in two studies, SBE was used which may influence the analyzing results.
In conclusion, the results of this meta-analysis may help decide the management of patients with OGIB and negative BAE. Our analysis shows that a negative BAE in patients with OGIB implies a subsequently low risk of rebleeding. Such patients can be closely followed up without further evaluation unless there is a rebleeding episode. Further investigation should be considered after rebleeding even if the initial BAE results are negative, as the diagnostic yield of further evaluation is more than 70% in these patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
XDS: conception and design and administrative support. YT, LW, and XDS: provision of study materials or patients. HTS, YGZ, LW, and XDS: collection and assembly of data, data analysis, and interpretation. HTS, YGZ, LW, YT, and XDS: manuscript writing and final approval of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.772954/full#supplementary-material
References
1. Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. (2001) 53:216–20. doi: 10.1067/mge.2001.112181
2. AGA: American Gastroenterological Association medical position statement: evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. (2000). 118:197–201. doi: 10.1016/S0016-5085(00)70429-X
3. Gralnek IM. Obscure-overt gastrointestinal bleeding. Gastroenterology. (2005) 128:1424-30. doi: 10.1053/j.gastro.2005.03.067
4. Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population based study. Am J Gastroenterol. (1997) 92:419–24.
5. Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am. (2005) 34:679–98. doi: 10.1016/j.gtc.2005.08.005
6. McLoughlin MT, Tham TC. Long-term follow-up of patients with iron deficiency anaemia after a negative gastrointestinal evaluation. Eur J Gastroenterol Hepatol. (2009) 21:872–6. doi: 10.1097/MEG.0b013e328321836c
7. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral. nutrition information system, 1993–2005. Public Health Nutr. (2009) 12:444–54. doi: 10.1017/S1368980008002401
8. van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, et al. Random comparison of guaiac and immunochemicalfecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. (2008) 135:82–90. doi: 10.1053/j.gastro.2008.03.040
9. Matsumoto T, Moriyama T, Esaki M, Nakamura S, Lida M. Performance of antegrade double-balloon enteroscopy: comparison with push enteroscopy. Gastrointest Endosc. (2005) 62:392–8. doi: 10.1016/j.gie.2005.04.052
10. May A, Nachbar L, Schneider M, Neumann M, EII C. Push-and-pull enteroscopy using the double-balloon technique: method of assessing depth of insertion and training of the enteroscopy technique using the Erlangen Endo-Trainer. Endoscopy. (2005) 37:66–70. doi: 10.1055/s-2004-826177
11. Wadhwa V, Sethi S, Tewani S, Garg SK, Pleskow DK, Chuttani R, et al. A meta-analysis on efficacy and safety: single-balloon vs. double-balloon enteroscopy. Gastroenterol Rep (Oxf). (2015) 3:148–55. doi: 10.1093/gastro/gov003
12. Zhi FC, Yue H, Jiang B, Xu ZM, Bai Y, Zhou DY, et al. Diagnostic value of double balloon enteroscopy for small-intestinal disease: experience from China. Gastrointest Endosc. (2007) 66:S19–21. doi: 10.1016/j.gie.2007.03.1047
13. Zhong J, Ma T, Zhang C, Sun B, Chen S, Cao Y, et al. A retrospective study of the application on double-balloon enteroscopy in 378 patients with suspected small-bowel diseases. Endoscopy. (2007) 39:208–15. doi: 10.1055/s-2007-966190
