- 1Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan
- 2Department of Regenerative Microbiology, Juntendo University School of Medicine, Tokyo, Japan
- 3Department of Microbiota Research, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 4Department of Gastroenterology and Hepatology, The Jikei University Kashiwa Hospital, Chiba, Japan
Inflammatory bowel disease (IBD) is a chronic intestinal mucosal inflammatory disease with complex etiology. Traditional anti-inflammatory treatment regimens have yielded unsatisfactory results. As research continues to deepen, it has been found that the gut microbiota of patients with IBD is generally altered. The presence of microorganisms in the human gastrointestinal tract is inextricably linked to the regulation of health and disease. Disruption of the microbiotic balance of microbiota in the gastrointestinal tract is called dysbiosis, which leads to disease. Therefore, in recent years, the exploration of therapeutic methods to restore the homeostasis of the gut microbiota has attracted attention. Moreover, the use of the well-established fecal microbiota transplantation (FMT) regimen for the treatment of Clostridioides difficile infection has attracted the interest of IBD researchers. Therefore, there are an increasing number of clinical studies regarding FMT for IBD treatment. However, a series of questions regarding FMT in the treatment of IBD warrants further investigation and discussion. By reviewing published studies, this review explored hot topics such as the efficacy, safety, and administration protocol flow of FMT in the treatment of IBD. Different administration protocols have generally shown reassuring results with significant efficacy and safety. However, the FMT treatment regimen needs to be further optimized. We believe that in the future, individual customized or standard FMT implementation will further enhance the relevance of FMT in the treatment of IBD.
Introduction
A growing number of studies have suggested that the presence of microbes in the human gastrointestinal tract is inextricably linked to the regulation of health and disease. Gut microbes ferment food into absorbable metabolites, synthesize essential vitamins, regulate the immune system, and act as a barrier to protect the gastrointestinal tract. Disruption of the gut microbiota balance, called dysbiosis, can lead to disease (1).
Inflammatory bowel disease (IBD) is an intestinal disease characterized by chronic inflammation of the intestinal mucosa that is prone to relapse. Common clinical types mainly include ulcerative colitis (UC), Crohn’s disease (CD), and pouchitis. The etiology of IBD is complex and diverse, which may be related to multiple interactive influences, such as environmental, microbial, genetic, and immune factors (2, 3). Traditional IBD treatment regimens have primarily focused on reducing inflammation. Although this treatment regimen has been continuously developed and updated, there are still drawbacks, such as easy relapse, immune tolerance, and drug resistance (4). Therefore, researchers continue to explore more effective treatment measures. It is generally accepted that the gut microbiota of patients with IBD is altered (3). The exploration of therapeutics to restore gut microbiota homeostasis has gained attention in recent years because the qualitative and quantitative profiles of the gastrointestinal microbiota in patients with IBD vary significantly compared to healthy individuals. Fecal microbiota transplantation (FMT) is an advanced microbial therapy that restores the gut microbiota and corrects the dysbiosis of the microbiota by providing full-spectrum microorganisms of healthy individuals to the patient so that the patient can obtain a complete functional ecosystem (5). In the Clostridioides difficile infection (CDI) treatment guidelines published in the United States and Europe, it is stated that FMT is a strongly recommended regimen for CDI with multiple recurrences (6, 7), with an effective rate of 92% (8). FMT has been implemented in a variety of disease fields (9–11), especially in improving the response of anti-PD-1 immunotherapy in metastatic melanoma (12, 13). Openbiome (14), a non-profit organization in the United States, is committed to providing an internationally standardized public stool bank for microbial treatment of various diseases. This provides the basic guarantee for FMT treatment. However, the use of FMT for the treatment of IBD is still progressing toward clinical application. In this review, we summarized hot topics such as efficacy, safety, and implementation of FMT for the treatment of IBD.
Efficacy
Since the two cases of using FMT to treat patients with UC in 1989 proved effective (15, 16), researchers have been increasingly enthusiastic about exploring the use of FMT for IBD treatment.
Efficacy of fecal microbiota transplantation in ulcerative colitis therapy
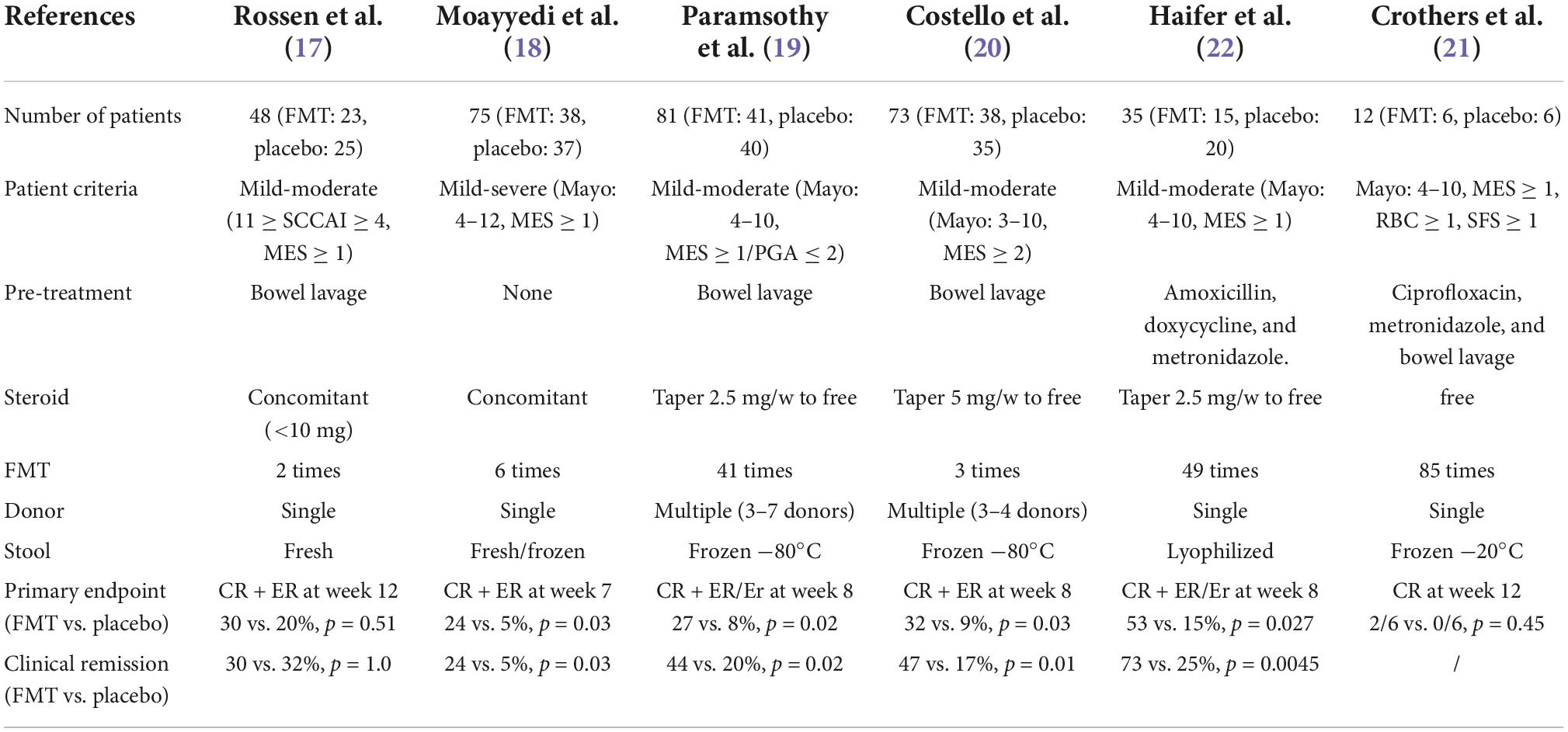
To date, six double-blinded, randomized controlled trials (RCTs) on the efficacy of FMT-induced remission in UC have been published (Table 1; 17–22). Moayyedi et al. recruited 75 patients with mild-severe UC (38 received FMT and 37 received placebo) and demonstrated that patients who received fecal enemas from donors (24%) had significantly higher rates of clinical remission at week 7 than those in the placebo enema group (5%) (p = 0.03). Two years later, Paramsothy et al. reported the results of their study of 81 patients with mild-moderate UC. Forty-one patients were included in the FMT group and 40 in the placebo group. At week 8, steroid-free clinical and endoscopic remission were achieved in 11 (27%) patients, which was significantly higher than that in the control group (3 patients [8%]) (p = 0.021). In an article published in 2019, Costello et al. enrolled 73 mild-moderate UC patients (38 in the FMT group and 35 in the placebo group). At week 8, steroid-free clinical and endoscopic remission were achieved in 12 (32%) of them. The treatment effect was significantly better than that observed in the placebo group, with only three of the 35 with complete remission (p = 0.03). In 2021, Haifer et al. also published the results of a RCT. Of the 35 mild-moderate UC patients recruited, 15 received FMT and 20 received a placebo. At week 8, the expected steroid-free clinical and endoscopic remission were achieved in 53% (n = 8) of patients in the FMT group, a significantly higher rate of remission than that in the placebo group of 15% (n = 3) (p = 0.027). Although positive results continued to emerge, as early as 2015, Rossen et al. reported contrary results. In 48 patients with mild-moderate UC, only seven of 23 patients receiving FMT achieved clinical and endoscopic remission at week 12, and five of 25 patients receiving placebo achieved remission, a result that was not significantly different (p = 0.51). Moreover, Crothers et al. published the results of a study with a small sample size (n = 12) in 2021. In the 12th week, only two of six patients in the FMT group achieved steroid-free clinical remission, while none in the placebo group achieved remission. There was no significant difference between the two groups (p = 0.45).
