- 1Department of Laboratory Medicine, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Laboratory Medicine, Medical Center Hospital of Qionglai City, Chengdu, China
Objective: We aim to analyze the distribution of Klebsiella pneumoniae in different specimen sources and its antibiotic resistance trends from the Antimicrobial Resistant Investigation Network of Sichuan Province (ARINSP) between 2017 and 2020.
Methods: According to the monitoring scheme, each participating hospital identified the bacteria and performed antimicrobial susceptibility tests using approved procedures. The data of non-repetitive isolates collected from outpatients and inpatients were submitted to ARINSP. The WHONET 5.6 software was used to analyze the results according to the Clinical and Laboratory Standards Institute (CLSI).
Results: Between 2017 and 2020, 833,408 non-repetitive clinical isolates of bacteria were isolated in total. The bacterial strains isolated from sputum and broncho-alveolar lavage accounted for 48.7, 56.4, 49.2, and 43.7% from 2017 to 2020 respectively, among all sources. The number of Klebsiella pneumoniae isolates from sputum and broncho-alveolar lavage increased from 18,809 in 2018, 19,742 in 2019, to 19,376 in 2020, playing a predominant role among all specimens. Meropenem-resistant K. pneumoniae occurrences (5.7% in 2017, 7.3% in 2018, 8.0% in 2019, and 7.5% in 2020) remained highest among carbapenems, and increased slightly over time. The resistance rate to tigecycline remained lowest, and declined from 2.4% in 2017, to 0.4% in 2018, and from 0.7% in 2019, to 0.6% in 2020.
Conclusion: The overall resistance rates of Klebsiella pneumoniae to carbapenems increased in Sichuan Province, giving a significant challenge to control K. pneumoniae related infections. Tigecycline has retained activity to against K. pneumoniae. Ongoing surveillance is essential. It can help for implementing intervention programs to reduce the occurrence of antimicrobial resistance and to provide with a rational use of antimicrobials.
Introduction
Klebsiella pneumoniae (K. pneumoniae) is an increasingly important gram-negative pathogen that can cause serious infections. According to the 2019 Antimicrobial Resistant Threats Report (1) from the Centers for Disease Control and Prevention (CDC), carbapenem-resistant Enterobacteriaceae (CRE), which commonly cause hard to treat infections among patients, were listed as “urgent threats” to public health. In the European Union and China, carbapenem-resistant Klebsiella pneumoniae (CRKP) strains account for ~64–87.8% of clinical CRE infections (2–4). Infections caused by CRE (CRKP most frequently) are associated with higher mortality (1) and increased healthcare burden (5, 6).
Nevertheless, due to the difference in resistance mechanisms, the resistance patterns in bacteria are various in different regions (3, 7–9). Carbapenemase is the primary carbapenem resistance mechanism among K pneumoniae isolates. In Greece, Italy, Portugal, the U.S., and China, Klebsiella pneumoniae carbapenemase (KPC) enzymes were the most detected carbapenemases in K pneumoniae (2, 3, 7, 10), whereas New Delhi Metallo-β-lactamase (NDM) enzymes were most frequent in Denmark, Montenegro, Serbia, and India (2, 11). According to a 20 Years follow-up of the SENTRY Antimicrobial Surveillance Program (12), the resistance of K. pneumoniae to carbapenems increased exponentially over time. In addition, they discovered that the endemicity of ESBLs-encoding genes in K. pneumoniae has changed from blaSHV toblaCTX−M in U.S. hospitals after 2013 (12). Hence, tracking the trends of drug resistance (especially carbapenems) of clinical isolates timely and regionally is essential to prevent the further spread of resistant bacteria and guide the rational use of antibiotics.
In China, bacterial resistance surveillance programs are implemented both regionally and provisionally. There are two national surveillance networks for bacterial resistance: the China Antimicrobial Resistance Surveillance System (CARSS) and the China Antimicrobial Surveillance Network (CHINET). CARSS monitors the different bacterial resistance profiles among disparate provinces and autonomous regions (8). CHINET mainly focuses on the bacterial resistance trends of major referral hospitals based on microdilution methods. The Antimicrobial Resistant Investigation Network of Sichuan Province (ARINSP), established in 2011, is the subordinate network of the China Antimicrobial Resistance Surveillance System (CARSS). It has the responsibility to capture the antibiotic resistance situation in the whole province. The overall resistance rates of different bacteria in different years were reported by CARSS and CHINET (13, 14). However, the resistance profiles of K. pneumoniae in Sichuan province were not reported in detail.
