- 1Department of Nuclear Medicine, Peking University First Hospital, Beijing, China
- 2Department of Medical Imaging, Peking University First Hospital, Beijing, China
- 3Department of Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Objective: Primary pulmonary nucleoprotein of the testis (NUT) carcinoma is very rare in the clinic. In this study, the clinicopathological manifestations and imaging features of the primary pulmonary NUT carcinoma were investigated to improve the diagnosis of this disease.
Methods: Six patients with pathologically diagnosed pulmonary NUT carcinoma were analyzed, including three males and three females, aged 19–64 (49.00 ± 16.40) years, with clinical manifestations of cough in two cases, hoarseness in one case, blood in sputum in one case, chest pain in one case, and physical examination findings in one case, with a disease duration of 5 days to 4 months. The clinical and imaging data including CT and PET/CT were retrospectively analyzed. Further literature reviews were analyzed in both pulmonary and extrapulmonary NUT carcinoma cases who performed 18F-FDG PET/CT.
Results: Most of the patients with pulmonary NUT carcinomas presented as heterogeneous lobulated masses (83.33%), four cases (66.67%) were located in the upper lobe of the left lung, one case (16.67%) in the middle lobe of the right lung, and one case (16.67%) in the lower lobe of the right lung, with the maximum diameter ranging from 1.30 to 8.90 cm and the median of 3.55 cm, most of them were irregularly shaped, with more lobulated margins and more heterogeneous density (83.33%), and the enhancement was mild. PET/CT showed increased 18F-FDG uptake in the lesion and metastatic areas. Both the pulmonary NUT patients in this study and literature reviews showed the SUVmax of the tumor ranged from 5 to 40 with an average value of 12.8, whereas that of extrapulmonary lesions had a range of SUVmax at 4.5–64.1 and a mean of 13.8.
Conclusion: In patients with central lung masses, rapid disease progression, and poor response to initial treatment, the possibility of NUT cancer should be considered and anti-NUT monoclonal antibody immunohistochemical staining, combined with genetic detection, if necessary, should be performed as soon as possible. CT and PET/CT imaging are essential for the staging, management, treatment response assessment, and monitoring of pulmonary NUT cancer.
Introduction
Primary nuclear protein in testis (NUT) carcinoma is a rare, aggressive, and malignant epithelial cell tumor characterized by an acquired rearrangement of the gene encoding NUT on chromosome 15q14 (1). NUT carcinoma is rare in clinical practice and arises preferentially in children and adolescents. It is most commonly seen in midline anatomical structures such as the mediastinum, head and neck, previously known as NUT carcinoma, but it can also involve non-midline sites such as the lung, kidney, bladder, and iliac bone (2–4). Due to the lack of characteristic histopathological features, it is frequently missed and misdiagnosed. In recent years, the application of anti-NUT immunohistochemical staining has improved the rate of accurate diagnosis. Primary pulmonary NUT carcinoma is very rare, and clinical diagnosis and differential diagnosis are still challenging for physicians (5). In this paper, we analyzed the clinical and imaging features of pulmonary NUT carcinoma and summarizes the novel treatment options to improve the understanding and awareness of it.
Methods
Patient selection and general information
The clinical and imaging data of six patients (three male and three female patients) with pathologically diagnosed pulmonary NUT carcinoma between January 2018 and August 2022 were retrospectively collected and analyzed. Their ages ranged from 19 to 64 years (49.00 ± 16.40) at the time of diagnosis. None of the patients had a history of smoking. There were two patients initially presented with cough, one with hoarseness, one with blood in sputum, one with chest pain, and one revealed by physical examination, and with a disease duration of 5 days to 4 months at diagnosis. Laboratory findings showed elevated alpha-fetoprotein (11.90–31.88 ng/mL) in two cases, elevated neuron-specific enolase (26.60–62.80 ng/mL) in two cases, elevated CA724 (8.15–9.97 U/ml) in two cases, elevated non-small cell lung cancer antigen (11.00–210.00 ng/mL) in two cases, elevated CA199 (343.00 U/ml) in one case, and elevated CA125 (39.30 U/ml) in one case. Chemotherapy was performed in three patients (One patient was treated with “pemetrexed + carboplatin + bevacizumab” and two patients were treated with “paclitaxel + carboplatin powder + sindilizumab”), targeted therapy in two patients and radical surgery combined with chemotherapy in one patient.
Imaging methods
All patients underwent chest CT examinations. CT scans were obtained by using a GE Discovery 750 HD CT scanner, USA, and were routinely performed from the apex of lung to the base of the diaphragm under inspiration with the following scan parameters: tube voltage, 120 kV; tube current using automatic mA technique; section thickness, 5 mm; matrix, 512 × 512; field of view, 18 cm × 18 cm. And one patient underwent whole-body 2-Deoxy-2-[fluorine-18]-fluoro-D-glucose (18F-FDG) PET/CT by using a Biograph TruePoint 64 (6) ring PET/CT instrument from Siemens, Germany, while CT was a 64-slice spiral CT. The HM-20 cyclotron and CFN-100 synthesis module from Sumitomo, Japan, were used to synthesize 18F-FDG by fully automated chemical method with automatic clinical quality control (radiochemical purity ≥98%). Patients were injected with 18F-FDG intravenously at 0.12 mCi/kg, and imaging was performed 50–60 min after injection.
Pathologic examination
All pathological specimens were fixed in 4% neutral formaldehyde, routinely dehydrated, embedded in paraffin, stained with hematoxylin-eosin (HE), and observed under a light microscope. Immunohistochemical staining (EnVision method) was performed strictly according to the instructions.
Image analysis
Images were interpreted by one imaging physician and one nuclear medicine physician both with more than 10 years of experience in diagnosis. They assessed the location (central or peripheral), number, density (homogeneous or not), shape (irregular or regular), size (maximum diameter), margins (lobulated, spiculated, or smooth) of the lesions, analyzed the enhancement patterns of the lesions (defining mild enhancement as an increase in CT value of the lesion after enhancement ≤20 HU, moderate enhancement as an increase ≤40 HU, and marked enhancement as an increase >40 HU), and observed the uptake of 18F-FDG in the lesions and the whole body to evaluate the metastasis.