El Hage Chehade et al. (23) conducted a meta-analysis of the different results of six double-blinded RCTs. A total of 324 patients were included in the analysis, and 30.43% of patients treated with FMT achieved clinical and endoscopic remission, significantly higher than 9.82% of patients in the placebo group who achieved clinical and endoscopic remission (p < 0.00001). In another non-double-blinded RCT (24), 90% of patients in the FMT group achieved the primary endpoint at week 8, compared with 50% in the placebo group. Considering the published conclusions so far, we believe that the efficacy of FMT for UC treatment is excellent.
Efficacy of fecal microbiota transplantation in Crohn’s disease therapy
Cohort studies showed that FMT for CD treatment is generally effective (25–29). However, a few reports also showed a less obvious effect (30, 31).
Currently, only one RCT study has evaluated the clinical effect of FMT in CD (32). In 2020, Sokol et al. published a multicenter, single-blinded RCT study. Twenty-one patients who achieved clinical remission after 3 weeks of prednisolone therapy were randomly assigned to the FMT or placebo groups. No patients in either the FMT or placebo groups achieved the primary outcome of successful gut colonization with the donor microbiota at 6 weeks. The steroid-free clinical remission rates in the FMT and placebo groups were 87.5 and 44.4% at week 10 and 50 and 33.3% at week 24, respectively. Both results were not statistically significant. In 2021, a meta-analysis of FMT for CD treatment reported that the pooled rate of clinical remission in patients with CD reached 0.62, and that of clinical response was 0.79 (33).
Because CD lesions extend into the small intestine, determining the treatment response is expected to be more challenging than for UC. Moreover, it is expected that the response to FMT treatment will differ depending on the site of the lesion and whether it is a small or large bowel type. The results of using FMT for the treatment of CD are still controversial; hence, more convincing RCT studies are required.
Efficacy of fecal microbiota transplantation in pouchitis therapy
Pouchitis is the most common complication of ileal pouch-anal anastomosis for refractory UC, with an incidence of up to 80% at 30-year follow-up (34). Some reports showed that 80% (35) of patients and 44% (36) with pouchitis achieved clinical remission after receiving FMT. A case report (37) also showed that antibiotic-refractory pouchitis improved significantly after FMT and persisted for more than 6 months. However, some other reports showed that (38–42) the efficacy was not very satisfactory, and no patient achieved clinical remission. Moreover, a recent RCT (43) report showed that FMT was not associated with relapse-free survival of pouchitis. In summary, the current results of the use of FMT in treating pouchitis are not satisfactory. Therefore, well-designed controlled studies are further needed.
Safety
For a new treatment regimen for IBD, the public is most concerned about safety and efficacy. Most patients experience only transient discomfort, such as diarrhea, abdominal pain, bloating, borborygmus, nausea, vomiting, and increase in C-reactive protein level (17, 21, 22, 25, 26, 29, 31, 32, 44–63), which are believed to be an immune response caused by the infused fecal microbiota. There are also a small number of patients who have narcolepsy, fatigue (61), skin pruritus (29, 52, 62), testicular pain, rectal abscess (18), perianal pain or fistula (26), blood in the stool (27, 57), herpes zoster (57), and other complaints (64). However, these symptoms have not been shown to be directly related to FMT. Serious adverse events of worsening colitis requiring colectomy and hospitalization have been reported in some patients (18–20, 22, 26, 30, 32, 45, 57, 65, 66). Some of these exacerbated conditions were observed in the placebo group, while those in the FMT group may have been associated with a change in treatment regimen or a disproportionate host immune response induced by the new microbiota of the incomplete mucosa and disease progression rather than FMT itself. In addition, the spread of infection is a problem that doctors are very concerned about. Cytomegalovirus infections (17, 67), and CDI (18, 51) have been reported in FMT for the treatment of IBD. However, Rossen et al. concluded that CMV infection was not associated with FMT because patients were randomly assigned to the placebo group (17). In addition, Suskind et al. speculated that C. difficile infection in two patients, which occurred 3 and 4 months after transplantation, may not be related to FMT because the feces used showed no abnormal results on microbiological examination (51). Some studies have also described the risk of bacteremia. However, most of the fever symptoms in patients suspected of bacteremia resolved spontaneously within a short period (17, 21, 25, 26, 28, 31, 45–47, 49, 50, 62, 63, 68–72). Blood cultures were used in some studies to test whether a patient had bacteremia but did not yield positive results (47, 50, 62). However, a report (73) described a patient with CD who had positive blood cultures for multidrug-sensitive Escherichia coli bacteremia after FMT. Moreover, Grewal et al. (66) reported a patient with UC progression and toxic megacolon after FMT, who died of sepsis after surgery. Although not treated for UC, in March 2020, the FDA issued a safety warning1 that two patients with CDI were infected with drug-resistant Escherichia coli as a result of FMT treatment, and one died due to bacteremia (74). Despite occasional infections, rigorous donor screening is believed to reduce the risk of bacteremia and infectious disease transmission to almost zero.