Here, we focused on analyzing the distribution of K. pneumoniae in different specimen sources and its antibiotic resistance trends from 2017 to 2020, from patients in ARINSP-participating hospitals in China.
Materials and Methods
Bacteria Isolates
The bacteria isolates were collected from outpatients and inpatients in ARINSP-participating hospitals from 2017 to 2020, and the annual number of hospitals included in the data analysis was 75, 86, 92, and 92, respectively. According to the monitoring scheme, only one isolate from the same species would be included, and thus the data of non-repetitive isolates were submitted. The isolation criteria of target bacteria from clinical specimens were as follows: (1) all non-contaminated bacteria from sterile site specimens (blood, cerebrospinal fluid, bone marrow, pleural fluid, bladder puncture, urine, ascites, and sterile space puncture fluid tissue); (2) bacteria from qualified specimens of open sites (sputum, pharynx, urine, and feces).
Identification of Bacteria Species
Species identification of the bacteria was conducted by established methods using the Vitek2 automated system, BD100 system, or matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Antimicrobial Susceptibility Test
Antimicrobial susceptibility test of the isolates was performed using VITEK2 and BD100 automated systems to determine minimum inhibitory concentrations (MICs). If the drug concentration range of the drug sensitivity test did not cover the cut-off point, a supplementary test was conducted according to the hospitals' clinical needs and the CARSS protocol's requirements (15). The drug sensitivity results confirmed by the additional tests were reported. Antimicrobial susceptibility was confirmed (if necessary, e.g., when imipenem is resistant using VITEK2 system) with the disc diffusion method or E-test. All results were interpreted according to the CLSI document except for tigecycline, which was interpreted according to the FDA criteria. The isolates were tested for ampicillin/sulbactam, cefoperazone/sulbactam, piperacillin/tazobactam, cefazolin, cefuroxime, ceftazidime, ceftriaxone, cefotaxime, cefepime, cefoxitin, ertapenem, imipenem, meropenem, amikacin, gentamycin, ciprofloxacin, trimethoprim-sulfamethoxazole, tigecycline, as recommended by the CARSS.
Quality Control
According to the CLSI, quality control test was performed routinely once a week. The reference strains were Klebsiella pneumoniae (ATCC 700603), Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922).
Statistical Analysis
The WHONET 5.6 software was used for data analysis. The actual resistance number and rate of each antibiotic were selected for statistical analysis in this monitoring.
Results
The Distribution Sites of the Specimen
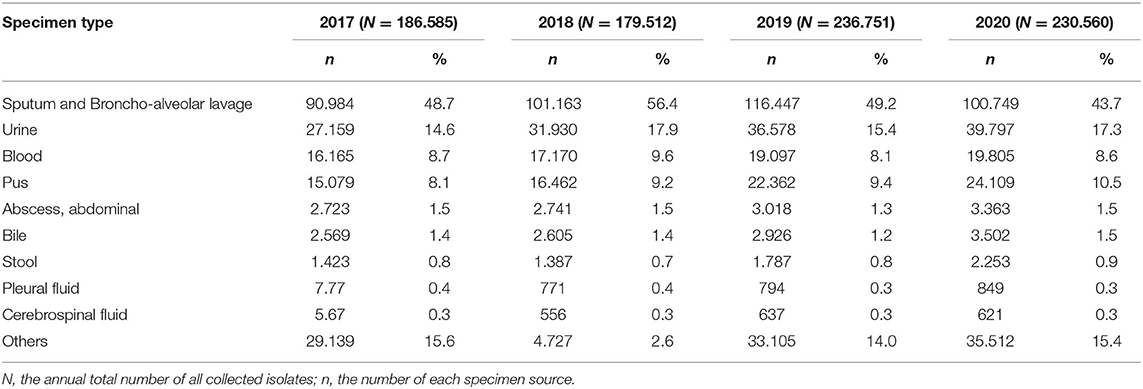
The specimens' type distribution is shown in Table 1. In total, 833,408 non-repetitive clinical isolates of bacteria were collected during the study period (2017–2020). The bacteria strains isolated from sputum and broncho-alveolar lavage accounted for 48.7, 56.4, 49.2, and 43.7% respectively from year 2017 to 2020. Isolates from urine made up the second population (14.6% in 2017, peaked in 2018 as 17.9%, 15.4% in 2019, and 17.3% in 2020) among all specimen types, followed by blood source (8.7% in 2017, peaked in 2018 as 9.6%, 8.1% in 2019, and 8.6% in 2020) and pus (annually increased from 8.1% in 2017, 9.2% in 2018, 9.4% in 2019, to 10.5% in 2020) (Table 1).
