
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 20 December 2022
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1092325
This article is part of the Research Topic Psychological Impact and Quality of Life in Rheumatic and Musculoskeletal Diseases View all 7 articles
 Julius Lindblom1
Julius Lindblom1 Sture Zetterberg1
Sture Zetterberg1 Sharzad Emamikia1
Sharzad Emamikia1 Alexander Borg1
Alexander Borg1 Gunilla von Perner2
Gunilla von Perner2 Yvonne Enman1
Yvonne Enman1 Emelie Heintz3
Emelie Heintz3 Malin Regardt4,5
Malin Regardt4,5 David Grannas6
David Grannas6 Alvaro Gomez1
Alvaro Gomez1 Ioannis Parodis1,7*
Ioannis Parodis1,7*Objectives: To investigate whether self-reported EQ-5D full health state (FHS) after therapeutic intervention for active systemic lupus erythematosus (SLE) is associated with a reduced risk to accrue organ damage. In a separate analysis, we sought to investigate associations between experience of “no problems” in each one of the five dimensions of EQ-5D and the risk to accrue damage.
Methods: Data from the open-label extension periods of the BLISS-52 and BLISS-76 trials of belimumab in SLE (NCT00724867; NCT00712933) were used (N = 973). FHS was defined as an experience of “no problems” in all five EQ-5D dimensions. Organ damage was assessed annually using the Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI). Associations between the three-level version of the EQ-5D (EQ-5D-3L) responses at open-label baseline and the first documented increase in organ damage were investigated using Cox regression accounting for age, sex, ancestry, SDI at baseline, and background therapy, and associations with SDI items were investigated using phi (φ) correlation analyses.
Results: A total of 147 patients (15.1%) accrued organ damage during follow-up, with the first increase in their SDI score occurring after a mean time of 29.1 ± 19.6 months. Lower proportions of FHS respondents accrued damage over a course of up to 7.9 years of open-label follow-up compared with no FHS respondents (p = 0.004; derived from the logrank test). FHS was associated with a reduced hazard to accrue subsequent organ damage (HR: 0.60; 95% CI: 0.38–0.96; p = 0.033) after adjustments, as was experience of “no problems” in mobility (HR: 0.61; 95% CI: 0.43–0.87; p = 0.006). “No problems” in mobility was negatively correlated with musculoskeletal damage accrual (φ = −0.08; p = 0.008) and associated with a lower hazard to accrue musculoskeletal damage in Cox regression analysis (HR: 0.38; 95% CI: 0.19–0.76; p = 0.006).
Conclusion: Experience of EQ-5D-3L FHS and “no problems” in mobility after therapeutic intervention heralded reduced hazard to accrue subsequent organ damage, especially musculoskeletal damage, suggesting that optimisation of these health-related quality of life aspects constitutes a clinically relevant treatment target in patients with SLE, along with clinical and laboratory parameters.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is characterised by considerable morbidity and impaired health-related quality of life (HRQoL) (1, 2). Organ damage is a particularly feared and irreversible consequence of the disease; approximately 50% of SLE patients accrue damage to some extent within 10 years of receiving their diagnosis, including damage in the musculoskeletal system, central nervous system (CNS), and kidneys (3). SLE is a highly heterogeneous disease (4); afflicted individuals differ not only in clinical presentation but also in how they experience their health, commonly experiencing a poor HRQoL, even after adequate clinical response to therapy (5–7).
During the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) IV consensus conference, four principal outcome domains were ratified for SLE clinical trials: (i) disease activity, (ii) HRQoL, (iii) adverse events, and (iv) organ damage (8). Because of the known discordance between SLE patients and physicians regarding concerns for disease features as well as perception of disease activity (9), optimal use of patient-reported outcomes is highly motivated both in clinical trial and real-life settings (10).
The three-level version of EQ-5D (EQ-5D-3L) is a patient-reported HRQoL measure that includes a questionnaire with five dimensions of health (11, 12). Each one of these dimensions yield patient responses at three severity levels i.e., level 1, 2, and 3, for experience of “no problems,” “some/moderate problems,” and “extreme/major problems,” respectively. Combinations of patient responses in this descriptive system represent the patient’s self-reported health status, which is referred to as an EQ-5D profile (12). These profile data can be supplemented by a weighting system which converts the EQ-5D profile into a single value, referred to as an EQ-5D index score, ranging from lower than 0 to 1, where 0 represents health state experience equal to death and 1 represents a health state equivalent to full health, also denoted as full health state (FHS) (12).
The relationship between health status reported using EQ-5D and SLE disease activity or organ damage was investigated in a meta-analysis by Shi et al. (13) which however, yielded inconclusive results. Aggarwal et al. (14) showed that EQ-5D was able to discriminate between patient subgroups of high and low disease activity but did not manage to differentiate between patient subgroups of high and low organ damage. Wang et al. (15) demonstrated a negative association between EQ-5D values and organ damage while a study by Chang et al. (16) showed a weaker association. The association between EQ-5D scores and organ damage in patients with SLE is thus not clear and warrants further research.
Three-level version of EQ-5D FHS was reported at a higher frequency among patients who received non-biological standard therapy plus belimumab than among patients who received standard therapy alone as well as in responders than in non-responders in a large clinical trial SLE population (17). However, the association between EQ-5D-3L FHS and long-term outcomes e.g., organ damage accrual in SLE remains unknown.
The aim of this study was to investigate whether experience of EQ-5D-3L FHS after therapy for active SLE is associated with deceleration of organ damage accrual in a diverse SLE population deriving from open-label studies of belimumab. In a separate analysis, we sought to investigate associations between experience of “no problems” in each one of the five dimensions of EQ-5D and the risk to accrue damage.
This study was conducted as a post hoc analysis of data collected in the setting of the BLISS-52 (NCT00424476) (18) and BLISS-76 (NCT00410384) (19) trials, and their open-label extension phases (NCT00724867; NCT00712933) (20, 21). A total of 973 participants with open-label extension phase data were included in the present study, comprising 528 and 445 participants from the BLISS-52 and BLISS-76 trials, respectively. Access to data was granted by GlaxoSmithKline (Uxbridge, UK) through the Clinical Study Data Request (CSDR) consortium.