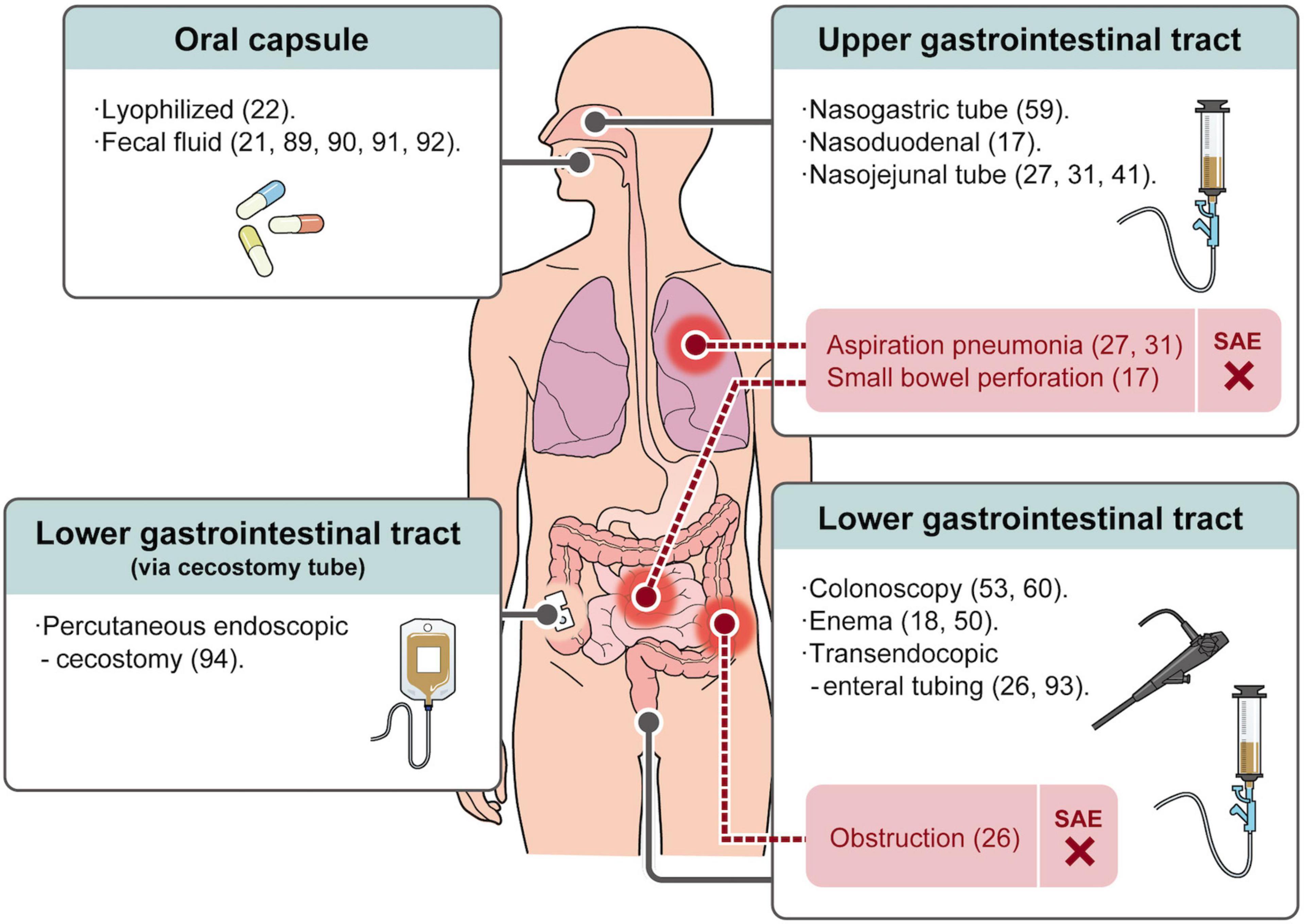
Small bowel perforation (17), obstruction (26), and aspiration pneumonia (27, 31) caused by improper handling of routes of administration in the upper gastrointestinal tract (nasogastric, nasoduodenal, or nasojejunal tube) and lower gastrointestinal tract (transendocopic enteral tubing) have also been reported. This has caused severe pneumonia and intestinal bleeding leading to the death of a patient (27). The occurrence of these adverse events makes every doctor distressed, and the operation regimen is constantly improving. Moreover, a recent meta-analysis article analyzed published RCTs using FMT for various diseases and no significant difference in the incidence of serious adverse events was observed between the FMT and placebo groups (75). This suggests that FMT is a safe treatment modality.
Implementation
There is still no unified standard protocol of FMT. The protocol of FMT affects the efficacy, safety, and patient acceptance of the treatment.
Dose intensity and antibiotic pre-treatment
Fecal microbiota transplantation attempts to reverse dysbiosis by colonizing patients with healthy microbiota. It is now known that a single FMT treatment can restore the abnormal microbiota environment in most patients with CDI for several years (76, 77). However, according to the current study results, the effect of administration intensity on efficacy in patients with IBD is unstable.
Published articles showing the effect of a single FMT administration on clinical outcomes are controversial (69, 78). In addition, the lack of a control group in these articles makes it impossible to rule out other factors that may have contributed to the biased results. However, Mocanu et al. statistically analyzed that repeated FMT administrations were higher than single administrations in both clinical response (70 vs. 53%) and clinical remission rates (43 vs. 30%) (79).
Some researchers have conducted some double-blinded RCTs on multiple administrations of FMT. In 2015, Moayyedi et al. (18) published an article involving six administrations of FMT per patient. The remission rate of patients in the FMT group was significantly higher than that in the placebo group, which led to interest in the negative results of a study involving two administrations published by Rossen et al. (17) in the same year. Were the negative results of Rossen et al. related to the frequency of FMT use? The study by Paramsothy et al. (19), Haifer et al. (22), and Crothers et al. (21) performed 41, 49, and 85 FMTs on each patient, respectively, and the effect of using FMT was significantly better in the FMT group than in the placebo group. However, in 2019, Costello et al. (20) used a similar FMT implementation protocol as Paramsothy et al. (19); however, they only performed three FMT administrations, obtaining similar clinical outcomes as Paramsothy’s 41-administration study. This result raises the question of if more than 40 administrations are meaningful. Furthermore, how many administrations can give the best results? In a subgroup analysis of the number of administrations by Paramsothy et al. the pooled proportion of patients with UC who received more than 10 administrations and achieved clinical remission was 49%, significantly higher than the remission rate (27%) for patients with UC who received fewer than 10 administrations (p = 0.001) (54). There have been reports that there was no significant difference in adverse events (both severe and common adverse events) between the FMT and placebo groups in RCT studies involving the use of either single or multiple FMT administrations (75). However, too many administrations of FMT will bring inconvenience and psychological burden to patients; therefore, getting the best therapeutic effect under the premise of the least number of administrations is a topic worthy of further study. To the best of our knowledge, in addition to the effectiveness of antibiotic cocktail therapy in the treatment of patients with UC (80, 81), recent studies have shown that pre-treatment with antibiotics prior to FMT can improve FMT treatment efficacy by aiding microbiota colonization (82). We have previously reported (53, 60, 83) a clinical remission rate of approximately 35% with combined antibiotic pretreatment prior to the use of a single FMT, which is higher than the clinical remission rate observed by using multiple FMTs as reported by Rossen et al. (30%) (17) and Moayyedi et al. (24%) (18). Moreover, a case report showed that patients with refractory CD who received a single dose of FMT after pre-treatment with antibiotics had significantly improved symptoms (84). More RCTs are needed to verify the potentiating ability of antibiotic pre-treatment on FMT.