The Distribution of Klebsiella pneumoniae Among Specimens
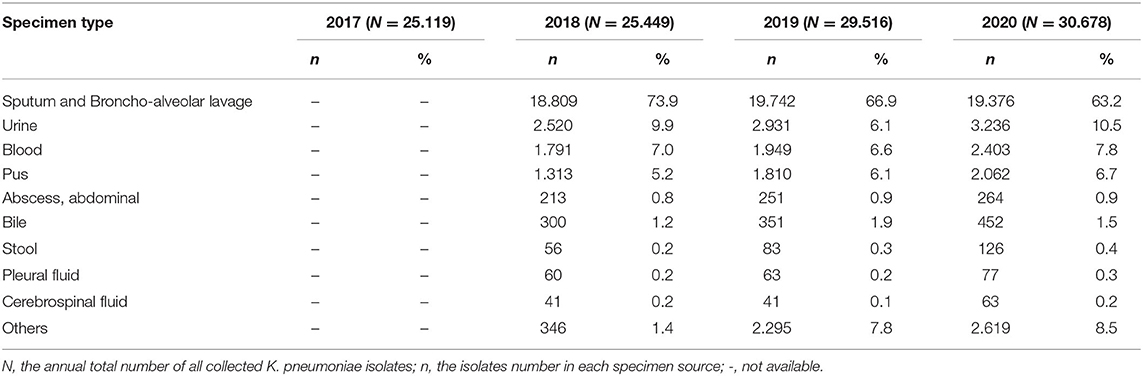
The distribution of K. pneumoniae among specimens is summarized in Table 2. Although the number of K. pneumoniae isolates from sputum and broncho-alveolar lavage increased from 18,809 in 2018, to 19,742 in 2019 and 19,376 in 2020 (the distribution data in 2017 were not available), the proportion was annually decreased from 73.9% in 2018, 66.9% in 2019, to 63.2% in 2020, making up a predominant proportion among all the specimen sources at all times. The proportions of K. pneumoniae isolates from urine were found to be 9.9% in 2018, 6.1% in 2019, and 10.5% in 2020. K. pneumoniae isolates from blood (7.0% in 2018, 6.6% in 2019, and 7.8% in 2020) and pus (5.2% in 2018, 6.1% in 2019, and 6.7% in 2020) sources increased slightly over time.
