
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 17 November 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1065350
This article is part of the Research Topic Update in Microcirculation in Dermatology View all 6 articles
Context: Tumor-associated cutaneous vascular disorder induced by PPGL was extremely rare, and the cutaneous manifestations could disappear after removal of the tumors. However, the definite pathological diagnosis and the potential mechanism remained unidentified. We presented a severe cutaneous vascular lesion manifested as diffuse erythema with ulceration and necrosis over the limbs in a female patient with metastatic paraganglioma. Skin biopsy was performed on her for defining the pathological diagnosis and potential mechanism. The patient was diagnosed as vascular disease according to the obvious angioectasia in dermis on cutaneous pathology, which might be caused by PPGL-induced hypercoagulability. We used the antiplatelet therapy with aspirin to treat the PPGL-associated cutaneous vascular disease for the first time, and the cutaneous lesions were relieved and healed gradually, further supporting the diagnosis of vascular disease.
Conclusion: For metastatic PPGL patients like the case we reported, the definite diagnosis by skin biopsy and the early antiplatelet therapy might be effective to the cutaneous lesions caused by the hypercoagulable state of PPGL.
Pheochromocytoma/paraganglioma (PPGL) is a neuroendocrine tumor with various protean manifestations (1). There may be several undetected and unraveled manifestations of this disease. Tumor-associated cutaneous vascular disorder induced by PPGL was first described in the 1970s, and to date, only 16 cases of PPGL-related cutaneous involvement have been reported. Removal of the tumors can lead to complete regression of the cutaneous lesions in these cases (2). However, the definite pathological diagnosis and the evidence for clinical treatment were both limited. Until now, there is no report yet on the effective treatment for severe cutaneous lesions in metastatic PPGL patients who could not receive surgery.
In this report, we presented a metastatic paraganglioma patient with severe diffuse erythema and localized ulcers and curst over the limbs, with suspected association with the hypercoagulability of the tumor. We noted that the cutaneous lesions were relieved and eventually healed gradually, after an antiplatelet therapy with aspirin. We used the antiplatelet therapy with aspirin to treat the PPGL-associated cutaneous vascular disease for the first time, and the potential mechanism behind this and the choice of treatment for PPGL-related cutaneous lesions have been discussed at length in the manuscript.
In October 2016, a 19-year-old Chinese girl was admitted to a local hospital with an elevated blood pressure (180/140 mmHg), accompanied by paroxysmal headache and sweating. Her 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) scan revealed an abdominal tumor of size 5.3 × 4.8 cm and SUVmax 8.5, along with multiple liver metastases. The abdominal mass was diagnosed as paraganglioma on biopsy pathology. She had not received any other therapy than long-term phenethylamine for controlling the symptoms. From December 2019 to October 2020, she underwent chemotherapy with cisplatin for 10 cycles intermittently, but there was little change in the abdominal tumor or liver metastases. In April 2021, cutaneous lesions manifested as diffuse erythema with ulcers, obvious pain, and tenderness appeared over the limbs (Figure 1), but without swelling, fever, or joint pain. Upon treatment with methylprednisolone at the dose of 16 mg qd for 1 month and subsequently, with 20 mg qd for another 1 month, the cutaneous lesions showed no improvement, and hence glucocorticoid was discontinued.