14. Cazzato IA, Cammarota G, Nista EC, Cesaro P, Sparano L, Bonomo V, et al. Diagnostic and therapeutic impact of double-balloon enteroscopy (DBE) in a series of100 patients with suspected small bowel diseases. Dig Liver Dis. (2007) 39:483–7. doi: 10.1016/j.dld.2007.01.019
15. Nakamura M, Niwa Y, Ohmiya N, Miyahara R, Ohashi A, Itoh A, et al. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy. (2006) 38:59–66. doi: 10.1055/s-2005-870446
16. Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, et al. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. (2006) 64:740–50. doi: 10.1016/j.gie.2006.05.022
17. Manabe N, Tanaka S, Fukumoto A, Nakao M, Kamino D, Chayama K. Double-balloon enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. (2006) 64:135–40. doi: 10.1016/j.gie.2005.12.020
18. Arakawa D, Ohmiya N, Nakamura M, Honda W, Shirai O, Itoh A, et al. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and video capsule endoscopy. Gastrointest Endosc. (2009) 69:866–74. doi: 10.1016/j.gie.2008.06.008
19. Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. (2011) 26:796–801. doi: 10.1111/j.1440-1746.2010.06530.x
20. Xin L, Liao Z, Jiang YP, Li ZS. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. (2011) 74:563–70. doi: 10.1016/j.gie.2011.03.1239
21. Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. (2007) 66:304–9. doi: 10.1016/j.gie.2007.02.044
22. May A, Friesing-Sosnik T, Manner H, Ell C. Long-term outcome after argon plasma coagulation of small-bowel lesions using double-balloon enteroscopy in patients with mid-gastrointestinal bleeding. Endoscopy. (2011) 43:759–65. doi: 10.1055/s-0030-1256388
23. Samaha E, Rahmi G, Landi B, Lorenceau-Savale C, Malamut G, Canard JM, et al. Long-term outcome of patients treated with double balloon enteroscopy for small bowel vascular lesions. Am J Gastroenterol. (2012) 107:240–6. doi: 10.1038/ajg.2011.325
24. Gerson LB, Batenic MA, Newsom SL, Ross A, Semrad CE. Long-term outcomes after double-balloon enteroscopy for obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. (2009) 7:664–9. doi: 10.1016/j.cgh.2009.01.021
25. Park JJ, Cheon JH, Kim HM, Park HS, Moon CM, Lee JH, et al. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc. (2010) 71:990–7. doi: 10.1016/j.gie.2009.12.009
26. Koh SJ, Im JP, Kim JW, Kim BG, Lee KL, Kim SG, et al. Long-term outcome in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastroenterol. (2013) 19:1632–8. doi: 10.3748/wjg.v19.i10.1632
27. Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. (2008) 68:1122–7. doi: 10.1016/j.gie.2008.06.054
28. Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. (2006) 101:1224–8. doi: 10.1111/j.1572-0241.2006.00565.x
29. Riccioni ME, Urgesi R, Cianci R, Rizzo G, D'Angelo L, Marmo R, et al. Negative capsule endoscopy in patients with obscure gastrointestinal bleeding reliable: recurrence of bleeding on long-term follow-up. World J Gastroenterol. (2013) 19:4520–5. doi: 10.3748/wjg.v19.i28.4520
30. Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. (2004) 36:1067–73. doi: 10.1055/s-2004-826034
31. Shinozaki S, Yamamoto H, Yano T, Sunada K, Hayashi Y, ShinhataH, et al. Favorable long term outcomes of repeat endotherapy for small-intestine vascular lesions by double-balloon endoscopy. Gastrointest Endosc. (2014) 80:112–7. doi: 10.1016/j.gie.2013.11.029
