
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 12 January 2023
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1065045
 Duc Trong Quach1,2*
Duc Trong Quach1,2* Bang Hong Mai3*
Bang Hong Mai3* Mien Kieu Tran1
Mien Kieu Tran1 Long Van Dao4
Long Van Dao4 Huy Van Tran5
Huy Van Tran5 Khanh Truong Vu6
Khanh Truong Vu6 Khien Van Vu3
Khien Van Vu3 Ho Thi-Thu Pham4
Ho Thi-Thu Pham4 Hoang Huu Bui1
Hoang Huu Bui1 Dung Dang-Quy Ho7
Dung Dang-Quy Ho7 Dung Tuan Trinh6
Dung Tuan Trinh6 Vinh Thuy Nguyen8
Vinh Thuy Nguyen8 Thai Hong Duong9
Thai Hong Duong9 Tuong Thi-Khanh Tran10
Tuong Thi-Khanh Tran10 Ha Thi-Viet Nguyen4
Ha Thi-Viet Nguyen4 Thinh Tien Nguyen3
Thinh Tien Nguyen3 Thang Duy Nguyen11
Thang Duy Nguyen11 Long Cong Nguyen12
Long Cong Nguyen12 Hang Viet Dao4
Hang Viet Dao4 Ky Doan Thai3
Ky Doan Thai3 Nam Trung Phan5
Nam Trung Phan5 Ly Thanh Le7
Ly Thanh Le7 Cong Hong-Minh Vo2
Cong Hong-Minh Vo2 Phat Tan Ho7
Phat Tan Ho7 Tung Lam Nguyen3
Tung Lam Nguyen3 Quang Dinh Le1
Quang Dinh Le1 Nho Viet Le13
Nho Viet Le13 Hoan Quoc Phan3
Hoan Quoc Phan3 Binh Canh Nguyen3
Binh Canh Nguyen3 Trung Thien Tran1
Trung Thien Tran1 Tu Viet Tran14
Tu Viet Tran14 Long Ta3
Long Ta3Helicobacter pylori (H. pylori) infection is prevalent and has a rapidly increasing antibiotic resistance rate in Vietnam. Reinfection is quite common, and gastric carcinoma remains one of the most common malignancies, which is not uncommon to develop after successful eradication. The purpose of this consensus is to provide updated recommendations on the management of H. pylori infection in the country. The consensus panel consisted of 32 experts from 14 major universities and institutions in Vietnam who were invited to review the evidence and develop the statements using the Delphi method. The process followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. The consensus level was defined as ≥80% for agreement on the proposed statements. Due to the limited availability of high-quality local evidence, this consensus was also based on high-quality evidence from international studies, especially those conducted in other populations in the Asia–Pacific region. The panel finally reached a consensus on 27 statements after two voting rounds, which consisted of four sections (1) indications for testing and selection of diagnostic tests (2), treatment regimens, (3) post-treatment confirmation of H. pylori status, and (4) reinfection prevention methods and follow-up after eradication. Important issues that require further evidence include studies on third-line regimens, strategies to prevent H. pylori reinfection, and post-eradication follow-up for precancerous gastric lesions. We hope this consensus will help guide the current clinical practice in Vietnam and promote multicenter studies in the country and international collaborations.
Helicobacter pylori (H. pylori) is one of the most common causes of bacterial infections in humans, accounting for over 50% of the world’s population (1). Currently, H. pylori gastritis is considered an infectious disease even when it does not cause symptoms or complications (2). Vietnam is one of the countries with the highest rate of H. pylori infection and H. pylori-induced gastrointestinal diseases in Southeast Asia (3). In Vietnam, the first consensus on managing H. pylori infection was developed in 2012 (published in Vietnamese) with recommendations focused on diagnosis and treatment. In the past 10 years, the prevalence of antibiotic-resistant H. pylori species has been increasing rapidly, and gastric cancer remains one of the most common and deadly cancers in the country, with the majority of patients being diagnosed at advanced stages (3, 4). Therefore, updating the consensus is an urgent need to guide local clinical practice. This consensus provides recommendations on H. pylori diagnosis and treatment as well as reinfection prevention and follow-up strategies after H. pylori eradication.