Results
Imaging findings
The CT findings of six patients are summarized in Table 1. Most of them presented as heterogeneous lobulated masses (83.33%), four cases (66.67%) were located in the upper lobe of the left lung, one case (16.67%) in the middle lobe of the right lung, and one case (16.67%) in the lower lobe of the right lung, with the maximum diameter ranging from 1.30 to 8.90 cm and the median of 3.55 cm. Most of them were irregularly shaped, with lobulated margins and heterogeneous density (83.33%), and all tumors showed mild enhancement. Mediastinal lymph node metastasis was observed in five patients (83.33%) and bone metastasis, liver metastasis, and brain metastasis occurred as well. PET/CT was performed in one patient and the lesion with increased glucose metabolism was observed near the hilum in the upper lobe of the left lung, as well as multiple enlarged mediastinal lymph nodes with avid FDG uptake considered as metastasis. In addition, the shape of the left vocal cord was irregular, indicating left recurrent laryngeal nerve was involved (Figures 1, 2).
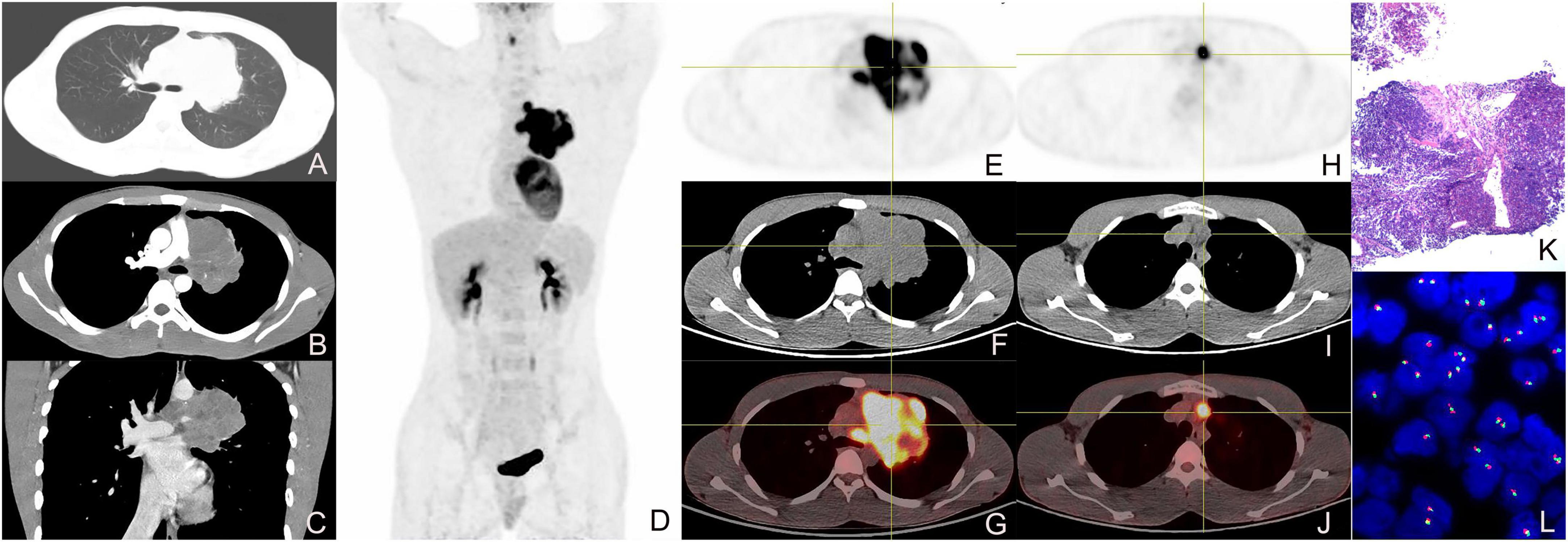
Figure 1. Enhanced CT, 18F-FDG PET/CT, and pathological images of a 19-year-old male with left upper lobe central nucleoprotein of the testis (NUT) carcinoma. (A) pulmonary window transverse CT demonstrated irregular lobulated paramediastinal mass in the upper lobe of the left lung; (B) arterial phase transverse image showed the CT value of the lesion was about 43 HU and tiny vessels could be seen within it. (C) Venous phase coronal image showed the CT value of the lesion was about 54 HU, presenting heterogeneous mild enhancement; (D) the whole-body maximum intensity projection image of PET/CT showed FDG-avid lesions in the left upper lobe and left hilum of the lung; (E–G) tomographic images showed the soft tissue mass with heterogeneous radioactive concentration, and SUVmax was about 22.2; (H–J) transverse images showed enlarged lymph nodes in mediastinal with radioactive accumulation, and SUVmax was about 14.5; (K) pathological image (HE staining, magnification × 40); (L) fluorescence in situ hybridization (FISH) showed the separation of red and green signals in the nuclei of the tumor cells, suggesting the disruption of the NUT gene (magnification × 1,000).