Route administration
At present, the widely used FMT administration routes are mainly divided into upper gastrointestinal tract, lower gastrointestinal tract, and oral capsule-based FMT (Figure 1). There are meta-analysis statistics on the therapeutic effect of the FMT administration route on IBD, and the conclusions are inconsistent (54, 85). However, we believe it is challenging to assess the effect of the administration route on efficacy due to the use of different FMT protocols between studies. However, several routes of administration in the upper gastrointestinal tract (nasogastric, nasoduodenal, and nasojejunal tube) are inevitably affected by the distance from inflammation and the influence of proximal gastrointestinal secretions. Furthermore, in addition to the inherent risks of endoscopy, such as perforation, they may lead to symptoms such as aspiration pneumonia (31), vomiting (17, 31), runny nose, sore throat (59), and reflux (86).
Lower gastrointestinal administration routes mainly include enema and colonoscopy routes. Although patients can perform FMT with self-enema at home, possible related adverse events such as rectal abscess (18) and left-sided abdominal fullness (50) have been reported. The administration of FMT via colonoscopy has the advantage of transporting more stool to the site of inflammation (87). Moreover, it can detect the inflammatory state of the intestinal mucosa and compare the mucosal healing after treatment (88). However, frequent colonoscopies can also bring mental stress to patients. Therefore, an oral capsule-based FMT has recently attracted attention. In previous studies, oral capsule FMT was generally used as an adjunctive therapy (21, 89–91). A small sample-sized open-label study showed that oral capsule FMT can temporarily improve patients’ quality of life and reduce calprotectin (92). A double-blinded RCT (22) report in 2021 showed that oral lyophilized capsule FMT combined with antibiotic pre-treatment was significantly more effective than the placebo treatment (p = 0.027). In addition, no significant difference in FMT maintenance between an enema and oral capsule delivery was observed (91). Therefore, oral FMT capsules are a promising drug delivery option for long-term use to maintain a stable gut microbiota structure (21). More RCTs on oral capsule-administered FMT with high acceptability are required.
There are also some less frequently used methods, such as transendocopic enteral tubing (26, 93) and ercutaneous endoscopic cecostomy (94). It is also important to choose a method acceptable to the patient because patient compliance is the key to treatment.
Treatment maintenance
Currently, the long-term maintenance effect of FMT in the treatment of IBD is unclear. Researchers have tried to maintain the diversity of gut microbiota through post-intervention of FMT to achieve the long-term efficacy of FMT in treating IBD. Repeating FMT several times after reaching remission to stabilize the intestinal environment is one method (95). An RCT study published in 2015 showed that of the nine patients who achieved clinical remission at week 7, eight were still in remission at week 52 with a monthly FMT interval (18). The article published by He et al. showed that the clinical remission rate (52%) after the initial FMT decreased slowly with the sustained remission rate after multiple FMT boosters, and 22.7% of patients were still in remission at 18 months (26). An RCT study randomly assigned 61 patients in remission to FMT to receive FMT or placebo administrations every 8 weeks for 48 weeks to determine the long-term maintenance effect of FMT. The results showed that FMT administration during the maintenance phase of UC patients could prolong the clinical, endoscopic, and histological remission of patients (96). It was further investigated that a second course of FMT consolidation therapy within 1 month could maintain the benefits of FMT in CD patients (65).
There are also attempts to maintain patient treatment outcomes in more light-hearted ways. For example, Wei et al. achieved the effect of slowing the loss of colonized microbiota by the oral administration of pectin that can be fermented into short-chain fatty acids and beneficial to intestinal microbiota (71). In our research group, we are conducting a double-blinded controlled RCT study to consolidate the efficacy of FMT in patients with UC by giving patients oral alginic acid (97). It is hoped that further research on the maintenance of efficacy will increase patients’ expectations and confidence in FMT for the treatment of IBD.
Donor stool
The first major hurdle in FMT treatment is donor stool selection and preparation. Not only the transmission of pathogens can occur during FMT, as the impact of intestinal microbiota on patients with mental and endocrine diseases has been reported (9, 11). Hence, the screening of healthy fecal providers is currently a primary task. Many institutions also propose and continuously improve screening criteria according to the living background and the occurrence of epidemics in their respective regions (6, 14, 98, 99). Donor screening can be performed using questionnaires, blood tests, and stool tests. The basic questionnaire section should exclude infection risk factors such as HIV infection, exposure to viral hepatitis, high-risk sexual behavior, tattooing or piercing within 6 months, history of incarceration, travel history to areas endemic for infectious diseases, known history of infection, and risk factors for multi-drug resistant organisms. There are also potential microbiota-mediated conditions which should be determined, such as whether the donor has gastrointestinal disease, atopic disease, autoimmune disease, chronic pain syndrome, malignancy, and surgical history, and questions about the donor’s metabolic system, neurological system, mental, and medication conditions. Blood tests should mainly include complete blood count with differential, hepatic function, HIV, hepatitis, treponema pallidum, and parasite testing. Fecal testing should mainly include C. difficile toxin A/B, Campylobacter, Salmonella, Shigella, Vibrio, Escherichia coli, Helicobacter pylori, rotavirus, norovirus, adenovirus, COVID-19, and monkeypox. A more detailed screening should ensure patient safety but will reduce screening pass rates and increase screening costs. Therefore, maintaining a balance between the three methods is a question that needs to be considered. Of course, the relationship between FMT efficacy and donor feces is also a problem to be explored.
Relationship between patients and donor
As far as we know, there are mainly two ways to obtain feces: one is from relatives or friends recommended by the patient and the other is from undirected stranger donors. Since some ethical, esthetic, and psychological barriers can be avoided by accepting stool from a donor recommended by the patient, the patient may be more receptive to the treatment. In addition, we previously reported higher long-term non-relapse rates for the treatment of UC with the stools of siblings compared to the stools of parents and offspring (p = 0.007) (60). The gut microbiota of siblings may be similar to the healthy microbiota state of the patient before IBD (100), and species originally present in the recipient’s microbiota are more likely to colonize the patient’s intestinal mucosa stably.
However, a meta-analysis showed no difference in the efficacy of feces from undirected stranger donors or patient-recommended donors for patients with CDI (101). Compared with patient-recommended donors, the undirected donor format has the advantages of avoiding screening time and starting treatment quickly, protecting the privacy of donor candidates, and saving costs for serving multiple patients after the successful screening. Therefore, doctors are more inclined to use the undirected donation of stranger feces.
Fresh, frozen, or lyophilized stool
Using frozen stool can reduce the cost of FMT and increase the timeliness and safety of treatment. In addition, it has been reported that although freezing reduced the overall viability of the fecal microbiota by approximately 25%, the live microbiota composition was not significantly different from that of fresh feces (102). Cryopreservation of fecal samples for 6 months did not affect colony forming unit counts for some bacterial groups (E. coli, total coliforms, Bifidobacteria, total aerobes, Lactobacilli, or total anaerobic bacteria) (103). Therefore, frozen feces did not affect the efficacy of FMT in the treatment of CDI (103–105). However, there are meta-analysis statistics that the preservation status before FMT has an unstable impact on IBD (85, 106). UC patients treated with fresh donor stool had a lower pooled clinical remission rate (15%) than those with frozen stool (42%). Moreover, for CD patients, the remission rate for FMT with fresh stool was 36% higher than that with frozen stool (28%). Recently, the use of oral-fecal lyophilized capsules is a new method of drug delivery and storage. This delivery method requires that the capsules are always stored at −20°C and should not be directly transferred between refrigerators. If transfer is required, it should be kept on dry ice at all times to maintain the microbiota’s viability (21). It is difficult to link these three stool processes before drug delivery to IBD efficacy without RCTs that control for other potentially confounding variables.
