
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 04 January 2023
Sec. Obstetrics and Gynecology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1081782
This article is part of the Research TopicEditors' Showcase: Obstetrics and GynecologyView all 18 articles
 Qi Wan1,2,3†
Qi Wan1,2,3† Ming-Xing Chen4†
Ming-Xing Chen4† Xuejiao Wang1
Xuejiao Wang1 Li Tan1
Li Tan1 Hui-Jun Yu1
Hui-Jun Yu1 Xing-Yu Lv1
Xing-Yu Lv1 Zhao-Hui Zhong5
Zhao-Hui Zhong5 Xiao-Jun Tang5
Xiao-Jun Tang5 Yu-Bin Ding4
Yu-Bin Ding4 Min Xia6*
Min Xia6* Yuan Li1*
Yuan Li1*Objectives: Resuscitation transfer of embryos after elective cryopreservation has been widely applied in in vitro fertilization-embryo transfer (IVF-ET) therapy for human infertility or sterility owing to higher embryo implantation rates. This method separates oocyte retrieval from embryo transfer. The optimal time for frozen embryo transfer (FET) remains unknown. Therefore, this study mainly compares the advantages and disadvantages of delayed FET and immediate FET through retrospective analysis.
Methods: We analyzed real world data of patients who underwent resuscitation transplantation between October 2019 and July 2021 at the Reproductive Center of Chengdu Jinjiang Hospital for Women’s and Children’s Health. Propensity score matching was applied to control potential confounding factors. A total of 5,549 patients who received at least one FET were analyzed. Patients undergoing transplantation within 60 days of oocyte retrieval were included in the immediate FET group (n = 1,265) and those undergoing transplantation > 60 days after retrieval were included in the delayed FET group (n = 4,284).
Results: Live birth rates between the two groups were comparable (45.25% vs. 45.76%, p = 0.757). Moreover, no difference was observed in the rates of biochemical pregnancy (64.50% vs. 66.80%), clinical pregnancy (55.24% vs. 56.83%), ectopic pregnancy (1.47% vs. 1.39%), early miscarriage (14.41% vs. 16.20%), late miscarriage (2.21% vs. 2.09%), singleton premature delivery (16.67% vs. 18.29%), and neonatal deformity (1.97% vs. 1.80%). After stratifying the patients based on the type of embryo transferred, number of embryos transferred, FET protocol, and good prognosis criteria, live birth rates remained comparable between the two groups (p > 0.05).
Conclusion: Pregnancy outcomes were comparable between the immediate and delayed FET groups.
Controlled ovarian hyperstimulation (COH) is one of key processes in assisted reproductive technology (ART) therapy. COH may lead to ovarian hyperstimulation syndrome (OHSS), cause endometrial synchronicity and other adverse factors that could reduce pregnancy rates, such as elevated progesterone and tubal effusion. Nearly half of the patients receiving COH undergo resuscitation transplantation after embryo freezing (1–4). Patients undergoing in vitro fertilization (IVF) are eager to get pregnant with shortened interval between egg retrieval and transplantation. However, there is no uniform regulation regarding the timing of post-COH resuscitation transplantation (5, 6). However, since the ovaries are enlarged during COH and the risk of ovarian torsion are increased, physicians of reproductive medicine tend to start embryo resuscitation and transplantation preparations until at least the second menstrual cycle.
In addition, more and more young cancer patients accept fertility preservation services at present since they receive certain types of cancer surgery which lead to removal of organs needed for a pregnancy, and certain therapy might increase hormone levels or cause damage to a female’s eggs (7). Previous studies have not come to a definitive conclusion on how immediate or delayed resuscitation will benefit pregnancy rates (8, 9). Additionally, many of the retrospective studies on this issue were conducted in years ago, and the results of these studies may be biased against changes in the existing COH protocols (1, 10, 11). Therefore, owing to the advantage of large data volumes at our hospital, we retrospectively analyzed the clinical data of patients who underwent COH and resuscitation transplantation from October 2019 to August 2021. We compared clinical data from first, second, and successive menstrual cycle resuscitations thereafter involving full embryo freezing.
This retrospective cohort study reviewed the data of patients who underwent IVF/intracytoplasmic sperm injection (ICSI) between October 2019 and July 2021 at the Reproductive Center of Chengdu Jinjiang Hospital for Women’s and Children’s Health, Sichuan, China. This study was approved by the Ethics Committee of the Chengdu Jinjiang Hospital for Women’s and Children’s Health. And all methods were performed in accordance with the relevant guidelines and regulations. The informed consent was obtained from all participants of the study.
Patients aged 20–38 years who underwent IVF/ICSI cycles, patients who froze all embryos, and patients who subsequently received FET after failure of fresh embryo transplantation were included in the study. Further, patients who underwent rescue ICSI cycles, with an endometrial thickness of <8 mm before embryo transfer, and patients with endometriosis, genital malformation, or uterine abnormality were excluded.
