- 1Department of Hematology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Xiyuan Clinical Medical College, Beijing University of Traditional Medicine, Beijing, China
Objectives: The currently recommended aspirin regimen appears inadequate for thromboprophylaxis in essential thrombocythemia (ET). This study aimed not only to evaluate the curative effect of aspirin but also to explore the coagulation status and determinants of aspirin resistance (AR) of ET patients.
Methods: A total of 80 ET patients who underwent coagulation tests, thromboelastography (TEG), and next-generation sequencing (NGS) were involved in the study. Patients were divided into the aspirin sensitivity (AS) group and AR group according to the arachidonic acid inhibition rate. Their clinical features and coagulation function were analyzed.
Results: The incidence of AR was 53.75% (43/80) in 80 ET patients. Fbg was significantly higher in coagulation tests in AR patients compared with AS patients (P < 0.05), while the differences in other variables (D-D, PT, PTA, INR, APTT, TT, FDP, and AT-III) were not statistically significant (P > 0.05). Compared with AS patients, the K values, α angles, MA values, and CI values of TEG in AR patients were statistically smaller (P < 0.05), but there was no significant difference in R value between them (P > 0.05). Univariate and multivariate logistic regression analysis showed that age, irregular use of aspirin, smoking, dyslipidemia, and hypertension increased the risk of AR (P < 0.05). In the routine NGS, the driver gene and non-driver gene had no effect on AR in ET patients.
Conclusion: Compared with AS patients, AR patients have enhanced platelet aggregation function, are in a relatively hypercoagulable state, and haveelevated fibrinogen function/levels, all of which cause a worse coagulation status. ET patients with increasing age, irregular use of aspirin, smoking, dyslipidemia, and hypertension are possibly at higher risk of AR. The routine NGS may not be helpful for the prediction of AR, therefore we recommend adding relevant drug-resistance genes to NGS.
1 Introduction
Essential thrombocythemia (ET) is a Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) characterized by highly proliferative megakaryocytes in the bone marrow and markedly elevated platelet counts in peripheral blood (1). The arterial and venous thrombosis rate in MPN patients has been estimated as 3-fold and 10-fold increased, respectively, compared with the general population (2). Therefore, it is very important to prevent thrombosis in the treatment of these patients (3).