This consensus was developed following the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (5). The recommendations in this consensus, prepared by an expert from the Vietnam Association of Gastroenterology, cover issues in four areas: (1) indication and selection of diagnostic tests; (2) H. pylori eradication therapies; (3) testing H. pylori status after eradication; and (4) reinfection prevention and follow-up after eradication. The drafted statements and supporting evidence were revised by core members and emailed to all panel members 4 weeks before the first virtual meeting. The Delphi method was used to develop consensus. All panel members graded the level of evidence, evaluated the level of agreement and the strength of recommendations for all statements based on the GRADE system, and voted via an electronic voting system. They might also suggest additional key references to assess the level of evidence. Regarding the level of consensus, each member will choose one of the following six levels: (1) accept completely (2), accept with some reservation (3), accept with major reservation (4), reject with some reservation (5), reject with major reservation; or (6) reject completely. A statement was approved if the consensus level (calculated based on the total votes at levels 1 and 2) reached ≥80%. The voting members were requested to explain the reasons for the votes that were not at level 1 or 2. Statements that had not reached consensus were revised and discussed in two virtual meetings held on 3 April 2022 and 29 May 2022. Those reaching consensus ≥80% after two voting rounds were used to develop the consensus. The strength of recommendations was rated on two levels: Strong and weak. Statements that received ≥80% of the votes as strong recommendations were considered strong, and the remaining statements were considered weak.
There were 27 consensus statements which are summarized in Table 1. The algorithms for diagnosing and eradicating H. pylori infection are also proposed in this consensus (Figures 1, 2).
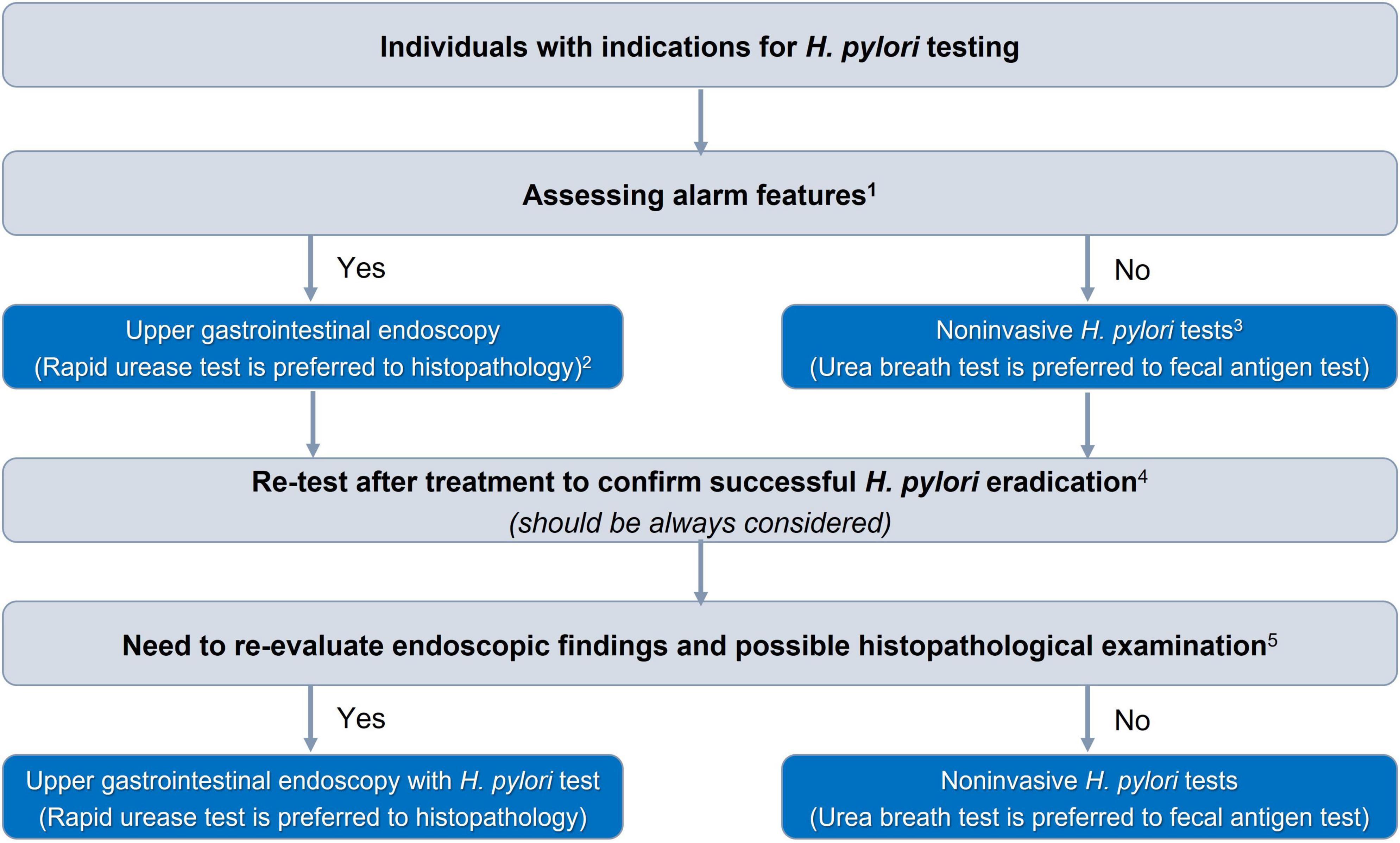