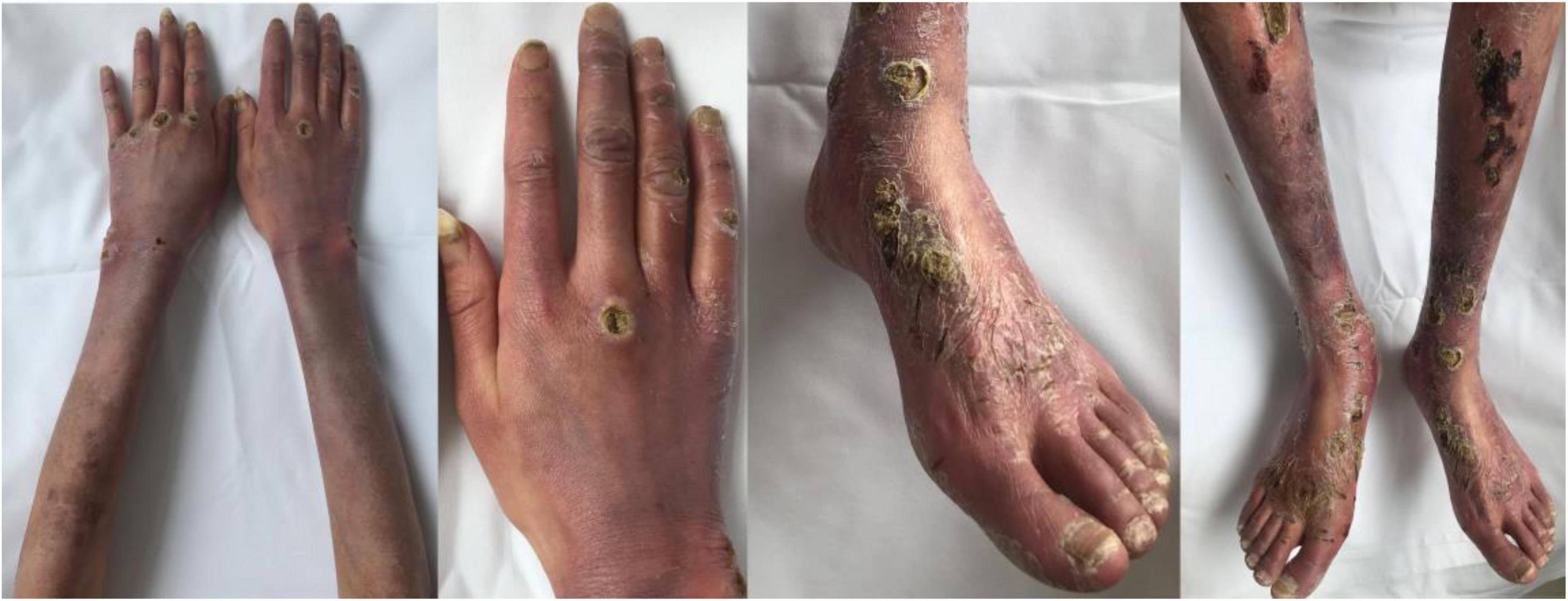
Figure 1. Diffuse erythema with severe ulceration and necrosis on the patient’s arms, hands, legs, and feet.
In September 2021, the patient was referred to our hospital for further examination. Her physical examination indicated a blood pressure of 140–150/90–100 mmHg. Her body mass index (BMI) was 16.7 kg/m2. The cutaneous lesions manifested as diffuse erythema with severe ulceration and necrosis on her limbs. Her laboratory tests indicated a WBC count of 10.5*109/L [(3.5–9.5)*109/L], HGB of 106 g/L [(110–150) g/L], a high platelet (PLT) count of 673*109/L [(100–350)*109/L], abnormal coagulation function with a high D-Dimer level of 596 ng/ml [(0–253) ng/ml]. Endocrine-related hormone tests indicated significantly increased catecholamine levels [NMN: 399.38 nmol/L (<0.9 nmol/L); MN: 0.61 nmol/L (<0.5 nmol/L); 24-h urinary NE: 9567.1 μg/24 h (<76.9 μg/24 h); 24-h urinary E: 2.3 μg/24 h (<11 μg/24 h), 24-h urinary DA: 5354.1 μg/24 h (<459.9 μg/24 h)]. A skin biopsy taken from the lesions on her left leg revealed significant hyperkeratosis and acanthosis. There was obvious angioectasia in the dermal papilla and mild lymphocytic infiltrate around the dermal vessels (Figure 2). The characteristics of cutaneous lesions were consistent with that of cutaneous vascular disease, and the patient accordingly received anticoagulant therapy.