Donor microbiota characteristics
Donor biomarkers which are best for IBD have not been definitively reported. However, it has been reported that the microbial diversity of donor feces is associated with the efficacy of FMT in the treatment of IBD (31, 107). While testing the relationship between the abundance of the single donor’s gut microbial species and the therapeutic effect, some studies have also attempted to transplant the mixed feces of multiple people into the patient’s gut and achieved a significant effect compared to the placebo group (19, 20). However, there seems to be a super-donor phenomenon in the treatment of UC with FMT in previous studies. In 2015, Moayyedi et al. found that seven of nine patients with UC who achieved remission after FMT received stool from the same donor (18). Moreover, the efficacy rate of the multiple donors’ fecal microbiota transplant containing the donor number D054 was higher than that in patients who received multiple donors’ fecal transplant that did not contain the donor D054′s feces (p = 0.054) (19). From the current evidence, increasing the abundance of microbiota may not be the only condition for inducing remission. Further analysis of the study showed that a high abundance of specific species of Bacteroides (B. fragilis and B. finegoldii) in mixed donor feces was associated with the efficacy of FMT in patients with UC (108).
Fecal microbiota in patients with IBD is not only less diverse (109) but also often lacks commensal bacteria (110). For example, the bacterial phylum Bacteroidota (83, 111, 112), which produces zwitterionic capsular polysaccharides that suppress inflammation by regulating T cells, and Bacillota, which produces host-beneficial short chain fatty acids (SCFAs), are lacking. Therefore, some of the special bacteria carried in the guts of super-donors may colonize the guts of IBD patients if they supplemented the lost bacteria, and restoring the microbiota to a pre-morbid state could be beneficial. Reports showed that the presence of the bacterial genus Ruminococcus in the feces of the donors was associated with the induction of remission (18, 107). UC patients who achieved long-term FMT maintenance response showed a similar profile of microbiota to donors, especially Bacteroidetes species (60). In accordance with our previous report that dysbiosis of fecal microbiota in patients with UC is associated with loss of Bacteroides species diversity (83), we identified a relative abundance of 12 key Bacteroidetes species inversely associated with UC activity (112). The proportion of Bacteroidetes in feces was significantly increased in patients who underwent FMT (53). Therefore, the enrichment of Bacteriodetes in donor feces is one of our future research directions. In addition, different reports have shown that the intestinal microbiota of patients with CD has undergone inconsistent changes, such as a decrease of Bacillota (113), Bididobacterium (114), Enterobacteriaceae (115), or Lactobacillus (116), or an increase of Helicobacter species (117). In patients with UC and pouchitis, decreases of Roseburia hominis and Faecalibacterium prausnitzii (118) and absence of Streptococcus species (119) were also found. Therefore, determining the change of intestinal microbiota in IBD patients is a prerequisite for FMT treatment for IBD that cannot be ignored.
Although the results of the current study have not been able to establish the best donor guidelines for FMT, we can predict that in the future, the stool for the treatment of IBD will be selective and even customized.
Conclusion
From the current research results, the effectiveness and safety of FMT in treating IBD are beyond doubt. However, the details of the entire execution process are still up for debate. New techniques for FMT are constantly being updated, and study has suggested that Sterile Fecal Filtrate Transfer (which only contains bacterial debris, proteins, antimicrobial compounds, metabolites, and oligonucleotides/DNA) can also eliminate symptoms and restore normal bowel habits in patients with CDI (120). It is unknown which substance in the gut produces this therapeutic effect. SCFA-producing bacteria are typically reduced in the gut of patients with IBD compared to healthy individuals (121). However, butyrate was increased in patients with UC who responded to FMT (122). Whether butyrate plays a major role in the treatment of FMT is unknown due to the lack of relevant clinical research data. Therefore, it is necessary to interpret the mechanism of FMT in the treatment of IBD from the perspectives of microbiology, immunology, and metabolism and propose a one-to-one customization scheme with a narrow-spectrum. Finally, while continuously optimizing the curative effect and maintaining the therapeutic outcome, it is essential to find the most acceptable route of administration for patients. In conclusion, more results from future studies are needed to obtain a perfect treatment of IBD using FMT.
Author contributions
All authors contributed to the generation of the concept, wrote and edited the manuscript, and approved the submitted version.
Funding
This research was funded by Japan Agency for Medical Research and Development (Issue tracking number: 22ae0121038h0002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse-events-likely
References
1. Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters. Trends Microbiol. (2018) 26:563–74. doi: 10.1016/j.tim.2017.11.002
2. Nishida A, Nishino K, Sakai K, Owaki Y, Noda Y, Imaeda H. Can control of gut microbiota be a future therapeutic option for inflammatory bowel disease? World J Gastroenterol. (2021) 27:3317–26. doi: 10.3748/wjg.v27.i23.3317
3. Akutko K, Stawarski A. Probiotics, prebiotics and synbiotics in inflammatory bowel diseases. J Clin Med. (2021) 10:2466. doi: 10.3390/jcm10112466
4. Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Dev Ther. (2011) 5:185–210. doi: 10.2147/DDDT.S11290
5. Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. (2013) 15:337. doi: 10.1007/s11894-013-0337-1
6. Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. (2017) 66:569–80. doi: 10.1136/gutjnl-2016-313017
7. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. (2018) 66:e1–48. doi: 10.1093/cid/cix1085
8. Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. (2017) 46:479–93. doi: 10.1111/apt.14201
9. Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. (2012) 143:913–6.e7. doi: 10.1053/j.gastro.2012.06.031
10. Halkjaer SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. (2018) 67:2107–15. doi: 10.1136/gutjnl-2018-316434
11. Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. (2019) 9:5821. doi: 10.1038/s41598-019-42183-0
12. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. (2021) 371:602–9. doi: 10.1126/science.abb5920
13. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. (2021) 371:595–602. doi: 10.1126/science.abf3363
15. Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. (1989) 1:164. doi: 10.1016/s0140-6736(89)91183-5
16. Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. (1989) 150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x
17. Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. (2015) 149:110–8.e4. doi: 10.1053/j.gastro.2015.03.045
18. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. (2015) 149:102–9.e6. doi: 10.1053/j.gastro.2015.04.001
19. Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. (2017) 389:1218–28. doi: 10.1016/S0140-6736(17)30182-4
20. Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. (2019) 321:156–64. doi: 10.1001/jama.2018.20046
21. Crothers JW, Chu ND, Nguyen LTT, Phillips M, Collins C, Fortner K, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. (2021) 21:281. doi: 10.1186/s12876-021-01856-9
22. Haifer C, Paramsothy S, Kaakoush NO, Saikal A, Ghaly S, Yang T, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (Lotus): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. (2022) 7:141–51. doi: 10.1016/S2468-1253(21)00400-3