32. Rahmi G, Samaha E, Vahedi K, Delvaux M, Gay G, LamouliatteH, et al. Long-term follow-up of patients under-going capsule and double-balloon enteroscopy for identification and treatment of small bowel vascular lesions: a prospective, multicenter study Endoscopy. (2014) 46:591–7. doi: 10.1055/s-0034-1365514
33. Jeon SR, Byeon JS, Jang HJ, Park SJ, Im JP, Kim ER, et al. Clinical outcome after enteroscopy for small bowel angioectasia bleeding: a Korean Association for the Study of Intestinal Disease (KASID) multicenter study. J Gastroenterol Hepatol. (2017) 32:388–94. doi: 10.1111/jgh.13479
34. Pinho R, Mascarenhas-Saraiva M, Mão-de-Ferro S, Ferreira S, Almeida N, Figueiredo P, et al. Multicenter survey on the use of device-assisted enteroscopy in Portugal. United Eur Gastroenterol J. (2016) 4:264–74. doi: 10.1177/2050640615604775
35. Pinho R, Ponte A, Rodrigues A, Pinto-Pais T, Fernandes C, Ribeiro I, et al. Long-term rebleeding risk following endoscopic therapy of small-bowel vascular lesions with device-assisted enteroscopy. Eur J Gastroenterol Hepatol. (2016) 28:479–85. doi: 10.1097/MEG.0000000000000552
36. Ponte A, Pérez-Cuadrado Robles E, Pinho R, Rodrigues A, Delgado PE, Silva J, et al. High short-term rebleeding rate in patients undergoing a second endoscopic therapy for small-bowel angioectasias after recurrent bleeding. Rev Esp Enferm Dig. (2018) 110:88–93. doi: 10.17235/reed.2017.4872/2017
37. Shinozaki S, Yamamoto H, Yano T, Sunada K, Miyata T, Hayashi Y, et al. Long-term outcome of patients with obscure gastrointestinal bleeding investigated by double-balloon endoscopy. Clin Gastroenterol Hepatol. (2010) 8:151–8. doi: 10.1016/j.cgh.2009.10.023
38. Sun B, Rajan E, Cheng S, Shen R, Zhang C, Zhang S, et al. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am J Gastroenterol. (2006) 101:2011–5. doi: 10.1111/j.1572-0241.2006.00664.x
39. Shinozaki S, Yano T, Sakamoto H, Sunada K, Hayashi Y, Sato H, et al. Long-term outcomes in patients with overt obscure gastrointestinal bleeding after negative double balloon endoscopy. Dig Dis Sci. (2015) 60:3691–6. doi: 10.1007/s10620-015-3792-8
40. Hashimoto R, Matsuda T, Nakahori M. False-negative double-balloon enteroscopy in overt small bowel bleeding: long-term follow-up after negative results. Surg Endosc. (2019) 33:2635–41. doi: 10.1007/s00464-018-6561-x
41. Kushnir VM, Tang M, Goodwin J, Hollander TG, Hovis CE, Murad FM, et al. Long-term outcomes after single-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Dig Dis Sci. (2013) 58:2572–9. doi: 10.1007/s10620-013-2588-y
42. Fujimori S, Seo T, Gudis K, Tanaka S, Mitsui K, Kobayashi T, et al. Diagnosis and treatment of obscure gastrointestinal bleeding using combined capsule endoscopy and double balloon endoscopy: 1-year follow-up study. Endoscopy. (2007) 39:1053–8. doi: 10.1055/s-2007-967014
43. Hsu CM, Chiu CT, Su MY, Lin WP, Chen PC, Chen CH. The outcome assessment of double-balloon enteroscopy for diagnosing and managing patients with obscure gastrointestinal bleeding. Dig Dis Sci. (2007) 52:162–6. doi: 10.1007/s10620-006-9554-x
44. Madisch A, Schmolders J, Brückner S, Aust D, Miehlke S. Less favorable clinical outcome after diagnostic and interventional double balloon enteroscopy in patients with suspected small-bowel bleeding? Endoscopy. (2008) 40:731–4. doi: 10.1055/s-2008-1077521
45. Fujita M, Manabe N, Honda K, Tarumi K, Murao T, Katada S, et al. Long-term outcome after double-balloon endoscopy in patients with obscure gastrointestinal bleeding. Digestion. (2010) 82:173–8. doi: 10.1159/000313360