All patients included in the BLISS-52 and BLISS-76 trials fulfilled the American College of Rheumatology (ACR) revised criteria for SLE (22), were adults, had an ANA titre ≥1:80 and/or serum antibodies against double-stranded DNA (anti-dsDNA) antibody level ≥30 IU/mL at screening, and a Safety of Estrogens in Lupus National Assessment-SLEDAI (SELENA-SLEDAI) score ≥6 (23). All patients were on stable non-biological standard therapy for ≥30 days before the baseline of the double-blinded phases of the BLISS-52 and BLISS 76 trials, including glucocorticoids, antimalarial agents, and/or immunosuppressants. Patients were randomised to receive add-on belimumab 1 mg/kg, belimumab 10 mg/kg, or placebo as intravenous infusions at weeks 0, 2, 4, and thereafter every 4th week. Participants from the BLISS-52 and BLISS-76 clinical trials all received belimumab 10 mg/kg intravenously every 4th week on top of non-biological standard therapy during the open-label phase of the studies and formed the study population of the present investigation.
The EQ-5D-3L descriptive system incorporates five HRQoL dimensions i.e., Mobility, Self-care, Usual activities, Pain/discomfort, and Anxiety/depression. Respondents may report no problems, some/moderate, or extreme/major problems in each one of these dimensions. As per the EQ-5D-3L user guide, we defined FHS as a response of “no problems” in all five dimensions, which yields an EQ-5D-3L index score of 1 (12) at the open-label baseline, which corresponded to the end of follow-up in the BLISS-52 trial at week 52 and the BLISS-76 trial at week 76. In subgroup analyses, we also analysed patient responses of “no problems” in each one of the five EQ-5D dimensions separately.
Systemic lupus erythematosus disease activity was assessed using the SLEDAI 2000 (SLEDAI-2K) (24) and organ damage was assessed using the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (SDI) (25). The SDI includes 39 items grouped into 12 domains i.e., ocular, neuropsychiatry, renal, pulmonary, cardiovascular, peripheral vascular, gastrointestinal, musculoskeletal, skin, premature gonadal failure, diabetes, and malignancy. Organ damage accrual was defined as the first documented increase in the patient’s SDI score from the open-label baseline over the open-label extension study period, based on yearly assessments for a total duration of up to 8 years.
Proportional hazards (Cox) regression was used to investigate associations between EQ-5D-3L FHS at the open-label baseline and organ damage accrual, both overall and stratified by organ domain, during the open-label extension period. In addition, associations between experience of “no problems” in each one of the five HRQoL dimensions of EQ-5D and organ damage accrual were assessed. Kaplan–Meier survival curves were used for illustrating the time to SDI increase, and the logrank test was used for unadjusted comparisons between groups. Adjustments for possible confounders were conducted using multivariable Cox regression models; covariates in these models included age, sex, ancestry, open-label baseline degree of organ damage, and background therapy i.e., antimalarial agent and immunosuppressant use at the open-label baseline, and mean prednisone or equivalent dose during open-label follow-up. Correlations between FHS or experience of “no problems” in each one of the five EQ-5D HRQoL dimensions and damage accrual were assessed using phi (φ) correlation coefficients. This analysis included stratification into SDI domains. Data are presented in the form of numbers (percentage) or means (standard deviation) while medians (interquartile range) are indicated in the case of non-normal distributions. Comparisons of unrelated continuous data were made using the Mann–Whitney U test, and associations between unrelated binomial variables were investigated using Pearson’s chi squared (χ2) or Fisher’s exact tests as appropriate. All p-values < 0.05 were considered statistically significant. Analyses were performed using the R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
The study complied with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants prior to enrolment in BLISS-52 and BLISS-76 and their open-label extension phases. The BLISS study protocols were reviewed and approved by regional ethics review boards for all participating centres, and the study protocol for this post hoc analysis was reviewed and approved by the Swedish Ethical Review Authority (2019-05498).
Patient research partners were involved in the study concept and design, interpretation of data, and editing of the manuscript.
Patient characteristics and clinical data at the open-label baseline, as well as comparisons between patients who experienced an increase in SDI during follow-up (N = 147) and patients who did not (N = 826) are presented in Table 1. The mean observation time for the entire open-label patient population was 49.7 ± 25.5 months. A total of 147 patients (15.1%) accrued organ damage during follow-up, with the first increase in their SDI score occurring after a mean time of 29.1 ± 19.6 months. In the group of patients who experienced an increase in SDI scores, a greater proportion had organ damage at the open-label baseline compared with their counterparts (56.5% versus 39.7%; p < 0.001). Among patients who reported FHS at the open-label baseline, the proportion of patients who experienced an increase in SDI scores during the subsequent follow-up was lower compared with that of patients who did not accrue damage (15.0% versus 26.6%; p = 0.004).
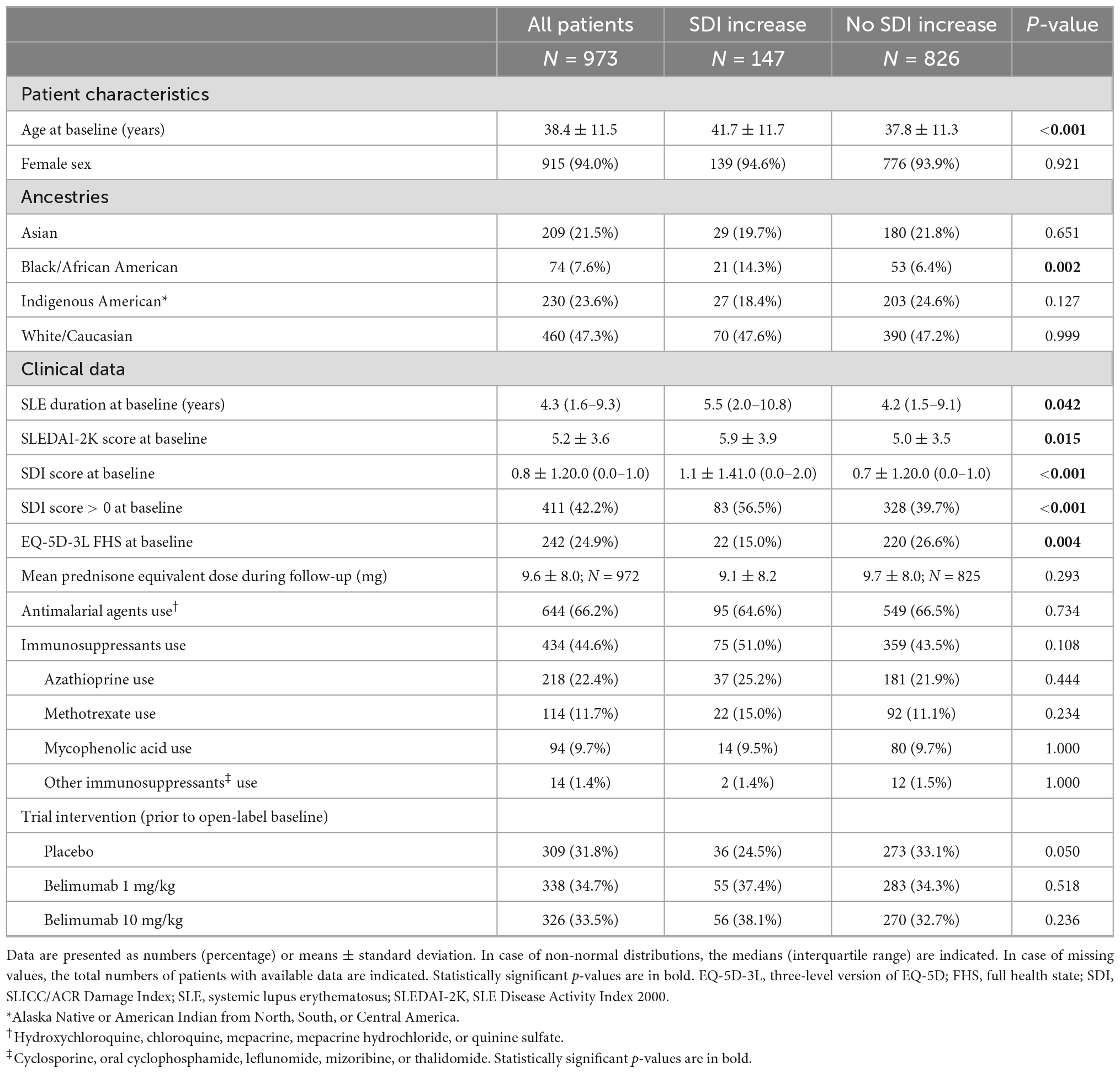
Table 1. Characteristics and comparisons between patients who displayed an increase in SDI during follow-up and patients who did not.