Four ovulation stimulation protocols were used. (I) The antagonist protocol, involving ovulation induction with follicle stimulating hormone (FSH) (Gonal-F, Merck Serono, Puregon, Organon) from days 2–5 of menstruation, followed by gonadotropin releasing hormone antagonist (GnRH-a) (Cetrorelix, Merck Serono, or Orgalutran Organon) at a daily dose of 0.25 mg, which was commenced when the largest follicle exceeded 12–14 mm. (II) The long ovulation stimulation protocol, involving 3.75 mg GnRH-a (Triptorelin, Ferring) injections on the 2nd–5th day of menstruation and FSH ovulation induction after 28–30 days. (III) The luteal phase improvement long protocol, involving 0.1 mg GnRH-a (Triptorelin, Ferring) injection during the luteal phase followed by FSH administration starting on the 3rd day of menstruation to initiate ovulation. (IV) The micro stimulation protocol, involving administration of FSH or Menotrophins (Lebaode, LiZhu) on the 2nd–3rd day of menstruation was used to promote egg excretion. Urinary human chorionic gonadotropin 2000–6000 and GnRH-a 0.2 mg or recombinant human choriogonadotropin alfa (AiZe, Merck Serono, Darmstadt, Germany) were then injected when the target follicle was 18–20 mm. Egg retrieval was carried out 36–38 h after triggering, and 90 mg/d of crinone (Merck Serono, Darmstadt, Germany) and 20 mg/d of dydrogesterone (Abbott Biologicals, Beijing, China) were administered as luteal support after egg retrieval. All frozen embryos were vitrified with open system.
Patients who underwent transplantation within 60 days of oocyte retrieval were included in the immediate FET group and those who underwent transplantation > 60 days after retrieval were included in the delayed FET group. The endometrial preparation program included natural cycle, hormone replacement cycle, ovulation-promoting cycle, and down-regulation cycle. The natural cycle was determined by monitoring follicular development using transvaginal ultrasonography and hormone levels. For the hormone replacement cycle, on days 2–4 of the menstrual cycle, 4 mg estradiol valerate tablets (Progynova, Berlin, Germany) were administered daily for 10 days. For the ovulation-promoting cycle, on days 2–4 of menstruation, 2.5–5 mg of letrozole, 20–40 mg of tamoxifen, or 50 mg of clomiphene was administered for 5 days. The down-regulation cycle involved injection of 3.75 mg GnRH-a (Triptorelin, Ferring) on days 2–3 of menstruation.
Luteal transformation was achieved with 90 mg/d of crinone or 600 mg/d of soft capsule progesterone (Cyndea Pharma, S.L.) and 40 mg/d of dydrogesterone, after the endometrium reached 8 mm thickness. Cleavage-stage embryos or blastocysts were transferred 3–5 days after transformation. Luteal support was provided after embryo transfer.
The primary outcome was the live birth rate, defined as the delivery of a living baby at ≥28 weeks of pregnancy after the first embryo transfer. The secondary outcomes were the rates of biochemical pregnancy, clinical pregnancy, ectopic pregnancy, early miscarriage, late miscarriage, singleton premature delivery, and neonatal deformity.
Propensity score matching (PSM) was used to make the baseline characteristics between the immediate and delayed FET groups balanced and comparable. The variables for PSM included female age, FSH, progesterone, fertilization method, COS protocol, FET protocol, the number of top-quality embryos transferred, and the type of embryo transferred. The (1:3) nearest neighbor caliper matching method without replacement was used to match data between the two groups, and the caliper was 0.05. The Kolmogorov–Smirnov test and the Shapiro–Wilk test were used to test the normality of the variables. Continuous variables are presented as mean ± SD or median (IQR). Categorical variables are presented as numbers and percentages. Continuous variables were compared between the groups using the t-test or Mann–Whitney U test. Categorical variables were compared using the Chi-square test. Multiple logistic regression models, odds ratio (OR), and 95% confidence intervals (CI) were calculated after adjusting for confounders. A P-value < 0.05 was considered statistically significant. All analyses were performed using SPSS software, version 25.0 and R software version 3.3.0.
All demographic data are shown in Table 1. A total of 5,549 patients who received at least one FET were analyzed in the study. The immediate FET group consisted of 1,265 patients, and the delayed FET group consisted of 4,284 patients. The interval between oocyte retrieval and embryo recovery was significantly shorter in the immediate FET group than in the delayed FET group [days: 34 (30–56) vs. 83 (67–111)]. Additionally, there were significant differences in maternal age, basal FSH, basal P, fertilization method, COS protocol, FET protocol, the number of top-quality embryos transferred, and the type of embryo transferred between the two groups (p < 0.05). After PSM, a total of 1,231 immediate FET patients were successfully matched to 3,280 delayed FET patients. The interval between oocyte retrieval and embryo recovery was still significantly shorter in the immediate FET group than in the delayed FET group [days: 30 (27, 33) vs. 69 (59, 94)]. After PSM, there were no significant differences in maternal age, basal FSH, basal progesterone, fertilization method, FET protocol, and number of top-quality embryos transferred between the two groups (p > 0.05). However, the COS protocol and type of embryo transferred were significantly different between the two groups (p < 0.05).