46. Shishido T, Oka S, Tanaka S, Imagawa H, Takemura Y, Yoshida S, et al. Outcome of patients who have undergone total enteroscopy for obscure gastrointestinal bleeding. World J Gastroenterol. (2012) 18:666–72. doi: 10.3748/wjg.v18.i7.666
47. Zhao L, Yin A, Liao F, Ding Y, Yu H. Inspecting the total gastrointestinal tract by consecutive bidirectional double-balloon enteroscopy in patients with suspected small bowel bleeding. Turk J Gastroenterol. (2020) 31:688–94. doi: 10.5152/tjg.2020.19387
48. Gomes C, Rubio Mateos JM, Pinho RT, Ponte A, Rodrigues A, Fosado Gayosso M, et al. The rebleeding rate inpatients evaluated for obscure gastrointestinal bleeding after negative small bowel findings by device assisted enteroscopy. Rev Esp Enferm Dig. (2020) 112:262–8. doi: 10.17235/reed.2020.6833/2019
49. Aniwan S, Viriyautsahakul V, Rerknimitr R, AngsuwatcharakonP, Kongkam P, Treeprasertsuk S, et al. Urgent double balloon endoscopy provides higher yields than non-urgent double balloon endoscopy in overt obscure gastrointestinal bleeding. Endosc Int Open. (2014) 2:E90–5. doi: 10.1055/s-0034-1365543
50. Pinto-Pais T, Pinho R, Rodrigues A, Fernandes C, Ribeiro I, FragaJ, et al. Emergency single-balloon enteroscopy in overt obscure gastrointestinal bleeding: efficacy and safety. United Eur Gastroenterol J. (2014) 2:490–6. doi: 10.1177/2050640614554850
51. Monkemuller K, Neumann H, Meyer F, Kuhn R, Malfertheiner P, Fry LC, et al. retrospective analysis of emergency double-balloon enteroscopy for small-bowel bleeding. Endoscopy. (2009) 41:715–7. doi: 10.1055/s-0029-1214974
52. Chu Y, Wu S, Qian Y, Wang Q, Li J, Tang Y, et al. Complimentary imaging modalities for investigating obscure gastrointestinal bleeding: capsule endoscopy, double balloon enteroscopy, and computed tomographic enterography. Gastroenterol Res Pract. (2016) 2016:8367519. doi: 10.1155/2016/8367519
53. Agrawal JR, Travis AC, Mortele KJ, Silverman SG, Maurer R, Reddy SI, et al. Diagnostic yield of dual-phase computed tomography enterography in patients with obscure gastrointestinal bleeding and a non-diagnostic capsule endoscopy. J Gastroenterol Hepatol. (2012) 27:751–9. doi: 10.1111/j.1440-1746.2011.06959.x
54. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. (2010) 16:3957–63. doi: 10.3748/wjg.v16.i31.3957
55. Lorenceau-Savale C, Ben-Soussan E, Ramirez S, Antonietti M, Lerebours E, Ducrotte P. Outcome of patients with obscure gastrointestinal bleeding after negative capsule endoscopy: results of a one-year follow-up study. Gastroenterol Clin Biol. (2010) 34:606–11. doi: 10.1016/j.gcb.2010.06.009
Keywords: obscure gastrointestinal bleeding, small intestine, enteroscopy, rebleeding, follow-up
Citation: Shao XD, Shao HT, Wang L, Zhang YG and Tian Y (2022) Clinical Outcomes of Negative Balloon-Assisted Enteroscopy for Obscure Gastrointestinal Bleeding: A Systematic Review and Meta-Analysis. Front. Med. 9:772954. doi: 10.3389/fmed.2022.772954
Received: 09 September 2021; Accepted: 31 January 2022;
Published: 04 March 2022.
Edited by:
Murat Toruner, Ankara University, TurkeyReviewed by:
Shubhra Mishra, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaDong Wu, Peking Union Medical College Hospital (CAMS), China
Copyright © 2022 Shao, Shao, Wang, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Dong Shao, sxdsys608@189.cn
†These authors have contributed equally to this work and share first authorship
 Xiao Dong Shao
Xiao Dong Shao Hao Tian Shao2†
Hao Tian Shao2†