Low-dose aspirin (75–100 mg/day) is widely used to prevent thrombosis in patients with ET (4, 5). In recent years, studies have shown that patients’ responses to aspirin are different (6–8). In the clinic, even if some patients regularly take aspirin for a long time, thrombosis will still occur. This may be because aspirin has insufficient inhibitory effect on platelets. This phenomenon is called aspirin resistance (AR), while the opposite is called aspirin sensitivity (AS) (9, 10). The purpose of this study was not only to evaluate the curative effect of aspirin in ET patients, but also to explore the coagulation status and determinants of AR.
2 Materials and methods
2.1 Patients
We collected data from 80 ET patients in the Xiyuan Hospital from June 2019 to December 2021. All patients were diagnosed according to World Health Organization (WHO) diagnostic criteria (11) and underwent the next-generation sequencing (NGS), coagulation test, and thromboelastography (TEG). All patients were over 18 years old and had been taking aspirin (100 mg once a day) for at least 1 month. Patients could not take other drugs that affect coagulation function within one month before being examined. This study was approved by the medical ethics committee of the hospital (Reference number: 2019XLA024-3) and by the Chinese Clinical Trial Registry (Registry number: ChiCTR2200057736).
2.2 Clinical and laboratory data
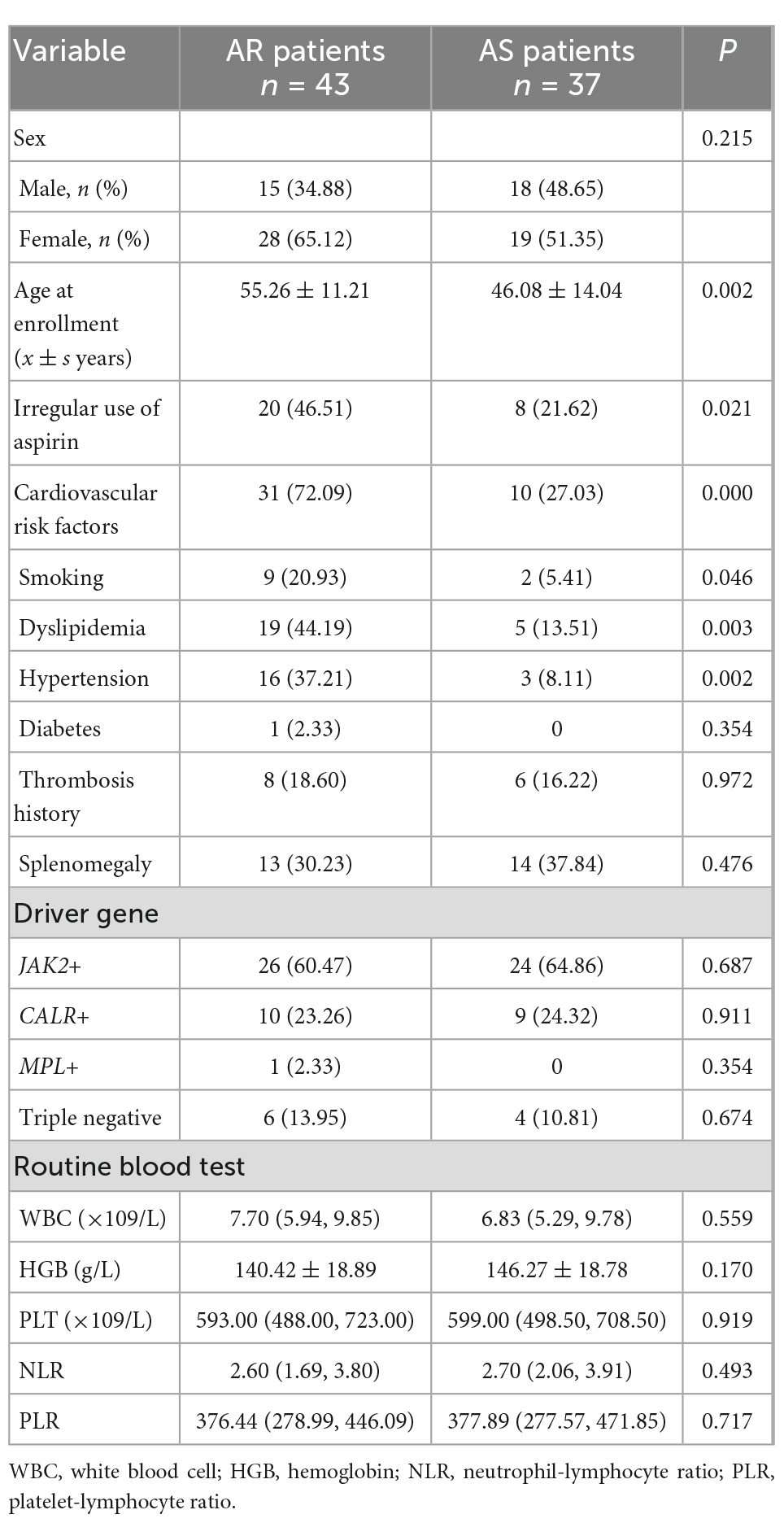
Laboratory data included sex, age, aspirin use, cardiovascular risk factors (smoking, dyslipidemia, hypertension, and diabetes), history of thrombosis, presence or absence of splenomegaly, driver gene types (JAK2, CALR, MPL, and Triple negative), presence or absence of non-driver genes, routine blood test (WBC, HGB, PLT, NLR, and PLR), coagulation test (Fbg, D-D, PT, PTA, INR, APTT, TT, FDP, and AT-III), and TEG (R, K, α angle, MA, and CI). Referring to the relevant evaluation criteria (12–16), AR is defined as the inhibition rate of arachidonic acid (AA) <50%, and AS is defined as the inhibition rate of AA ≥50%.
2.3 Statistical analysis
SPSS 26.0 statistical software was used for analysis. The measurement data conforming to the normal distribution adopted the mean ± standard deviation (x ± s) and two-sample t-tests. If the measurement data did not conform to the normal distribution, M (P25 and P75) was used to express it, and the rank sum test was adopted. The risk factors of AR were analyzed by univariate and multivariate logistic regression. The enumeration data were statistically analyzed with the Chi-squared test. P < 0.05 meant statistically significant.