Before PSM, the live birth rate was not significantly different between the immediate FET and the delayed FET groups (45.22 vs. 45.38%, p = 0.920) (Table 2). Additionally, after PSM the live birth rates between the groups remained comparable (45.25 vs. 45.76%, p = 0.757) (Table 2). Moreover, no significant differences were observed in the rates of biochemical pregnancy (64.50 vs. 66.80%), clinical pregnancy (55.24 vs. 56.83%), ectopic pregnancy (1.47 vs. 1.39%), early miscarriage (14.41 vs. 16.20%), late miscarriage (2.21 vs. 2.09%), singleton premature delivery (16.67 vs. 18.29%), and neonatal deformity (1.97 vs. 1.80%) between the immediate and delayed FET groups, respectively.
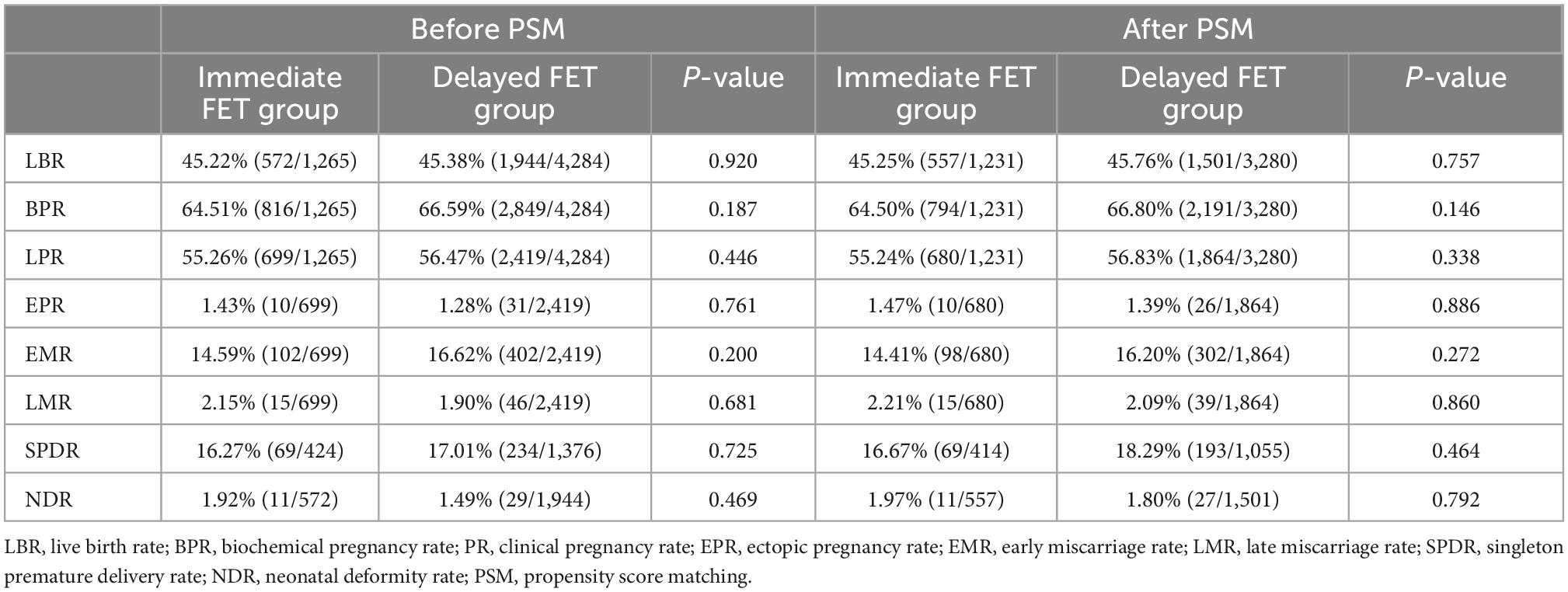
Table 2. Comparison of pregnancy outcomes between the immediate and delayed groups before and after PSM.
The COS protocol and type of embryo transferred are displayed in Table 3. The interval between oocyte retrieval and embryo recovery had no significant effect on pregnancy outcomes. The findings in Table 3 after PSM are consistent with the results from multivariate regression analysis adjusted for potential confounding factors, including maternal age, basal FSH, basal P, fertilization method, COS protocol, FET protocol, number of top-quality embryos transferred, and type of embryo transferred.
