- 1Department of Hospice, Sheng Jing Hospital of China Medical University, Shenyang, China
- 2Department of Ultrasound, Sheng Jing Hospital of China Medical University, Shenyang, China
Background: Uterine cystic adenomyosis is a very rare type of adenomyosis which can be easily misdiagnosed in clinical practice. In the past, cases have been mostly treated with surgical resection of the uterine lesion.
Case Presentation: We report the case of a 25-year-old woman who presented with severe dysmenorrhea for more than 1 year. Physical examination showed that the uterus was enlarged. The transvaginal ultrasound showed a cystic mass of about 5.0 × 3.6 × 3.6 cm in the posterior myometrium, with dense echo spots and no blood flow signal in the cystic part. Magnetic resonance imaging (MRI) indicated hemorrhages within the cystic mass, suggesting the possibility of uterine cystic adenomyosis. The lower abdominal pain and severe dysmenorrhea were not alleviated after a 6-month trial of oral contraceptives. Subsequently, she underwent ultrasound-guided transvaginal aspiration and sclerotherapy for uterine cystic adenomyosis. Approximately 90 mL of chocolate-colored fluid was aspirated from the mass and 20 mL of lauromacrogol was injected in the cyst. The reduction rates of the mass 3 and 12 months after the procedure were 92.01 and 99.10%, respectively. Her dysmenorrhea completely resolved. One and half year after the operation, she had a successful pregnancy and gave birth to a healthy baby through vagina.
Conclusion: The rare entity of uterine cystic adenomyosis can be treated safely and effectively by ultrasound-guided transvaginal aspiration and sclerotherapy.
Introduction
Adenomyosis is a common gynecological condition characterized by the abnormal presence of endometrial glands and stroma within the myometrium (1). Menstrual bleeding within the ectopic endometrial tissue can lead to cystic foci. These cystic foci are usually small, with the largest diameter usually being <5 mm (2). However, in very rare cases, the cystic foci acquire diameters >1 cm thus constituting uterine cystic adenomyosis (3). The endometrial-like tissue in uterine cystic adenomyosis sheds with the menstrual cycle, leading to hemorrhagic infarction of the adjacent smooth muscle, and accumulation of bloody fluid that increases the volume of the cyst. Enlarged cysts can cause symptoms such as menorrhagia, infertility, pelvic pain, and severe dysmenorrhea (3). These symptoms are often not effectively controlled pharmacologically and need to be treated by surgical removal of the uterine lesion.