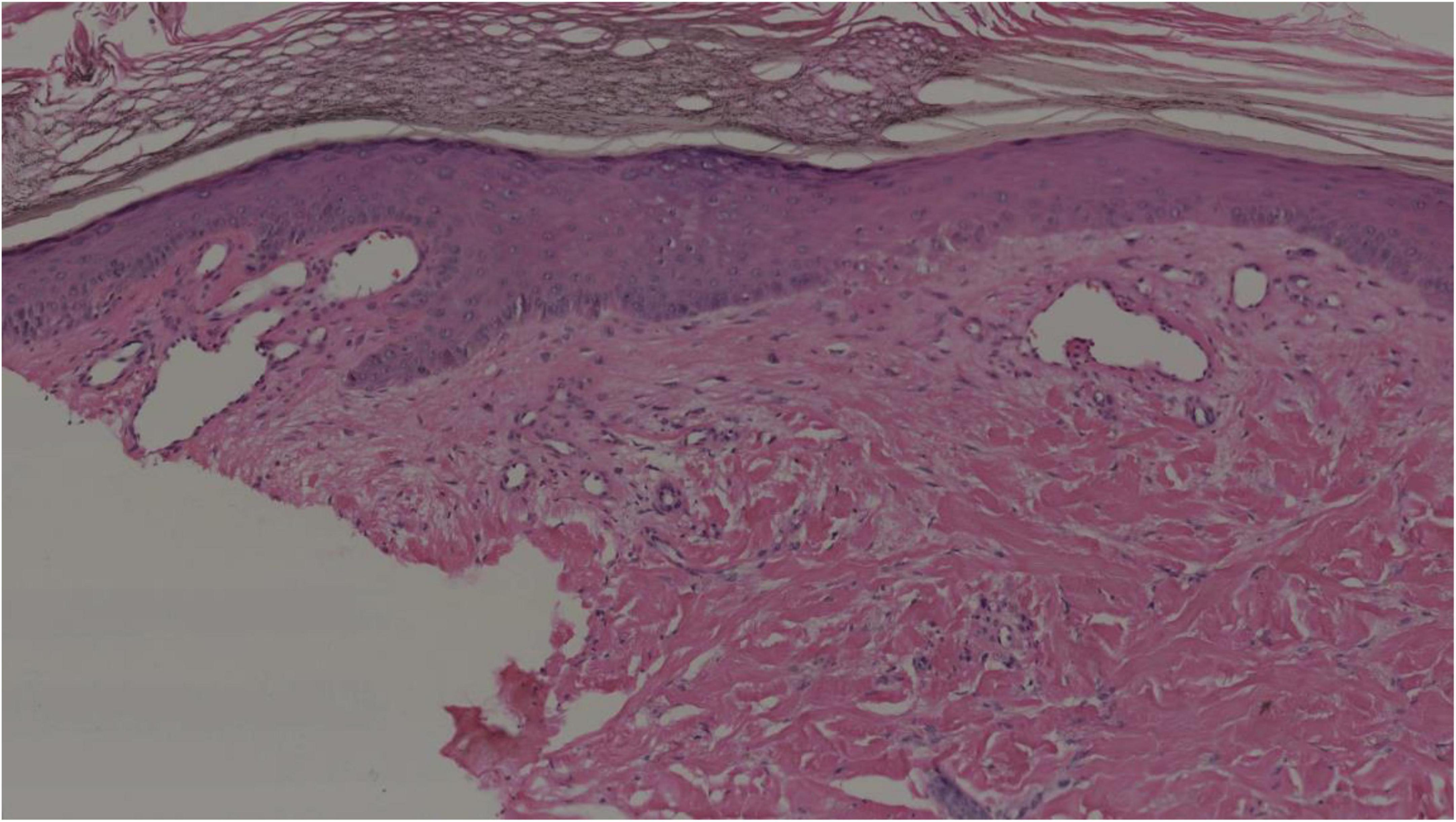
Figure 2. Histopathological findings of the cutaneous lesions on the left leg. Hyperkeratosis, acanthosis, and obvious angioectasia in the dermal papilla and mild lymphocytic infiltration around the dermal vessels (hematoxylin–eosin, original magnification 10×).
The patient took rivaroxaban, but epistaxis and ulcer bleeding occurred soon after. Therefore, rivaroxaban was withdrawn and switched to the antiplatelet drug aspirin at the dose of 25 mg qd. After the treatment with aspirin for 2 months, her cutaneous ulcer healed and the diffuse erythema disappeared, with only atrophie blanche and skin hyperpigmentation left (Figure 3).