Klebsiella pneumoniae
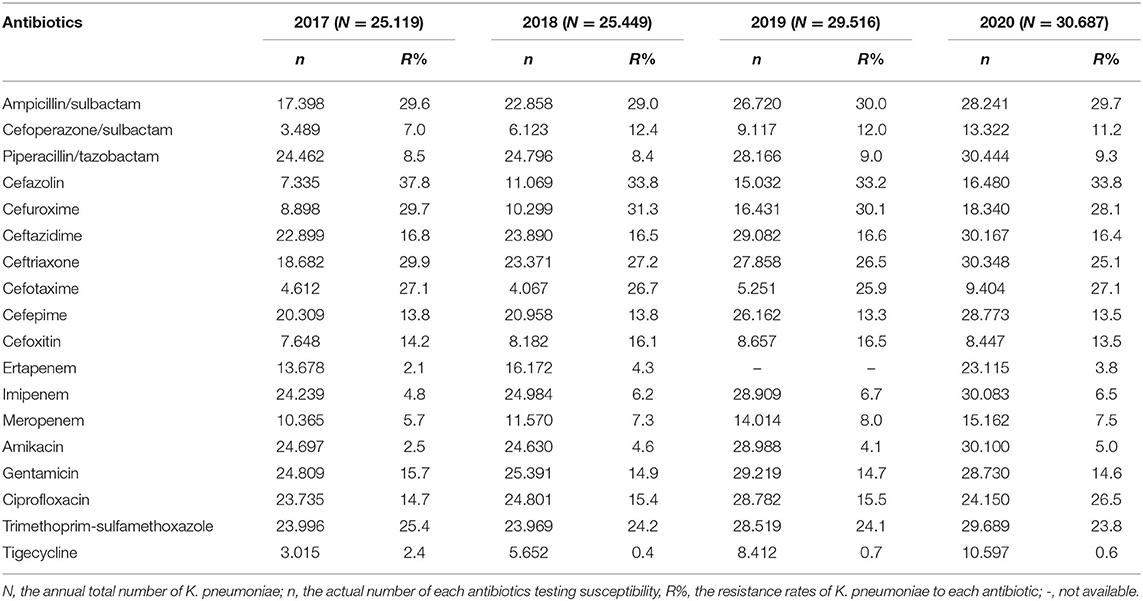
The antimicrobial susceptibility results of K. pneumoniae to antibiotics commonly used are shown in Table 3. The tested number of K. pneumoniae isolates was 25,115 in 2017, 25,449 in 2018, 29,516 in 2019, and 30,687 in 2020, respectively. The resistance rates of K. pneumoniae to ertapenem (2.1% in 2017, 4.3% in 2018, and 3.8% in 2020), imipenem (4.8% in 2017, 6.2% in 2018, peaked in 2019 as 6.7%, and 6.5% in 2020), and meropenem (5.7% in 2017, 7.3% in 2018, peaked in 2019 as 8.0%, and 7.5% in 2020) increased slightly over time (Figure 1). Tigecycline resistance level remained lowest, and the trend declined from 2.4% in 2017 to 0.4% in 2018, and from 0.7% in 2019, to 0.6% in 2020 (Figure 1). A marked increase of resistance was seen for ciprofloxacin from 14.7% in 2017, 15.4% in 2018, 15.5% in 2019, to 26.5% in 2020. The resistance levels of Ampicillin/sulbactam (29.6% in 2017, 29.0% in 2018, 30.0% in 2019, and 29.7% in 2020), ceftazidime (16.8% in 2017, 16.5% in 2018, 16.6% in 2019, and 16.4% in 2020), and cefepime (13.8% in 2017, 13.8% in 2018, 13.3% in 2019, and 13.5% in 2020) were stable during the 4 years. Resistance rate to cefoperazone/sulbactam was found to be 7.0% in 2017, 12.4% in 2018, 12.0% in 2019, and 11.2% in 2020, respectively. The resistance rates of K. pneumonia to piperacillin/tazobactam (8.5% in 2017, 8.4% in 2018, 9.0% in 2019, and 9.3% in 2020) and amikacin (2.5% in 2017, 4.6% in 2018, 4.1% in 2019, and 5.0% in 2020) increased slightly over time. The resistance level of K. pneumoniae to cefazolin remained highest among all antibiotics tested but decreased from 37.8% in 2017 to 33.8% in 2018 and remained stable in the next 2 years. Resistance rates to ceftriaxone (29.9, 27.2, 26.5, and 25.1% from 2017 to 2020), gentamicin (15.7, 14.9, 14.7, and 14.6% from 2017 to 2020), and trimethoprim-sulfameth (25.4, 24.2, 24.1, and 23.8% from 2017 to 2020) declined slightly over time. Cefuroxime and cefotaxim resistance rates fluctuated around 29.8 and 26.5%, respectively.