23. El Hage Chehade N, Ghoneim S, Shah S, Chahine A, Mourad FH, Francis FF, et al. Efficacy of fecal microbiota transplantation in the treatment of active ulcerative colitis: a systematic review and meta-analysis of double-blind randomized controlled trials. Inflam Bowel Dis. (2022) [Epub ahead of print]. doi: 10.1093/ibd/izac135
24. Fang H, Fu L, Li X, Lu C, Su Y, Xiong K, et al. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Fact. (2021) 20:18. doi: 10.1186/s12934-021-01513-6
25. Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. (2015) 30:51–8. doi: 10.1111/jgh.12727
26. He Z, Li P, Zhu J, Cui B, Xu L, Xiang J, et al. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn’s disease complicated with inflammatory mass. Sci Rep. (2017) 7:4753. doi: 10.1038/s41598-017-04984-z
27. Xiang L, Ding X, Li Q, Wu X, Dai M, Long C, et al. Efficacy of faecal microbiota transplantation in Crohn’s disease: a new target treatment? Microb Biotechnol. (2020) 13:760–9. doi: 10.1111/1751-7915.13536
28. Zou M, Jie Z, Cui B, Wang H, Feng Q, Zou Y, et al. Fecal microbiota transplantation results in bacterial strain displacement in patients with inflammatory bowel diseases. FEBS Open Bio. (2020) 10:41–55. doi: 10.1002/2211-5463.12744
29. Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis. (2016) 22:2182–90. doi: 10.1097/MIB.0000000000000893
30. Gutin L, Piceno Y, Fadrosh D, Lynch K, Zydek M, Kassam Z, et al. Fecal microbiota transplant for Crohn disease: a study evaluating safety, efficacy, and microbiome profile. U Eur Gastroenterol J. (2019) 7:807–14. doi: 10.1177/2050640619845986
31. Vermeire S, Joossens M, Verbeke K, Wang J, Machiels K, Sabino J, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis. (2016) 10:387–94. doi: 10.1093/ecco-jcc/jjv203
32. Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome. (2020) 8:12. doi: 10.1186/s40168-020-0792-5
33. Cheng F, Huang Z, Wei W, Li Z. Fecal microbiota transplantation for Crohn’s disease: a systematic review and meta-analysis. Tech Coloproctol. (2021) 25:495–504. doi: 10.1007/s10151-020-02395-3
34. Lightner AL, Mathis KL, Dozois EJ, Hahnsloser D, Loftus EV, Raffals LE, et al. Results at UP to 30 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Inflamm Bowel Dis. (2017) 23:781–90. doi: 10.1097/MIB.0000000000001061
35. Stallmach A, Lange K, Buening J, Sina C, Vital M, Pieper DH. Fecal microbiota transfer in patients with chronic antibiotic-refractory pouchitis. Am J Gastroenterol. (2016) 111:441–3. doi: 10.1038/ajg.2015.436
36. Kousgaard SJ, Michaelsen TY, Nielsen HL, Kirk KF, Brandt J, Albertsen M, et al. Clinical results and microbiota changes after faecal microbiota transplantation for chronic pouchitis: a pilot study. Scand J Gastroenterol. (2020) 55:421–9. doi: 10.1080/00365521.2020.1748221
37. Fang S, Kraft CS, Dhere T, Srinivasan J, Begley B, Weinstein D, et al. Successful treatment of chronic Pouchitis utilizing fecal microbiota transplantation (FMT): a case report. Int J Colorectal Dis. (2016) 31:1093–4. doi: 10.1007/s00384-015-2428-y
38. Nishida A, Imaeda H, Inatomi O, Bamba S, Sugimoto M, Andoh A. The efficacy of fecal microbiota transplantation for patients with chronic pouchitis: a case series. Clin Case Rep. (2019) 7:782–8. doi: 10.1002/ccr3.2096
39. Herfarth H, Barnes EL, Long MD, Isaacs KL, Leith T, Silverstein M, et al. Combined endoscopic and oral fecal microbiota transplantation in patients with antibiotic-dependent pouchitis: low clinical efficacy due to low donor microbial engraftment. Inflamm Intest Dis. (2019) 4:1–6. doi: 10.1159/000497042
40. Landy J, Walker AW, Li JV, Al-Hassi HO, Ronde E, English NR, et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci Rep. (2015) 5:12955. doi: 10.1038/srep12955
41. Selvig D, Piceno Y, Terdiman J, Zydek M, Umetsu SE, Balitzer D, et al. Fecal microbiota transplantation in pouchitis: clinical, endoscopic, histologic, and microbiota results from a pilot study. Dig Dis Sci. (2020) 65:1099–106. doi: 10.1007/s10620-019-05715-2
42. Schmid M, Frick JS, Malek N, Goetz M. Successful treatment of pouchitis with vedolizumab, but not fecal microbiota transfer (FMT), after proctocolectomy in ulcerative colitis. Int J Colorectal Dis. (2017) 32:597–8. doi: 10.1007/s00384-017-2761-4
43. Karjalainen EK, Renkonen-Sinisalo L, Satokari R, Mustonen H, Ristimäki A, Arkkila P, et al. Fecal microbiota transplantation in chronic pouchitis: a randomized, parallel, double-blinded clinical trial. Inflamm Bowel Dis. (2021) 27:1766–72. doi: 10.1093/ibd/izab001
44. Karolewska-Bochenek K, Grzesiowski P, Banaszkiewicz A, Gawronska A, Kotowska M, Dziekiewicz M, et al. A two-week fecal microbiota transplantation course in pediatric patients with inflammatory bowel disease. Adv Exp Med Biol. (2018) 1047:81–7. doi: 10.1007/5584_2017_123
45. Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis. (2018) 24:410–21. doi: 10.1093/ibd/izx035
46. Vandenplas Y, Veereman G, van der Werff Ten Bosch J, Goossens A, Pierard D, Samsom JN. Fecal microbial transplantation in Early-onset colitis: caution advised. J Pediatr Gastroenterol Nutr. (2015) 61:e12–4. doi: 10.1097/MPG.0000000000000281
47. Kumagai H, Yokoyama K, Imagawa T, Inoue S, Tulyeu J, Tanaka M, et al. Failure of fecal microbiota transplantation in a three-year-old child with severe refractory ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr. (2016) 19:214–20. doi: 10.5223/pghn.2016.19.3.214
48. Damman CJ, Brittnacher MJ, Westerhoff M, Hayden HS, Radey M, Hager KR, et al. Low Level Engraftment and Improvement following a Single Colonoscopic Administration of Fecal microbiota to Patients with Ulcerative Colitis. PLoS One. (2015) 10:e0133925. doi: 10.1371/journal.pone.0133925
49. Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. (2015) 13:298. doi: 10.1186/s12967-015-0646-2
50. Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. (2013) 56:597–601. doi: 10.1097/MPG.0b013e318292fa0d
51. Suskind DL, Singh N, Nielson H, Wahbeh G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. (2015) 60:27–9. doi: 10.1097/MPG.0000000000000544
52. Zhang T, Cui B, Li P, He Z, Long C, Wei L, et al. Short-term surveillance of cytokines and C-reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS One. (2016) 11:e0158227. doi: 10.1371/journal.pone.0158227
53. Ishikawa D, Sasaki T, Osada T, Kuwahara-Arai K, Haga K, Shibuya T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflamm Bowel Dis. (2017) 23:116–25. doi: 10.1097/MIB.0000000000000975
54. Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. (2017) 11:1180–99. doi: 10.1093/ecco-jcc/jjx063
55. Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. (2016) 92:117–27. doi: 10.1016/j.jhin.2015.10.024
56. Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. (2016) 11:e0161174. doi: 10.1371/journal.pone.0161174
57. Wang H, Cui B, Li Q, Ding X, Li P, Zhang T, et al. The safety of fecal microbiota transplantation for Crohn’s disease: findings from A long-term study. Adv Ther. (2018) 35:1935–44. doi: 10.1007/s12325-018-0800-3
58. Yang Z, Bu C, Yuan W, Shen Z, Quan Y, Wu S, et al. Fecal microbiota transplant via endoscopic delivering through small intestine and colon: no difference for Crohn’s disease. Dig Dis Sci. (2020) 65:150–7. doi: 10.1007/s10620-019-05751-y
59. Suskind DL, Brittnacher MJ, Wahbeh G, Shaffer ML, Hayden HS, Qin X, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. (2015) 21:556–63. doi: 10.1097/MIB.0000000000000307
60. Okahara K, Ishikawa D, Nomura K, Ito S, Haga K, Takahashi M, et al. Matching between donors and ulcerative colitis patients is important for long-term maintenance after fecal microbiota transplantation. J Clin Med. (2020) 9:1650. doi: 10.3390/jcm9061650
61. Chen M, Liu XL, Zhang YJ, Nie YZ, Wu KC, Shi YQ. Efficacy and safety of fecal microbiota transplantation by washed preparation in patients with moderate to severely active ulcerative colitis. J Dig Dis. (2020) 21:621–8. doi: 10.1111/1751-2980.12938
62. Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. (2013) 108:1620–30. doi: 10.1038/ajg.2013.257
63. Ren R, Sun G, Yang Y, Peng L, Zhang X, Wang S, et al. [A pilot study of treating ulcerative colitis with fecal microbiota transplantation]. Zhonghua Nei Ke Za Zhi. (2015) 54:411–5.