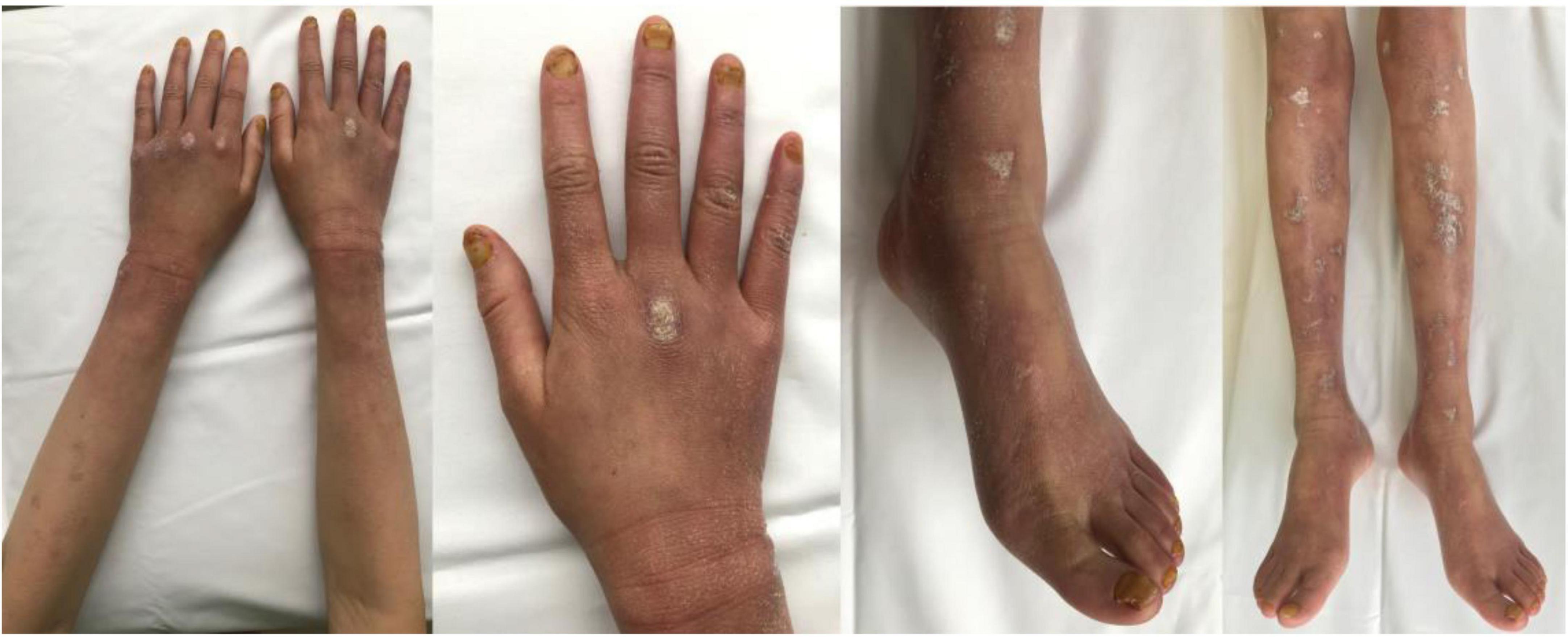
Figure 3. Evident regression of the cutaneous lesions of the patient’s arms, hands, legs, and feet after the antiplatelet therapy.
Genetic screening of the genomic DNA from peripheral blood leukocytes of the patient was conducted through targeted next-generation sequencing (NGS) involving 20 genes (NF1, VHL, RET, SDHA, SDHB, SDHC, SDHD, SDHAF2, MAX, TMEM127, FH, KIF1B, BAP1, IDH1, EPAS1, EGLN2, EGLN1, EGLN3, HRAS, and MDH2). A pathogenic heterozygous variation of SDHB (NM_003000.3), c.C136T (p.Arg46Ter) was detected by using targeted NGS. The patient was prescribed chemotherapy with temozolomide (TMZ) after the cutaneous lesions cured. Before the treatment was performed, catecholamines and metabolites were tested to reveal the following results: NMN: 891.39 nmol/L; MN: 0.52 nmol/L; 24-h urinary NE: 27255.7 μg/24 h; 24-h urinary E: 3.6 μg/24 h, 24-h urinary DA: 6907.8 μg/24 h. The patient was found to demonstrate multifocal abdomen, liver, and bone lesions on contrast-enhanced CT and 68Ga-DOTATATE-PET/CT. She was then put on TMZ at 150 mg/m2/day on days 1–5 in a 28-day cycle. In the second and subsequent cycles, she took TMZ at 200 mg/m2/day (on a 5/28-day regimen). After 6 cycles, the levels of catecholamines and metabolites decreased significantly (NMN: 286.35 nmol/L; MN: 0.21 nmol/L; 24-h urinary NE: 7487.5 μg/24 h; 24-h urinary E: 3.8 μg/24 h, 24-h urinary DA: 323.7 μg/24 h), and CT suggested that the lesions have diminished in some degree. The patient tolerated TMZ well, and only suffered from mild nausea during the medication. No bone marrow suppression and abnormal liver and kidney functions were recorded.