3 Results
3.1 The incidence of AR in ET patients
Among the 80 ET patients, 43 (53.75%) developed AR, with an average AA inhibition rate of 25.91%. There were 37 patients with AS, and the average inhibition rate of AA was 65.05%.
3.2 Comparison of clinical characteristics between AR and AS patients
Compared with AS patients, AR patients were significantly older, took aspirin irregularly, and had smoking, dyslipidemia, and hypertension (P < 0.05). There was no significant difference (P > 0.05) between the two groups in gender, thrombosis history, splenomegaly, driver gene type, white blood cell count (WBC), hemoglobin (HGB), platelet count (PLT), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR), as shown in Table 1.
3.3 Comparison of coagulation status between AR patients and AS patients
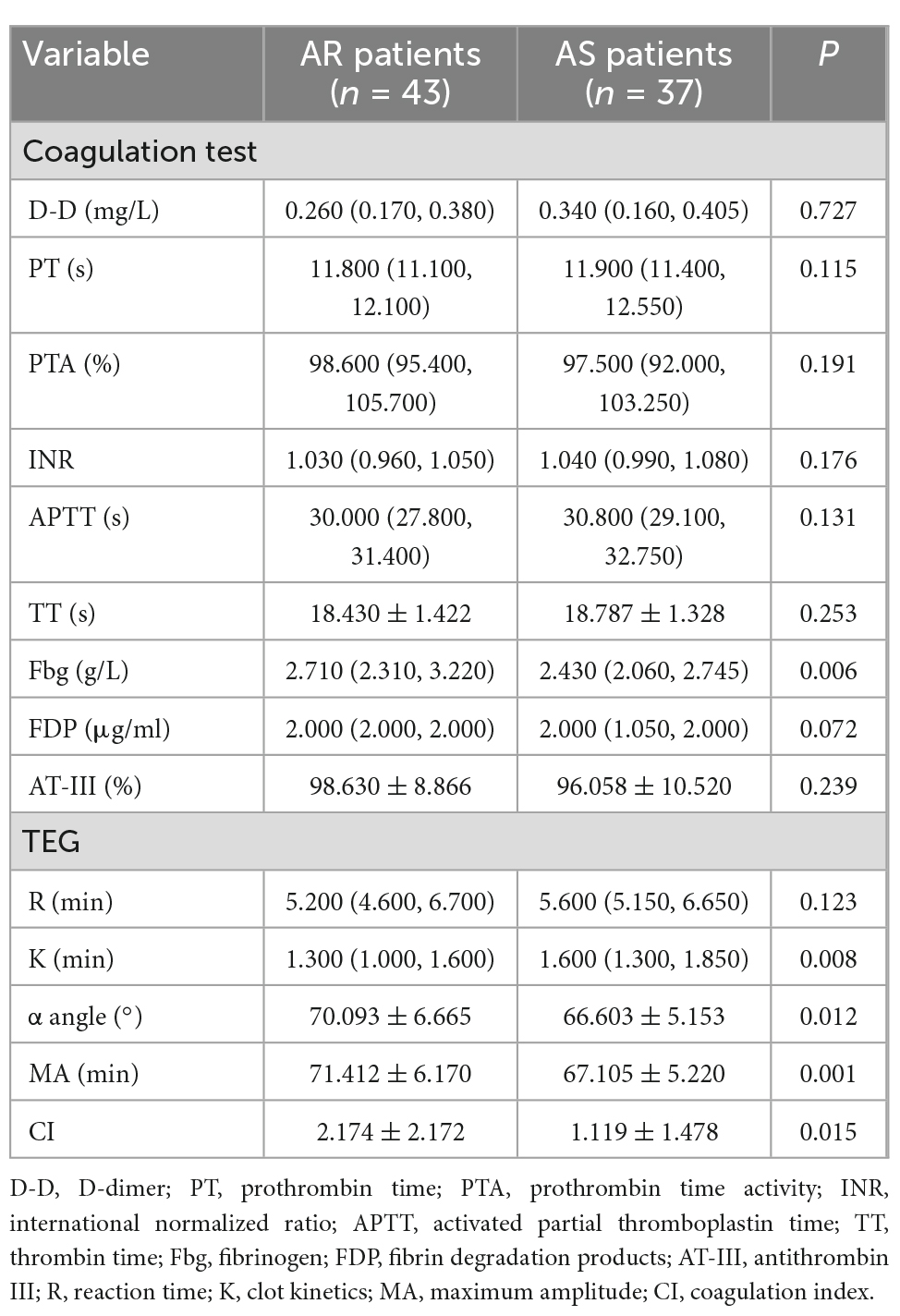
In the coagulation test, the fibrinogen (Fbg) of 5% (4/80, 3 cases of AR and 1 case of AS) ET patients exceeded the normal upper limit, while other indexes (D-D, PT, PTA, INR, APTT, TT, FDP, and AT-III) did not. On TEG, 7.5% (6/80, 5 cases of AR and 1 case of AS) of patients had enhanced coagulation factor function (R < 4 min), 30.0% (24/80, 18 cases of AR and 6 cases of AS) of patients had increased fibrinogen function/level (K < 1 min or α angle >72°), 45.0% (36/80, 24 cases of AR and 12 cases of AS) of patients had enhanced platelet aggregation function (MA >70 mm), and 26.3% (21/80, 17 cases of AR and 4 cases of AS) of patients were in hypercoagulable state (CI >3).
Specifically, in the coagulation test, the Fbg of AR patients was significantly higher than that of AS patients (P < 0.05), while the differences in other variables (D-D, PT, PTA, INR, APTT, TT, FDP, and AT-III) were not statistically significant (P > 0.05). Compared with AS patients, the K values, α angles, MA values, and CI values of TEG in AR patients were lower (P < 0.05). But the difference of R values was not statistically significant (P > 0.05). See Table 2 for greater detail.
3.4 Risk factors for AR: Univariate logistic regression analysis
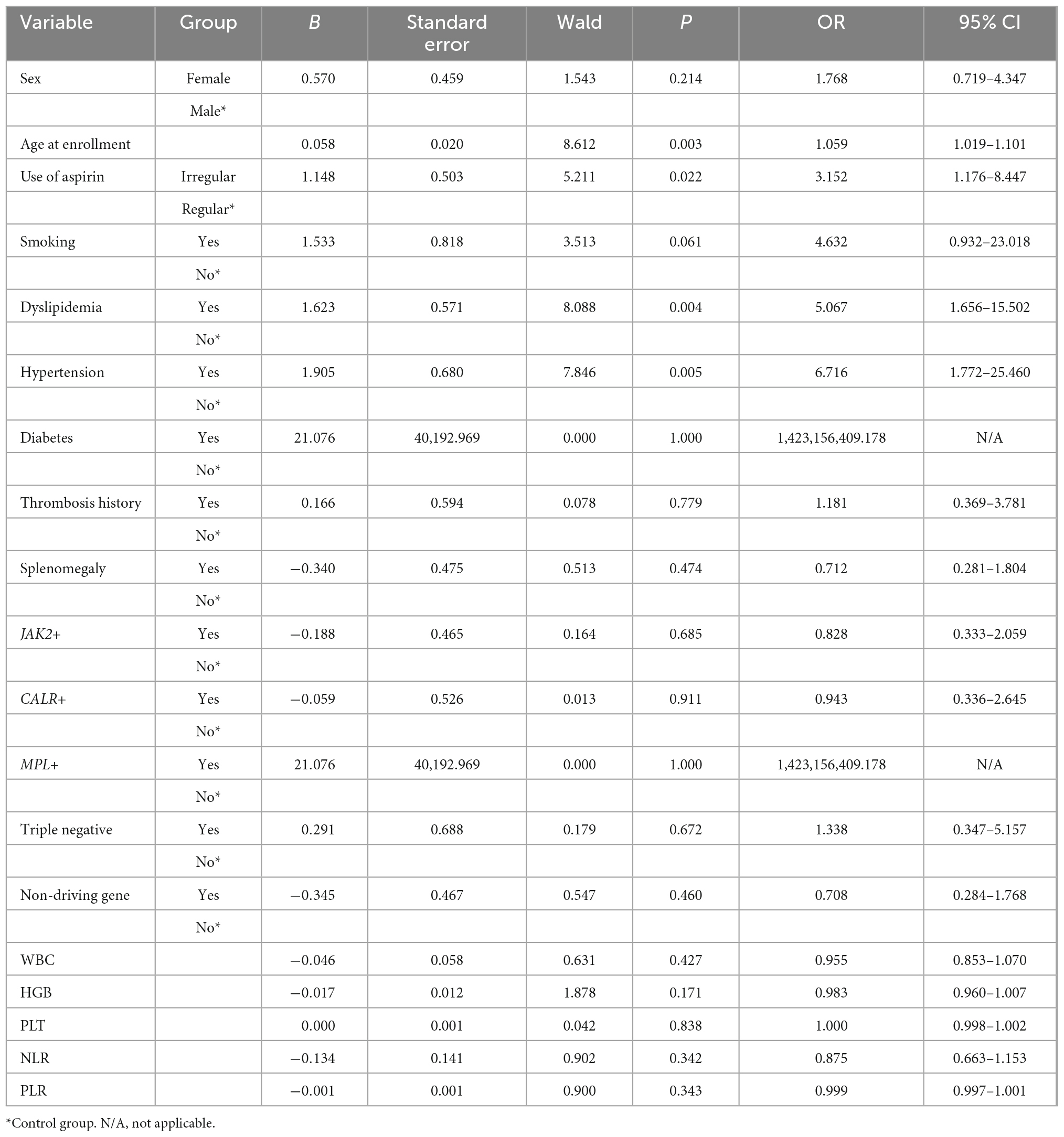
Aspirin resistance was used as the dependent variable, as shown in Table 3, and 19 influencing factors were analyzed by univariate logistic regression. The results showed that patients with older age (OR = 1.059, 95% CI 1.019–1.101, P = 0.003), irregular aspirin use (OR = 3.152, 95% CI 1.176–8.447, P = 0.022), dyslipidemia (OR = 5.067, 95% CI 1.656–15.502, P = 0.004), and hypertension (OR = 6.716, 95% CI 1.772–25.460, P = 0.005) had a higher risk of AR.