Figure 1. Algorithm for selection of Helicobacter pylori diagnostic test. 1The alarm features are presented in Table 2. 2In patients with acute upper gastrointestinal bleeding, rapid urease test and histopathology can be falsely negative, and H. pylori infection should be confirmed after stabilizing gastrointestinal bleeding. 3Serum antibody test should not be used. 4Patients must stop taking antibiotics or bismuth for at least 4 weeks and PPIs for at least 2 weeks. 5Patients diagnosed with gastric ulcers, suspected malignant gastric lesions, or gastric precancerous lesions need further evaluation of their extent and severity.
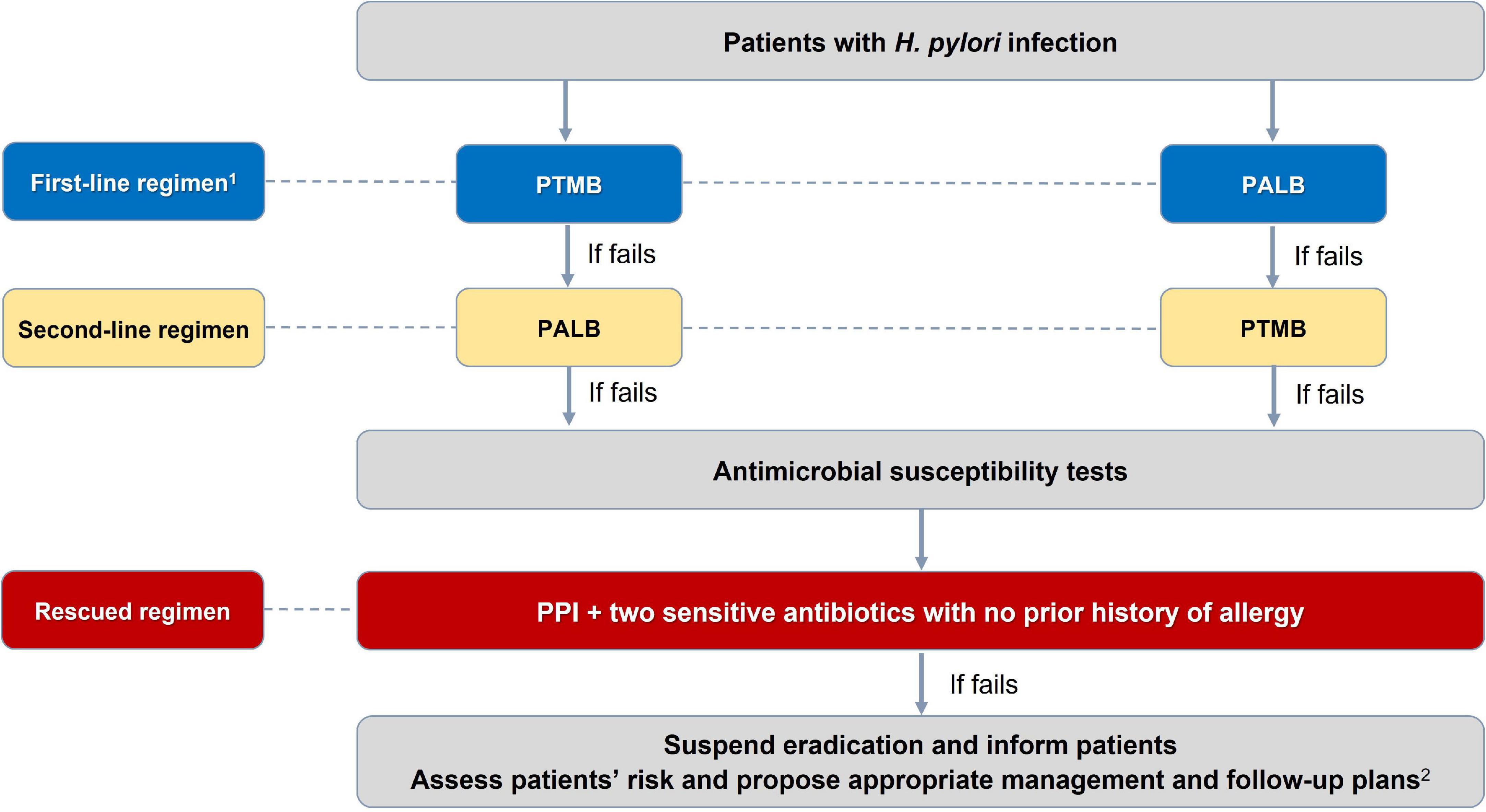
Figure 2. Algorithm for selection of Helicobacter pylori eradication regimen. 1PTMB is preferred over PALB because of its more stable and higher efficacy. PALB is also not used for people allergic to penicillin. 2For patients with H. pylori-induced peptic ulcer diseases in whom H. pylori have not been successfully eradicated, maintenance anti-secretory therapy is needed. And those with gastric precancerous lesions need appropriate endoscopic follow-up plans to detect early gastric cancer. PTMB: PPI + Tetracycline + Metronidazole + Bismuth; PALB: PPI + Amoxicillin + Levofloxacine + Bismuth. All treatment regimens are in 14 days.
I. Indications and selection of diagnostic tests
Statement 1. In clinical practice, diagnostic testing for H. pylori should be indicated only when eradication therapy is intended.
Evidence level: Low,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: H. pylori infection is a common bacterial infection, but only approximately 10% of patients with the infection progress to peptic ulcer disease, and 1–3% progress to gastric cancer (6). Vietnam has a very high rate of H. pylori infection, with almost two-thirds of the population having positive H. pylori serum antibody test results (3, 7). Meanwhile, antibiotic resistance tends to rapidly increase, and there are currently no effective measures to prevent reinfection (4). Consequently, population-based massive screening and eradication are not currently considered in the country.
Statement 2. H. pylori infection diagnosis is recommended in subjects with the following conditions (Table 1).