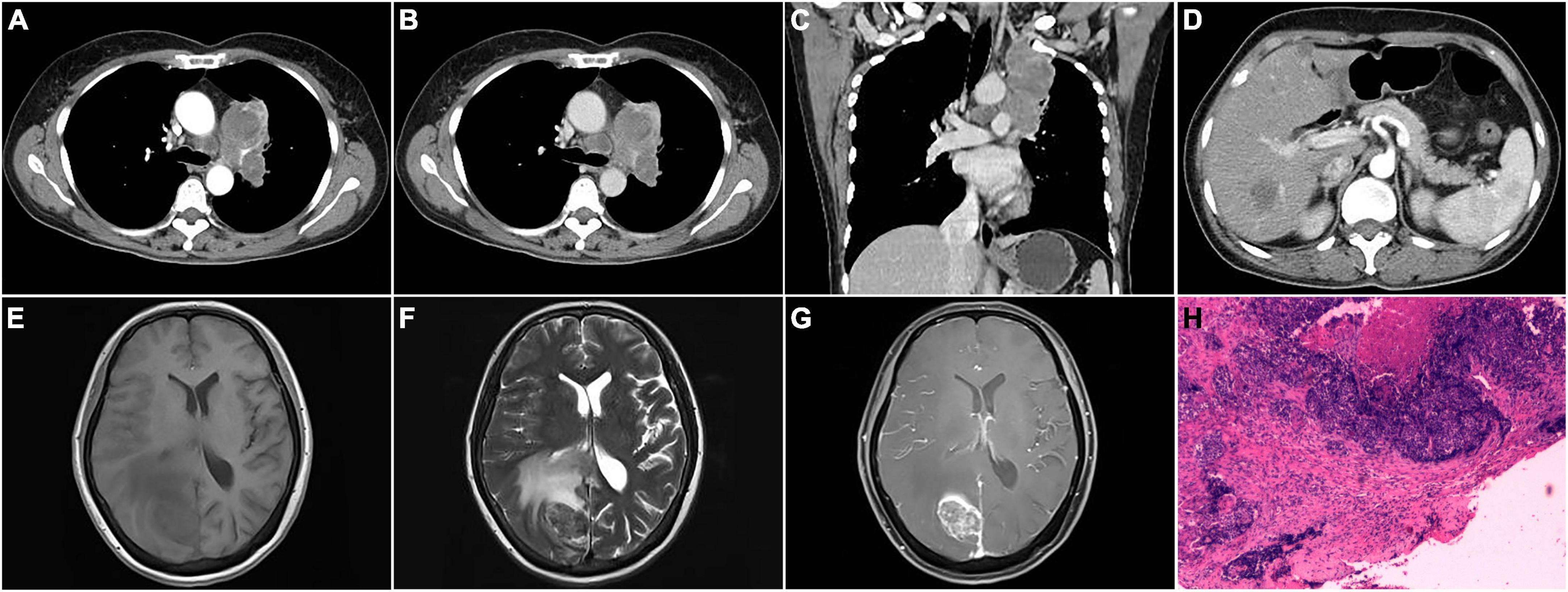
Figure 2. CT, MRI, and pathological images of a 60-year-old female with the left upper lobe central nucleoprotein of the testis (NUT) carcinoma invading pleural, and metastatic to mediastinal lymph node, liver, and brain. (A) Arterial phase transverse CT image showed an irregular mass in the left upper lobe with heterogeneous density and displayed enlarged lymph nodes in the mediastinum; (B) venous phase transverse image showed heterogeneous mild enhancement of the lesion; (C) venous phase coronal image showed the lesion invaded the upper lobe pleura; (D) arterial phase transverse image demonstrated metastatic liver lesion; (E–G) T1WI, T2WI, and enhanced axial images of brain metastases; (H) pathological image (HE staining, magnification × 40).
Pathologic manifestations
Histopathologic results revealed that the tumors showed diffuse nests of small to medium-sized, completely undifferentiated or poorly differentiated tumor cells with scanty cytoplasm. Partial foci were accompanied by obvious interstitial connective tissue hyperplasia, irregular nuclear contours, prominent nucleoli, and nuclear fission, and some foci showed squamous differentiation and necrosis. The results of immunohistochemistry are shown in Table 2.
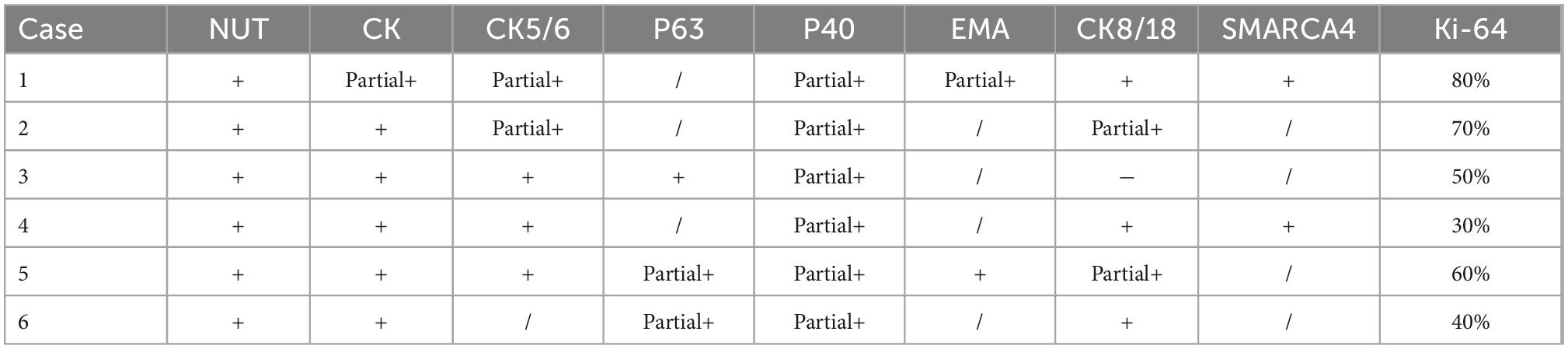
Table 2. Immunohistochemical manifestations of primary lung nucleoprotein of the testis (NUT) carcinoma.
Discussion
Nucleoprotein of the testis carcinoma is a highly aggressive malignant epithelial cell tumor that accounts for approximately 0.22% of primary lung tumors (7, 8). It harbors unique molecular genetic changes, that is, rearrangements or fusions of the NUT gene on chromosome 15q14 with bromodomain-containing 4 (BRD4), BRD3, nuclear receptor binding SET domain protein 3 (NSD3) or other genes, among which BRD4-NUT fusion is the most common. In 2015, WHO listed it as an independent solid tumor of the chest and classified it as undifferentiated carcinoma (9). NUT carcinoma has no gender preference and can occur at any age, while more prevalent in children and young adults (10), with the median age of lung NUT patients being 30 years (21–68 years) (7, 11). None of the patients in this study had a history of smoking, and the mean age was 49 years, which was higher than that in previous reports. The clinical manifestations of pulmonary NUT carcinoma are closely related to the size and location of the tumor, and the presence of complications or metastasis. And common symptoms include cough, chest pain, hemoptysis, wheezing, dyspnea, fever, weight loss, etc. (7, 12). As for the laboratory tests, it lacks specific tumor markers.