3.5 Risk factors for AR: Multivariate logistic regression analysis
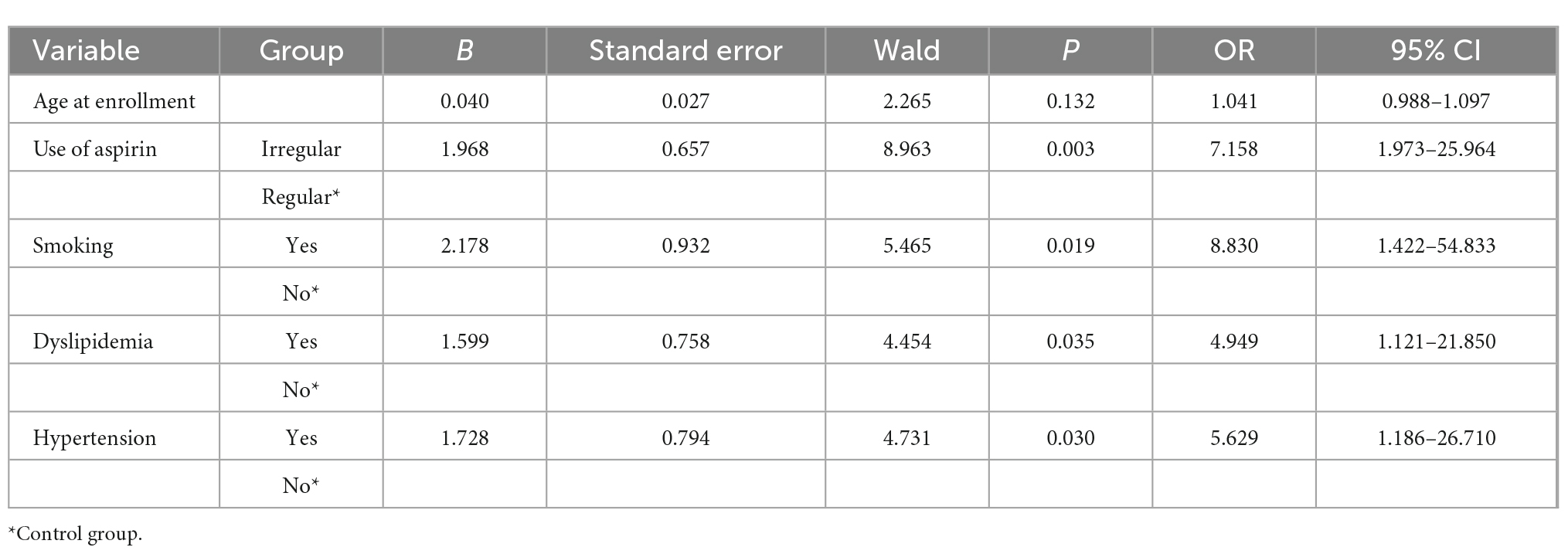
Variables with P < 0.10 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis. A total of five variables were eligible (age, irregular aspirin use, smoking, dyslipidemia, and hypertension). The results showed that patients who took aspirin irregularly (OR = 7.158 95% CI 1.973–25.964, P = 0.003), smoked (OR = 8.830, 95% CI 1.422–54.833, P = 0.019), had dyslipidemia (OR = 4.949, 95% CI 1.121–21.850, P = 0.035), and had hypertension (OR = 5.629, 95% CI 1.186–26.710, P = 0.030) had a higher risk of AR, as shown in Table 4.