In this report, we present a rare case of uterine cystic adenomyosis that was treated by ultrasound-guided transvaginal aspiration and sclerotherapy. The diagnosis and treatment strategies for uterine cystic adenomyosis are discussed by summarizing and analyzing relevant literature over the past 30 years.
Case Presentation
A 25-year-old woman presented to our hospital with “severe dysmenorrhea for more than 1 year” as the main complaint. She had a regular menstrual cycle of 28 days. During her menstrual period, she experienced lower abdominal pain and occasional back pain. In the past year, her dysmenorrhea had gradually deteriorated. Routine blood tests were normal, and serum tumor markers were not tested. She denied having a history of malignancy, endometriosis, genetic and psychosocial diseases, or prior surgeries.
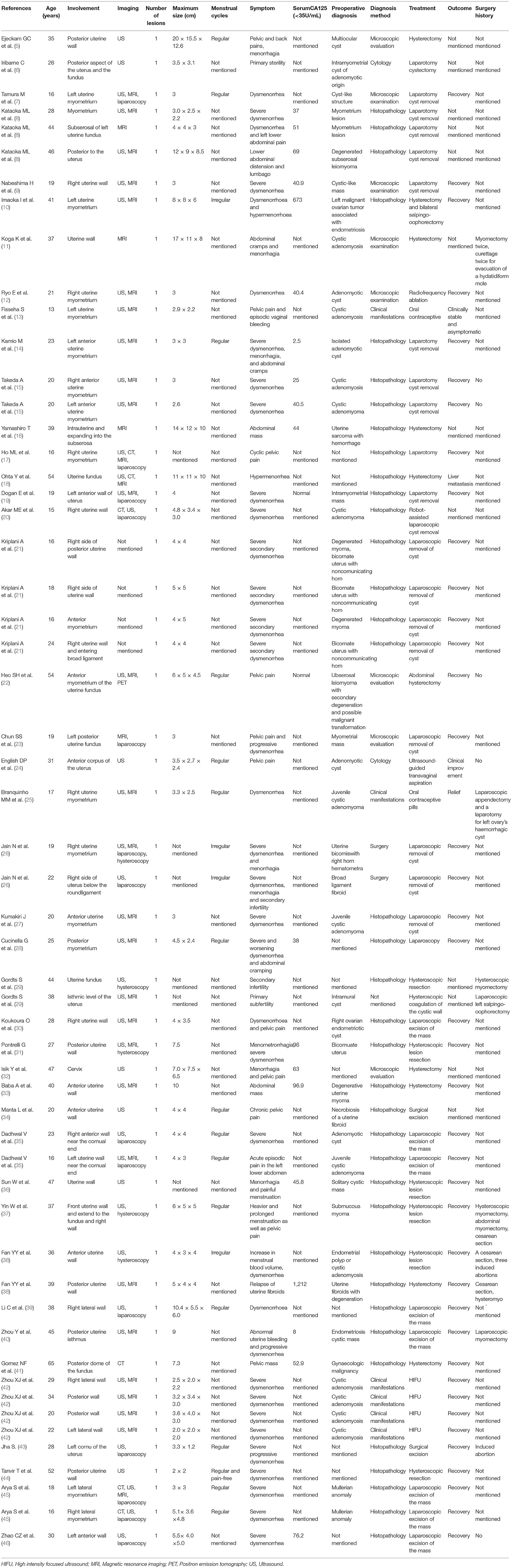
Physical examination showed that the uterus was enlarged and slightly hard on palpation, and the posterior wall protruded outward locally. Transabdominal ultrasound in another hospital suggested a mass, not otherwise specified, in the uterine wall; therefore, a transvaginal ultrasound examination was scheduled. Transvaginal ultrasound showed that the uterus was enlarged, and a cystic mass, well-circumscribed and ellipsoid, ~5.0 × 3.6 × 3.6 cm in size was identified in the posterior uterine myometrium (Figure 1). Dense echo spots were observed in the liquid. Color Doppler flow detected weak blood flow in the cystic wall, but no blood flow within the cyst. The myometrium around the mass was compressed and thinned. The shape of the uterine cavity was normal, and the cystic mass was well separated from the uterine cavity. Bilateral ovaries were normal without space-occupying lesions. We considered the uterine cystic mass to be the result of prior hemorrhage. We, therefore, proceeded with magnetic resonance imaging (MRI) to further clarify the nature of the mass. MRI showed that the oval-like cystic mass was located in the posterior wall of the uterus, with a regular shape and smooth and clear boundaries (Figure 2). The cystic part showed a hyperintense signal on both T1-weighted and T-2 weighted images, with a few flocculent equal signals. There was no obvious enhancement in the mass on enhanced scanning. MRI also indicated hemorrhages within the cystic mass, suggesting the possibility of uterine cystic adenomyosis. Combined with the patient's history of severe dysmenorrhea, the characteristics of transvaginal ultrasound and MRI, a diagnosis of uterine cystic adenomyosis was established.
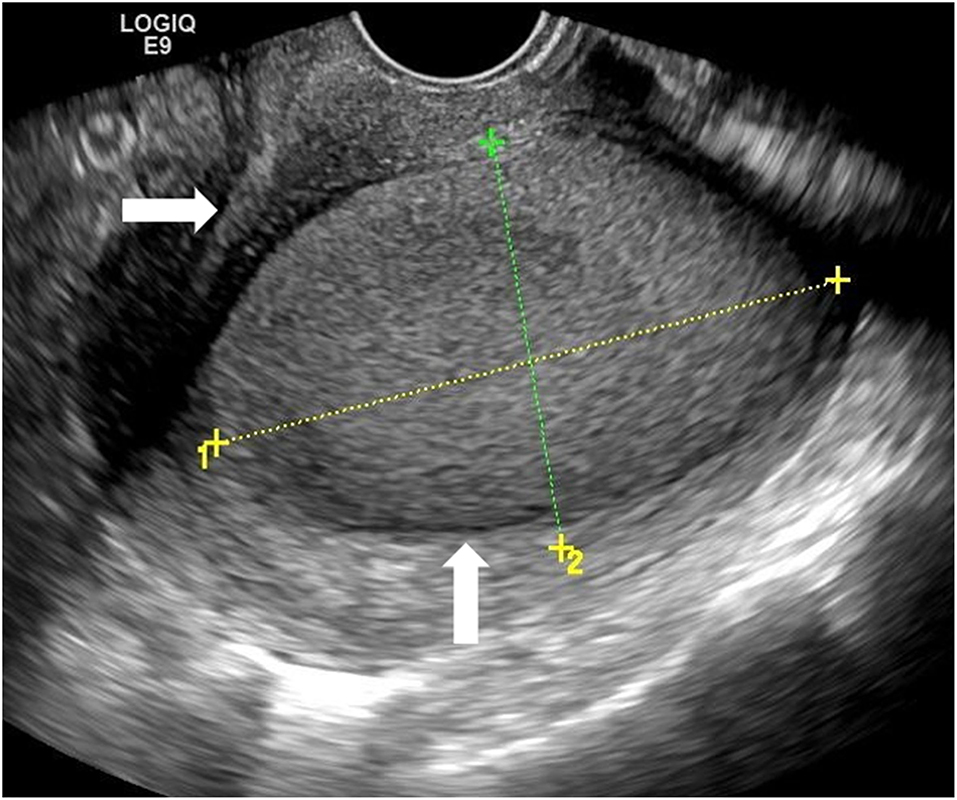
Figure 1. Transvaginal ultrasound showed a cystic mass (vertical arrow) in the posterior myometrium, accompanied by dense echo spots. The uterine cavity (horizontal arrow) did not communicate with the mass.