Generally, all indications for H. pylori diagnostic testing with a high level of evidence from the Maastricht V consensus and the Bangkok consensus on H. pylori management in ASEAN countries have been adopted in this consensus (8, 9). These indications include peptic ulcer disease, prior history of peptic ulcer disease but never tested for H. pylori infection, uninvestigated dyspepsia, precancerous gastric lesions, after endoscopic resection of early gastric cancer, low-grade MALT lymphoma, having first-degree relatives diagnosed with gastric cancer or starting and being planned to use long-term non-steroidal anti-inflammatory drugs (Table 1).
Long-term low-dose aspirin therapy: Peptic ulcer disease occurs in approximately 10% of patients taking low-dose aspirin, most of whom are asymptomatic. Risk factors include age ≥60 years and H. pylori infection (10). The risk of peptic ulcer bleeding is almost doubled in patients with H. pylori infection, but the number needed to treat to prevent one bleeding case per year is very high, ranging from 100 to 1,000 (11). In patients with a prior history of gastrointestinal bleeding, H. pylori eradication markedly reduces recurrent gastrointestinal bleeding and should be considered (12). However, for those who have never had such a history, the costs and benefits of treatment should be carefully considered.
Gastroesophageal reflux disease requiring long-term maintenance therapy with proton pump inhibitors (PPIs): Gastric cancer is one of the most common malignancies in Vietnam, and the majority of H. pylori infections in Vietnam are virulent strains (3, 13). Long-term treatment with PPIs in patients with H. pylori infection may promote corpus-predominant gastritis and atrophy, the two high-risk conditions for gastric cancer development that can be effectively prevented if H. pylori is eradicated early (14).
Unexplained iron deficiency anemia: A meta-analysis of 16 randomized controlled trials compared the outcomes of two groups of iron-deficient H. pylori-infected patients treated with H. pylori eradication plus iron supplementation and with iron supplementation alone. The study showed that hemoglobin levels and iron deficiency improved significantly in the former group, especially in patients with moderate or severe anemia (15).