In the pooled BLISS study population, lower proportions of FHS respondents accrued damage over the course of the open-label extension period compared with no FHS respondents (p = 0.004; derived from the logrank test; Figure 1). Experience of FHS at the open-label baseline was associated with a lower probability of and/or longer time to subsequent organ damage accrual during the open-label extension period in unadjusted Cox regression analysis [hazard ratio (HR): 0.52; 95% confidence interval (CI): 0.33–0.81; p = 0.004; Supplementary Table 1]. This association remained significant after adjustment for potential confounders in multivariable analysis (HR: 0.60; 95% CI: 0.38–0.96; p = 0.033; Figure 2 and Supplementary Table 2). However, no associations were seen between experience of FHS at open-label baseline and subsequent organ damage accrual in analysis stratified by SDI organ domain (Supplementary Tables 3, 4).
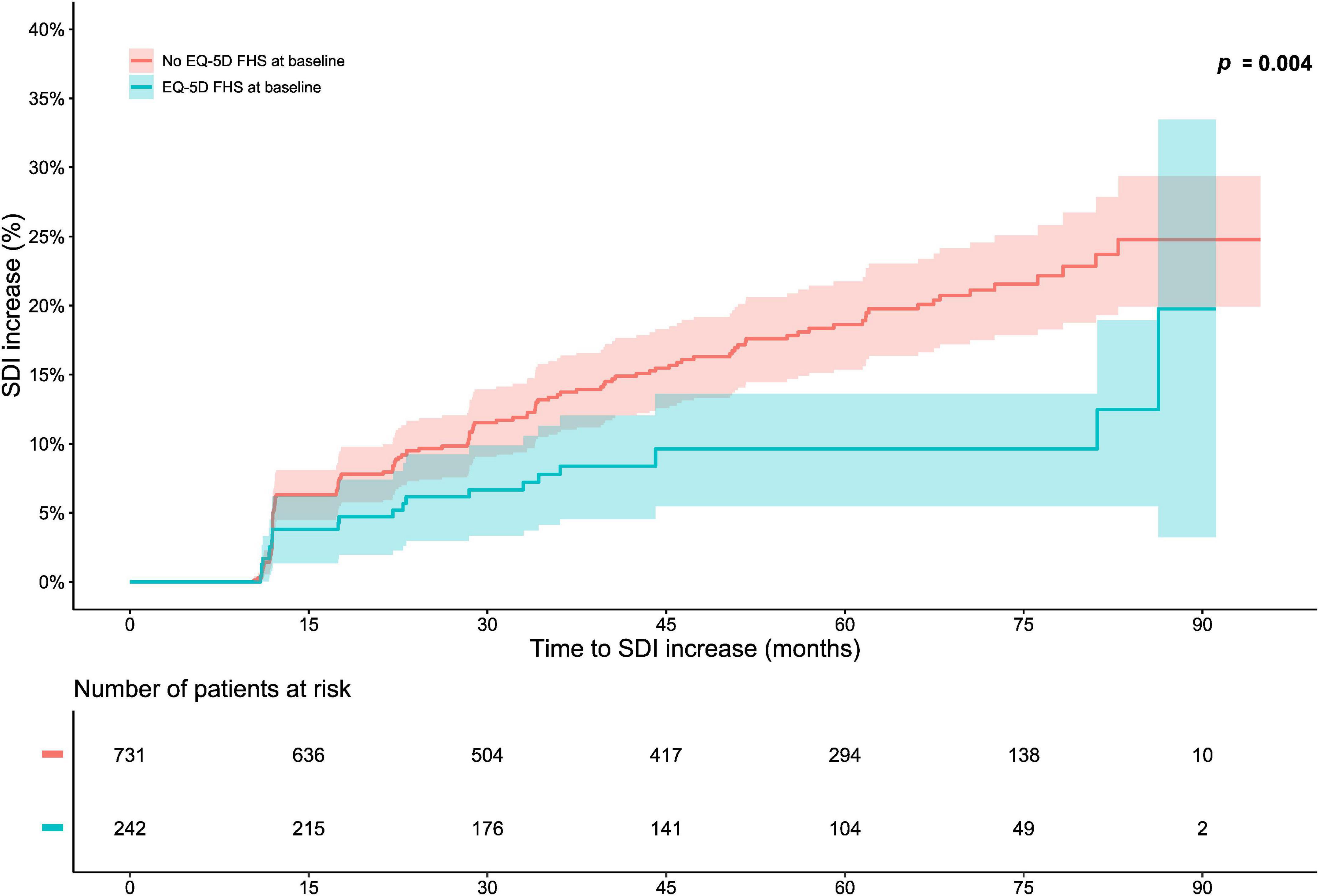
Figure 1. Full health state (FHS) in relation to organ damage accrual. The graphs in the upper panel delineate proportions of patients who accrued organ damage over the course of the open-label study period, stratified into patients who experienced EQ-5D-3L FHS at the open-label baseline and patients who did not. The lower panel shows numbers of participants in the two groups over time, decreasing due to documentation of SDI increase or data censoring. EQ-5D-3L, three-level version of EQ-5D; FHS, full health state; SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index.