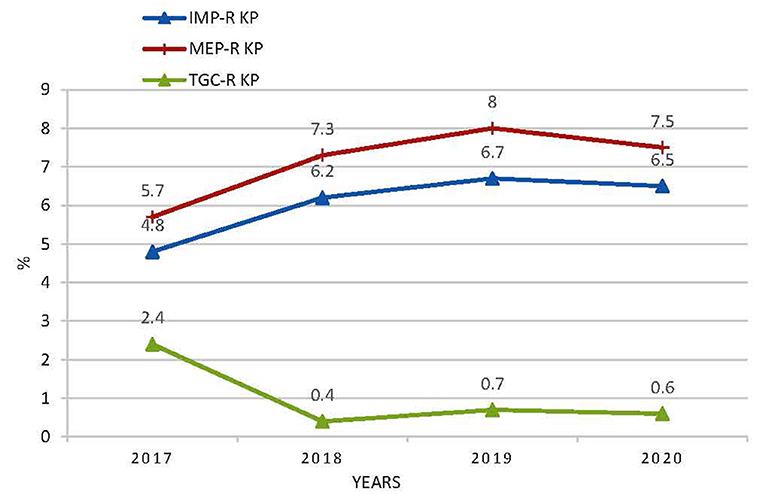
Figure 1. The resistance rates of K. pneumoniae to imipenem, meropenem, and tigecycline. IMP KP, imipenem-resistant K. pneumonia; MEP-R, meropenem-resistant K. pneumonia; TGC-R KP, tigecycline- resistant K. pneumonia.
Discussion
This study aimed to analyze the distribution of K. pneumoniae among different specimen sources and its antimicrobial resistance profiles. The annual total number of all collected isolates increased, and specimens from the sputum and broncho-alveolar lavage played the dominant role (48.7% in 2017, 56.4% in 2018, 49.2% in 2019, and 43.7% in 2020) in the study period (Table 1). These results are higher than the domestic level reported by CARSS (14) and the China Antimicrobial Surveillance Network (CHINET) (16). There are still controversies regarding the clinical value of sputum cultures in the management of pneumonia. Saukkoriipi et al. (17) reported that the culture of all sputum samples (either high-quality or low-quality) would add value to the pneumococcal community-acquired pneumonia (CAP)-diagnosis in elderly patients (≥65 years). Another study (18) demonstrated that sputum cultures had no clinical or economic benefits for both CAP and healthcare-associated pneumonia (HCAP) patients. However, cultures can reduce costs and shorten the overall length of hospital stay under some circumstances (e.g., empirical antibiotics therapy). Therefore, clinicians should make decisions based on the traits of patients.
K. pneumoniae can cause community-acquired and hospital-acquired infections (HAIs) (19, 20), both of which presents unique challenges for clinicians. In addition, Studies identified K. pneumoniae pathogens as a leading cause of HCAP (21, 22). In this study, the most common source of K. pneumoniae isolates was sputum and broncho-alveolar lavage (73.9% in 2018, 66.9% in 2019, and 63.2% in 2020), followed by urine (9.9% in 2018, 6.1% in 2019, and 10.5% in 2020), blood (7.0% in 2018, 6.6% in 2019, and 7.8% in 2020), and pus (5.2% in 2018, 6.1% in 2019, and 6.7% in 2020) (Table 2). The distribution and drug susceptibility profiles of K. pneumoniae in community-acquired infections and HAIs could be further analyzed if the information of outpatients and inpatients was available.
K. pneumoniae, which belongs to the Enterobacteriaceae, is one of the most threatening pathogens and a significant source of antibiotic resistance (23). In the last decade, CRE has spread rapidly and caused great public health concerns (24), of which CRKP was one of the most important pathogens. A study (13) reported that the rate of CRKP was increased from 2.9% in 2005 to 10.0% in 2012 and 25.3% in 2019, an ~8-fold increase. Moreover, the rate of CRKP rose from 0.7 to 14.2% in Europe and from 0.5% to 6.1% in APAC during 1997–2016 (25), yet the rate of CRKP (meropenem, 5.7% in 2017, 7.3% in 2018, peaked in 2019 at 8.0%, 7.5% in 2020, Table 3) in Sichuan province was much lower than the domestic level (13). Europe was reported (25) with both increased CRKP's number and enhanced resistance rate. This scenario presents significant challenges for clinicians. Although some countermeasures such as guidelines (26) and surveillance networks were applied to curb these pathogens, the result remains dissatisfied. The reasons for the failure in curbing CRKP are not well-understood (21). However, several critical factors, such as the overcrowding and shortage of staff, the excessive use of carbapenems, and the absence of a network to share patient information, may contribute to their spread. Further measures should be taken to curb the spread. Additionally, with the rapid increase in CRKP prevalence, antibiotic treatment therapy for CRKP is extremely limited in clinical practice. Ceftazidime-avibactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam, cefiderocol, or tigecycline were considered the last line agents for treating infections caused by CRE (27). Only ceftazidime-avibactam and tigecycline are marketed in China. Ceftazidime-avibactam, first approved by the US Food and Drug Administration (FDA) in 2015 (28) and marketed in China in 2019 (29), is a promising drug for treating infections caused by carbapenem-resistant gram-negative bacilli (28, 30, 31). However, it developed resistance rapidly (30), further diminishing the limited options for antibiotic treatments. Therefore, the microbiological laboratory staff should contact the clinical to add potentially practical antibiotic tests (e.g., ceftazidime-avibactam, tigecycline) once CRKP is detected.