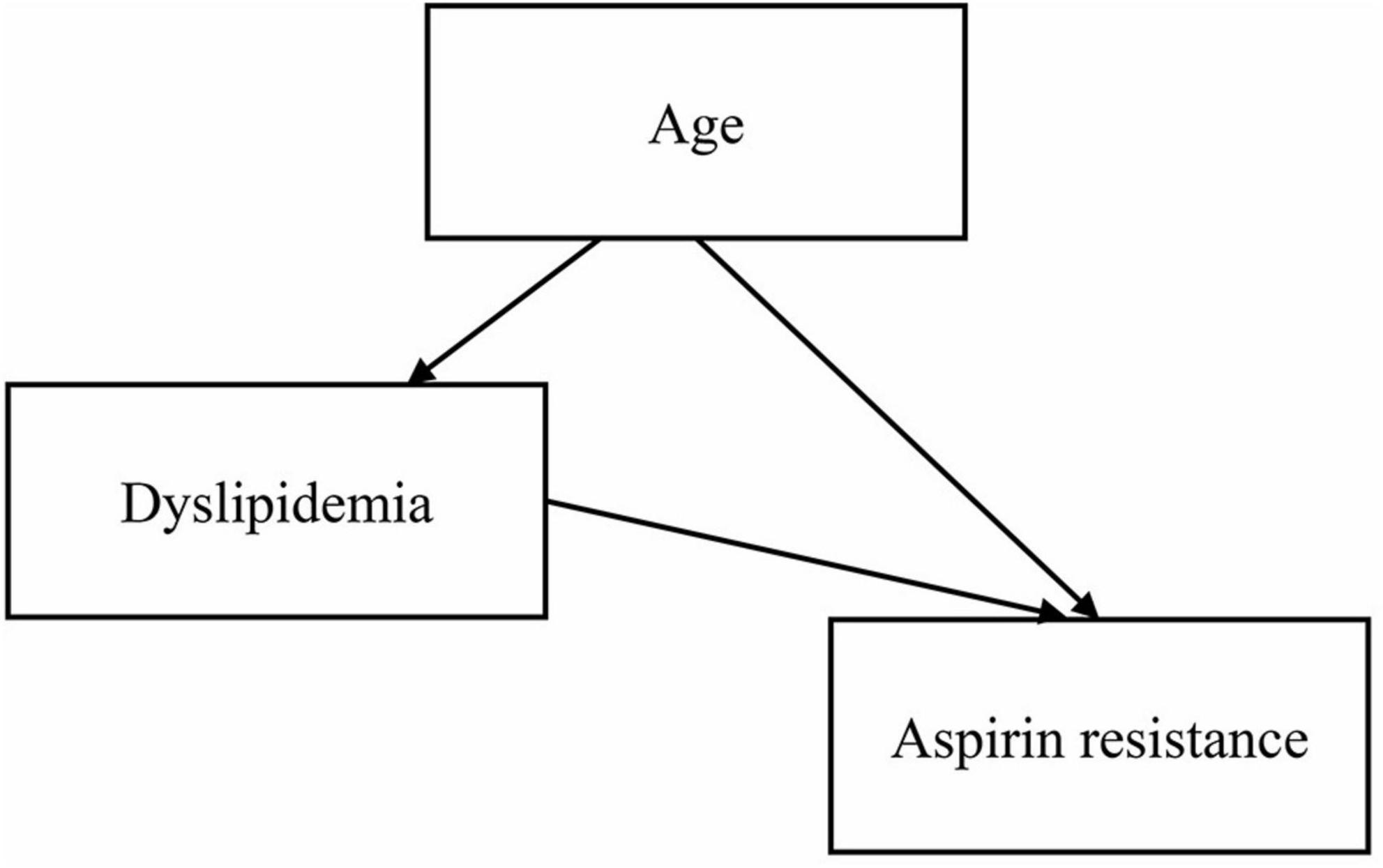
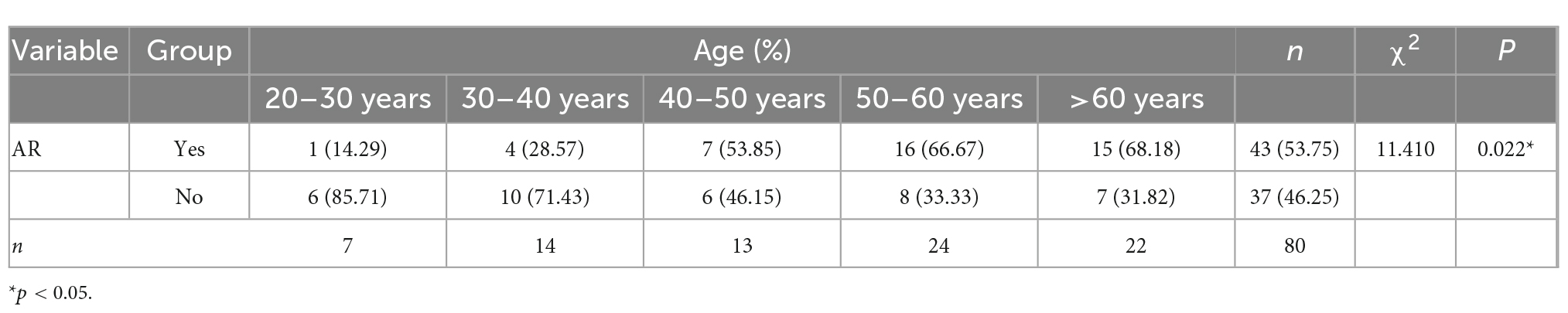
Age was statistically significant in the univariate logistic regression analysis, but not in the multivariate logistic regression analysis. The main reason could be the presence of intermediate or confounding variables. We used the two-factor model approach to explore the reasons for the inconsistent results. We established several regression models, each with the dependent variable “aspirin resistance,” the independent variable “age” and one other independent variable. Only in the two-factor regression model of “age + dyslipidemia,” age was found to be statistically insignificant, so “dyslipidemia” was considered to be an interfering factor affecting age. Studies have shown that age is a risk factor for dyslipidemia (17, 18), and a directed acyclic graph (DAG) showed that dyslipidemia is an intermediate variable of age (Figure 1). After excluding dyslipidemia, the other four variables (age, irregular aspirin use, smoking, and hypertension) were analyzed by multivariate logistic regression. The results showed that the older the patients were, the higher the risk of AR was (OR = 1.067, 95% CI 1.017–1.119, P = 0.008). The Chi-square test was used to further analyze the relationship between different age segments and AR, and there were significant differences (χ2 = 11.410, P = 0.022). It could be seen that the AR resistance rate gradually increased with age increase, especially after the age of 40, the proportion of AR patients was significantly higher than that of AS patients (as shown in Table 5).