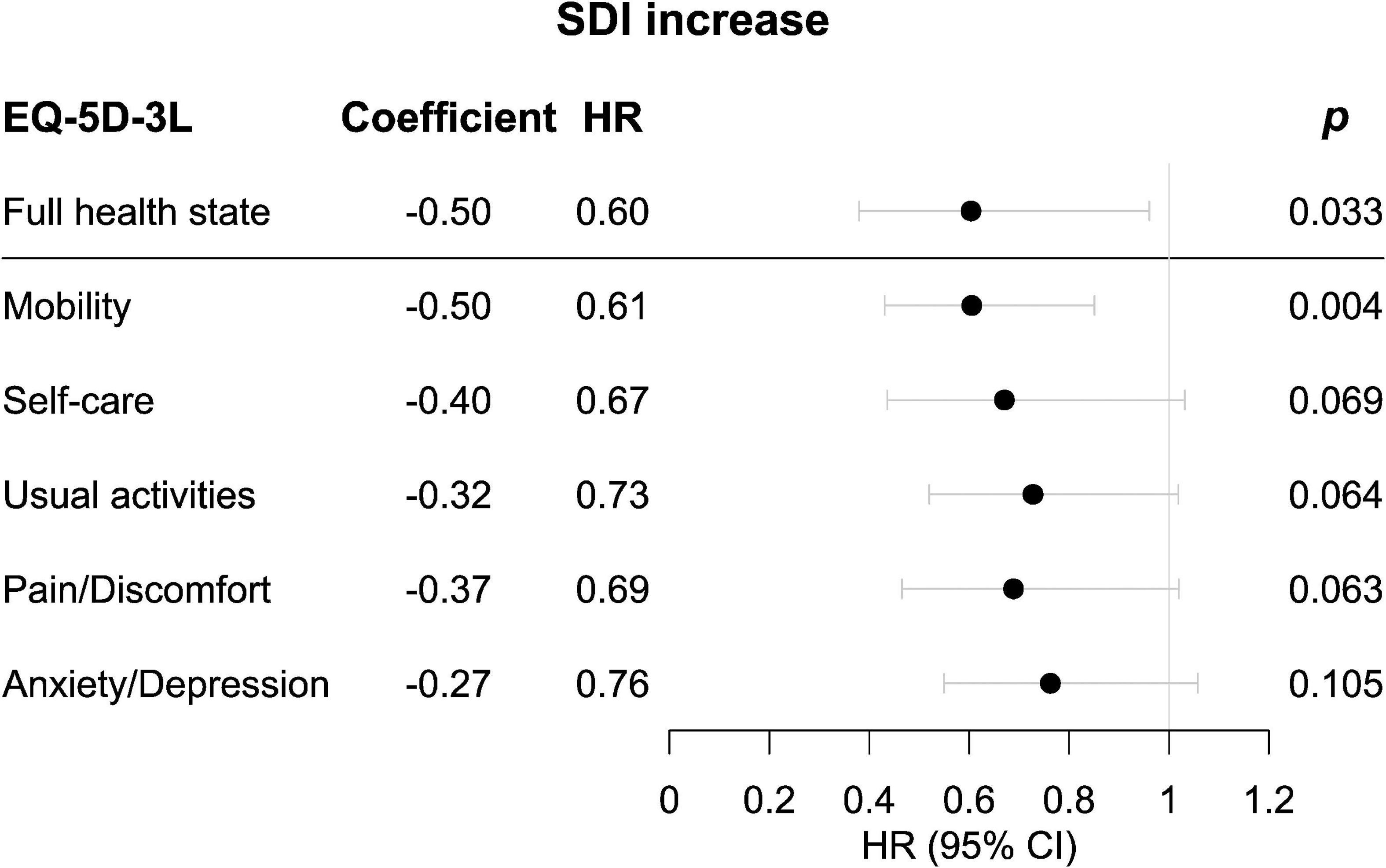
Figure 2. Associations of experience of full health state (FHS) or “no problems” in the separate dimensions of EQ-5D-3L in relation to organ damage accrual. Forest plots illustrating results from multivariable proportional hazards (Cox) regression analysis. Circles represent HRs and whiskers denote the 95% CI. CI, confidence interval; EQ-5D-3L, three-level version of EQ-5D; FHS, full health state; HR, hazard ratio; SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index.
Figure 3 delineates patients reporting “no problems” (severity level 1) and “problems” (severity level 2–3) in each one of the five EQ-5D dimensions at open-label baseline in relation to organ damage accrual throughout the open-label follow-up. A lower proportion of level 1 respondents accrued organ damage during the course of the open-label extension than did other respondents regarding mobility (p < 0.001; derived from the logrank test; Figure 3A), self-care (p = 0.013; Figure 3B), usual activities (p = 0.010; Figure 3C), and pain/discomfort (p = 0.011; Figure 3D). Moreover, an association between experience of a level 1 response at open-label baseline and a lower probability of and/or longer time to organ damage accrual was seen with regard to mobility (HR: 0.54; 95% CI: 0.39–0.75; p < 0.001), self-care (HR: 0.59; 95% CI: 0.38–0.90; p = 0.014), usual activities (HR: 0.66; 95% CI: 0.48–0.91; p = 0.011), and pain/discomfort (HR: 0.61; 95% CI: 0.42–0.90; p = 0.012; Supplementary Table 1). Of these associations, only the association for mobility remained significant after adjustment for potential confounders in multivariable Cox regression analysis (HR: 0.61; 95% CI: 0.43–0.85; p = 0.004; Figure 2 and Supplementary Tables 5–9).