The resistance rate of K. pneumoniae to tigecycline remained lowest among all tested antibiotics, which declined from 2.4% in 2017 to 0.4% in 2018 and from 0.7% in 2019 to 0.6% in 2020, suggesting that tigecycline has retained high activity over K. pneumoniae. These results were lower than the tigecycline resistance level in Europe (5% according to its EUCAST recommended breakpoint) (2). However, the microbiological laboratory technicians should notice that when tigecycline susceptibility was moderately sensitive or resistant (measured by paper dispersion or automated systems method), an additional test using the micro broth dilution method should be conducted to confirm the susceptibility. Many factors can affect the in vitro activity of tigecycline, such as the media type, medium detection method, and breakpoint selection (32). Currently, the underlying resistance mechanisms of K. pneumoniae to tigecycline have not been fully understood (33, 34). However, it is mainly related to the upregulation of resistance-nodulation-division (RND) efflux pump AcrAB and OqxAB, which was regulated by the mutations of transcriptional genes ramR and acrR and the upregulation of ramA (35, 36), acrB, rarA, and oqxB (33).
A marked increase of resistance to ciprofloxacin was noted from 14.7% in 2017, 15.4% in 2018, 15.5% in 2019, to 26.5% in 2020, similar to the trends (from 7.3% in 1997 to 27.9% in 2016) reported by the SENTRY Antimicrobial Surveillance Program (12). The blaCTX−M gene was demonstrated to be responsible for the increased resistance to ciprofloxacin in US hospitals. Besides, blaCTX−M ESBL is the most common genotype in China (37). Urgent measures should be taken to reserve the drug susceptibility.
Our study has several limitations. Firstly, due to patients' information not being available, we did not analyze the antimicrobial resistance rates among outpatients and inpatients. Secondly, not all hospitals conform to the standards (e.g., personnel, equipment, facilities, methodology) to participate in the ARINSP Program to ensure monitoring accuracy. Therefore, we are not able to capture all in this study. Thirdly, the testing methods used in some hospitals are not identical. Uniformity of the methodology applied in some hospitals is not there that may affect the result.
In conclusion, the increasing trend of K. pneumoniae's antimicrobial resistance to carbapenems exists, while tigecycline has retained activity to against K. pneumoniae. Since the resistance mechanisms of K. pneumoniae could be different in various populations from different regions (38), future surveillance is essential. It can help for implementing intervention programs/plans to reduce the occurrence of antimicrobial resistance and to provide with a rational use of antimicrobials.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The study was conducted on retrospective data. Ethical approval was obtained from the Institutional Review Board of Sichuan Provincial People's Hospital, and University of Electronic Science and Technology of China (Number: 2021-511).
Author Contributions
HY designed the study. JZ and DL contributed to manuscript writing, revised, and supervised the project. SL and XH checked the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81702064).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. CDC. Antibiotic Resistance Threats in the United States. (2019). Available online at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed October 22, 2021).
2. Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasevi ć AT, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and escherichia coli in the European survey of carbapenemase-producing enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. (2017) 17:153–63. doi: 10.1016/S1473-3099(16)30257-2
3. Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBio Med. (2017) 19:98–106. doi: 10.1016/j.ebiom.2017.04.032
4. Li J, Huang Z, Tang M, Min C, Xia F, Hu Y, et al. Clonal dissemination of multiple carbapenemase genes in carbapenem-resistant enterobacterales mediated by multiple plasmids in China. Infect Drug Res. (2021) 14:3287–95. doi: 10.2147/IDR.S327273
5. Avendano EE, Raman G, Chan J, McCann E. Burden of carbapenem non-susceptible infections in high-risk patients: systematic literature review and meta-analysis. Antimicro Res Infect Control. (2020) 9:193. doi: 10.1186/s13756-020-00858-8
6. Zhen X, Stålsby Lundborg C, Sun X, Zhu N, Gu S, Dong H. Economic burden of antibiotic resistance in China: a national level estimate for inpatients. Antimicro Res Infect Control. (2021) 10:5. doi: 10.1186/s13756-020-00872-w
7. Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. (2013) 13:785–96. doi: 10.1016/S1473-3099(13)70190-7
8. Hu F, Zhu D, Wang F, Wang M. Current status and trends of antibacterial resistance in China. Clin Infect Dis. (2018) 67(Suppl. 2):S128–34. doi: 10.1093/cid/ciy657
9. Chen H-Y, Jean S-S, Lee Y-L, Min C, Ko W-C, Liu P-Y, et al. Carbapenem-resistant enterobacterales in long-term care facilities: a global and narrative review. Front Cell Infect Microbiol. (2021) 11:601968. doi: 10.3389/fcimb.2021.601968
10. Lai C-C, Yu W-L. Klebsiella pneumoniae harboring carbapenemase genes in Taiwan: its evolution over 20 years, 1998-2019. Int J Antimicro Agents. (2021) 58:106354. doi: 10.1016/j.ijantimicag.2021.106354
11. Mojica MF, Rossi M-A, Vila AJ, Bonomo RA. The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. Lancet Infect Dis. (2022) 22:e28–e34. doi: 10.1016/S1473-3099(20)30868-9
12. Castanheira M, Deshpande LM, Mendes RE, Canton R, Sader HS, Jones RN. Variations in the occurrence of resistance phenotypes and carbapenemase genes among isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis. (2019) 6(Suppl 1):S23–33. doi: 10.1093/ofid/ofy347
13. Hu F, Guo Y, Zhu D, Wang F. CHINET surveillance of bacterial resistance across tertiary hospitals in 2019. Chin J Infect Chemother. (2020) 20:11. doi: 10.16718/j.1009-7708.2020.03.001
14. CARSS. Surveillance on antimicrobial resistance of bacteria in different levels of hospitals: surveillance report from China antimicrobial resistance surveillance system in 2014−2019. Chin J Infect Control. (2021) 2. doi: 10.12138/j.issn.1671-9638.20216180
15. CARSS. The Technical Scheme of CARSS. (2021). Available online at: http://www.carss.cn/Download/Details/657 (accessed October 23, 2021).
16. Hu F, Guo Y, Yang Y, Zheng Y, Wu S, Jiang X, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. (2019) 38:2275–81. doi: 10.1007/s10096-019-03673-1
17. Saukkoriipi A, Palmu AA, Jokinen J. Culture of all sputum samples irrespective of quality adds value to the diagnosis of pneumococcal community-acquired pneumonia in the elderly. Eur J Clin Microbiol Infect Dis. (2019) 38:1249–54. doi: 10.1007/s10096-019-03536-9
18. Asti L, Bartsch SM, Umscheid CA, Hamilton K, Nachamkin I, Lee BY. The potential economic value of sputum culture use in patients with community-acquired pneumonia and healthcare-associated pneumonia. Clin Microbiol Infect. (2019) 25 1038.e1031–1038.e1039. doi: 10.1016/j.cmi.2018.11.031
19. Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. (2019) 32:e00001–19. doi: 10.1128/CMR.00001-19
20. Choby JE, Howard-Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. J Intern Med. (2020) 287:283–300. doi: 10.1111/joim.13007
21. Zong Z, Wu A, Hu B. Infection control in the era of antimicrobial resistance in China: progress, challenges, and opportunities. Clin Infect Dis. (2020) 71(Suppl. 4):S372–8. doi: 10.1093/cid/ciaa1514
22. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. (2014) 370:1198–208. doi: 10.1056/NEJMoa1306801
23. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. (2017) 41:252–75. doi: 10.1093/femsre/fux013
24. Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. (2017) 43:1464–75. doi: 10.1007/s00134-017-4878-x
25. Sader HS, Castanheira M, Arends SJR, Goossens H, Flamm RK. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: results from 20 years of the SENTRY antimicrobial surveillance program (1997-2016). J Antimicro Chemother. (2019) 74:1595–606. doi: 10.1093/jac/dkz074