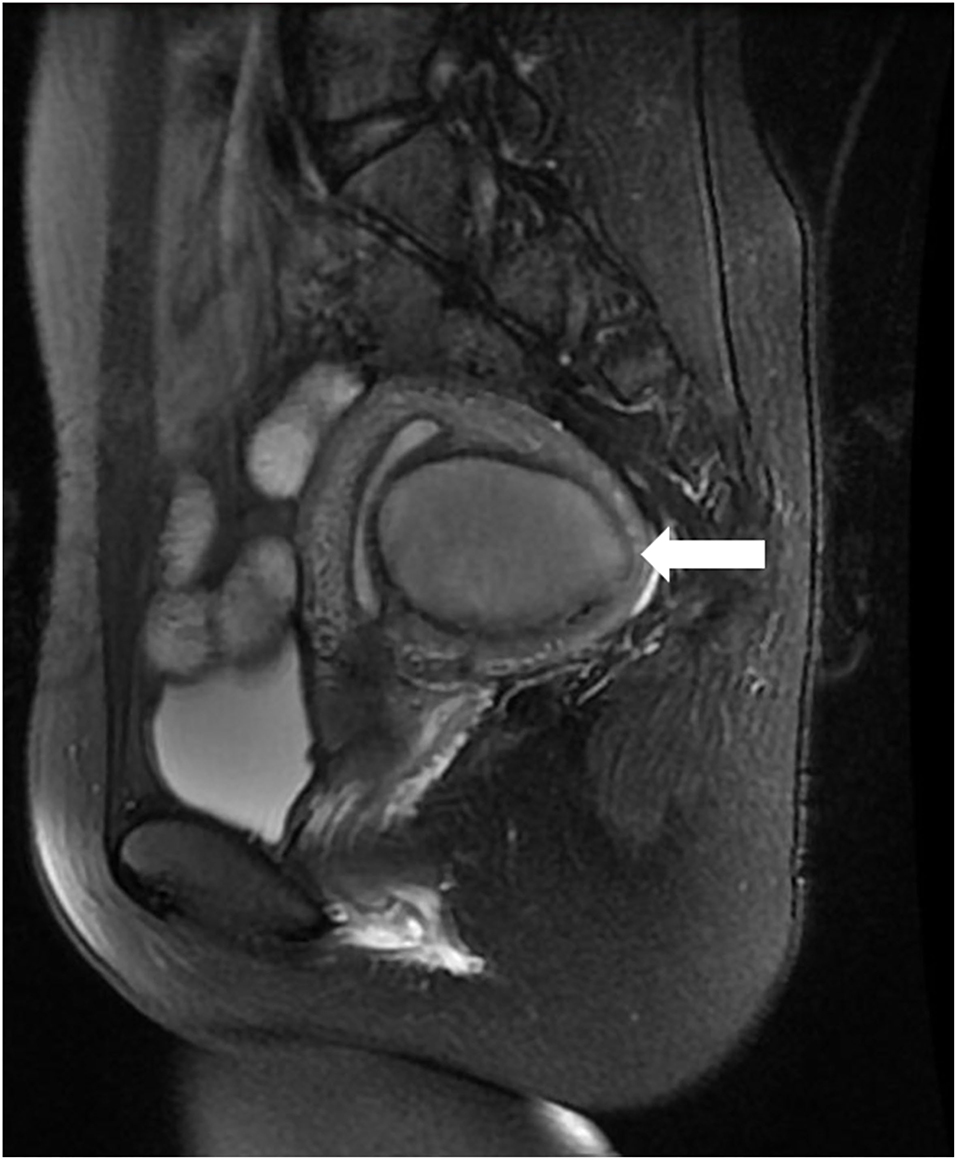
Figure 2. The mass (horizontal arrow) showed hyperintense signal on T-2 weighted Magnetic resonance imaging (MRI) image. It was protruding outward compressing the endometrium but not communicating with the uterine cavity.
The patient was started on continuous oral contraceptive (OC) pills for 6 months, without, however, improvement of her lower abdominal pain and severe dysmenorrhea. Six months later, ultrasound examination showed that the mass had increased to 7.5 × 5.4 × 4.8 cm. At that point surgical treatment was recommended. Considering that the patient was a young woman who might wish to become pregnant in the future, several uterine-preserving treatment methods were discussed with her in detail, among whom she opted for the method of ultrasound-guided transvaginal aspiration and sclerotherapy and provided informed consent. The research protocol was approved by the medical ethics committee of our hospital and participants gave written informed consent, according to CARE guidelines and in compliance with the Declaration of Helsinki principles. Transvaginal sclerotherapy was performed for uterine cystic adenomyosis under ultrasound guidance on the 10th day of the menstrual cycle. The patient was placed in the lithotomy position, and 2% lidocaine was administered locally. A percutaneous transhepatic cholangiography (PTC) needle (18G) was inserted into the cystic mass, and the position of the needle tip was verified by ultrasound. Approximately 90 mL of chocolate-colored fluid was aspirated from the mass, and part of the fluid was sent for pathological examination. The cyst cavity was repeatedly flushed with normal saline until the color of the aspiration fluid was clear, and all the fluid from the cyst was drained. While flushing the cyst cavity, we observed that there was no leakage of normal saline in the uterus, thus indicating lack of communication between the cyst and the uterine cavity. Lauromacrogol injection (10 ml, 0.1 g) was used for sclerotherapy treatment. Two branches of Lauromacrogol injections (20 ml) were injected into the cyst through a PTC needle and finally retained. The procedure lasted 45 min and the patient was observed for about 4 hours after the operation. During and after the injection, the patient had no discomfort and no adverse events.
Cytological examination of the aspirate revealed hemosiderin-laden macrophages, without tumor cells, epithelial or mesenchymal components. The chocolate-like appearance and cytological characteristics of the aspirated contents confirmed the diagnosis of uterine cystic adenomyosis.