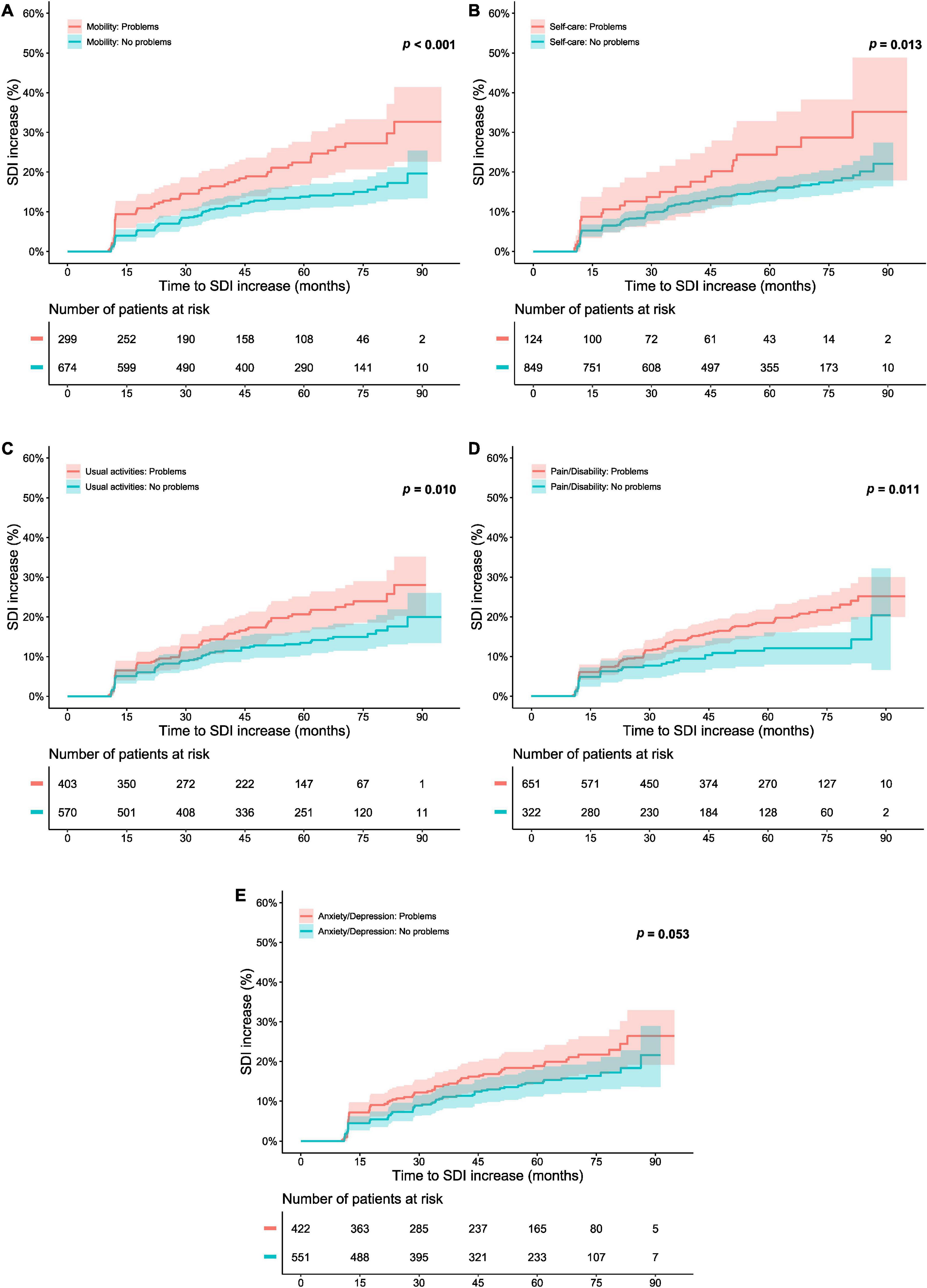
Figure 3. Responses of “no problems” in the separate dimensions of EQ-5D-3L in relation to organ damage accrual. The graphs in the upper panels delineate proportions of patients who accrued organ damage over the course of the open-label study period, stratified into patients who experienced “no problems” with regard to (A) mobility, (B) self-care, (C) usual activities, (D) pain/discomfort, and (E) anxiety/depression at the open-label baseline, and patients who did not. The lower panel shows numbers of participants in the different groups over time, decreasing due to documentation of SDI increase or data censoring. EQ-5D-3L, three-level version of EQ-5D; FHS, full health state; SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index.
The analysis of associations between “no problems” within EQ-5D dimensions and organ-specific damage accrual revealed that a lower proportion of level 1 respondents accrued musculoskeletal damage during the course of the open-label phase than did level 2–3 respondents regarding the mobility dimension (p = 0.004; derived from the logrank test; Figure 4). Experience of “no problems” regarding mobility was associated with reduced subsequent musculoskeletal damage accrual in time-dependent Cox regression analysis (HR: 0.38; 95% CI: 0.19–0.76; p = 0.006). No other associations were documented between “no problems” within the EQ-5D dimensions and organ-specific damage accrual (Supplementary Table 3). Similarly, experience of “no problems” within mobility was negatively correlated with musculoskeletal damage accrual during the open-label follow-up, irrespective of when in time this occurred (φ = −0.08; p = 0.008; Supplementary Table 4). We observed no such correlations between “no problems” within the other EQ-5D dimensions and organ-specific damage accrual (Supplementary Table 4).
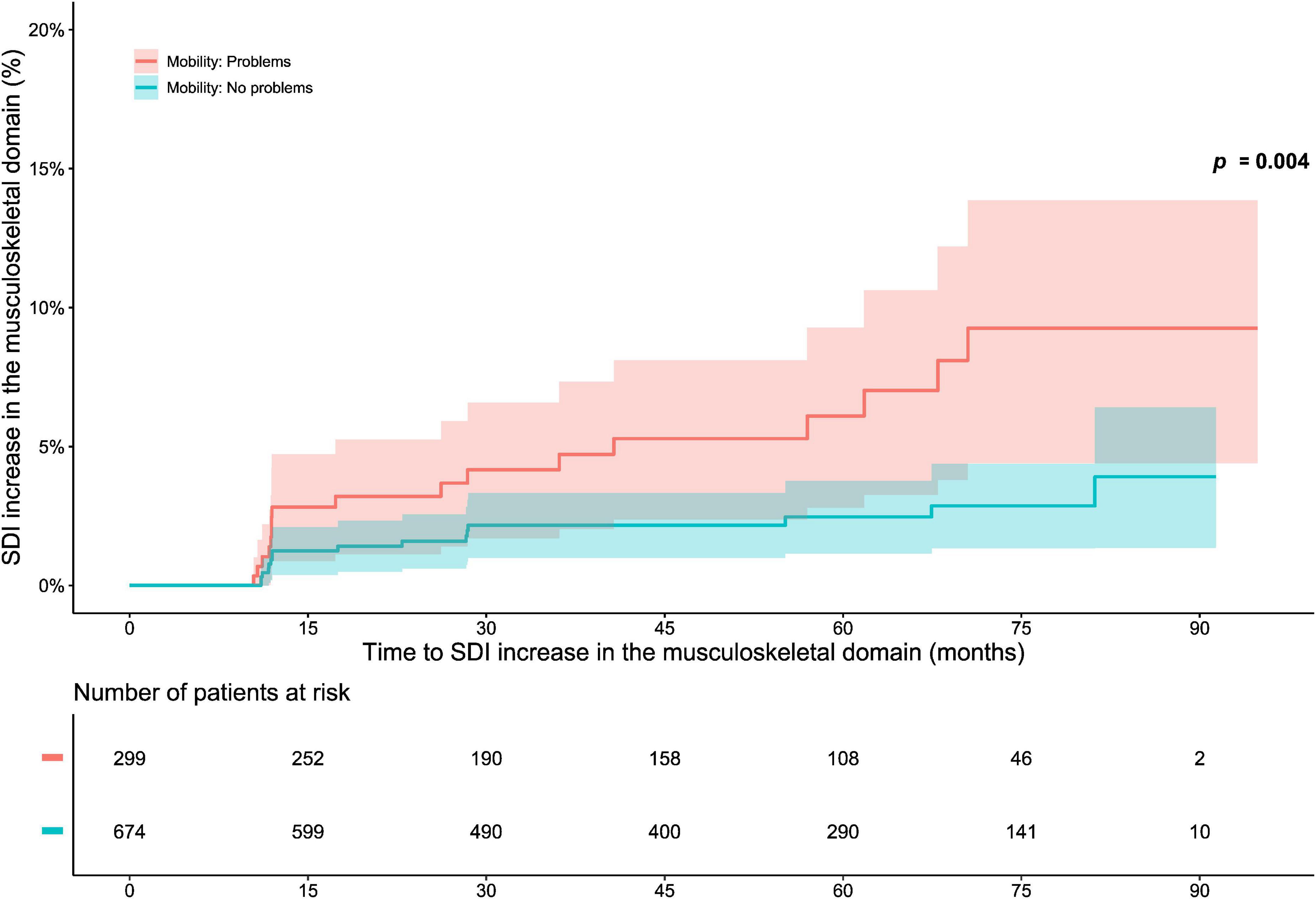
Figure 4. EQ-5D-3L mobility in relation to musculoskeletal damage accrual. The graphs in the upper panel delineate proportions of patients who accrued musculoskeletal damage over the course of the open-label study period, stratified into patients who experienced “no problems” with regard to mobility at the open-label baseline, and patients who did not. The lower panel shows numbers of participants in the two groups over time, decreasing due to documentation of SDI increase or data censoring. EQ-5D-3L, three-level version of EQ-5D; FHS, full health state; SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index.