26. WHO. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities. Geneva: WHO (2017).
27. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious diseases society of America guidance on the treatment of extended-spectrum beta-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. (2021) 72:e169–83. doi: 10.1093/cid/ciaa1478
28. Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. (2019) 68:355–64. doi: 10.1093/cid/ciy492
29. Kuang H, Zhong C, Wang Y, YeH, Ao K, Zong Z, Lv X, et al. Clinical characteristics and outcomes of patients with multidrug-resistant Gram-negative bacterial infections treated with ceftazidime/avibactam. J Glob Antimicrob Resist. (2020) 23:404–7. doi: 10.1016/j.jgar.2020.10.023
30. Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. (2018) 62:e02497–17. doi: 10.1128/AAC.02497-17
31. Sousa A, Pérez-Rodríguez MT, Soto A, Rodríguez L, Pérez-Landeiro A, Martínez-Lamas L, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. (2018) 73:3170–75. doi: 10.1093/jac/dky295
32. Healthcare CM. Expert consensus on polymyxins, tigecycline and ceftazidime/avibactam susceptibility testing. Chin J Lab Med. (2020) 43:964–72. doi: 10.3760/cma.j.cn114452-20200719-00619
33. Sheng Z-K, Hu F, Wang W, Guo Q, Chen Z. Mechanisms of tigecycline resistance among Klebsiella pneumoniae clinical isolates. Antimicro Agents Chemother. (2014) 58:6982–5. doi: 10.1128/AAC.03808-14
34. Lv L, Wan M, Wang C, Gao X, Yang Q, Partridge SR, et al. Emergence of a plasmid-encoded resistance-nodulation-division efflux pump conferring resistance to multiple drugs, including tigecycline, in Klebsiella pneumoniae. mBio. (2020) 11:e02930–19. doi: 10.1128/mBio.02930-19
35. Rosenblum R, Khan E, Gonzalez G, Hasan R, Schneiders T. Genetic regulation of the ramA locus and its expression in clinical isolates of Klebsiella pneumoniae. Int J Antimicro Agents. (2011) 38:39–45. doi: 10.1016/j.ijantimicag.2011.02.012
36. Wang X, Chen H, Zhang Y, Wang Q, Zhao C, Li H, et al. Genetic characterisation of clinical Klebsiella pneumoniae isolates with reduced susceptibility to tigecycline: role of the global regulator RamA and its local repressor RamR. Int J Antimicro Agents. (2015) 45:635–40. doi: 10.1016/j.ijantimicag.2014.12.022
37. Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, antimicrobial resistance. Antimicro Agents Chemother. (2016) 60:6115–20. doi: 10.1128/AAC.01127-16
Keywords: antimicrobial resistance, Klebsiella pneumoniae, surveillance, carbapenem-resistant, antimicrobial susceptibility
Citation: Zhang J, Li D, Huang X, Long S and Yu H (2022) The Distribution of K. pneumoniae in Different Specimen Sources and Its Antibiotic Resistance Trends in Sichuan, China From 2017 to 2020. Front. Med. 9:759214. doi: 10.3389/fmed.2022.759214
Received: 22 September 2021; Accepted: 24 January 2022;
Published: 15 February 2022.
Edited by:
Nicola Petrosillo, Policlinico Universitario Campus Bio-Medico, ItalyReviewed by:
Dinesh Sriramulu, Independent Researcher, Chennai, IndiaRam Prasad Adhikari, Nepal Medical College, Nepal
Copyright © 2022 Zhang, Li, Huang, Long and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yu, eXZodWEyMDAyJiN4MDAwNDA7MTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jie Zhang
Jie Zhang Dan Li
Dan Li Xiangning Huang
Xiangning Huang Shanshan Long1
Shanshan Long1