Three months after the procedure, ultrasound examination showed that the mass had decreased to 3.4 × 2.2 × 2.0 cm, corresponding to a volume reduction rate of 92.01%. A small fluid collection was still visible. One year after the operation, the mass had decreased to 1.4 × 1.2 × 1.0 cm, corresponding to a volume reduction rate of 99.10%, and it was moderately echogenic, with no fluid present, appearing similar to a scar-like structure (Figure 3). The thickness and echo of the myometrium around the mass were normal, and the thickness of the endometrium was within the normal range. After the operation, the patient had regular menstruation and no symptoms of dysmenorrhea or abdominal pain. Based on the improvement of the patient's clinical symptoms and imaging manifestations, the operator believed that the effect of this treatment was very significant. One and half year after the operation, the patient became pregnant and successfully delivered a healthy baby through vagina at 40 weeks of pregnancy in our hospital. We recommended that the patient undergo MRI of the uterus before and after delivery, but she refused. Ultrasonography showed no cyst formation in the uterine wall. The patient never experienced delivery complications and any sclerotherapy-related adverse events, she was very satisfied with the treatment.
Figure 3. Transvaginal ultrasound showed that the mass (horizontal arrow) was significantly decreased 1 year after operation, with moderate echo and no fluid collection.
Discussion
In 1908, Cullen first described uterine cystic lesions filled with chocolate-colored fluid (4). As a special type of endometriosis, the incidence of uterine cystic adenomyosis is low, and related literature reports in the past 30 years are shown in Table 1 (5–46). Terms often used in the literature include juvenile cystic adenomyosis (JCA), cystic myometrial lesions, juvenile adenomyotic cysts, uterine cystic adenomyosis, adenomyotic cyst of the uterus, adenomyotic cyst, intramyometrial cystic adenomyosis, or intrauterine cystic adenomyosis. Compared with adenomyosis, the age of onset of this cystic form is younger, with the average age in the literature being 29.5 years, and 60.71% (34/56) of the patients being younger than 30 years. The most common symptoms are severe dysmenorrhea and pelvic pain, while some patients also experience irregular menstruation. Dysmenorrhea in women with uterine cystic adenomyoma can be explained by the progressive increase in cyst size resulting from repeated intra-cystic bleeding during menstruation.
The cause of uterine cystic adenomyosis is unclear, and researchers have proposed different theories. Uterine cystic adenomyosis is divided into two types according to the age of onset: juvenile and adult, with different etiologies. Some researchers have suggested that JCA results from developmental defects of the Mullerian ducts, leading to duplication or persistence of paramesonephric tissue (15, 47). Takeuchi et al. considered it to be a cystic variant of adenomyosis (48). The pathogenesis of the adult type is different from that of the juvenile type, and one hypothesis, accepted by most researchers, is the endometrial injury invagination theory (49). Previous uterine surgery and injury to the endometrial-myometrial junction may be the pathological basis of the disease (43). A previous history of miscarriage, parity, and curettage is associated with high risk of endometrial and myometrial injury. The resultant damage in the junction between endometrium and myometrium can cause secondary adenomyosis, which occasionally evolves into uterine cystic adenomyosis (39). Previous reports have identified eight cases of uterine cystic adenomyosis developing in the presence of the above-mentioned high-risk factors (11, 29, 37, 38, 40, 43). However, the present case had not previously undergone any surgery.
All cases of uterine cystic adenomyosis involved single lesions. At present, there are no reports of multiple lesions in the literature. The lesions can involve the uterine body, cervix, and fundus, with the uterine body and especially the right wall being the most common (41/56, 73.21% and 13/41, 31.71%, respectively), The size of the lesions varies from 2 to 20 cm, with an average diameter of ~5.5 cm. The present case was also a single lesion involving the posterior wall of the uterus, with a diameter greater than the average.
Transvaginal ultrasonography is the preferred method of examination for gynecological diseases; MRI also plays an important role in the evaluation of these cystic lesions, and was used in ~59% of cases (33/56 cases). Transvaginal ultrasonography can determine the location of the mass, distinguish between the cystic and solid components, and whether they are separated from the normal uterine cavity. The cystic part of the uterine cystic adenomyosis is mostly accompanied by dense echo spots, with moderate echoes in the cyst wall. The typical MRI findings in uterine cystic adenomyosis are a well-defined cystic lesion filled with hemorrhagic fluid in the myometrium. The liquid part of the cyst shows a hyperintense signal on both T1-weighted and T-2 weighted images, and the cystic wall shows low signal on T2 weighted images. The rim of hemosiderin in uterine cystic adenomyosis is represented by a hypointense signal on both T1-weighted and T-2 weighted images (25). The differential diagnosis includes congenital anomaly with hematometra in a Non-communicating horn, congenital uterine cysts, intramyometrial hydrosalpinx, and fibroid degeneration. In hemorrhagic hysteromyoma, methemoglobin accumulates in the periphery, producing a T1-hyperintense and T2-hypointense rim, which is different from the hypointense rim of hemosiderin in uterine cystic adenomyosis. Adipose tissue can be distinguished by a fat suppression sequence to exclude the possibility of steatosis of leiomyoma (50). Congenital uterine cysts and hydrosalpinx in the myometrium can also present as cystic lesions in the myometrium. However, the fluid composition of these lesions is simple, and the cysts lack a hemosiderin rim. MRI is also helpful in identifying complex uterine malformations (51). In cases of cystic masses of the uterine myometrium, without adequate visualization of both uterine horns on MRI or ultrasonography, it is necessary to rule out isolated congenital anomalies with hematometra in a Non-communicating horn. Hysterosalpingography may be useful, if necessary.