3.6 Comparison of driver genes mutational load and non-driver genes types between AR and AS patients based on NGS
Table 1 demonstrated that there was no significant difference in the type of driver genes between AR and AS patients. As shown in Table 6, our further analysis showed that there was no significant difference in driver gene mutational load and non-driver gene types between AR and AS patients (P > 0.05).
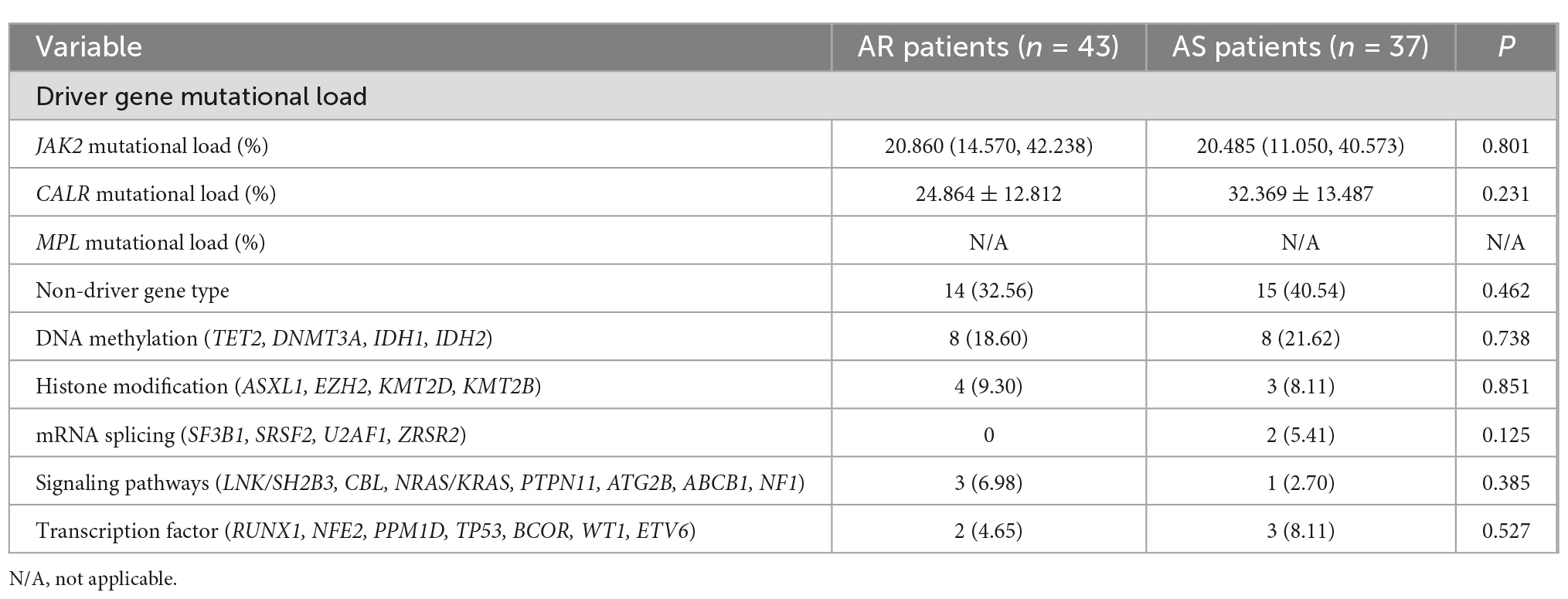
Table 6. Comparison of driver genes mutational load and non-driver genes types between AR and AS patients.
4 Discussion
Thrombosis prevention is an important therapeutic goal of ET. Aspirin is widely used for the primary and secondary prevention of thrombosis in ET patients. A study showed that the widely promoted low-dose (100 mg mg/day) aspirin regimen could not effectively reduce platelet activation (19). Therefore, exploring coagulation status and determinants of AR has important clinical significance for thromboprophylaxis of ET.
Thrombosis occurs as a result of a combination of changes in the vascular endothelial cells, platelets, coagulation, fibrinolytic system, and blood rheology. Studies have shown that all these factors have changed to varying degrees before thrombosis (3, 20, 21). TEG can dynamically monitor the process of coagulation, and it can help identify the prethrombotic state of patients in combination with a coagulation test (22–24).
In the study, the incidence of AR was 53.75% in 80 patients. Our study found that 45.0% of ET patients had enhanced platelet aggregation (MA >70 mm), and 26.3% had hypercoagulability (CI >3). Specifically, the incidence of enhanced platelet aggregation was higher in AR patients than in AS patients (55.81 > 32.43%, P < 0.05), and the incidence of hypercoagulable state was also higher in AR patients than in AS patients (39.53 > 10.81%, P < 0.05). This means that even in AS patients, 1/3 of them still have enhanced platelet aggregation function, and 1/10 of them are in a hypercoagulable state. This indicates that the current antiplatelet treatment scheme is really inadequate and needs to be improved. And compared to AS patients, AR patients had significantly higher fibrinogen values, significantly lower K values, and significantly larger alpha angles (p < 0.05). Therefore, it is considered that hypercoagulability is not only related to the enhancement of platelet aggregation function, but also related to the function/level of fibrinogen. The R value can reflect the activity of coagulation factors. In this study, 7.5% of patients had R values below normal, which means that there is no general abnormality of coagulation factors in ET patients. And there was no difference in R values between AR patients and AS patients, which implies that aspirin has little effect on coagulation factors.