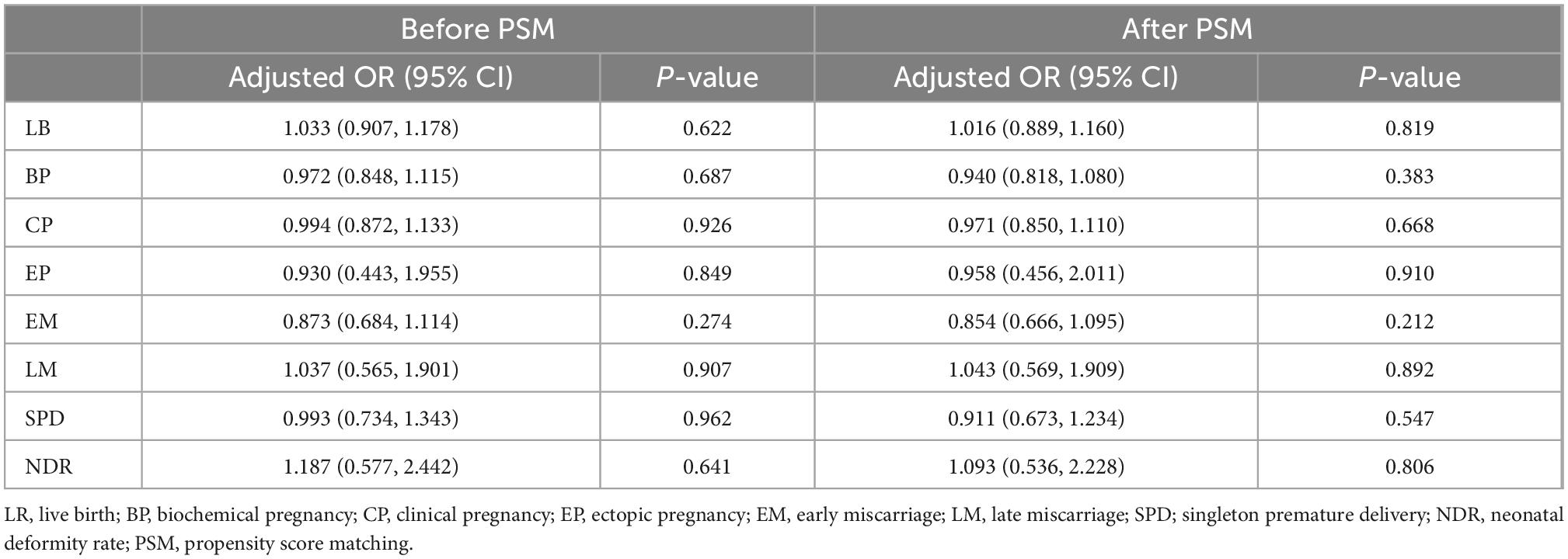
Table 3. Multivariate logistic regression analysis of pregnancy outcomes for immediate FET and delayed FET.
To compare the live birth rates between the immediate and delayed FET groups in patients with different characteristics, we carried out further analysis by stratifying the patients according to the type of embryo transferred, number of embryos transferred, FET protocol, and criteria for good prognosis (AMH > 1.1 ng/ml and AFC > 8) (Tables 4, 5). After PSM, live birth rates were comparable among the groups for each stratification (p > 0.05). Additionally, after adjusting for the COS protocol and type of embryo transferred, multivariate logistic analysis on the four stratified groups revealed no significant correlation between oocyte retrieval and embryo recovery with live birth rates (Tables 4, 5).
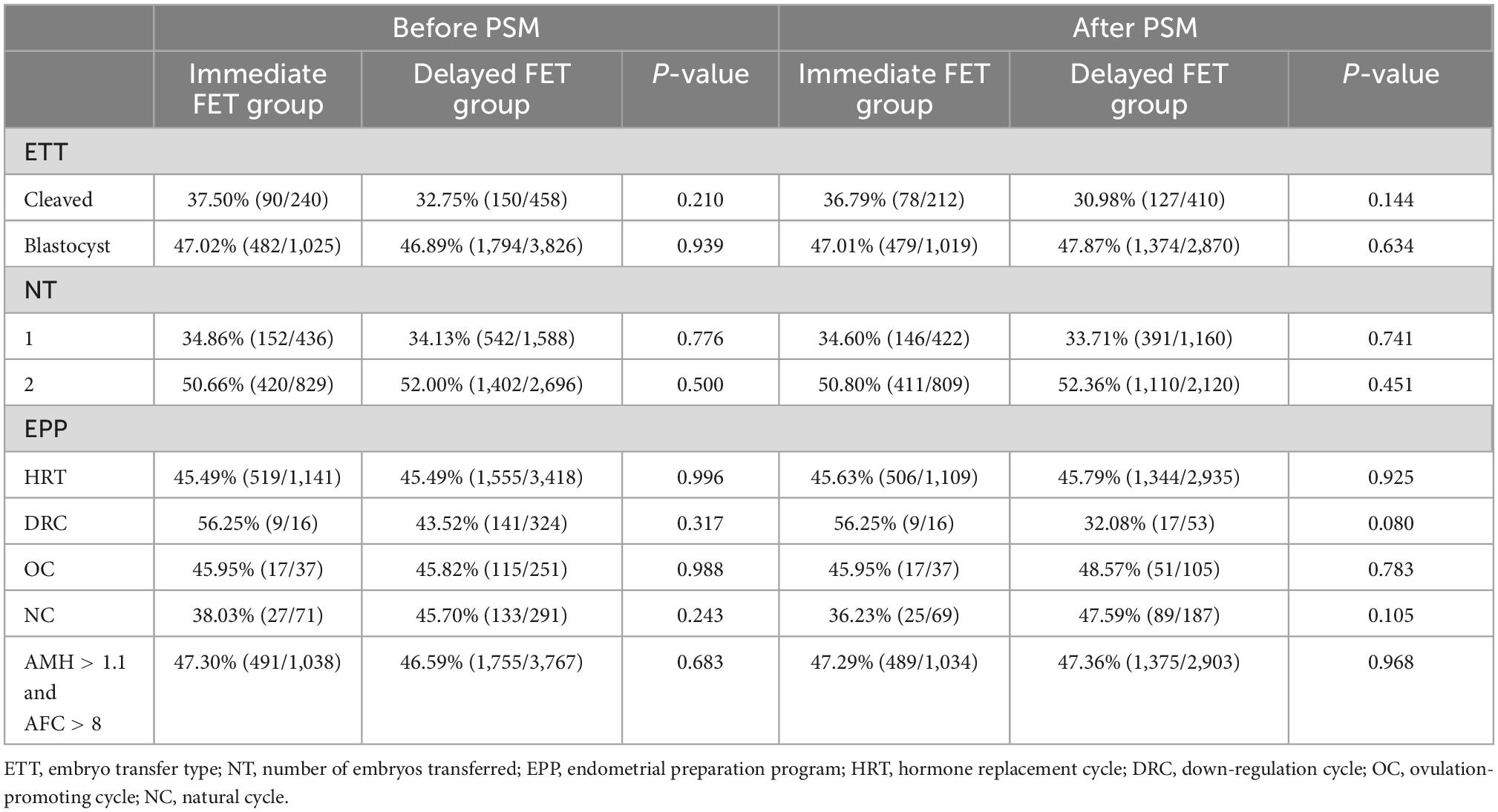
Table 4. Stratified analysis comparing the live birth rate between the immediate FET and delayed FET groups.
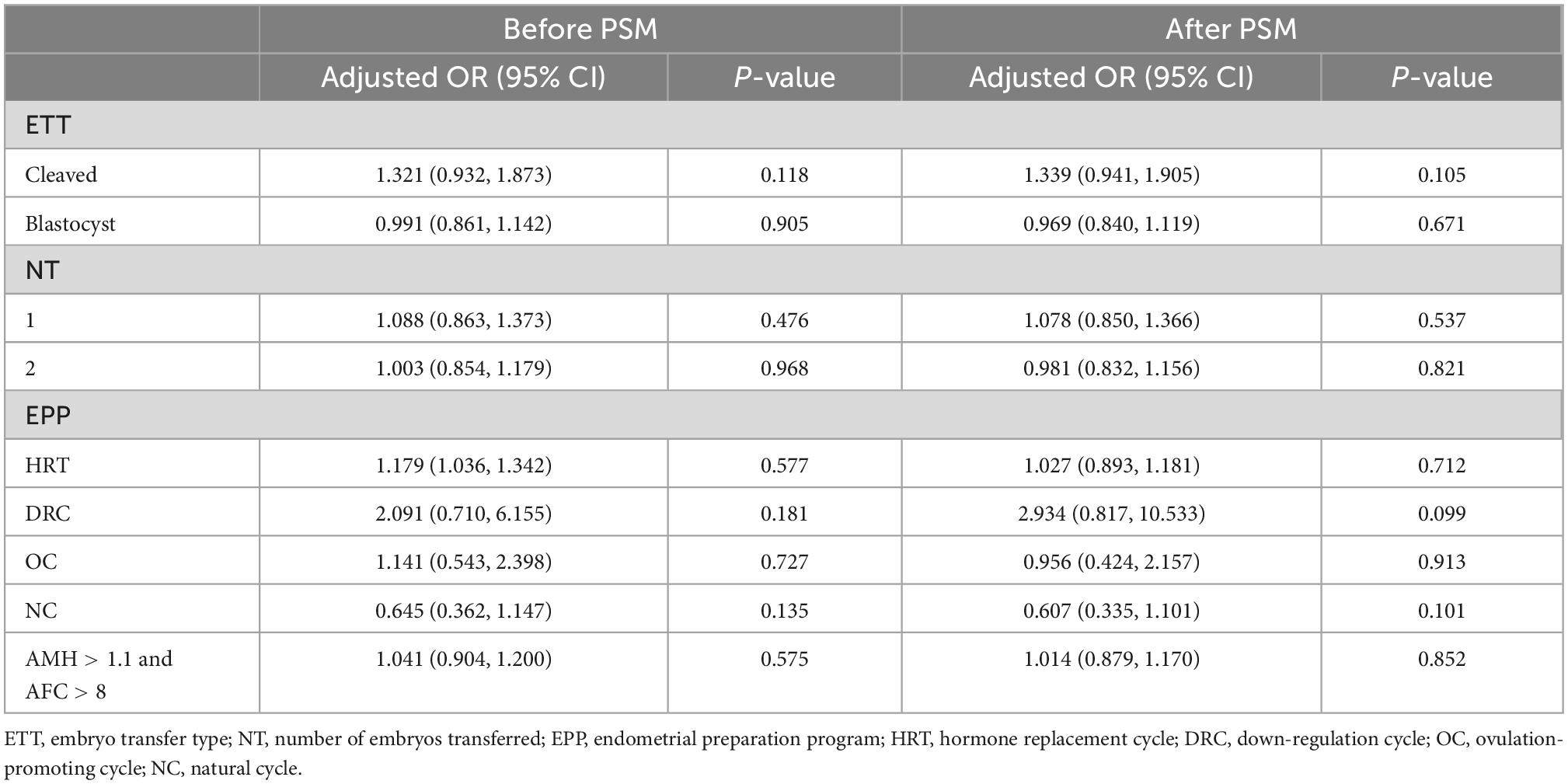
Table 5. Multivariate logistic regression analysis of live birth rates for immediate FET and delayed FET.
Patients undergoing embryo transplantation during fresh cycles have elevated estrogen and progesterone levels and unpredictable OHSS may occur. Although, there are some studies suggest that the administration of a rescue double GnRH antagonist dose at 1 day before hCG trigger may represent a safe alternative preventive strategy for preventing early OHSS without affecting the reproductive outcomes (12). However, more studies are pointing to embryo transfer in resuscitation cycles to improve clinical pregnancy rates and live birth rates and to reduce the occurrence of OHSS (13, 14). There are many randomized controlled trials comparing the advantages and disadvantages of conventional whole embryo freezing and fresh cycle transfer strategies (15). Although there is no favorable