Idiopathic thrombocytopenic purpura: A meta-analysis of 6 controlled trials found that H. pylori eradication was effective in significantly increasing platelet counts (16). The study, however, was performed on only 241 patients, and the original trials did not clearly describe the randomization process. Therefore, well-designed studies with larger sample sizes are needed.
Individuals who wish to receive H. pylori eradication after careful explanation that the treatment is unnecessary. A meta-analysis mainly based on Asian studies reported that H. pylori eradication in asymptomatic subjects with H. pylori infection reduced the incidence of gastric cancer in regions with high gastric cancer prevalence (17). The best effect is achieved if eradication therapy is performed before mucosal atrophy and gastric metaplasia have occurred (2, 18). Given that gastric cancer is prevalent in Vietnam, individuals with H. pylori infection who wish to receive H. pylori eradication regimen are justified for the treatment. However, there are still important issues that need further investigation, including the age at which gastric precancerous lesions take off and the long-term adverse consequences on the gut microbiota due to early H. pylori eradication in Vietnamese individuals (8).
Statement 3. Patients with uninvestigated dyspepsia aged ≥35 years (in females) or ≥40 years (in males) and, or with alarming symptoms should undergo upper gastrointestinal endoscopy. The diagnosis of H. pylori infection in these patients should be performed using biopsy-based tests.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 87.5%,
Alarm symptoms (Table 2) are not highly sensitive in detecting upper gastrointestinal malignancies (UGIMs) (19, 20). Patient age is an important factor in identifying patients requiring upper gastrointestinal endoscopy. According to the 2011 Asian consensus on the management of dyspepsia, the age threshold to decide upper gastrointestinal endoscopy significantly varies across countries depending on the local prevalence of gastric cancer (21). A recent endoscopic database review of 472,744 Vietnamese patients with upper gastrointestinal symptoms found that there were 2,198 (0.4%) patients with UGIMs. In this study, there were 145 patients with UGIMs whose age was <40 years. Of these patients, 138 (95.2%) were diagnosed with gastric cancer. The age threshold of 35 in women and 40 in men helped to avoid missing UGIMs by 7.1% (95% CI, 5.2–9.4%) and 6.6% (95% CI, 3.3–5.4%), respectively. The age threshold of ≥40 years in women only helped to avoid missing UGIMs by 12.6% (95% CI, 10.1–15.5%) (22). Hormonal factors are suggested to explain why early onset gastric cancer (e.g., age <40 years) is more common in women than in men, but a clear explanation remains unresolved (23).
Statement 4. In patients with indications for H. pylori infection testing who undergo upper gastrointestinal endoscopy, the rapid urease test is the test of choice.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: Among the biopsy-based diagnostic tests available in Vietnam, the local rapid urease tests that have been validated should be the test of choice (24, 25). Compared to histopathology, culture, and polymerase chain reaction, these tests are more rapid, much cheaper and have acceptable sensitivity and specificity (except for culture) (26). The other diagnostic methods also require facilities and human resources that are not available in many Vietnamese hospitals.
Statement 5. Among non-invasive diagnostic tests for H. pylori infection, the urea breath test is considered the first choice.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: One meta-analysis showed that the urea breath test has a sensitivity and specificity of 96 and 93%, respectively (27). Local studies have shown that the urea breath test is as accurate as the rapid urease test (25, 28). There are currently few local data regarding the value of fecal antigen testing. One study in adult patients showed that the test had a sensitivity of 85.7% and a specificity of 71.4% (29). Another study in pediatric patients showed that the test was highly accurate, with a sensitivity and specificity of 96.6 and 94.9%, respectively (30).
Statement 6. It is necessary to ensure that patients have not taken any antibiotics or bismuth within 4 weeks or PPIs within 2 weeks before performing diagnostic tests for H. pylori.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 93.7%,
Comments: Diagnostic tests for H. pylori infection, which include the urea breath test, rapid urease test, and stool antigen test, can be false negative if patients have recently taken drugs that inhibit the growth of H. pylori, such as antibiotics, proton pump inhibitors, and bismuth (31, 32). It should be noted that antibiotics used to treat infections of other organs can also result in false negative tests.