In the present post hoc analysis of data from the BLISS-52 and BLISS-76 open-label extension studies, we found an association between EQ-5D-3L FHS experience at baseline (i.e., post therapeutic intervention with belimumab or placebo on top of non-biological standard therapy) and reduced subsequent organ damage accrual over the course of up to 8 years of open-label follow-up of SLE patients receiving add-on belimumab. Furthermore, experience of “no problems” regarding mobility was associated with a lower hazard to accrue organ damage, particularly musculoskeletal damage.
To the best of the authors’ knowledge, the association between EQ-5D FHS and organ damage accrual has not been studied to date. Several studies have found associations between patient-reported HRQoL and long-term outcomes in people with SLE, but the data have been conflicting (26–29). In cross-sectional studies, there has been a lack of consensus as to whether EQ-5D scores associate with already established organ damage (14–16). Importantly, associations between patient-reported HRQoL experience and SDI increase over time have not been reported in the literature and hence served as the scope of the present work, aiming at an understanding of how SLE patients’ perceptions of health state relate to disease evolution.
Self-reported experience of FHS indicates a high level of wellbeing in all five EQ-5D dimensions. We found that FHS was associated with a reduced hazard to accrue subsequent organ damage, as was “no problems” within the mobility dimension, the latter thus presumably comprising the main driver of the association found for FHS. Interestingly, experience of “no problems” within the mobility dimension of EQ-5D was negatively associated with progression of organ damage in the musculoskeletal SDI domain in particular. The intuitive direction of this association, with unhampered mobility presumably facilitating physical activity and preservation of musculoskeletal function, points to the reliability of the self-reported patient perspective, hence its usefulness in treatment and disease evaluation. Of course, patient-reported outcomes are not meant to substitute but rather be complemental to clinical and laboratory parameters. The findings of this study advocate for their potential usefulness also as a proxy for prognostication.
Several self-assessment instruments of HRQoL exist that have specifically been developed for SLE. Inevitably, the more precise the instrument is, the more time and effort it requires from the respondent to fill in the instrument, which may also result in decreased response frequencies or recruitment of participants in studies, incomplete responses, and incorrect completion (30). For this reason, the briefness of the EQ-5D format paired with its known-group validity (17) and its satisfactory psychometric properties (14) in SLE patients along with its ability to predict long-term disease outcome as shown in the present study suggest that EQ-5D is a feasible and clinically relevant HRQoL instrument to use in SLE practices and studies.
During the past few decades, treatment of common chronic illnesses such as hypertension and diabetes has evolved from symptomatic to target-based (31). In the wake of this trend, the principle of treat-to-target has evolved during the past decade as a strategy for the management of rheumatic diseases e.g., rheumatoid arthritis (RA) (32). In later years, it has also been successfully applied in SLE, where remission (33, 34) and Lupus Low Disease Activity State (LLDAS) (35, 36) have been demonstrated to associate with deceleration of organ damage accrual (37) and favourable HRQoL experience (38, 39), and hence receive increasing endorsement as treatment targets (31). However, in contrast to the Disease Activity Score (DAS)-28-based definition of remission used in RA (40), which encompasses patient-reported global health, common definitions of remission and low disease activity in SLE do not include patient-reported components. While consensus upon how patient-reported outcomes should be integrated in SLE to serve as outcome measures used in studies and clinical practice has yet to be achieved (10), our findings advocate that patient-reported HRQoL by means of EQ-5D holds promise as a complemental component to current definitions. However, it is important to note that addition of patient-reported components to already validated outcome measures is a highly speculative notion until their added value has been thoroughly investigated. Nevertheless, the need of adequately capturing the patient perspective is imperative, as highlighted by the apparent discrepancies between patients’ and clinicians’ priorities (9) and perceptions of health state (41–43). Along the same lines, a recent study showed that substantial proportions of patients who had attained adequate responses to treatment still experienced poor HRQoL (7). Altogether, the prospect of including patient-reported components in definitions of response to therapy, remission, or low disease activity may form an essential constituent of the treat-to-target research agenda, with the present investigation lending support for EQ-5D as a useful tool for capturing SLE patients’ HRQoL experience.
Limitations included the post hoc nature of our study design, the lack of EQ-5D-3L data during the open-label phase, as well as the lack of adjustments for potentially confounding comorbidities, such as fibromyalgia. Another important limitation was the different follow-up times of 52 and 76 weeks in the BLISS-52 and BLISS-76 clinical trials, respectively, which may have favoured attainability of “no problems” in the different EQ-5D dimensions or FHS in BLISS-76 where the patients were followed for approximately 50% longer time than in BLISS-52 before entering the open-label phase. However, a previous analysis from our group showed that proportions of EQ-5D-3L FHS responses plateaued from week 52 through week 76 in BLISS-76 (17). Furthermore, the selected population of the trials consisting mainly of articular and/or mucocutaneous SLE and excluding severe active renal and CNS lupus may not be considered fully representative of real-life clinical settings. It is also worth noting that relatively few patients accrued organ damage during the open-label extension studies, as indicated herein as well as by the work of others (20). Particular strengths of the study included the large and ethnically diverse SLE population of the BLISS trials, and the follow-up period of up to 8 years.