evidence to prove that whole embryo freezing has better advantages, clinicians are increasingly using this method (16). A recent review mentioned that in the long-term follow-up of newborns, it was found that the perinatal incidence rate and neonatal congenital malformation rate of patients undergoing FET were similar to those of patients undergoing fresh embryo transfer, and in some specific nervous systems, newborns who had frozen embryo pregnancy even had better cognitive function. Therefore, it is suggested that frozen embryo resuscitation transfer is a safe and reliable choice in clinical practice (17).
If whole embryo freezing is selected despite the risks of OHSS and increase of progesterone during COH, it is suggested that the preparation for resuscitation and transplantation can start immediately. However, patients may need to prolong the time before FET, owing to several reasons, such as endometrial abnormalities and hydrosalpinx. Of note, the optimal time to perform resuscitation and transfer after embryo freezing is yet unclear. Existing studies are primarily based on retrospective analysis, and high quality prospective randomized controlled studies are needed.
In previous retrospective studies, they found that immediate FET had higher live birth rates than delayed FET (18, 19). However, other studies concluded that immediate and delayed FET did not differ in clinical pregnancy and live birth rates (20–22). In those studies, the use of natural cycles in endometrial preparation protocols was excluded from the cycles due to ovulatory disturbances because of the impact of COH. Our study included natural cycle endometrial preparation, which means data is more comprehensive; however, the immediate FET group had fewer natural cycles than the delayed FET group, that might due to the patients in the immediate FET group have ovulation disorders and were not suitable for the natural cycle scheme. In