Statement 7. In patients with acute upper gastrointestinal bleeding, H. pylori testing results using rapid urease test and histopathology can be falsely negative. If these tests are negative, the infection should be confirmed using another highly reliable test after stabilizing gastrointestinal bleeding.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 81.2%,
Comments: A meta-study showed that the sensitivity of biopsy-based tests, such as rapid urease test, histopathology, and culture, was significantly decreased in patients presenting with acute upper gastrointestinal bleeding (33). A positive result of H. pylori serology does not indicate an active infection, and unintended H. pylori eradication was reported in about 11% of infected individuals (34). Therefore, the test can be used as a screening tool only. Interestingly, this meta-analysis showed that the accuracy of the urea breath test was still above 90%. Another meta-study showed that repeating diagnostic tests for H. pylori at ≥4 weeks after stabilizing gastrointestinal bleeding detected significantly more patients with H. pylori infections (35). A local study of 171 patients presenting with peptic ulcer bleeding that used multiple sequential H. pylori tests reported that the rate of H. pylori infection was 94% (36).
II. Treatment regimens for H. pylori eradication
Statement 8. A regimen is considered effective and recommended only when its eradication rate is at least 80% (intention to treat). The choice of regimen by this consensus was based on: (1) the results from local clinical trials, (2) the results of high-quality studies conducted in other countries, and (3) the local experts’ experience.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: The Bangkok consensus states that the ideal eradication rate should be at least 90% (intention to treat) (9). However, local clinical trials in the last 5 years have shown that it is rare for eradication treatment to achieve such a high rate. The required eradication rate of ≥80% according to the WGO Guidelines is probably more appropriate for Vietnam (37). The choice of the regimen was mainly based on the findings of local clinical trials and recent international high-quality trials. The local experts’ experience was considered adjunctive support, especially in situations where the benefits may not outweigh the potential risks, such as the use of a rifabutin-based regimen as tuberculosis is frequent in Vietnam.
Statement 9. The primary resistance rates of clarithromycin and metronidazole are very high. The primary resistance rates of amoxicillin and levofloxacin are on the rise. However, the tetracycline resistance rate is still low and stable.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: The results of clinical trials conducted during the last 5 years in Vietnam showed that the primary resistance rates of clarithromycin and metronidazole were very high, up to 34.1 and 69.4%, respectively (4, 38). Recent data also show that the resistance rates of amoxicillin and levofloxacin tend to increase significantly (39, 40). More importantly, there is an emerging increase in multidrug-resistant H. pylori strains, which poses a considerable challenge for eradication therapy in clinical practice (41, 42).
Statement 10. Non-adherence to treatment is one of the main causes leading to eradication failure. Spending time counseling and explaining the possible side effects of drugs in eradication regimens can help improve treatment adherence and, consequently, the successful eradication rate.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: Non-adherence to treatment is one of the leading causes of treatment failure (43, 44). With the same regimen, the eradication rate in non-adherent patients was more than 25% lower than that of adherents (43). Factors affecting the patient’s adherence include complex regimen, long duration of treatment, and lack of instructions and information, especially about the side effects of drugs in the regimen (45). The 2021 WGO guidelines emphasize the use of medication leaflets with the information presented in both text and pictures (37).
Statement 11. Patients should be advised to neither smoke nor drink alcohol during H. pylori eradication therapy to avoid reducing eradication efficacy.
Evidence level: Moderate,
Strength of recommendation: Weak,
Consensus level: 93.7%,
Comments: A meta-analysis showed that smoking while on H. pylori eradication therapy could reduce the eradication rate of the same regimen by up to 8.4%. However, the exact mechanism was still not understood (46). Studies on the effect of alcohol consumption on the eradication of H. pylori have conflicting results (47, 48). However, it is not uncommon that some Vietnamese people to have a habit of drinking large amounts of alcohol, and being drunk may affect treatment adherence. In particular, when treated with regimens containing metronidazole or tinidazole, patients who drank alcohol during the treatment period are prone to severe side effects such as nausea, vomiting, headache, and flushing (49).