It is still unclear about the specific etiology, pathogenesis, and risk factors of NUT carcinoma (13). The occurrence of it may be related to MYC, P63, and MED24 as well as Wnt, MAPK, and PI3K signaling pathways, but it has no connection with environmental factors and viral infection, such as Epstein-Barr virus and human papillomavirus (7, 14). The tissue origin of NUT carcinoma is likewise unrevealed. Some studies speculate that it may originate from cells derived from primitive neural grooves (15), while others believe that it may arise from pluripotent stem cells (16).
The morphology of lung NUT carcinoma is difficult to distinguish from other poorly differentiated tumors such as Ewing’s sarcoma and small cell lung cancer, and the diagnosis is challenging due to the possibility of misdiagnosis. However, it shows unique alterations in the NUT gene. As for the histological features, small to medium-sized completely undifferentiated or poorly differentiated tumor cells are diffusely arranged in sheets, with sparse, eosinophilic or double eosinophilic cytoplasm, and some foci are accompanied by obvious interstitial connective tissue proliferation, with irregular nuclear contours, most of which are naked nuclei, prominent nucleoli, and frequent nuclear fission (3, 7). What is more, local squamous differentiation can be observed in 33–40% of cases (4, 10), and extensive necrosis sometimes exists. Lung cancer with adenocarcinoma morphology should be excluded from the differential diagnosis of NUT carcinoma because NUT carcinoma is considered a non-glandular epithelioid tumor (13). NUT carcinoma can simultaneously express markers of squamous cell carcinoma and neuroendocrine carcinoma (1, 3), and immunohistochemical detection demonstrates that tumor cells are positive for NUT staining, with a specificity of 100% and a sensitivity of 87% (17). And staining of epithelial markers such as AE1/AE3, CK5/6, P63, P40, and EMA is usually positive (18). The diagnosis relies on molecular tests such as immunohistochemical staining, FISH, and RT-PCR (5), but these methods have not been routinely used in clinical practice, resulting in misdiagnosis or delayed diagnosis of NUT carcinoma.
Chest CT is often used to evaluate the characteristics of lung lesions and abnormalities of lymph nodes, pleura, and adjacent bone. Combined with the results of our study and related literature review, the imaging manifestations of NUT carcinoma are summarized as follows: (1) It was reported that pulmonary NUT carcinoma is mainly located in the right lower lobe (19, 20), while in our group it is mostly located in the left upper lobe, which is inconsistent with previous reports. The tumor is large, mostly central-located, with aggressive and infiltrating growth, irregular shape, and mostly lobulated margins. It can also be manifested as massive pleural effusion, lobar collapse, obstructive atelectasis, and mediastinal widening or displacement (21, 22). (2) The tumor showed low-density soft tissue mass with the low-density necrotic area and occasional calcification in it on plain scan (19), and heterogeneous enhancement on enhanced images. (3) NUT carcinoma can invade the bronchial wall or blood vessels (7, 23), and is frequently associated with ipsilateral mediastinal lymph node metastasis and pleural involvement in the form of pleural thickening or nodules (13, 21), while some cases presenting with supra clavicular, contralateral mediastinal and subcarinal lymph node metastasis. The location of extrathoracic metastasis is mainly bone, with osteolytic destruction manifestation on CT, and liver, adrenal gland, and subcutaneous soft tissue involvement has also been reported (7). However, brain metastasis is rare, and we speculate that it is due to the rapid progression of tumors and the short survival time of patients, which is not enough to develop brain metastasis. Mediastinal and left supraclavicular lymph node metastasis, bone metastasis, and liver metastasis occurred in our patients, and brain metastasis occurred in one patient during disease progression. Therefore, there is no specific metastatic route for lung NUT cancer.
18F-FDG PET has been widely used for staging, restaging, and response evaluation of lung cancer. Considering lung NUT cancer is highly aggressive and usually accompanied by symptoms and signs caused by extrathoracic distant metastasis, it is recommended that patients undergo PET/CT examination to comprehensively evaluate whether they have developed other invaded sites, which is useful for the staging, early detection of metastasis, and evaluation of the metabolic and anatomical characteristics of the tumor. We searched the literature in the PubMed and Google Scholar databases using the keywords containing lung, NUT and PET. The PET/CT manifestations of total 18 cases from 2014 to 2022 are summarized in Table 3 (12, 22, 24–29). The most frequently involved lobe is right upper lobe of the lung among these cases (8 in 17) and the mean max diameter of the lung lesions is 5.0 cm (1.4–12.0 cm). The feature of lung NUT on PET/CT is the increased uptake of 18F-FDG in the lesion and the metastatic areas. In the reported cases we summarized in Table 3, the SUVmax of the tumor could range from 5 to 40 and the mean value is 12.8, and the number of patients with a SUVmax of more than 10.0 was up to 10 in these 14 available cases. And the most common metastatic sites are lymph nodes and bones, with FDG-avid presentation on PET/CT as well. We additionally searched for the extrapulmonary NUT for more extensive analysis using the keywords including NUT and PET and screened out the primary lung NUT. Totally 22 cases containing descriptions of PET/CT are included and summarized in Table 4 (30–50). Extrapulmonary sites involved include commonly the mediastinum and nasal sinuses and sometimes the neck and abdomen. The presentation of extrapulmonary lesions on PET/CT is similar to that of pulmonary lesions with a range of 4.5–64.1 and a mean of 13.8. Interestingly, the age of onset of extrapulmonary NUT is significantly younger than that of pulmonary NUT (23.9 ± 4.2 of extrapulmonary lesions vs. 42.7 ± 3.8 of pulmonary lesions, P = 0.002).