the immediate FET group, the hormone replacement cycle was not affected by ovarian cysts. Additionally, hormone replacement can inhibit the growth of follicles, thereby reducing physiological cysts after egg retrieval. It is also the preferred option. We did not record the cycles that were canceled due to ovulation disorders. Our study concluded that immediate FET and delayed FET showed comparable live birth rates for different embryo types, embryo numbers, and different endometrial preparation protocols, which is consistent with the findings of other studies (1, 6). Therefore, there is no evidence to support the necessity for routine delay of at least one menstrual cycle after IVF/ICSI before FET.
However, patients with breast cancer or leukemia need fertility preservation before tumor therapy, embryo or egg freezing is their main choice for fertility preservation and they have to delayed FET. A retrospective study showed that 43% of breast cancer patients decided to preserve their fertility (7), and another study shows that whether performing COH before, or ART following anticancer treatment in young women with breast cancer does not seem to be associated with detrimental prognostic effect in terms of breast cancer recurrence, mortality or event-free survival (23). Therefore, the delayed resuscitation is also a good choice for tumor patients. Egg freezing is similar to embryo freezing, since 2013, egg cryopreservation has no longer been considered experimental by the American, clinical application faces some ethical challenges (24, 25), such as the effect on tumor recurrence of patients. For some patients, such as patients with endometrial cancer, there have been some studies on molecular level to find out whether the patients are suitable for fertility preservation and whether these treatments have an impact on the prognosis of patients (26). These studies are very meaningful for guiding our treatment.