In this study, patient-reported experience of EQ-5D-3L full health state and “no problems” in the mobility dimension after therapeutic intervention heralded a reduced hazard to accrue subsequent organ damage, especially in the musculoskeletal domain. In the era of treat-to-target strategies, our findings canvass the use of EQ-5D in a discretised manner as a clinically relevant treatment target in SLE, captured by the patients themselves, complemental to clinical and laboratory parameters. Presumably, more favourable long-term outcomes may be achieved for SLE patients if we target towards the best possible patient-reported HRQoL experience, along with the lowest possible disease activity and, when possible, remission.
Patient research partners were involved in the study concept and design, interpretation of data, and editing of the manuscript.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The BLISS study protocols were reviewed and approved by regional Ethics Review Boards for all participating centres, and the study protocol for this post-hoc analysis was reviewed and approved by the Swedish Ethical Review Authority (2019-05498). Written informed consent was obtained from all study participants prior to enrolment in BLISS-52 and BLISS-76 and their OL extension phases.
JL, SZ, YE, DG, AG, and IP: conception and design of the work. JL, SZ, SE, DG, AG, and IP: data management. JL, SZ, GP, YE, EH, MR, DG, AG, and IP: statistical analysis and interpretation of data. GP and YE: patient research partners. JL, SZ, SE, AB, GP, YE, EH, MR, DG, AG, and IP: critical revision of the manuscript for important intellectual content. All authors reviewed and approved the final version of the manuscript prior to submission and agreed to be accountable for all aspects of the work.
This work was supported by the GlaxoSmithKline Investigator-Sponsored Studies (ISS) programme, and grants from the Swedish Rheumatism Association (R-941095), King Gustaf V’s 80-year Foundation (FAI-2020-0741), Professor Nanna Svartz Foundation (2020-00368), Swedish Society of Medicine (SLS-974449), Ulla and Roland Gustafsson Foundation (2021–26), Region Stockholm (FoUI-955483), and Karolinska Institutet. JL, EH, MR, and IP have received research funding from the EuroQol Research Foundation. EH has received honoraria from the EuroQol Research Foundation. IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis, Otsuka Pharmaceutical, and F. Hoffmann-La Roche AG. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
The authors thank GlaxoSmithKline (Uxbridge, United Kingdom) for sharing the data from the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials, and their open-label extension studies BEL112233 (NCT00724867) and BEL112234 (NCT00712933), through the Clinical Study Data Request (CSDR) portal, as well as all participating patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1092325/full#supplementary-material
1. Kaul A, Gordon C, Crow M, Touma Z, Urowitz M, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. (2016) 2:16039. doi: 10.1038/nrdp.2016.39
2. Dey M, Parodis I, Nikiphorou E. Fatigue in systemic lupus erythematosus and rheumatoid arthritis: a comparison of mechanisms, measures and management. J Clin Med. (2021) 10:3566. doi: 10.3390/jcm10163566
3. Chambers S, Allen E, Rahman A, Isenberg D. Damage and mortality in a group of british patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology. (2009) 48:673–5. doi: 10.1093/rheumatology/kep062
4. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. (2019) 96:1–13. doi: 10.1016/j.jaut.2018.11.001
5. Parodis I, Lopez Benavides A, Zickert A, Pettersson S, Moller S, Welin Henriksson E, et al. The impact of belimumab and rituximab on health-related quality of life in patients with systemic lupus erythematosus. Arthritis Care Res. (2019) 71:811–21. doi: 10.1002/acr.23718
6. Gomez A, Soukka S, Johansson P, Akerstrom E, Emamikia S, Enman Y, et al. Use of antimalarial agents is associated with favourable physical functioning in patients with systemic lupus erythematosus. J Clin Med. (2020) 9:1813. doi: 10.3390/jcm9061813
7. Gomez A, Qiu V, Cederlund A, Borg A, Lindblom J, Emamikia S, et al. Adverse health-related quality of life outcome despite adequate clinical response to treatment in systemic lupus erythematosus. Front Med. (2021) 8:651249. doi: 10.3389/fmed.2021.651249
8. Strand V, Gladman D, Isenberg D, Petri M, Smolen J, Tugwell P. Endpoints: consensus recommendations from omeract iv. outcome measures in rheumatology. Lupus. (2000) 9:322–7. doi: 10.1191/096120300678828424
9. Golder V, Ooi J, Antony A, Ko T, Morton S, Kandane-Rathnayake R, et al. Discordance of patient and physician health status concerns in systemic lupus erythematosus. Lupus. (2018) 27:501–6. doi: 10.1177/0961203317722412
10. Parodis I, Studenic P. Patient-reported outcomes in systemic lupus erythematosus. can lupus patients take the driver’s seat in their disease monitoring? J Clin Med. (2022) 11:340. doi: 10.3390/jcm11020340
11. Euroqol Group. Euroqol - a new facility for the measurement of health-related quality of life. Health Policy. (1990) 16:199–208. doi: 10.1016/0168-8510(90)90421-9
12. Euroqol Research Foundation [ERF]. EQ-5d-3l user guide. (2018). Available online at: https://Euroqol.Org/Publications/User-Guides (accessed July 30, 2020).