Elevated serum CA-125 has been proposed as a diagnostic tool for uterine cystic adenomyosis, but its specificity and sensitivity are low (38). Only 16 of the published cases reported serum CA-125 levels which were slightly increased.
It should be noted that although uterine cystic adenomyosis is a benign disease, there are three reports in the literature of malignant tumors originating from this disease (18, 33, 41). Among the three cases of cystic mass, one case had a solid component, one was nodular, and one had multiple excrescences (18, 33, 41). The patients were 40, 54, and 65 years of age, respectively. Therefore, the possibility of malignant tumors needs to be ruled out especially in older patients with solid components of cystic lesions. In addition to MRI and ultrasound, positron emission tomography (PET) can assist in the differential diagnosis by providing information on the metabolic activity of the lesion. With 18F- fluorodeoxyglucose (FDG) PET, FDG generally accumulates in malignant lesions due to high glucose metabolism. Most malignant uterine tumors, such as endometrial cancer, cervical cancer, and uterine sarcoma, usually show intense FDG uptake (52).
Inhibition of menstruation with continuous OC, gonadotropin-releasing hormone analogs, and Non-steroidal anti-inflammatory drugs may provide temporary and partial pain relief in uterine cystic adenomyosis, but symptoms may relapse after withdrawal. Because many patients with uterine cystic adenomyosis are young and wish pregnancy, minimally invasive surgery to preserve the uterus is desirable. Laparoscopic surgical resection is suitable for lesions in the myometrium, close to the serosal layer. Laparoscopic resection can significantly improve related dysmenorrhea and increase the possibility of successful pregnancy (39, 46). Robot-assisted laparoscopic management allows an optimal view and more efficient multilayer closure of the hysterotomy, thereby increasing the safety of future pregnancies (20). Hysteroscopic surgery is another minimally invasive treatment. Hysteroscopic resection of the lesion is the preferred mode of treatment for the submucosal subtype (38). However, laparoscopic or hysteroscopic surgery destroys the muscle layer surrounding the lesion in isolated cystic adenomyosis, thus increasing the risk of obstetric complications, including an increased risk of uterine rupture during pregnancy. It is also difficult to avoid the occurrence of new iatrogenic endometriosis during surgery (42). Zhou et al. reported four cases of cystic adenomyosis treated with high-intensity focused ultrasound, with resultant disappearance of dysmenorrhea and high patient satisfaction (42). Koga et al. reported a case treated with four vaginal aspirations, followed by infusions of ethanol, minocycline, and danazol without cure (11). Ryo et al. described a case treated with radiofrequency ablation with disappearance of the cystic lesion and symptomatic improvement (12).
Ethanol sclerotherapy of ovarian endometrioma has been proven to be a safe and effective minimally invasive procedure (53, 54). The pathological feature of cystic adenomyosis is endometriosis. Therefore, the successful use of ethanol sclerotherapy for ovarian endometrioma can be extended to sclerotherapy for uterine cystic adenomyosis. The reported incidence of abdominal pain during ethanol sclerotherapy for endometriotic ovarian cysts is 1.8–15.3% (55). The major ingredient of lauromacrogol is polyoxyethylene lauryl ether, in addition to ethanol and sterilized water. It is not only a type of foam sclerotherapy, but it also functions as a local anesthetic (56), which is widely used clinically. Therefore, the drug not only has a therapeutic effect, but also alleviates discomfort during treatment. Furthermore, the injection of lauromacrogol is easier and safer than the injection of absolute alcohol. Xu et al. indicated that lauromacrogol sclerotherapy is safe and effective in patients with hepatic cysts (57). A preliminary experimental study showed that lauromacrogol injection produced significant regression of endometrial foci (58). Ultrasound-guided aspiration sclerotherapy using lauromacrogol is a successful and effective treatment for refractory long-course ovarian endometrial cysts (59). In the present case, the functional endometrial tissue within the lesion was completely ablated by lauromacrogol, and the muscle layer surrounding the lesion was not damaged, retaining the integrity of the uterine wall. In previous literature the follow-up time was relatively short, and information on pregnancy and childbirth was generally missing. Therefore, we cannot judge whether the operation has an impact on the uterus during pregnancy. In our case, the long-term follow-up of our patient from procedure to pregnancy and childbirth proved that lauromacrogol is safe and effective in the treatment of uterine cystic adenomyosis. Additionally, the cost of aspiration and sclerotherapy is very low, which makes it a good choice for patients who want to reduce healthcare expenses.
Our report is limited by the fact that the cystic lesion was not surgically removed; therefore, immunohistochemical examination could not be performed. However, the combination of clinical symptoms, imaging findings and cytology are in agreement with previous literature and fully support a diagnosis of uterine cystic adenomyosis.