64. Blanchaert C, Strubbe B, Peeters H. Fecal microbiota transplantation in ulcerative colitis. Acta Gastroenterol Belg. (2019) 82:519–28.
65. Li P, Zhang T, Xiao Y, Tian L, Cui B, Ji G, et al. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl Microbiol Biotechnol. (2019) 103:349–60. doi: 10.1007/s00253-018-9447-x
66. Grewal CS, Sood A, Mehta V, Sood N, Midha V, Mahajan R, et al. Role of Fecal Microbiota Transplantation in Patients with Steroid Dependant Ulcerative Colitis: 2455. Am J Gastroenterol. (2016) 111:S1252–61. doi: 10.1038/ajg.2016.385
67. Hohmann EL, Ananthakrishnan AN, Deshpande V. Case records of the Massachusetts general hospital. case 25-2014. a 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med. (2014) 371:668–75. doi: 10.1056/NEJMcpc1400842
68. Uygun A, Ozturk K, Demirci H, Oger C, Avci IY, Turker T, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine. (2017) 96:e6479. doi: 10.1097/MD.0000000000006479
69. Jacob V, Crawford C, Cohen-Mekelburg S, Viladomiu M, Putzel GG, Schneider Y, et al. Single delivery of high-diversity fecal microbiota preparation by colonoscopy is safe and effective in increasing microbial diversity in active ulcerative colitis. Inflamm Bowel Dis. (2017) 23:903–11. doi: 10.1097/MIB.0000000000001132
70. Kump PK, Gröchenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. (2013) 19:2155–65. doi: 10.1097/MIB.0b013e31829ea325
71. Wei Y, Gong J, Zhu W, Tian H, Ding C, Gu L, et al. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. (2016) 16:255. doi: 10.1186/s12866-016-0869-2
72. Wei Y, Zhu W, Gong J, Guo D, Gu L, Li N, et al. Fecal microbiota transplantation improves the quality of life in patients with inflammatory bowel disease. Gastroenterol Res Pract. (2015) 2015:517597. doi: 10.1155/2015/517597
73. Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis. (2014) 8:252–3. doi: 10.1016/j.crohns.2013.10.002
74. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. (2019) 381:2043–50. doi: 10.1056/NEJMoa1910437
75. Chen C, Chen L, Sun D, Li C, Xi S, Ding S, et al. Adverse events of intestinal microbiota transplantation in randomized controlled trials: a systematic review and meta-analysis. Gut Pathog. (2022) 14:20. doi: 10.1186/s13099-022-00491-3
76. Staley C, Kaiser T, Vaughn BP, Graiziger C, Hamilton MJ, Kabage AJ, et al. Durable long-term bacterial engraftment following encapsulated fecal microbiota transplantation to treat clostridium difficile infection. mBio. (2019) 10:e01586–19. doi: 10.1128/mBio.01586-19
77. Jalanka J, Mattila E, Jouhten H, Hartman J, de Vos WM, Arkkila P, et al. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med. (2016) 14:155. doi: 10.1186/s12916-016-0698-z
78. Mizuno S, Nanki K, Matsuoka K, Saigusa K, Ono K, Arai M, et al. Single fecal microbiota transplantation failed to change intestinal microbiota and had limited effectiveness against ulcerative colitis in Japanese patients. Intest Res. (2017) 15:68–74. doi: 10.5217/ir.2017.15.1.68
79. Mocanu V, Rajaruban S, Dang J, Kung JY, Deehan EC, Madsen KL. Repeated fecal microbial transplantations and antibiotic pre-treatment are linked to improved clinical response and remission in inflammatory bowel disease: a systematic review and pooled proportion meta-analysis. J Clin Med. (2021) 10:959. doi: 10.3390/jcm10050959
80. Nishikawa Y, Sato N, Tsukinaga S, Uchiyama K, Koido S, Ishikawa D, et al. Long-term outcomes of antibiotic combination therapy for ulcerative colitis. Ther Adv Chronic Dis. (2021) 12:20406223211028790. doi: 10.1177/20406223211028790
81. Ohkusa T, Kato K, Terao S, Chiba T, Mabe K, Murakami K, et al. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol. (2010) 105:1820–9. doi: 10.1038/ajg.2010.84
82. Ji SK, Yan H, Jiang T, Guo CY, Liu JJ, Dong SZ, et al. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front Microbiol. (2017) 8:1208. doi: 10.3389/fmicb.2017.01208
83. Ishikawa D, Sasaki T, Takahashi M, Kuwahara-Arai K, Haga K, Ito S, et al. The microbial composition of Bacteroidetes species in ulcerative colitis is effectively improved by combination therapy with fecal microbiota transplantation and antibiotics. Inflamm Bowel Dis. (2018) 24:2590–8. doi: 10.1093/ibd/izy266
84. Kao D, Hotte N, Gillevet P, Madsen K. Fecal microbiota transplantation inducing remission in Crohn’s colitis and the associated changes in fecal microbial profile. J Clin Gastroenterol. (2014) 48:625–8. doi: 10.1097/MCG.0000000000000131
85. Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. BioMed Res Int. (2018) 2018:8941340. doi: 10.1155/2018/8941340
86. Wang Y, Ren R, Sun G, Peng L, Tian Y, Yang Y. Pilot study of cytokine changes evaluation after fecal microbiota transplantation in patients with ulcerative colitis. Int Immunopharmacol. (2020) 85:106661. doi: 10.1016/j.intimp.2020.106661
87. Narula N, Kassam Z, Yuan Y, Colombel JF, Ponsioen C, Reinisch W, et al. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. (2017) 23:1702–9. doi: 10.1097/MIB.0000000000001228
88. Allegretti J, Eysenbach LM, El-Nachef N, Fischer M, Kelly C, Kassam Z. The current landscape and lessons from fecal microbiota transplantation for inflammatory bowel disease: past, present, and future. Inflamm Bowel Dis. (2017) 23:1710–7. doi: 10.1097/MIB.0000000000001247
89. Adler E, Tabaa A, Kassam Z, Zydek M, Terdiman J, El-Nachef N. Capsule-delivered fecal microbiota transplant is safe and well tolerated in patients with ulcerative colitis. Dig Dis Sci. (2019) 64:2452–4. doi: 10.1007/s10620-019-05596-5
90. Chu ND, Crothers JW, Nguyen LTT, Kearney SM, Smith MB, Kassam Z, et al. Dynamic colonization of microbes and their functions after fecal microbiota transplantation for inflammatory bowel disease. mBio. (2021) 12:e00975–21. doi: 10.1128/mBio.00975-21
91. Smith BJ, Piceno Y, Zydek M, Zhang B, Syriani LA, Terdiman JP, et al. Strain-resolved analysis in a randomized trial of antibiotic pretreatment and maintenance dose delivery mode with fecal microbiota transplant for ulcerative colitis. Sci Rep. (2022) 12:5517. doi: 10.1038/s41598-022-09307-5
92. Cold F, Browne PD, Günther S, Halkjaer SI, Petersen AM, Al-Gibouri Z, et al. Multidonor FMT capsules improve symptoms and decrease fecal calprotectin in ulcerative colitis patients while treated - An open-label pilot study. Scand J Gastroenterol. (2019) 54:289–96. doi: 10.1080/00365521.2019.1585939