Statement 12. Good inhibition of acid secretion is one of the critical factors determining the effectiveness of H. pylori eradication regimens.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: PPIs are effective in eradicating H. pylori and enhancing the effectiveness of antibiotics (50). Recent studies have shown that regimens using esomeprazole and rabeprazole had a better eradication rate of H. pylori than first-generation PPIs (i.e., omeprazole, lansoprazole, and pantoprazole) (51, 52). The advantage of rabeprazole and esomeprazole compared to the first-generation PPIs may be due to their higher relative potencies on 24-h gastric pH (53). Indeed, these two PPIs are not concerned by the rapid metabolism due to the hepatic enzyme CYP-2C19, which occurs in some patients due to the genetic polymorphism of the enzyme (54). Dual therapy (PPI plus amoxicillin taken 3–4 times per day) has been reported to be effective in some regions. However, its efficacy needs to be further studied in Vietnam (55). There is emerging evidence that eradication regimens containing potassium-competitive acid secretion inhibitors (PCABs) may be more effective than PPI-based regimens (56, 57). However, PCABs are currently unavailable in Vietnam.
Statement 13. The optimal duration of all H. pylori eradication regimens recommended by this consensus is 14 days.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 93.7%,
Comments: For all H. pylori eradication regimens, it is recommended to prescribe in 14 days for the best eradication effect (9). Most local trials used 14-day regimens, which showed higher and more stable eradication rates than the shorter regimens (Supplementary material).
Statement 14. Selecting the first-line H. pylori eradication regimens.
A- The first choice regimen is PPI + Tetracycline + Metronidazole + Bismuth (PTMB).
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 100%,
B- The alternative first-line regimen is PPI + Amoxicillin + Levofloxacin + Bismuth (PALB).
Evidence level: Low,
Strength of recommendation: Strong,
Consensus level: 84.4%,
Comments: Vietnam has very high primary resistance rates to clarithromycin and metronidazole (4). There is strong evidence to use PTMB as a first-line regimen (8). Metronidazole resistance does not affect the eradication results in clinical practice if the antibiotic is used at a dose ≥1,500 mg/day and in combination with bismuth (58). Most clinical trials conducted during the last 5 years in Vietnam have shown that the success rate of the 14-day PTMB regimen was ≥90% (Supplementary material). This regimen is also suitable for patients who are allergic to penicillin. Some local trials also showed that the successful eradication rate of the 14-day levofloxacin-based triple regimen was ≥80%. The eradication rate of this regimen improved when bismuth was added, as reported in previous studies worldwide (59). The recommended doses of antibiotics and PPIs in eradication regimens are summarized in Table 3. Concomitant and sequential regimens are not recommended as first-line regimens, as the eradication rate was lower than that of the two abovementioned regimens, and the evidence level was very low (Supplementary material).
C- The clarithromycin-based triple regimen should not be used due to the high failure rate.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: In Vietnam, the eradication rate of this regimen dropped from more than 90% in the 2000s to less than 70% in the 2010s (4). It may further decrease due to the increasing prevalence of clarithromycin-resistant strains. Consequently, clarithromycin should not be considered in the eradication regimen unless antibiotic susceptibility tests show it is sensitive.
Statement 15. Selecting the second-line H. pylori eradication regimens.
A- Use the PTMB regimen if it has not been used as a first-line regimen.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 100%,
B- Use the PALB regimen if PTMB has been used as a first-line regimen.
Evidence level: Low,
Strength of recommendation: Strong,
Consensus level: 90.6%,
Comments: Several local clinical trials showed that the eradication rate (per protocol) of the 14-day PTMB regimen ranged from 86.7 to 97.6% (Supplementary material). There are few trials on the 14-day PALB regimen. However, the findings suggest that it is also a good option, with an eradication rate of 93.1% in patients whose eradication was unsuccessful with PTMB. Studies on dual regimens have yielded inconsistent results, and further studies are needed (Supplementary material).
Statement 16. Selecting salvage regimens after two failed eradication attempts.
A- The PTMB regimen should be considered if it has not been used.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 100%,
B- If the PTMB regimen has been used, antibiotic susceptibility tests should be performed.
Evidence level: Low,
Strength of recommendation: Strong,
Consensus level: 84.3%,
Comments: Most of the clinical trials in Vietnam showed that the 14-day PTMB regimen was still effective, with an eradication rate of over 90% if it had not been used. However, one clinical trial reported that the 10-day PTMB regimen has an eradication rate of only 78.9% (Supplementary material). In another open-label non-randomized control trial conducted on patients who had failed eradication twice and PTMB had not been used, two arms of treatment were compared: One with an empiric 14-day PTMB regimen and the other with a tailored 14-day regimen based on antibiotic susceptibility test results. The per-protocol eradication of the former was significantly higher than that of the latter (95.2 vs. 82.8%, p < 0.001) (60). The empiric approach was also less expensive.