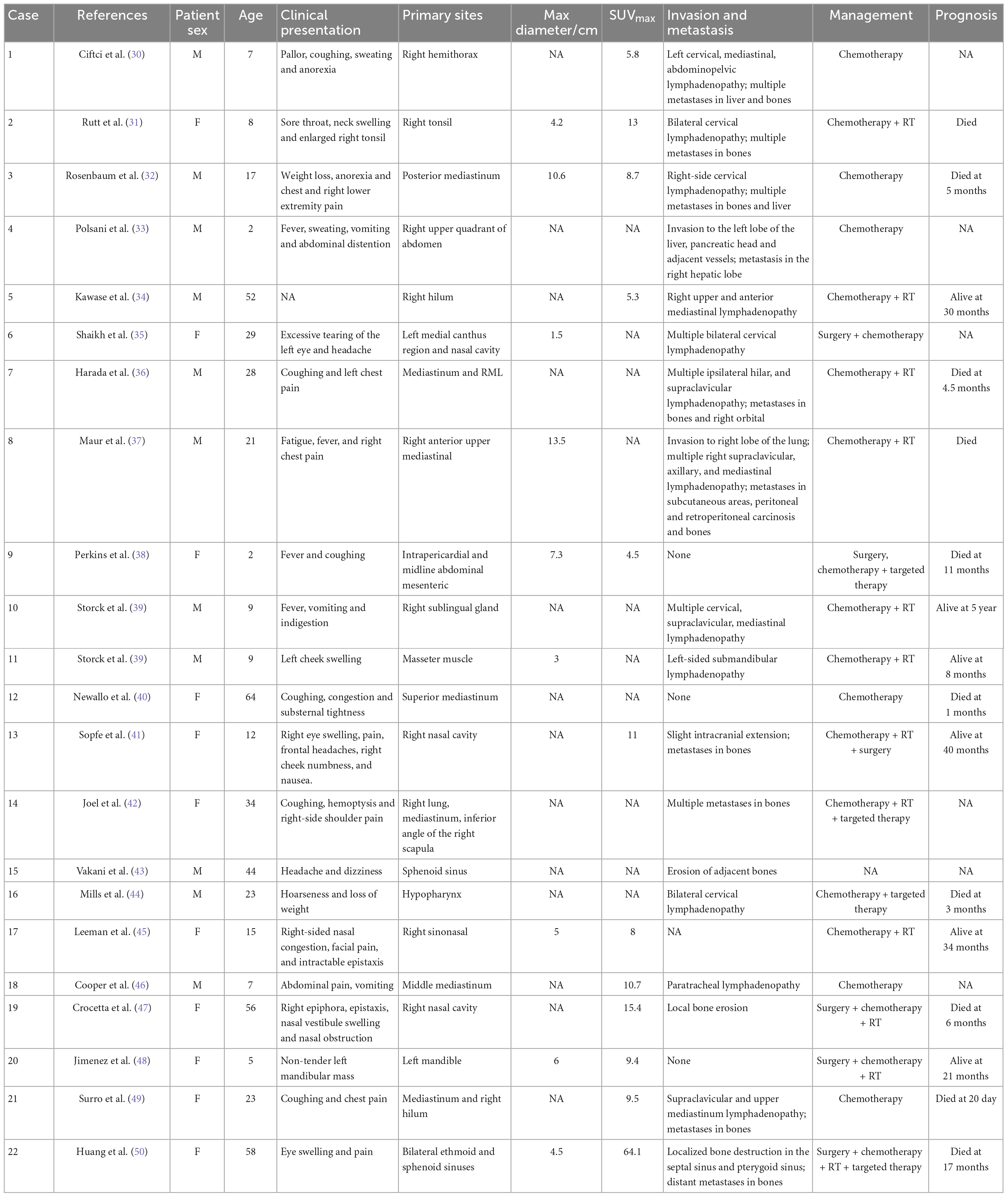
Table 4. 18F-FDG PET/CT manifestations of extrapulmonary nucleoprotein of the testis (NUT) carcinoma.
Through the analysis of our cases and the literature review, we concluded that PET/CT plays a vital role in the diagnosis, staging and treatment monitoring of NUT. The abnormally elevated FDG uptake on PET/CT is beneficial to distinguish between benign and malignant diseases. Rutt et al. (31) reported an 8-year-old girl with a neck mass mimicking tonsillitis. The presentation on CT indicated a tonsillar abscess while the SUV of 13 on PET scans was suggestive of a malignant disease, and the results of histopathological examination confirmed the diagnosis of NUT. Additionally, with the advantages of the whole body scan and available information of tissue function and biological metabolism, PET/CT is a superior imaging technique of choice for detection and staging of NUT, especially for distant metastases (30, 49). PET scans can accurately reveal the most common bony metastases with significant hypermetabolic activity while conventional imaging modalities such as bone scintigraphy will substantially underestimate the degree of bone involvement (7). Rosenbaum et al. (32) reported a patient developing extensive lytic bone metastases at the later stage of treatment. They found that whole-body bone scintigraphy neglected some lesions revealed by PET scans. Moreover, some studies suggested that PET/CT should be used to monitor treatment response and guide the therapy regimen (34, 36). A patient reported by Niederkohr et al. (51) received 18F-FDG PET/CT examination for four times after chemotherapy and the PET results clearly demonstrated the process of remission after initial chemotherapy and aggravated disease burden several months later. PET/CT can reveal both the transient chemotherapeutic response prior to disease progression and residual lesion after operation. Furthermore, the decreased uptake in PET at the center of the mass indicates necrosis within the lesion (19, 32, 52), therefore, PET/CT is an excellent imaging modality of choice for directing percutaneous tissue biopsy (22).
It is difficult to distinguish NUT from other pulmonary tumors based solely on PET/CT findings. Previous literature reported that the SUVmax of pulmonary squamous cell carcinoma ranged from 3.1 to 30.2 (6), the SUVmax of lung adenocarcinoma ranged from 2.2 to 18.1 (53), the SUVmax of pulmonary primary lymphoma ranged from 2.58 to 22.6 (54), the SUVmax of small cell lung cancer ranged from 3.3 to 29.9 (55). And there is no obvious difference compared with the data for lung NUT we summarized above. Although lack of pathognomonic imaging findings, NUT should be taken into consideration for differential diagnosis with aggressive clinical presentations, exponential interval growth of the tumor and extensive metastasis (22, 33).