Now the embryo freezing has been well development and utilized in IVF therapy or fertility preservation, and the frozen embryo is mainly carried out through open and closed carriers. Some scholars believe that the closed carrier for embryo freezing can reduce the risk of sample pollution, and the closed vitrification system may be the future development trend (27). However, although it is theoretically believed that there is a risk of sample contamination in the freezing of open carriers, there is no such report in the world at present, and some studies believe that whether open vitrification system or closed vitrification system have no significant impact on the freezing outcome of embryos (28). We have to admit that embryo freezing technology has helped countless infertile couples. In the future, whether for fertility preservation or for reducing OHSS risk, embryo freezing may be an increasing choice for clinicians. Therefore, perhaps we need more prospective research to explore which is safer, open, or closed vitrification system?
In conclusion, our study further confirmed patients undergoing immediate FET and delayed FET had comparable live birth rates and other pregnancy outcomes with a large amount of patients’ population. However, we failed to record reasons from patients why they chose immediate FET or delayed FET. And therefore, further high quality, randomized, controlled trials are needed to obtain more accurate results and conclusions.
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Chengdu Jinjiang Hospital for Women’s and Children’s Health. The patients/participants provided their written informed consent to participate in this study.
QW and M-XC designed the study and were responsible for the conception of the study and for manuscript drafting. X-JW, LT, H-JY, X-YL, Z-HZ, X-JT, and Y-BD contributed to the manuscript drafting and statistical analysis. YL and MX contributed to the revision and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Medical Science and Technology Project of Sichuan Provincial Health Committee (21PJ166) and supported by the grants from Natural Science Foundation of Chongqing (Nos. cstc2019jcyj-msxmX0749, cstc2019jxjl130030, and cstc2018jxjl130065).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen S, Yao Y, Luo Y, Mao Y, Liu H, Du H, Kang X, Li L., et al. Effect of the time for embryo transfer from oocyte retrieval on clinical outcomes in freeze-all cycles: a retrospective cohort study. Arch Gynecol Obstet. (2020) 301:303–8.
2. Wong K, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to h fertilization success rates. Fertil Steril. (2014) 102:19–26.
3. Shapiro B, Daneshmand S, Garner F, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. (2014) 102:3–9. doi: 10.1016/j.fertnstert.2014.04.018
4. Doody K. Cryopreservation and delayed embryo transfer-assisted reproductive technology registry and reporting implications. Fertil Steril. (2014) 102:27–31. doi: 10.1016/j.fertnstert.2014.04.048
5. Huang J, Lu X, Xie Q, Lin J, Cai R, Kuang Y. Timing of frozen-thawed embryo transfer after controlled ovarian stimulation in a non-elective freeze-all policy. Ann Transl Med. (2019) 7:752. doi: 10.21037/atm.2019.11.74
6. Lattes K, Checa M, Vassena R, Brassesco M, Vernaeve V. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod. (2017) 32:368–74. doi: 10.1093/humrep/dew306
7. Zaami S, Melcarne R, Patrone R, Gullo G, Negro F, Napoletano G, et al. Oncofertility and reproductive counseling in patients with breast cancer: a retrospective study. J Clin Med. (2022) 11:1311.
8. Matorras R, Pijoan J, Perez-Ruiz I, Lainz L, Malaina I, Borjaba S. Meta-analysis of the embryo freezing transfer interval. Reprod Med Biol. (2021) 20:144–58.