Aspirin can prevent the production of thromboxane A2 (TXA2) in platelets by irreversibly acetylating a serine residue at position 529 of the cyclooxygenase-1 (COX-1) isoform (25). Aging affects AR probably associated with some reduction in the first-pass metabolism and bioavailability of aspirin, which is due to decreased liver mass and perfusion (26, 27). Taking aspirin irregularly will weaken its efficacy, which is an important factor of AR (25). In smokers, the biosynthesis of TXA2 is increased. Their serum C-reactive protein (CRP) also increases, while in the inflammatory state, the risk of AR increases (28, 29). Hypercholesterolemia and hypertension can lead to overexpression of COX-1, which enhances AR (30–32). After univariate and multivariate logistic regression analysis, the risk of AR was higher in ET patients with increasing age, irregular aspirin use, smoking, dyslipidemia, and hypertension. It suggests that ET patients should take aspirin regularly, quit smoking, and control blood lipid and blood pressure. However, there are too few diabetic patients among these patients to accurately judge the relationship between diabetes and AR, which is still worthy of attention.
Polymorphisms in some genes (COX-1, COX-2, GPIba, etc.) are strongly associated with AR, but it is still unknown whether the ET’s driver and non-driver genes affect it (33, 34). Unfortunately, our study showed that the driver and non-driver genes do not assist in predicting AR in ET patients. This reminds us of the need to add relevant drug-resistance genes to the routine NGS.
5 Conclusion
Compared with AS patients, AR patients have enhanced platelet aggregation function, are in a relatively hypercoagulable state, and have elevated fibrinogen function/levels, all of which cause a worse coagulation status. ET patients with increasing age, irregular aspirin use, smoking, dyslipidemia, and hypertension may have a higher risk of AR. The routine NGS may not be helpful for the prediction of AR, therefore we recommend adding relevant drug-resistance genes to NGS.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of China Academy of Chinese Medical Sciences Xiyuan Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EY and XH designed the study. ZW collected the data. YuL, DW, YS, and YZ performed the analysis. JN and ZC normalized the pictures. EY, YaL, and WL wrote the original draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82174360), and the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A01702 and CI2021A01708).
Acknowledgments
The authors would like to thank China Academy of Chinese Medical Sciences Xiyuan Hospital for supporting that work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
APTT, activated partial thromboplastin time; AR, aspirin resistance; AS, aspirin sensitivity; AT-III, antithrombin III; CALR, calreticulin; CI, coagulation index; COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; CRP, C-reactive protein; D-D, D-dimer; ET, essential thrombocythemia; Fbg, fibrinogen; FDP, fibrin degradation products; GPIba, glycoprotein 1b, alpha polypeptide; HGB, hemoglobin; INR, international normalized ratio; JAK2, janus kinase 2; K, clot kinetics; MA, maximum amplitude; MPL, myeloproliferative leukemia virus; MPN, myeloproliferative neoplasm; NGS, next-generation sequencing; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; PLT, platelet; PT, prothrombin time; PTA, prothrombin time activity; R, reaction time; TEG, thromboelastography; TT, thrombin time; TXA2, thromboxane A2; WBC, white blood cell; WHO, World Health Organization; N/A, not applicable.
References
1. Tefferi A, Pardanani A. Essential thrombocythemia. N Engl J Med. (2019) 381:2135–44. doi: 10.1056/NEJMcp1816082
2. Hultcrantz M, Björkholm M, Dickman P, Landgren O, Derolf ÅR, Kristinsson S, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. (2018) 168:317–25. doi: 10.7326/M17-0028
3. Falanga A, Marchetti M, Schieppati F. Prevention and management of thrombosis in BCR/ABL-negative myeloproliferative neoplasms. Hamostaseologie. (2021) 41:48–57. doi: 10.1055/a-1334-3259
4. Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. (2004) 350:114–24. doi: 10.1056/NEJMoa035572
5. Barbui T, Vannucchi A, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. (2015) 5:e369. doi: 10.1038/bcj.2015.94
6. Perrier-Cornet A, Ianotto J, Mingant F, Perrot M, Lippert E, Galinat H. Decreased turnover aspirin resistance by bidaily aspirin intake and efficient cytoreduction in myeloproliferative neoplasms. Platelets. (2018) 29:723–8. doi: 10.1080/09537104.2017.1361018
7. Gillet B, Ianotto J, Mingant F, Didier R, Gilard M, Ugo V, et al. Multiple Electrode Aggregometry is an adequate method for aspirin response testing in myeloproliferative neoplasms and differentiates the mechanisms of aspirin resistance. Thromb Res. (2016) 142:26–32. doi: 10.1016/j.thromres.2016.04.006
8. Hankey G, Eikelboom J. Aspirin resistance. Lancet. (2006) 367:606–17. doi: 10.1016/S0140-6736(06)68040-9
9. Jing Y, Yue X, Yang S, Li S. Association of aspirin resistance with increased mortality in ischemic stroke. J Nutr Health Aging. (2019) 23:266–70. doi: 10.1007/s12603-019-1168-z
10. Yi X, Zhou Q, Lin J, Chi L. Aspirin resistance in Chinese stroke patients increased the rate of recurrent stroke and other vascular events. Int J Stroke. (2013) 8:535–9. doi: 10.1111/j.1747-4949.2012.00929.x
11. Arber D, Orazi A, Hasserjian R, Thiele J, Borowitz M, Le Beau M, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
12. Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. (2010) 14:R55. doi: 10.1186/cc8948
13. Kang L, Jian L, Cheng-bin W, Hai W, Li Y, Yu-long C. Application of PFA-100 and thromboelastograph for monitoring clinical efficacy of aspirin in elderly patients with cardiovascular disease. Chin J Misdiagnostics. (2011) 11:3789–91.