Researches showed that paraneoplastic vascular disorders could be found on PPGL patients. We reviewed the cases of PPGL demonstrating cutaneous involvement in the previous studies (Table 1). In half a century, reports on PPGL-associated cutaneous disorders have been scanty across the globe, and the cutaneous lesions manifesting as purpura, erythema, or even partial necrosis tend to involve limbs, hands, and feet. Some studies reported that the outcomes of patients after removal of the PPGL tumors were remarkably favorable with a complete regression of the cutaneous lesions (3–7).
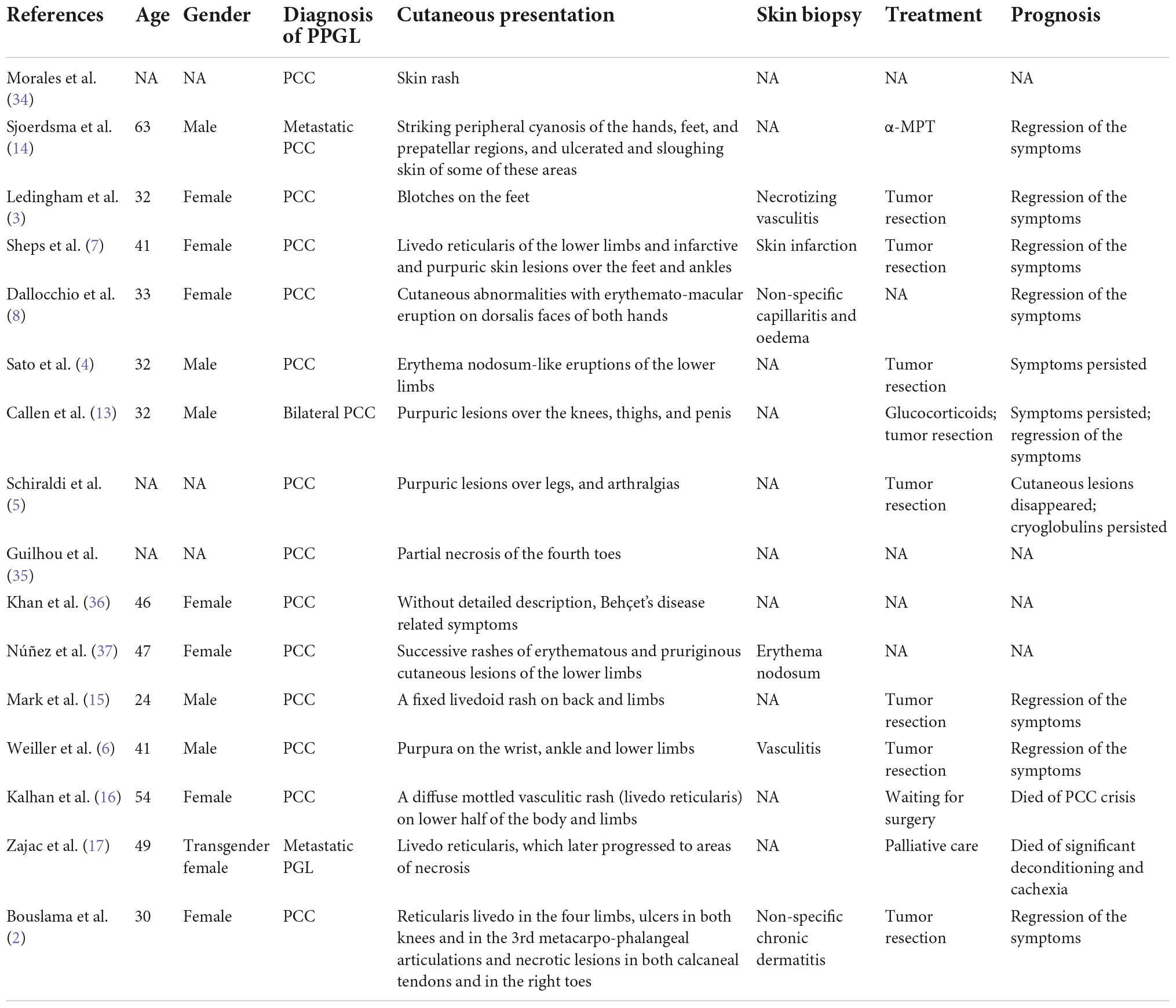
Table 1. Summary of the cases of pheochromocytoma/paraganglioma with cutaneous involvement in the literatures.