13. Shi Y, Li M, Liu L, Wang Z, Wang Y, Zhao J, et al. Relationship between disease activity, organ damage and health-related quality of life in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev. (2021) 20:102691. doi: 10.1016/j.autrev.2020.102691
14. Aggarwal R, Wilke C, Pickard A, Vats V, Mikolaitis R, Fogg L, et al. Psychometric properties of the euroqol-5d and short form-6d in patients with systemic lupus erythematosus. J Rheumatol. (2009) 36:1209–16. doi: 10.3899/jrheum.081022
15. Wang S, Wu B, Zhu L, Leng L, Bucala R, Lu L. Construct and criterion validity of the euro QOL-5d in patients with systemic lupus erythematosus. PLoS One. (2014) 9:e98883. doi: 10.1371/journal.pone.0098883
16. Chang S, Cho J, Shin N, Oh H, Choi B, Yoon M, et al. Depression and quality of life in patients with systemic lupus erythematosus. J Rheum Dis. (2015) 22:346–55. doi: 10.4078/jrd.2015.22.6.346
17. Lindblom J, Gomez A, Borg A, Emamikia S, Ladakis D, Matilla J, et al. Eq-5d-3l full health state discriminates between drug and placebo in clinical trials of systemic lupus erythematosus. Rheumatology. (2021) 60:4703–16. doi: 10.1093/rheumatology/keab080
18. Navarra S, Guzmán R, Gallacher A, Hall S, Levy R, Jimenez R, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. (2011) 377:721–31. doi: 10.1016/s0140-6736(10)61354-2
19. Furie R, Petri M, Zamani O, Cervera R, Wallace D, Tegzová D, et al. A phase iii, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits b lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. doi: 10.1002/art.30613
20. van Vollenhoven R, Navarra S, Levy R, Thomas M, Heath A, Lustine T, et al. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a phase iii study extension. Rheumatology. (2020) 59:281–91. doi: 10.1093/rheumatology/kez279
21. Furie R, Wallace D, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy-six-week phase iii parent study in the united states. Arthritis Rheumatol. (2018) 70:868–77. doi: 10.1002/art.40439
22. Hochberg M. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40:1725. doi: 10.1002/art.1780400928
23. Petri M, Kim M, Kalunian K, Grossman J, Hahn B, Sammaritano L, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. (2005) 353:2550–8. doi: 10.1056/NEJMoa051135
24. Gladman D, Ibanez D, Urowitz M. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29:288–91.
25. Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the systemic lupus international collaborating clinics/American college of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. (1996) 39:363–9. doi: 10.1002/art.1780390303
26. Fortin P, Abrahamowicz M, Neville C, du Berger R, Fraenkel L, Clarke A, et al. Impact of disease activity and cumulative damage on the health of lupus patients. Lupus. (1998) 7:101–7. doi: 10.1191/096120398678919813
27. Jolly M, Utset T. Can disease specific measures for systemic lupus erythematosus predict patients health related quality of life? Lupus. (2004) 13:924–6. doi: 10.1191/0961203304lu2034oa
28. Gladman D, Urowitz M, Ong A, Gough J, MacKinnon A. Lack of correlation among the 3 outcomes describing sle: disease activity, damage and quality of life. Clin Exp Rheumatol. (1996) 14:305–8.
29. Azizoddin D, Jolly M, Arora S, Yelin E, Katz P. Patient-reported outcomes predict mortality in lupus. Arthritis Care Res. (2019) 71:1028–35. doi: 10.1002/acr.23734
30. Mahieu M, Yount S, Ramsey-Goldman R. Patient-reported outcomes in systemic lupus erythematosus. Rheum Dis Clin North Am. (2016) 42:253–63. doi: 10.1016/j.rdc.2016.01.001
31. van Vollenhoven R, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrom K, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. (2014) 73:958–67. doi: 10.1136/annrheumdis-2013-205139
32. Golder V, Tsang A. Treatment targets in sle: remission and low disease activity state. Rheumatology. (2020) 59(Suppl. 5):v19–28. doi: 10.1093/rheumatology/keaa420
33. van Vollenhoven R, Voskuyl A, Bertsias G, Aranow C, Aringer M, Arnaud L, et al. A framework for remission in sle: consensus findings from a large international task force on definitions of remission in sle (Doris). Ann Rheum Dis. (2017) 76:554–61. doi: 10.1136/annrheumdis-2016-209519
34. van Vollenhoven R, Bertsias G, Doria A, Isenberg D, Morand E, Petri M, et al. 2021 doris definition of remission in sle: final recommendations from an international task force. Lupus Sci Med. (2021) 8:e000538. doi: 10.1136/lupus-2021-000538
35. Franklyn K, Lau C, Navarra S, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and initial validation of a lupus low disease activity state (Lldas). Ann Rheum Dis. (2016) 75:1615–21. doi: 10.1136/annrheumdis-2015-207726
36. Golder V, Kandane-Rathnayake R, Huq M, Nim H, Louthrenoo W, Luo S, et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatol. (2019) 1:e95–102. doi: 10.1016/s2665-9913(19)30037-2
37. Ugarte-Gil M, Mendoza-Pinto C, Reátegui-Sokolova C, Pons-Estel G, van Vollenhoven R, Bertsias G, et al. Achieving remission or low disease activity is associated with better outcomes in patients with systemic lupus erythematosus: a systematic literature review. Lupus Sci Med. (2021) 8:e000542. doi: 10.1136/lupus-2021-000542
38. Tsang A, Bultink I, Heslinga M, van Tuyl L, van Vollenhoven R, Voskuyl A. The relationship between remission and health-related quality of life in a cohort of sle patients. Rheumatology. (2019) 58:628–35. doi: 10.1093/rheumatology/key349
39. Emamikia S, Oon S, Gomez A, Lindblom J, Borg A, Enman Y, et al. Impact of remission and low disease activity on health-related quality of life in patients with systemic lupus erythematosus. Rheumatology. (2022) 61:4752–62. doi: 10.1093/rheumatology/keac185
40. Prevoo M, van ’t Hof M, Kuper H, van Leeuwen M, van de Putte L, van Riel P. Modified disease activity scores that include twenty-eight-joint counts. development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. (1995) 38:44–8. doi: 10.1002/art.1780380107
41. Yen J, Neville C, Fortin P. Discordance between patients and their physicians in the assessment of lupus disease activity: relevance for clinical trials. Lupus. (1999) 8:660–70. doi: 10.1191/096120399680411362
42. Leong K, Chong E, Kong K, Chan S, Thong B, Lian T, et al. Discordant assessment of lupus activity between patients and their physicians: the singapore experience. Lupus. (2010) 19:100–6. doi: 10.1177/0961203309345748
Keywords: systemic lupus erythematosus, organ damage, patient-reported outcomes, patient perspective, outcomes research, health-related quality of life
Citation: Lindblom J, Zetterberg S, Emamikia S, Borg A, von Perner G, Enman Y, Heintz E, Regardt M, Grannas D, Gomez A and Parodis I (2022) EQ-5D full health state after therapy heralds reduced hazard to accrue subsequent organ damage in systemic lupus erythematosus. Front. Med. 9:1092325. doi: 10.3389/fmed.2022.1092325
Received: 07 November 2022; Accepted: 02 December 2022;
Published: 20 December 2022.
Edited by:
Cong-Qiu Chu, Oregon Health and Science University, United StatesReviewed by:
Graciela Alarcon, University of Alabama at Birmingham, United StatesCopyright © 2022 Lindblom, Zetterberg, Emamikia, Borg, von Perner, Enman, Heintz, Regardt, Grannas, Gomez and Parodis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Parodis, ✉ aW9hbm5pcy5wYXJvZGlzQGtpLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.