Conclusion
In conclusion, uterine cystic adenomyosis is rare and can be easily misdiagnosed. Transvaginal ultrasound and MRI are of great value for diagnosis. Ultrasound-guided transvaginal aspiration and sclerotherapy for uterine cystic adenomyosis can not only effectively treat the lesion, but also preserve the integrity of the uterine wall and minimize the risk of uterine rupture during pregnancy. The present case is the first report of ultrasound-guided transvaginal aspiration and lauromacrogol sclerotherapy for uterine cystic adenomyosis with long term follow-up, and proves that it is a safe and effective minimally invasive treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the participant for the publication of this case report. Ethical approval was given by the Medical Ethics Committee of our hospital.
Author Contributions
YY diagnosed and treated the patient. All authors wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the patient for agreeing and providing her case history. We would like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.764523/full#supplementary-material
References
1. Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. (2016) 23:164–85. doi: 10.1016/j.jmig.2015.09.018
2. Protopapas A, Milingos S, Markaki S, Loutradis D, Haidopoulos D, Sotiropoulou M, et al. Cystic uterine tumors. Gynecol Obstet Invest. (2008) 65:275–80. doi: 10.1159/000113871
3. Brosens I, Gordts S, Habiba M, Benagiano G. Uterine cystic adenomyosis: a disease of younger women. J Pediatr Adolesc Gynecol. (2015) 28:420–6. doi: 10.1016/j.jpag.2014.05.008
5. Ejeckam GC, Zeinab OA, Salman M, Bobeck HE. Giant adenomyotic cyst of the uterus. Br J Obstet Gynaecol. (1993) 100:596–8. doi: 10.1111/j.1471-0528.1993.tb15318.x
6. Iribarne C, Plaza J, de la Fuente P, Garrido C, Garzon A, Olaizola JI. Intramyometrial cystic adenomyosis. J Clin Ultrasound. (1994) 22:348–50. doi: 10.1002/jcu.1870220511
7. Tamura M, Fukaya T, Takaya R, Ip CW, Yajima A. Juvenile adenomyotic cyst of the corpus uteri with dysmenorrhea. Tohoku J Exp Med. (1996) 178:339–44. doi: 10.1620/tjem.178.339
8. Kataoka ML, Togashi K, Konishi I, Hatabu H, Morikawa K, Kojima N, et al. MRI of adenomyotic cyst of the uterus. J Comput Assist Tomogr. (1998) 22:555–9. doi: 10.1097/00004728-199807000-00010
9. Nabeshima H, Murakami T, Terada Y, Noda T, Yaegashi N, Okamura K. Total laparoscopic surgery of cystic adenomyoma under hydroultrasonographic monitoring. J Am Assoc Gynecol Laparosc. (2003) 10:195–9. doi: 10.1016/S1074-3804(05)60298-8
10. Imaoka I, Kaji Y, Kobashi Y, Wada A, Honjo G, Hayashi M, et al. Cystic adenomyosis with florid glandular differentiation mimicking ovarian malignancy. Br J Radiol. (2005) 78:558–61. doi: 10.1259/bjr/82283833
11. Koga K, Osuga Y, Hiroi H, Oishi H, Kugu K, Yano T, et al. Images in reproductive medicine. A case of giant cystic adenomyosis. Fertil Steril. (2006) 85:748–9. doi: 10.1016/j.fertnstert.2005.11.028
12. Ryo E, Takeshita S, Shiba M, Ayabe T. Radiofrequency ablation for cystic adenomyosis: a case report. J Reprod Med. (2006) 51:427–30
13. Fisseha S, Smith YR, Kumetz LM, Mueller GC, Hussain H, Quint EH. Cystic myometrial lesion in the uterus of an adolescent girl. Fertil Steril. (2006) 86:716–8. doi: 10.1016/j.fertnstert.2006.03.019
14. Kamio M, Taguchi S, Oki T, Tsuji T, Iwamoto I, Yoshinaga M, et al. Isolated adenomyotic cyst associated with severe dysmenorrhea. J Obstet Gynaecol Res. (2007) 33:388–91. doi: 10.1111/j.1447-0756.2007.00543.x
15. Takeda A, Sakai K, Mitsui T, Nakamura H. Laparoscopic management of juvenile cystic adenomyoma of the uterus: report of two cases and review of the literature. J Minim Invasive Gynecol. (2007) 14:370–4. doi: 10.1016/j.jmig.2007.01.005
16. Yamashiro T, Gibo M, Utsunomiya T, Murayama S. Huge uterine leiomyoma with adenomyotic cysts mimicking uterine sarcoma on MR imaging. Radiat Med. (2007) 25:127–9. doi: 10.1007/s11604-006-0106-2
17. Ho ML, Ratts V, Merritt D. Adenomyotic cyst in an adolescent girl. J Pediatr Adolesc Gynecol. (2009) 22:e33–8. doi: 10.1016/j.jpag.2008.05.011
18. Ohta Y, Hamatani S, Suzuki T, Ikeda K, Kiyokawa K, Shiokawa A, et al. Clear cell adenocarcinoma arising from a giant cystic adenomyosis: a case report with immunohistochemical analysis of laminin-5 gamma2 chain and p53 overexpression. Pathol Res Pract. (2008) 204:677–82. doi: 10.1016/j.prp.2008.02.005
19. Dogan E, Gode F, Saatli B, Seçil M. Juvenile cystic adenomyosis mimicking uterine malformation: a case report. Arch Gynecol Obstet. (2008) 278:593–5. doi: 10.1007/s00404-008-0618-3
20. Akar ME, Leezer KH, Yalcinkaya TM. Robot-assisted laparoscopic management of a case with juvenile cystic adenomyoma. Fertil Steril. (2010) 94:e55–6. doi: 10.1016/j.fertnstert.2010.06.001
21. Kriplani A, Mahey R, Agarwal N, Bhatla N, Yadav R, Singh MK. Laparoscopic management of juvenile cystic adenomyoma: four cases. J Minim Invasive Gynecol. (2011) 18:343–8. doi: 10.1016/j.jmig.2011.02.001
22. Heo SH, Lee KH, Kim JW, Jeong YY. Unusual manifestation of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis of the uterus: emphasis on MRI and positron emission tomography CT findings. Br J Radiol. (2011) 84:e210–2. doi: 10.1259/bjr/24318075
23. Chun SS, Hong DG, Seong WJ, Choi MH, Lee TH. Juvenile cystic adenomyoma in a 19-year-old woman: a case report with a proposal for new diagnostic criteria. J Laparoendosc Adv Surg Tech A. (2011) 21:771–4. doi: 10.1089/lap.2011.0014
24. English DP, Verma U, Pearson JM. Uterine cyst as a cause of chronic pelvic pain: a case report. J Reprod Med. (2012) 57:446–8.