The diagnosis of cutaneous lesions on some patients was PPGL-related cutaneous vasculitis, which suggested to be induced by catecholamine excess (6, 8). In addition to the skin involvement, catecholamine has been reported to be associated with the pathogenesis of large vessel vasculitis, such as aortoarteritis and the central nervous system (CNS) vasculitis (9–12). The potential mechanism behind this may be that the catecholamine excess status results in direct damage to the vessel wall as well as triggering of autoimmune mechanism, both of which act in conjunction to produce vasculitis in large and small blood vessels (11, 13). However, the cutaneous pathology of most cases was absent, and only two patients we reviewed presented with vasculitis by the skin biopsy.
Except for the surgical removal of the tumor, drugs that inhibit catecholamine secretion or treat vasculitis may have a certain effect on the cutaneous lesions. Engelman et al. have presented a metastatic PPGL patient with cutaneous lesions responded well to alpha-methyltyrosine (α-MPT), an inhibitor of catecholamine biosynthesis (14). In the previous studies, cases with catecholamine-induced vasculitis have been treated with glucocorticoids or/and cyclophosphamide and achieved improvement in the symptoms, although their final resolution was obtained from the excision of the PPGL tumors (9, 12).
However, there were still some cases with cutaneous lesions that were difficult to explain by catecholamine-induced vasculitis. Researchers reported several PPGL cases suffered from the livedo reticularis (2, 7, 15–17), and the potential mechanism was considered as the hypercoagulability and platelet aggregation which induced by the tumors of PPGL (18, 19). However, the evidence to support the diagnosis was limited. Except for two patients with pathological description as non-specific chronic dermatitis and skin infarction, respectively, there was no definite pathological diagnosis of other patients’ cutaneous lesions. Meanwhile, no experience of drug treatment in how to treat the PPGL-related cutaneous lesions caused by hypercoagulability has been found.
In the previous studies, arteriovenous thrombosis, pulmonary embolism, and ventricular thrombus have also occurred in PPGL patients, wherein PPGL-related hypercoagulability was considered as the causative mechanism (20–26). And after anticoagulant dabigatran or heparin therapy, the thrombus could disappear, showing that anticoagulant drugs might be effective on the hypercoagulability of PPGL (27–33).
The cutaneous biopsy pathology of the present patient was definite and it showed that there was obvious angioectasia in the dermal without leukocytoclasis or true vasculitis, which was considered as the vascular disease caused by hypercoagulability and platelet aggregation. On the other hand, patients with vasculitis may response to the treatment of glucocorticoids or immunosuppressants, but it is ineffective for the present patient. Her cutaneous lesions were eventually relieved and even healed gradually with the aspirin antiplatelet therapy, which was consistent with the therapeutic response of cutaneous vascular disease. We used the antiplatelet therapy with aspirin to treat the PPGL-associated cutaneous vascular disease for the first time, and for metastatic PPGL patients like the case we reported, the early antiplatelet therapy might be effective to the cutaneous lesions caused by the hypercoagulable state of PPGL.
In summary, we presented a metastatic paraganglioma patient with severe cutaneous vascular disease identified by skin pathology, who demonstrated gradual regression of the symptoms after the antiplatelet therapy. PPGL-related cutaneous vascular involvement is extremely rare, which may be resolved after tumor resection. For patients who could not receive the surgery, antiplatelet therapy is effective on cutaneous lesions induced by the hypercoagulable state of PPGL.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AT and YL designed the study. YG, YC, and ZH collected the clinical data. YC, YW, and TL participated in the diagnosis and treatment of the patient. YG and AT wrote the manuscript. All authors read and approved the final manuscript.
This work was supported by the CAMS Innovation Fund for Medical Sciences (grant numbers: 2021-I2M-C&T-B-002 and 2017-I2M-1-001) and National High Level Hospital Clinical Research Funding (2022-PUMCH-C-028).
The authors are grateful to the case patient who agreed to participate in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Manger WM. The protean manifestations of pheochromocytoma. Horm Metab Res. (2009) 41:658–63. doi: 10.1055/s-0028-1128139
2. Toujani S, Abida R, El Ouni A, Belhassen A, Abdelkefi C, Meddeb Z, et al. Pheochromocytoma presenting as an authentic small vessel vasculitis and complicated with pulmonary embolism: an original presentation. Ann Cardiol Angeiol (Paris). (2021) 70:168–70. doi: 10.1016/j.ancard.2021.04.006
3. Silburn M, Macmillan DC, Vickers HR, Ledingham JG. Phaeochromocytoma with livedo reticularis. Proc R Soc Med. (1971) 64:1193–4.
4. Oishi S, Koga B, Sasaki M, Umeda T, Sato T. Pheochromocytoma associated with Behçet’s disease. Jpn J Clin Oncol. (1989) 19:283–6.