9. Bergenheim SJ, Saupstad M, Pistoljevic N, Andersen AN, Forman JL, Løssl K, et al. Immediate versus postponed frozen embryo transfer after IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. (2021) 27:623–42. doi: 10.1093/humupd/dmab002
10. He Y, Zheng H, Du H, Liu J, Li L, Liu H, et al. Delayed frozen embryo transfer failed to improve live birth rate and neonatal outcomes in patients requiring whole embryo freezing. Reprod Biol Endocrinol. (2020) 18:1. doi: 10.1186/s12958-019-0560-1
11. Hu S, Xu B, Long R, Jin L. Pregnancy and perinatal outcomes in pregnancies resulting from time interval between a freeze-all cycle and a subsequent frozen-thawed single blastocyst transfer. BMC Pregnancy Childbirth. (2020) 20:161. doi: 10.1186/s12884-020-02858-3
12. Prapas Y, Ravanos K, Petousis S, Panagiotidis Y, Papatheodorou A, Margioula-Siarkou C, et al. GnRH antagonist administered twice the day before hCG trigger combined with a step-down protocol may prevent OHSS in IVF/ICSI antagonist cycles at risk for OHSS without affecting the reproductive outcomes: a prospective randomized control trial. J Assist Reprod Genet. (2017) 34:1537–45. doi: 10.1007/s10815-017-1010-7
13. Boynukalin FK, Turgut NE, Gultomruk M, Ecemis S, Yarkiner Z, Findikli N, et al. Impact of elective frozen vs. fresh embryo transfer strategies on cumulative live birth: do deleterious effects still exist in normal & hyper responders. PLoS One. (2020) 15:e0234481. doi: 10.1371/journal.pone.0234481
14. Fan L, Tang N, Yao C, Wei X, Tang Y, Li J, et al. Association between fresh embryo transfers and frozen-thawed embryo transfers regarding live birth rates among women undergoing long gonadotropin-releasing hormone antagonist protocols. Front Cell Dev Biol. (2022) 10:884677. doi: 10.3389/fcell.2022.884677
15. Maheshwari A, Bell JL, Bhide P, Brison D, Child T, Chong HY, et al. Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-freeze). Hum Reprod. (2022) 37:476–87.
16. Zaat T, Zagers M, Mol F, Goddijn M, van Wely M, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. (2021) 2:CD011184.
17. Gullo G, Scaglione M, Cucinella G, Chiantera V, Perino A, Greco ME, et al. Neonatal outcomes and long-term follow-up of children born from frozen embryo, a narrative review of latest research findings. Medicina. (2022) 58:1218. doi: 10.3390/medicina58091218
18. Huang J, Lu X, Xie Q, Lin J, Kuang Y. Timing of frozen-thawed embryo transfer after controlled ovarian stimulation in a non-elective freeze-all policy. Ann Translat Med. (2019) 7:752–752.
19. Higgins C, Healey M, Jatkar S, Vollenhoven B. Interval between IVF stimulation cycle and frozen embryo transfer: is there a benefit to a delay between cycles. Aust NZ J Obstet Gynecol. (2018) 58:217–21. doi: 10.1111/ajo.12696
20. Song J, Xiang S, Sun Z. Frozen embryo transfer at the cleavage stage can be performed within the first menstrual cycle following the freeze-all strategy without adversely affecting the live birth rate: a STROBE-compliant retrospective study. Medicine. (2019) 98:e17329. doi: 10.1097/MD.0000000000017329
21. Ozgur K, Bulut H, Berkkanoglu M, Humaidan P, Coetzee K. Frozen embryo transfer can be performed in the cycle immediately following the freeze-all cycle. J Assist Reprod Genet. (2018) 35:135–42.
22. Santos-Ribeiro S, Polyzos NP, Lan VT, Siffain J, Mackens S, Van Landuyt L, Tournaye H, Blockeel C, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod. (2016) 31:2541–8.
23. Arecco L, Blondeaux E, Bruzzone M, Ceppi M, Latocca MM, Marrocco C, et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum Reprod. (2022) 37:954–68. doi: 10.1093/humrep/deac035
24. Zaami S, Busardò F. Elective egg freezing: can you really turn back the clock. Eur Rev Med Pharmacol Sci. (2015) 19:3537–8.
25. Asplund K. Use of in vitro fertilization-ethical issues. Ups J Med Sci. (2020) 125:192–9. doi: 10.1080/03009734.2019.1684405
26. Cavaliere AF, Perelli F, Zaami S, D’Indinosante M, Turrini I, Giusti M, Gullo G, Vizzielli G, Mattei A, Scambia G, Vidiri A, Signore F, et al. Fertility sparing treatments in endometrial cancer patients: the potential role of the new molecular classification. Int J Mol Sci. (2021) 22:12248. doi: 10.3390/ijms222212248
27. Gullo G, Perino A, Cucinella G. Open vs. closed vitrification system: which one is safer. Eur Rev Med Pharmacol Sci. (2022) 26:1065–7. doi: 10.26355/eurrev_202202_28092
Keywords: immediate frozen embryo transfer, delayed frozen embryo transfer, live birth rate, clinical pregnancy, pregnancy outcome
Citation: Wan Q, Chen M-X, Wang X-J, Tan L, Yu H-J, Lv X-Y, Zhong Z-H, Tang X-J, Ding Y-B, Xia M and Li Y (2023) Effect of interval between oocyte retrieval and resuscitation embryo transfer on pregnancy outcomes. Front. Med. 9:1081782. doi: 10.3389/fmed.2022.1081782
Received: 27 October 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Giuseppe Gullo, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyReviewed by:
Marco Scaglione, University of Genoa, ItalyCopyright © 2023 Wan, Chen, Wang, Tan, Yu, Lv, Zhong, Tang, Ding, Xia and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Xia, ✉ NDkxNDA1MDk2QHFxLmNvbQ==; Yuan Li, ✉ bGx5eWRvY3RvckAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.