To date, there is no standard treatment for primary pulmonary NUT carcinoma and the prognosis is poor, associated with its rapid progression, susceptibility to recurrence, and unsatisfactory treatment outcome, with a median overall survival of 6.7 months (1, 8). If patients can be diagnosed early, surgery remains the treatment of choice for NUT carcinoma and will help improve the prognosis of patients (5). Due to the aggressive growth of the tumor, most patients already have metastases at the time of initial diagnosis and the outcome of the surgery will be unfavorable or the opportunity for surgery has been missed (56). One study reported a case of primary pulmonary NUT in which the progression of the tumor was inhibited by radiation therapy combined with anlotinib (57). It was suggested that approximately 40% of patients initially responded to chemotherapy and radiotherapy, but always relapsed rapidly and did not respond to subsequent therapeutic interventions (5, 13). Chemotherapeutic agents commonly used include vincristine, cyclophosphamide, etoposide, and adriamycin (21). The use of PD-1 inhibitors as second-line therapy has been reported in the literature to further improve survival (7). The overall outcome of primary lung NUT is unsatisfactory, and molecular targeted therapy is being investigated as a promising therapy, and two molecular targeted agents targeting the underlying pathogenic mechanism have emerged: bromodomain and extra-terminal protein inhibitors (BETi) and histone deacetylase inhibitors (HDACi) are applied in clinical trials and have proved efficacy in some patients, both of which can induce NUT carcinoma cell differentiation and inhibit their growth (4). BETis inhibit the function of BRD4-NUT protein, allowing cell differentiation to proceed. One study showed that two patients administered with a novel BET inhibitor (OTX015/MK-8628) had a significantly higher overall survival (19 and 18 months, respectively) than the previously reported median survival of 6.7 months (58). However, variant NUT may be insensitive to the treatment, and therefore identification of the molecular type is necessary for predicting responsiveness to Beti treatment (59). BRD4-NUT oncoprotein binds and activates histone acetyltransferases, resulting in chromatin acetylation and a feed-forward mechanism, and finally leading to tumorigenesis. HDACis can reverse the function of BRD4-NUT and restore normal cellular processes and is expected to be a new therapeutic target for lung NUT (4), but toxicity and side effects are still issues that need to be addressed (14).
Conclusion
In summary, the possibility of NUT should be considered in patients with central lung masses and rapidly progressive disease, and anti-NUT immunohistochemical staining should be performed as soon as possible, combined with genetic testing if necessary. CT and PET/CT imaging are essential for staging, management, treatment response assessment, and monitoring of pulmonary NUT cancer.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University and Peking University First Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WH: draft the manuscript. YZ: acquisition and analysis of the work and imaging data collection and analysis. QY and GG: imaging data collection and analysis and resources. YQ and LL: formal analysis and resources. LK: supervision and writing—review and editing. All authors met the requirements for authorship for the submitted version and agreed to its submission.
Funding
This study was funded by the Beijing Science Foundation for Distinguished Young Scholars (JQ21025), the Beijing Municipal Science and Technology Commission (Z221100007422027), and the Peking University Medicine Fund of Fostering Young Scholars’ Scientific and Technological Innovation (BMU2022PY006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moreno V, Saluja K, Pina-Oviedo S. Nut carcinoma: clinicopathologic features, molecular genetics and epigenetics. Front Oncol. (2022) 12:860830. doi: 10.3389/fonc.2022.860830
2. Lantuejoul S, Pissaloux D, Ferretti G, McLeer A. Nut carcinoma of the lung. Semin Diagn Pathol. (2021) 38:72–82. doi: 10.1053/j.semdp.2021.06.005
3. Claudia G, Alexandra G. Challenging diagnosis in nut carcinoma. Int J Surg Pathol. (2021) 29:722–5. doi: 10.1177/10668969211019532
4. Zhang H, Kong W, Liang W. Nut midline carcinoma: a rare solid tumour characterized by chromosome rearrangement. Evid Based Complement Alternat Med. (2022) 2022:3369895. doi: 10.1155/2022/3369895
5. Liu Y, Li Y, Ke X, Lu Y. The primary pulmonary nut carcinomas and some uncommon somatic mutations identified by next-generation sequencing: a case report. AME Case Rep. (2020) 4:24. doi: 10.21037/acr-19-168
6. Zhang M, Wang D, Sun Q, Pu H, Wang Y, Zhao S, et al. Prognostic significance of Pd-L1 expression and (18)F-Fdg Pet/Ct in surgical pulmonary squamous cell carcinoma. Oncotarget. (2017) 8:51630–40. doi: 10.18632/oncotarget.18257
7. Li X, Shi H, Zhang W, Bai C, He M, Ta N, et al. Immunotherapy and targeting the tumor microenvironment: current place and new insights in primary pulmonary nut carcinoma. Front Oncol. (2021) 11:690115. doi: 10.3389/fonc.2021.690115
8. Bauer D, Mitchell C, Strait K, Lathan C, Stelow E, Lüer S, et al. Clinicopathologic features and long-term outcomes of nut midline carcinoma. Clin Cancer Res. (2012) 18:5773–9. doi: 10.1158/1078-0432.Ccr-12-1153
9. Travis W, Brambilla E, Nicholson A, Yatabe Y, Austin J, Beasley M, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/jto.0000000000000630
10. French C. Pathogenesis of nut midline carcinoma. Annu Rev Pathol. (2012) 7:247–65. doi: 10.1146/annurev-pathol-011811-132438
11. Chau N, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (Nut) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. (2020) 4:kz094. doi: 10.1093/jncics/pkz094
12. Xie X, Wang L, Qin Y, Lin X, Xie Z, Liu M, et al. Clinical features, treatment, and survival outcome of primary pulmonary nut midline carcinoma. Orphanet J Rare Dis. (2020) 15:183. doi: 10.1186/s13023-020-01449-x
13. Xie M, Fu X, Wang W. Clinicopathological and molecular characterizations of pulmonary nut midline carcinoma. Cancer Med. (2021) 10:5757–64. doi: 10.1002/cam4.4096
14. Zhang Y, Han K, Dong X, Hou Q, Li T, Li L, et al. Case report and literature review: primary pulmonary nut-midline carcinoma. Front Oncol. (2021) 11:700781.