25. Branquinho MM, Marques AL, Leite HB, Silva IS. Juvenile cystic adenomyoma. BMJ Case Rep. (2012) 2012:bcr2012007006. doi: 10.1136/bcr-2012-007006
26. Jain N, Goel S. Cystic Adenomyoma simulates uterine malformation: a diagnostic dilemma: CASE report of two unusual cases. J Hum Reprod Sci. (2012) 5:285–8. doi: 10.4103/0974-1208.106342
27. Kumakiri J, Kikuchi I, Sogawa Y, Jinushi M, Aoki Y, Kitade M, et al. Single-incision laparoscopic surgery using an articulating monopolar for juvenile cystic adenomyoma. Minim Invasive Ther Allied Technol. (2013) 22:312–5. doi: 10.3109/13645706.2013.789060
28. Cucinella G, Billone V, Pitruzzella I, Lo Monte AI, Palumbo VD, Perino A. Adenomyotic cyst in a 25-year-old woman: case report. J Minim Invasive Gynecol. (2013) 20:894–8. doi: 10.1016/j.jmig.2013.04.022
29. Gordts S, Campo R, Brosens I. Hysteroscopic diagnosis and excision of myometrial cystic adenomyosis. Gynecol Surg. (2014) 11:273–8. doi: 10.1007/s10397-014-0861-5
30. Koukoura O, Kapsalaki E, Daponte A, Pistofidis G. Laparoscopic treatment of a large uterine cystic adenomyosis in a young patient. BMJ Case Rep. (2015) 2015:bcr2015210358. doi: 10.1136/bcr-2015-210358
31. Pontrelli G, Bounous VE, Scarperi S, Minelli L, di Spiezio Sardo A, Florio P. Rare case of giant cystic adenomyoma mimicking a uterine malformation, diagnosed and treated by hysteroscopy. J Obstet Gynaecol Res. (2015) 41:1300–4. doi: 10.1111/jog.12698
32. Isik Y, Dag ZO, Celik H. A giant adenomyotic cyst originating from the cervix. Int J Gynaecol Obstet. (2015) 131:205–6. doi: 10.1016/j.ijgo.2015.05.025
33. Baba A, Yamazoe S, Dogru M, Ogawa M, Takamatsu K, Miyauchi J. Clear cell adenocarcinoma arising from adenomyotic cyst: a case report and literature review. J Obstet Gynaecol Res. (2016) 42:217–23. doi: 10.1111/jog.12866
34. Manta L, Suciu N, Constantin A, Toader O, Popa F. Focal adenomyosis (intramural endometriotic cyst) in a very young patient - differential diagnosis with uterine fibromatosis. J Med Life. (2016) 9:180–2
35. Dadhwal V, Sharma A, Khoiwal K. Juvenile cystic adenomyoma mimicking a uterine anomaly: a report of two cases. Eurasian J Med. (2017) 49:59–61. doi: 10.5152/eurasianjmed.2017.17028
36. Sun W, Guo X, Zhu L, Fei X, Zhang Z, Li D. Hysteroscopic treatment of a uterine cystic adenomyosis. J Minim Invasive Gynecol. (2018) 25:374–5. doi: 10.1016/j.jmig.2017.07.015
37. Yin W, Zhang J, Xu L, Luo L. Intrauterine endometrial cyst after low uterine incision: a case report with literature review. Medicine. (2018) 97:e0376. doi: 10.1097/MD.0000000000010376
38. Fan YY, Liu YN, Li J, Fu Y. Intrauterine cystic adenomyosis: Report of two cases. World J Clin Cases. (2019) 7:676–83. doi: 10.12998/wjcc.v7.i5.676
39. Li C, Xu Y, Cong L. Laparoscopic treatment of a large cystic adenomyosis of the uterus: a case report. Int J Surg Case Rep. (2020) 71:179–82. doi: 10.1016/j.ijscr.2020.04.084
40. Zhou Y, Chen ZY, Zhang XM. Giant exophytic cystic adenomyosis with a levonorgestrel containing intrauterine device out of the uterine cavity after uterine myomectomy: a case report. World J Clin Cases. (2020) 8:188–93. doi: 10.12998/wjcc.v8.i1.188
41. Gomez NF, Still MA, Akki AS, Carbajal-Mamani SL, Cardenas-Goicoechea J. Uterine clear cell carcinoma arising from cystic adenomyosis: a case report. J Obstet Gynaecol. (2021) 22:1–4. doi: 10.1080/01443615.2020.1847056
42. Zhou XJ, Zhao ZM, Liu P, Zhao CY, Lin YJ, Liu Y, et al. Efficacy of high intensity focused ultrasound treatment for cystic adenomyosis: a report of four cases. Ann Palliat Med. (2020) 9:3742–9. doi: 10.21037/apm-20-1599
43. Jha S. Adenomyotic cyst mimicking a congenital Müllerian anomaly: diagnosis and treatment with laparoscopy. Clin Exp Reprod Med. (2021) 48:91–4. doi: 10.5653/cerm.2020.03867