5. Perrone A, Guida G, Leuci D, Schiraldi O. [Cutaneous vasculitis, mixed cryoglobulinemia in a patient with non-secreting monolateral pheochromocytoma. A likely paraneoplastic syndrome]. Recenti Prog Med. (1995) 86: 499–502.
6. Sovaila S, Belenotti P, Ené N, Sebag F, Serratrice J, Weiller PJ. [Favorable evolution of a cutaneous vasculitis after pheochromocytoma excision]. Presse Med. (2013) 42:113–4. doi: 10.1016/j.lpm.2012.01.017
7. Radtke WE, Kazmier FJ, Rutherford BD, Sheps SG. Cardiovascular complications of pheochromocytoma crisis. Am J Cardiol. (1975) 35:701–5. doi: 10.1016/0002-9149(75)90060-0
8. Dany F, Doutre MS, Coupillaud G, Beylot C, Dallocchio M. [Cutaneous manifestation of pheochromocytoma. Report of a case (author’s transl)]. Sem Hop. (1981) 57:1223–5.
9. Townsend RR, McGinnis PA, Tuan WM, Thrasher K. Case report: bilateral adrenal pheochromocytoma. Am J Med Sci. (1994) 308:123–5. doi: 10.1097/00000441-199408000-00013
10. Razavi M, Bendixen B, Maley JE, Shoaib M, Zargarian M, Razavi B, et al. CNS pseudovasculitis in a patient with pheochromocytoma. Neurology. (1999) 52:1088–90. doi: 10.1212/wnl.52.5.1088
11. Sarathi V, Lila AR, Bandgar TR, Shah NS. Aortoarteritis: could it be a form of catecholamine-induced vasculitis? Indian J Endocrinol Metab. (2013) 17:163–6. doi: 10.4103/2230-8210.107874
12. Rupala K, Mittal V, Gupta R, Yadav R. Atypical presentation of pheochromocytoma: central nervous system pseudovasculitis. Indian J Urol. (2017) 33:82–4. doi: 10.4103/0970-1591.195760
13. Kulp-Shorten CL, Rhodes RH, Peterson H, Callen JP. Cutaneous vasculitis associated with pheochromocytoma. Arthritis Rheum. (1990) 33:1852–6. doi: 10.1002/art.1780331215
14. Engelman K, Horwitz D, Jéquier E, Sjoerdsma A. Biochemical and pharmacologic effects of alpha-methyltyrosine in man. J Clin Invest. (1968) 47:577–94. doi: 10.1172/jci105754
15. Buckley SA, Lessing JN, Mark NM. Livedo reticularis in a patient with pheochromocytoma resolving after adrenalectomy. J Clin Endocrinol Metab. (2013) 98:439–40. doi: 10.1210/jc.2012-2842
16. Shrikrishnapalasuriyar N, Noyvirt M, Evans P, Gibson B, Foden E, Kalhan A. Livedo reticularis: a cutaneous clue to an underlying endocrine crisis. Endocrinol Diabetes Metab Case Rep. (2018) 2018:17–179. doi: 10.1530/edm-17-0170
17. Frydman AS, Nolan BJ, Zajac JD. Intestinal pseudo-obstruction and livedo reticularis: rare manifestations of catecholamine excess. Am J Med. (2020) 133:e526–7. doi: 10.1016/j.amjmed.2019.12.030
18. Nakada K, Enami T, Kawada T, Hoson M, Wakisaka M, Mochizuki A, et al. Characterization of platelet activity in neuroblastoma. J Pediatr Surg. (1994) 29:625–9. doi: 10.1016/0022-3468(94)90727-7
19. Danta G. Pre- and postoperative platelet adhesiveness in pheochromocytoma. Thromb Diath Haemorrh. (1970) 23:189–90.
20. Vindenes T, Crump N, Casenas R, Wood K. Pheochromocytoma causing cardiomyopathy, ischemic stroke and acute arterial thrombosis: a case report and review of the literature. Conn Med. (2013) 77:95–8.
21. Yang CY, Chou CW, Lin MB, Lin CN, Wang LW, Chen SY, et al. Recurrent pulmonary embolism in an elderly patient with Cushing’s syndrome, adrenocortical adenoma, pheochromocytoma and prostate adenocarcinoma. J Chin Med Assoc. (2004) 67:360–5.