15. French C. Demystified molecular pathology of nut midline carcinomas. J Clin Pathol. (2010) 63:492–6. doi: 10.1136/jcp.2007.052902
16. den Bakker M, Beverloo B, van den Heuvel-Eibrink M, Meeuwis C, Tan L, Johnson L, et al. Nut midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. (2009) 33:1253–8. doi: 10.1097/PAS.0b013e3181abe120
17. Bishop J. Newly described tumor entities in sinonasal tract pathology. Head Neck Pathol. (2016) 10:23–31. doi: 10.1007/s12105-016-0688-7
18. Saiki A, Sakamoto K, Bee Y, Izumo T. Nuclear protein of the testis midline carcinoma of the thorax. Jpn J Clin Oncol. (2022) 52:531–8. doi: 10.1093/jjco/hyac033
19. Sholl L, Nishino M, Pokharel S, Mino-Kenudson M, French C, Janne P, et al. Primary pulmonary nut midline carcinoma: clinical, radiographic, and pathologic characterizations. J Thorac Oncol. (2015) 10:951–9. doi: 10.1097/jto.0000000000000545
20. Harms A, Herpel E, Pfarr N, Penzel R, Heussel C, Herth F, et al. Nut carcinoma of the thorax: case report and review of the literature. Lung Cancer. (2015) 90:484–91. doi: 10.1016/j.lungcan.2015.10.001
21. Virarkar M, Saleh M, Ramani N, Morani A, Bhosale P. Imaging spectrum of nut carcinomas. Clin Imaging. (2020) 67:198–206. doi: 10.1016/j.clinimag.2020.07.025
22. Bair R, Chick J, Chauhan N, French C, Madan R. Demystifying nut midline carcinoma: radiologic and pathologic correlations of an aggressive malignancy. AJR Am J Roentgenol. (2014) 203:W391–9. doi: 10.2214/ajr.13.12401
23. Reddy R, Woods T, Allan R, Malhotra P, Mehta H, Sarkar P, et al. Nut (nuclear protein in testis) carcinoma: a report of two cases with different histopathologic features. Int J Surg Pathol. (2019) 27:225–9. doi: 10.1177/1066896918796606
24. Chang A, Kim T, Han J, Kim T, Choi J. Nut midline carcinoma of the lung: computed tomography findings in 10 patients. J Comput Assist Tomogr. (2021) 45:330–6. doi: 10.1097/rct.0000000000001133
25. Baras A, Naidoo J, Hann C, Illei P, Reninger C, Lauring J. Rediagnosis of lung cancer as nut midline carcinoma based on clues from tumor genomic profiling. J Natl Compr Canc Netw. (2018) 16:467–72. doi: 10.6004/jnccn.2017.7203
26. Numakura S, Saito K, Motoi N, Mori T, Saito Y, Yokote F, et al. P63-negative pulmonary nut carcinoma arising in the elderly: a case report. Diagn Pathol. (2020) 15:134. doi: 10.1186/s13000-020-01053-4
27. Gupta R, Mumaw D, Antonios B, Anusim N, Dhulipalla S, Stender M, et al. Nut midline lung cancer: a rare case report with literature review. AME Case Rep. (2022) 6:2. doi: 10.21037/acr-21-35
28. Gasljevic G, Matter M, Blatnik O, Unk M, Dirnhofer S. Nut carcinoma: a clinical, morphological and immunohistochemical mimicker-the role of Rna sequencing in the diagnostic procedure. Int J Surg Pathol. (2022) 30:273–7. doi: 10.1177/10668969211047981
29. Cavalieri S, Stathis A, Fabbri A, Sonzogni A, Perrone F, Tamborini E, et al. Uncommon somatic mutations in metastatic nut midline carcinoma. Tumori. (2017) 103(Suppl. 1):e5–8. doi: 10.5301/tj.5000685
30. Ciftci E, Demirsoy U, Anik Y, Gorur G, Corapcioglu F, Demir H. Staging and evaluation of neoadjuvant chemotherapy response with 18F-Fdg Pet/Ct in nut-midline carcinoma in a child: a case report and review of the literature. Rev Esp Med Nucl Imagen Mol. (2015) 34:53–5. doi: 10.1016/j.remn.2014.08.007
31. Rutt A, Poulik J, Siddiqui A, Konski A, Kalaf M, Madgy D, et al. Nut midline carcinoma mimicking tonsillitis in an eight-year-old girl. Ann Otol Rhinol Laryngol. (2011) 120:546–9. doi: 10.1177/000348941112000810
32. Rosenbaum D, Teruya-Feldstein J, Price A, Meyers P, Abramson S. Radiologic features of nut midline carcinoma in an adolescent. Pediatr Radiol. (2012) 42:249–52. doi: 10.1007/s00247-011-2288-8
33. Polsani A, Braithwaite K, Alazraki A, Abramowsky C, Shehata B. Nut midline carcinoma: an imaging case series and review of literature. Pediatr Radiol. (2012) 42:205–10. doi: 10.1007/s00247-011-2272-3
34. Kawase T, Naka G, Kubota K, Sakashita B, Takeda Y. Nut midline carcinoma in elderly patients: usefulness of 18f-Fdg Pet/Ct for treatment assessment. Clin Nucl Med. (2015) 40:764–5. doi: 10.1097/rlu.0000000000000795
35. Shaikh F, Pagedar N, Awan O, McNeely P. Sinonasal nut-midline carcinoma - a multimodality approach to diagnosis, staging and post-surgical restaging. Cureus. (2015) 7:e288. doi: 10.7759/cureus.288
36. Harada Y, Koyama T, Takeuchi K, Shoji K, Hoshi K, Oyama Y. Nut midline carcinoma mimicking a germ cell tumor: a case report. BMC Cancer. (2016) 16:895. doi: 10.1186/s12885-016-2944-3
37. Maur M, Toss A, Dominici M, Frassoldati A, Corradini P, Maiorana A, et al. Impressive Response to dose-dense chemotherapy in a patient with nut midline carcinoma. Am J Case Rep. (2015) 16:424–9. doi: 10.12659/ajcr.893879
38. Perkins C, Pucar D, McDonough C, Williams H. Nuclear protein in testis midline carcinoma presenting in an infant as a pericardial mass with staging by (18)F-fluorodeoxyglucose-positron emission tomography/computed tomography. World J Nucl Med. (2017) 16:247–50. doi: 10.4103/1450-1147.207284
39. Storck S, Kennedy A, Marcus K, Teot L, Vaughn J, Gnekow A, et al. Pediatric nut-midline carcinoma: therapeutic success employing a sarcoma based multimodal approach. Pediatr Hematol Oncol. (2017) 34:231–7. doi: 10.1080/08880018.2017.1363839
40. Newallo D, Shogbesan G, Chataigne M, Hall L. Metastatic nut midline carcinoma. Open J Clin Med Images. (2022) 2:1068.