44. Tanvir T, Meeta M, Singh A. Adenomyotic cyst at menopause transition: a combined treatment. J Minim Invasive Gynecol. (2021) 28:736–8. doi: 10.1016/j.jmig.2020.06.021
45. Arya S, Burks HR. Juvenile cystic adenomyoma, a rare diagnostic challenge: case reports and literature review. F S Rep. (2021) 2:166–171. doi: 10.1016/j.xfre.2021.02.002
46. Zhao CZ, Wang B, Zhong CY, Lu ST, Lei L. Management of uterine cystic adenomyosis by laparoscopic surgery: case report. BMC Womens Health. (2021) 21:263. doi: 10.1186/s12905-021-01341-1
47. Acién P, Sánchez del Campo F, Mayol MJ, Acién M. The female gubernaculum: role in the embryology and development of the genital tract and in the possible genesis of malformations. Eur J Obstet Gynecol Reprod Biol. (2011) 159:426–32. doi: 10.1016/j.ejogrb.2011.07.040
48. Takeuchi H, Kitade M, Kikuchi I, Kumakiri J, Kuroda K, Jinushi M. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: a review of nine cases. Fertil Steril. (2010) 94:862–8. doi: 10.1016/j.fertnstert.2009.05.010
49. Tan J, Yong P, Bedaiwy MA. A critical review of recent advances in the diagnosis, classification, and management of uterine adenomyosis. Curr Opin Obstet Gynecol. (2019) 31:212–21. doi: 10.1097/GCO.0000000000000555
50. Agostinho L, Cruz R, Osório F, Alves J, Setúbal A, Guerra A. MRI for adenomyosis: a pictorial review. Insights Imaging. (2017) 8:549–56. doi: 10.1007/s13244-017-0576-z
51. Krentel H, Cezar C, Becker S, di Spiezio Sardo A, Tanos V, Wallwiener M, et al. From clinical symptoms to MR imaging: diagnostic steps in adenomyosis. Biomed Res Int. (2017) 2017:1514029. doi: 10.1155/2017/1514029
52. Kitajima K, Murakami K, Kaji Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR Am J Roentgenol. (2010) 195:737–43. doi: 10.2214/AJR.09.4074
53. García-Tejedor A, Castellarnau M, Ponce J, Fernández ME, Burdio F. Ethanol sclerotherapy of ovarian endometrioma: a safe and effective minimal invasive procedure. Preliminary results. Eur J Obstet Gynecol Reprod Biol. (2015) 187:25–9. doi: 10.1016/j.ejogrb.2015.02.004
54. Nasab S, Bedrick BS, Christianson MS. Ethanol sclerotherapy for endometriomas: ready for prime time? Fertil Steril. (2021). 115:100–1. doi: 10.1016/j.fertnstert.2020.10.035
55. Cohen A, Almog B, Tulandi T. Sclerotherapy in the management of ovarian endometrioma: systematic review and meta-analysis. Fertil Steril. (2017) 108:117–24.e5. doi: 10.1016/j.fertnstert.2017.05.015
56. Gaballa D, Moyer M. Thoughts of the safety and efficacy of EUS-guided cyst ablation with the use of lauromacrogol. Gastrointest Endosc. (2018) 87:1367. doi: 10.1016/j.gie.2017.12.030
57. Xu S, Rao M, Pu Y, Zhou J, Zhang Y. The efficacy of laparoscopic lauromacrogol sclerotherapy in the treatment of simple hepatic cysts located in posterior segments: a refined surgical approach. Ann Palliat Med. (2020) 9:3462–71. doi: 10.21037/apm-20-1723
58. Liu W, Wang L, Guo CX. The effects of lauromacrogol injection into rat endometrial cysts: a preliminary experimental study. Arch Gynecol Obstet. (2016) 294:555–9. doi: 10.1007/s00404-016-4095-9
Keywords: adenomyosis, aspiration, cystic adenomyosis, sclerotherapy, ultrasound, uterine tumor
Citation: Zhao X and Yang Y (2022) Ultrasound-Guided Transvaginal Aspiration and Sclerotherapy for Uterine Cystic Adenomyosis: Case Report and Literature Review. Front. Med. 9:764523. doi: 10.3389/fmed.2022.764523
Received: 25 August 2021; Accepted: 09 February 2022;
Published: 03 March 2022.
Edited by:
Erol Tavmergen, Ege University, TurkeyCopyright © 2022 Zhao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Yang, NDUyNDY0MDlAcXEuY29t
 Xinxin Zhao
Xinxin Zhao Ye Yang
Ye Yang