23. Yebra Yebra M, Martín Asenjo R, Arrue I, Paz Yepes M, Bastante Valiente MT, Prieto S. [Acute myocardial ischemia and ventricular thrombus associated with pheochromocytoma]. Rev Esp Cardiol. (2005) 58:598–600.
24. Stevenson S, Ramani V, Nasim A. Extra-adrenal pheochromocytoma: an unusual cause of deep vein thrombosis. J Vasc Surg. (2005) 42:570–2. doi: 10.1016/j.jvs.2005.05.013
25. Stella P, Bignotti G, Zerbi S, Ciurlino D, Landoni C, Fazio F, et al. Concurrent pheochromocytoma, diabetes insipidus and cerebral venous thrombosis – a possible unique pathophysiological mechanism. Nephrol Dial Transplant. (2000) 15:717–8. doi: 10.1093/ndt/15.5.717
26. Shulkin BL, Shapiro B, Sisson JC. Pheochromocytoma, polycythemia, and venous thrombosis. Am J Med. (1987) 83:773–6. doi: 10.1016/0002-9343(87)90913-2
27. Chen R, Wang Y, Yang J, Cheng X, Wang J, Huang L. Recurrent pheochromocytoma with catecholamine cardiomyopathy and left ventricular thrombus: a case report. J Int Med Res. (2021) 49:3000605211007723. doi: 10.1177/03000605211007723
28. Shafiq A, Nguyen P, Hudson MP, Rabbani B. Paraganglioma as a rare cause of left ventricular thrombus in the setting of preserved ejection fraction: discussing the literature. BMJ Case Rep. (2013) 2013:bcr2013202001. doi: 10.1136/bcr-2013-202001
29. Buchbinder NA, Yu R, Rosenbloom BE, Sherman CT, Silberman AW. Left ventricular thrombus and embolic stroke caused by a functional paraganglioma. J Clin Hypertens (Greenwich). (2009) 11:734–7. doi: 10.1111/j.1751-7176.2009.00182.x
30. Hou R, Leathersich AM, Ruud BT. Pheochromocytoma presenting with arterial and intracardiac thrombus in a 47-year-old woman: a case report. J Med Case Rep. (2011) 5:310. doi: 10.1186/1752-1947-5-310
31. Heindel SW, Maslow AD, Steriti J, Mashikian JS. A patient with intracardiac masses and an undiagnosed pheochromocytoma. J Cardiothorac Vasc Anesth. (2002) 16:338–43. doi: 10.1053/jcan.2002.124144
32. Wiyono SA, Vletter WB, Soliman OI, ten Cate FJ, Geleijnse ML. Thrombus in a normal left ventricle: a cardiac manifestation of pheochromocytoma. Echocardiography. (2010) 27:195–7. doi: 10.1111/j.1540-8175.2009.01041.x
33. Zhou W, Ding SF. Concurrent pheochromocytoma, ventricular tachycardia, left ventricular thrombus, and systemic embolization. Intern Med. (2009) 48:1015–9. doi: 10.2169/internalmedicine.48.2022
34. Moorhead EL, II, Caldwell JR, Kelly AR, Morales AR. The diagnosis of pheochromocytoma. Analysis of 26 cases. JAMA. (1966) 13:1107–13. doi: 10.1001/jama.1966.03100260045014
35. Bessis D, Dereure O, Le Quellec A, Ciurana AJ, Guilhou JJ. [Pheochromocytoma manifesting as toe necrosis]. Ann Dermatol Venereol. (1998) 3:185–187.
Keywords: pheochromocytoma/paraganglioma, diffuse erythema, tumor-associated cutaneous vascular disorders, antiplatelet therapy, hypercoagulability
Citation: Gao Y, Cui Y, Hu Z, Wang Y, Li T, Liu Y and Tong A (2022) Case report: Antiplatelet therapy on metastatic paraganglioma-associated cutaneous vascular disease and literature review. Front. Med. 9:1065350. doi: 10.3389/fmed.2022.1065350
Received: 12 October 2022; Accepted: 28 October 2022;
Published: 17 November 2022.
Edited by:
Giusto Trevisan, University of Trieste, ItalyReviewed by:
Salvino Bilancini, Institute of Angiology Jean Francois Merlen, ItalyCopyright © 2022 Gao, Cui, Hu, Wang, Li, Liu and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anli Tong, dG9uZ2FsQHB1bWNoLmNu, dG9uZ2FubGlAaG90bWFpbC5jb20=; orcid.org/0000-0002-1418-1012; Yuehua Liu, eXVlaHVhbGl1NjNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.