93. Chen HT, Huang HL, Xu HM, Luo QL, He J, Li YQ, et al. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp Ther Med. (2020) 19:2650–60. doi: 10.3892/etm.2020.8512
94. Ni X, Fan S, Zhang Y, Wang Z, Ding L, Li Y, et al. Coordinated hospital-home fecal microbiota transplantation via percutaneous endoscopic cecostomy for recurrent steroid-dependent ulcerative colitis. Gut Liver. (2016) 10:975–80. doi: 10.5009/gnl15456
95. Li Q, Zhang T, Ding X, Xiang L, Cui B, Buch H, et al. Enhancing patient adherence to fecal microbiota transplantation maintains the long-term clinical effects in ulcerative colitis. Eur J Gastroenterol Hepatol. (2020) 32:955–62. doi: 10.1097/MEG.0000000000001725
96. Sood A, Mahajan R, Singh A, Midha V, Mehta V, Narang V, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. (2019) 13:1311–7. doi: 10.1093/ecco-jcc/jjz060
97. Ishikawa D, Zhang X, Nomura K, Seki N, Haraikawa M, Haga K, et al. A randomized placebo-controlled trial of combination therapy with post-triple-antibiotic-therapy fecal microbiota transplantation and alginate for ulcerative colitis: protocol. Front Med. (2022) 9:779205. doi: 10.3389/fmed.2022.779205
98. Costello SP, Tucker EC, La Brooy J, Schoeman MN, Andrews JM. Establishing a fecal microbiota transplant service for the treatment of clostridium difficile infection. Clin Infect Dis. (2016) 62:908–14. doi: 10.1093/cid/civ994
99. Zhang X, Ishikawa D, Nomura K, Fukuda N, Haraikawa M, Haga K, et al. Donor screening revisions of fecal microbiota transplantation in patients with ulcerative colitis. J Clin Med. (2022) 11:1055. doi: 10.3390/jcm11041055
100. Korpela K, Helve O, Kolho KL, Saisto T, Skogberg K, Dikareva E, et al. Maternal fecal microbiota transplantation in Cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell. (2020) 183:324–34.e5. doi: 10.1016/j.cell.2020.08.047
101. Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. (2013) 108:500–8. doi: 10.1038/ajg.2013.59
102. Papanicolas LE, Choo JM, Wang Y, Leong LEX, Costello SP, Gordon DL, et al. Bacterial viability in faecal transplants: which bacteria survive? EBiomedicine. (2019) 41:509–16. doi: 10.1016/j.ebiom.2019.02.023
103. Costello SP, Conlon MA, Vuaran MS, Roberts-Thomson IC, Andrews JM. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther. (2015) 42:1011–8. doi: 10.1111/apt.13366
104. Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. (2016) 315:142–9. doi: 10.1001/jama.2015.18098
105. Satokari R, Mattila E, Kainulainen V, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection–An observational cohort study. Aliment Pharmacol Ther. (2015) 41:46–53. doi: 10.1111/apt.13009
106. Takahashi M, Ishikawa D, Sasaki T, Lu YJ, Kuwahara-Arai K, Kamei M, et al. Faecal freezing preservation period influences colonization ability for faecal microbiota transplantation. J Appl Microbiol. (2019) 126:973–84. doi: 10.1111/jam.14167
107. Kump P, Wurm P, Gröchenig HP, Wenzl H, Petritsch W, Halwachs B, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther. (2018) 47:67–77. doi: 10.1111/apt.14387
108. Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. (2019) 156:1440–54.e2. doi: 10.1053/j.gastro.2018.12.001
109. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. (2006) 55:205–11. doi: 10.1136/gut.2005.073817
110. Stojek M, Jabłońska A, Adrych K. The role of fecal microbiota transplantation in the treatment of inflammatory bowel disease. J Clin Med. (2021) 10:4055. doi: 10.3390/jcm10184055
111. Neff CP, Rhodes ME, Arnolds KL, Collins CB, Donnelly J, Nusbacher N, et al. Diverse intestinal bacteria contain putative zwitterionic capsular polysaccharides with anti-inflammatory properties. Cell Host Microbe. (2016) 20:535–47. doi: 10.1016/j.chom.2016.09.002
112. Nomura K, Ishikawa D, Okahara K, Ito S, Haga K, Takahashi M, et al. Bacteroidetes species Are correlated with disease activity in ulcerative colitis. J Clin Med. (2021) 10:1749. doi: 10.3390/jcm10081749
113. Fujimoto T, Imaeda H, Takahashi K, Kasumi E, Bamba S, Fujiyama Y, et al. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J Gastroenterol Hepatol. (2013) 28:613–9. doi: 10.1111/jgh.12073
114. Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. (2011) 60:631–7. doi: 10.1136/gut.2010.223263
115. Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. (2003) 52:237–42. doi: 10.1136/gut.52.2.237
116. Favier C, Neut C, Mizon C, Cortot A, Colombel JF, Mizon J. Fecal beta-D-galactosidase production and Bifidobacteria are decreased in Crohn’s disease. Dig Dis Sci. (1997) 42:817–22. doi: 10.1023/a:1018876400528
117. Bartels LE, Jepsen P, Christensen LA, Gerdes LU, Vilstrup H, Dahlerup JF. Diagnosis of Helicobacter Pylori Infection is Associated with Lower Prevalence and Subsequent Incidence of Crohn’s Disease. J Crohns Colitis. (2016) 10:443–8. doi: 10.1093/ecco-jcc/jjv229
118. Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. (2014) 63:1275–83. doi: 10.1136/gutjnl-2013-304833
119. Segal JP, Oke S, Hold GL, Clark SK, Faiz OD, Hart AL. Systematic review: ileoanal pouch microbiota in health and disease. Aliment Pharmacol Ther. (2018) 47:466–77. doi: 10.1111/apt.14454
120. Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology. (2017) 152:799–811.e7. doi: 10.1053/j.gastro.2016.11.010
121. Dong LN, Wang M, Guo J, Wang JP. Role of intestinal microbiota and metabolites in inflammatory bowel disease. Chin Med J. (2019) 132:1610–4. doi: 10.1097/CM9.0000000000000290
Keywords: fecal microbiota transplantation, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, pouchitis
Citation: Zhang X, Ishikawa D, Ohkusa T, Fukuda S and Nagahara A (2022) Hot topics on fecal microbiota transplantation for the treatment of inflammatory bowel disease. Front. Med. 9:1068567. doi: 10.3389/fmed.2022.1068567
Received: 13 October 2022; Accepted: 21 November 2022;
Published: 02 December 2022.
Edited by:
Angel Lanas, University of Zaragoza, SpainReviewed by:
Jean-Pierre Routy, McGill University, CanadaJing Ouyang, Chongqing Public Health Medical Center, China
Copyright © 2022 Zhang, Ishikawa, Ohkusa, Fukuda and Nagahara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dai Ishikawa, ZGFpQGp1bnRlbmRvLmFjLmpw
 Xiaochen Zhang
Xiaochen Zhang Dai Ishikawa
Dai Ishikawa Toshifumi Ohkusa
Toshifumi Ohkusa Shinji Fukuda2
Shinji Fukuda2 Akihito Nagahara
Akihito Nagahara