41. Sopfe J, Greffe B, Treece A. Metastatic nut midline carcinoma treated with aggressive neoadjuvant chemotherapy, radiation, and resection: a case report and review of the literature. J Pediatr Hematol Oncol. (2021) 43:e73–5. doi: 10.1097/mph.0000000000001860
42. Joel S, Weschenfelder F, Schleussner E, Hofmann G, Weschenfelder W. Nut midline carcinoma in a young pregnant female: a case report. World J Surg Oncol. (2020) 18:290. doi: 10.1186/s12957-020-02065-6
43. Vakani P, Maheshwari J, Maheshwari M, Shah B. Sinonasal nut midline carcinoma: a new histological entity. Indian J Pathol Microbiol. (2020) 63:103–5. doi: 10.4103/ijpm.Ijpm_373_19
44. Mills A, Lanfranchi M, Wein R, Mukand-Cerro I, Pilichowska M, Cowan J, et al. Nut midline carcinoma: a case report with a novel translocation and review of the literature. Head Neck Pathol. (2014) 8:182–6. doi: 10.1007/s12105-013-0479-3
45. Leeman R, Pinkney K, Bradley J, Ruiz R, DuBois S, French C, et al. Nut carcinoma without upfront surgical resection: a case report. J Pediatr Hematol Oncol. (2021) 43:e707–10. doi: 10.1097/mph.0000000000001865
46. Cooper K, Hull N, Horst K, Kolbe A, Zingula S, Thacker P. Nut carcinoma of the thorax in a 7-year-old child. Radiol Case Rep. (2022) 17:1549–53. doi: 10.1016/j.radcr.2022.01.077
47. Crocetta F, Botti C, Fornaciari M, Castellucci A, Murri D, Santandrea G, et al. Sinonasal nut carcinoma: delayed diagnosis due to the Covid-19 pandemic and a review of the literature. Head Neck Pathol. (2021) 15:1409–14. doi: 10.1007/s12105-021-01311-x
48. Jimenez C, Stanton E, Kondra K, Nickels E, Jacob L, Shah R, et al. Nut carcinoma of the mandible in a child: case report and systematic review. Int J Oral Maxillofac Surg. (2022): [Epub ahead of print]. doi: 10.1016/j.ijom.2022.07.002
49. Surro A, Al Tarhuni M, Al-Katib S. Nut carcinoma resulting in svc syndrome. Clin Pulm Med. (2020) 27:54–7. doi: 10.1097/cpm.0000000000000339
50. Huang W, Gao G, Qiu Y, Yang Q, Song L, Chen Z, et al. Multimodality imaging and treatment of paranasal sinuses nuclear protein in testis carcinoma: a case report. World J Clin Cases. (2022) 10:12395–403. doi: 10.12998/wjcc.v10.i33.12395
51. Niederkohr R, Cameron M, French C. Fdg Pet/Ct imaging of nut midline carcinoma. Clin Nucl Med. (2011) 36:e124–6. doi: 10.1097/RLU.0b013e31821c9a23
52. Bruzzi J, Munden R. Pet/Ct imaging of lung cancer. J Thorac Imaging. (2006) 21:123–36. doi: 10.1097/00005382-200605000-00004
53. Lee Y, Chung J, Kim S, Kim T, Lee K. Adenosquamous carcinoma of the lung: Ct, Fdg Pet, and clinicopathologic findings. Clin Nucl Med. (2014) 39:107–12. doi: 10.1097/RLU.0b013e3182952c2d
54. Peng Y, Qi W, Luo Z, Zeng Q, Huang Y, Wang Y, et al. Role of 18f-Fdg Pet/Ct in patients affected by pulmonary primary lymphoma. Front Oncol. (2022) 12:973109. doi: 10.3389/fonc.2022.973109
55. Choi E, Park M, Im J, Chung Y, Oh J. Prognostic value of (18)F-Fdg Pet/Ct metabolic parameters in small cell lung cancer. J Int Med Res. (2020) 48:300060519892419. doi: 10.1177/0300060519892419
56. Baumgartner K, Lauer U, Horger M, Zender L, Kloth C. [Nuclear protein in testis (Nut) midline carcinoma]. Rofo. (2020) 192:303–6. doi: 10.1055/a-1026-6561
57. Jiang J, Ren Y, Xu C, Lin X. Nut midline carcinoma as a primary lung tumor treated with anlotinib combined with palliative radiotherapy: a case report. Diagn Pathol. (2022) 17:4. doi: 10.1186/s13000-021-01188-y
58. Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord J, de La Motte Rouge T, et al. Clinical response of carcinomas harboring the Brd4-nut oncoprotein to the targeted bromodomain inhibitor Otx015/Mk-8628. Cancer Discov. (2016) 6:492–500. doi: 10.1158/2159-8290.Cd-15-1335
Keywords: lung neoplasms, nuclear protein in testis carcinoma, computed tomography, positron emission tomography, clinical imaging
Citation: Huang W, Zhang Y, Yang Q, Gao G, Qiu Y, Li L and Kang L (2023) Clinical imaging of primary pulmonary nucleoprotein of the testis carcinoma. Front. Med. 9:1083206. doi: 10.3389/fmed.2022.1083206
Received: 28 October 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), SwitzerlandReviewed by:
Natale Quartuccio, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalySilvia Taralli, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
Copyright © 2023 Huang, Zhang, Yang, Gao, Qiu, Li and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Kang, ✉ a2FuZ2xlaUBiam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Wenpeng Huang
Wenpeng Huang Yongbai Zhang
Yongbai Zhang Qi Yang
Qi Yang Ge Gao2
Ge Gao2 Yongkang Qiu
Yongkang Qiu Liming Li
Liming Li Lei Kang
Lei Kang