14. Temperilli F, Rina A, Massimi I, Montemari A, Guarino M, Zicari A, et al. Arachidonic acid-stimulated platelet tests: identification of patients less sensitive to aspirin treatment. Platelets. (2015) 26:783–7. doi: 10.3109/09537104.2014.1003291
15. Olechowski B, Ashby A, Mariathas M, Khanna V, Mahmoudi M, Curzen N. Is arachidonic acid stimulation really a test for the response to aspirin? Time to think again? Expert Rev Cardiovasc Ther. (2017) 15:35–46. doi: 10.1080/14779072.2017.1266255
16. Gurbel P, Bliden K, DiChiara J, Newcomer J, Weng W, Neerchal N, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the aspirin-induced platelet effect (ASPECT) study. Circulation. (2007) 115:3156–64. doi: 10.1161/CIRCULATIONAHA.106.675587
17. Yu J, Cunningham J, Thouin S, Gurvich T, Liu D. Hyperlipidemia. Prim Care. (2000) 27:541–87. doi: 10.1016/S0095-4543(05)70164-0
19. Rocca B, Tosetto A, Betti S, Soldati D, Petrucci G, Rossi E, et al. A randomized double-blind trial of 3 aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood. (2020) 136:171–82. doi: 10.1182/blood.2019004596
20. Sixma J. The prethrombotic state. Br J Haematol. (1980) 46:515–22. doi: 10.1111/j.1365-2141.1980.tb06007.x
21. Bauer K, Rosenberg R. The pathophysiology of the prethrombotic state in humans: insights gained from studies using markers of hemostatic system activation. Blood. (1987) 70:343–50. doi: 10.1182/blood.V70.2.343.343
22. Yao Y, Zhang J, Tang X, He C, Ma Y, Xu J, et al. Head to head comparison of two point-of-care platelet function tests used for assessment of on-clopidogrel platelet reactivity in chinese acute myocardial infarction patients undergoing percutaneous coronary intervention. Chin Med J. (2016) 129:2269–74. doi: 10.4103/0366-6999.190664
23. Tang N, Yin S, Sun Z, Xu X, Qin J. The relationship between on-clopidogrel platelet reactivity, genotype, and post-percutaneous coronary intervention outcomes in Chinese patients. Scand J Clin Lab Invest. (2015) 75:223–9. doi: 10.3109/00365513.2014.993696
24. Trelinski J, Okonska M, Robak M, Chojnowski K. Assessment of rotation thromboelastometry parameters in patients with essential thrombocythemia at diagnosis and after hydroxyurea therapy. Blood Coagul Fibrinolysis. (2016) 27:205–9. doi: 10.1097/MBC.0000000000000421
25. Floyd C, Ferro A. Antiplatelet drug resistance: molecular insights and clinical implications. Prostaglandins Other Lipid Mediat. (2015) 120:21–7. doi: 10.1016/j.prostaglandins.2015.03.011
26. Zeeh J, Platt D. The aging liver: structural and functional changes and their consequences for drug treatment in old age. Gerontology. (2002) 48:121–7. doi: 10.1159/000052829
27. Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. (2011) 12:601–10. doi: 10.2174/138920011796504527
28. Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. (2012) 18:1478–93. doi: 10.2174/138161212799504731
29. Poudel K, Poudel-Tandukar K, Bertone-Johnson E, Pekow P, Vidrine D. Inflammation in relation to intensity and duration of cigarette smoking among people living with HIV. AIDS Behav. (2021) 25:856–65. doi: 10.1007/s10461-020-03048-0
30. Gendron M, Thorin-Trescases N, Villeneuve L, Thorin E. Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. Am J Physiol Heart Circ Physiol. (2007) 292:H451–8. doi: 10.1152/ajpheart.00551.2006
31. Wong W, Tian X, Huang Y. Endothelial dysfunction in diabetes and hypertension: cross talk in RAS, BMP4, and ROS-dependent COX-2-derived prostanoids. J Cardiovasc Pharmacol. (2013) 61:204–14. doi: 10.1097/FJC.0b013e31827fe46e
32. Gong X, Wang X, Xu Z, Zhu T, Zhang Q, Zhang J, et al. Over-expression of cyclooxygenase-2 in increased reticulated platelets leads to aspirin resistance after elective off-pump coronary artery bypass surgery. Thromb Res. (2017) 160:114–8. doi: 10.1016/j.thromres.2017.11.003
33. Li X, Cao J, Fan L, Wang Q, Ye L, Cui C, et al. Genetic polymorphisms of HO-1 and COX-1 are associated with aspirin resistance defined by light transmittance aggregation in Chinese Han patients. Clin Appl Thromb Hemost. (2013) 19:513–21. doi: 10.1177/1076029612444002
Keywords: essential thrombocythemia, aspirin resistance, clinical features, coagulation status, next-generation sequencing
Citation: Yang E, Lv Y, Wang Z, Wang D, Li Y, Sun Y, Zhang Y, Niu J, Chen Z, Liu W and Hu X (2022) Coagulation status and determinants of possible aspirin resistance in patients with essential thrombocythemia. Front. Med. 9:1092281. doi: 10.3389/fmed.2022.1092281
Received: 07 November 2022; Accepted: 05 December 2022;
Published: 20 December 2022.
Edited by:
Egidio Imbalzano, The University of Messina, ItalyReviewed by:
Mervat Mattar, Cairo University, EgyptAhmet Emre Eskazan, Istanbul University-Cerrahpaşa, Turkey
Copyright © 2022 Yang, Lv, Wang, Wang, Li, Sun, Zhang, Niu, Chen, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyi Liu, ✉ bGl1d2VpeWkwNTMwQGhvdG1haWwuY29t; Xiaomei Hu, ✉ aHV4aWFvbWVpXzJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Erpeng Yang
Erpeng Yang Yan Lv1†
Yan Lv1† Ziqing Wang
Ziqing Wang Dehao Wang
Dehao Wang Yan Sun
Yan Sun Yanyu Zhang
Yanyu Zhang Zhuo Chen
Zhuo Chen Weiyi Liu
Weiyi Liu Xiaomei Hu
Xiaomei Hu