Statement 17. The rifabutin-based eradication regimen should not be considered due to the complicated situation of antibiotic-resistant tuberculosis in Vietnam.
Evidence level: Very low,
Strength of recommendation: Strong,
Consensus level: 87.5%,
Comments: Vietnam has a high prevalence of tuberculosis, while the prevalence of H. pylori infection is also high, and the rate of secondary antibiotic resistance tends to increase rapidly (3, 4, 7, 61). Therefore, the rifabutin-based regimen should not be prescribed even if this drug is available in Vietnam. This statement has also been mentioned in the WGO guidelines 2021 and the Bangkok Consensus on the management of H. pylori in the Southeast Asia Nations 2018 (9, 37).
Statement 18. When patients have well adhered to the appropriate regimens but still do not have successful H. pylori eradication, eradication therapy should be suspended. Patients should be informed about appropriate management and follow-up plans until a new and effective eradication regimen is available.
Evidence level: Very low,
Strength of recommendation: Weak,
Consensus level: 81.2%,
Comments: As multidrug-resistant H. pylori strains have rapidly increased, the H. pylori infecting patients could be hardly eradicated (41, 60). Further eradication attempts in such patients are unlikely to be successful, potentially increasing secondary resistance and should be avoided. Instead, patients should be informed about appropriate management and follow-up plan until a new and effective regimen is available. For patients with H. pylori-induced peptic ulcer diseases in whom H. pylori have not been successfully eradicated, maintenance anti-secretory therapy is needed (37). Those with gastric precancerous lesions will need appropriate endoscopic follow-up plans to detect early gastric cancer (62).
III. Testing H. pylori status after eradication
Statement 19. Testing for H. pylori status should be performed in all patients who have received eradication therapy.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: In patients with H. pylori infection who fail eradication, H. pylori-induced gastric mucosal injuries may continue to progress, and complications such as peptic ulcer diseases, MALT lymphoma, and gastric carcinoma may eventually develop (2). In real-life practice, most cases of H. pylori infection are treated empirically without antibiotic susceptibility testing. Therefore, post-eradication confirmation helps to recognize changes in antibiotic resistance of the bacteria, thereby promptly re-evaluating the evidence and updating recommendations on empiric regimens.
Statement 20. Upper gastrointestinal endoscopy should be performed in patients with (1) gastric ulcers, (2) suspected malignant gastric lesions, or (3) gastric precancerous lesions, which need further evaluation of their extent and severity.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: Patients with H. pylori infection who have gastric ulcers or suspected malignant gastric lesions should have endoscopic re-evaluation after treatment. Approximately 5–10% of gastric ulcers that initially had benign biopsy results were confirmed to be malignant (63). Patients who need endoscopic follow-up include those with precancerous gastric lesions, post-endoscopic resection, early gastric cancer, and gastric adenoma. Rapid urease test has acceptable accuracy, and it is more rapid and much cheaper compared to other endoscopy-based H. pylori testing methods.
Statement 21. For individuals who have received H. pylori eradication but do not require endoscopic re-evaluation, urea breath tests should be the test of choice to confirm eradication.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: The 13C urea breath test is preferred to the 14C urea breath test for confirming H. pylori eradication in such situations. The latter test is cheaper but has the disadvantage of radiation exposure and cannot be used by children and pregnant women (8). The Maastricht V and the Bangkok consensuses recommend using stool antigen test as an alternative test (8, 9). However, there are few studies on the performance of fecal antigen testing in adult patients in Vietnam (29).
IV. Reinfection prevention and follow-up after eradication
Statement 22. H. pylori is a pathogenic bacterium capable of being transmitted from person to person, especially between members of the same family, through mouth-to-mouth, fecal-oral routes, and contaminated medical devices.
Evidence level: High,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: The prevalence of H. pylori infection in subjects living in the same Vietnamese family can be as high as 80% (61). The highest prevalence of H. pylori infection is in children <12 years of age and in subjects living in multigenerational families. A mother with H. pylori infection is the most important risk factor compared to other family members (64). However, other factors may also contribute to the risk of H. pylori infection, including living habits, environmental sanitation, and socioeconomic conditions (7, 65). The most common transmission routes are oral-oral, fecal-oral, and transmission among healthcare workers from contaminated equipment (66–68).
Statement 23. H. pylori reinfection and recrudescence are common in Vietnam.
Evidence level: Low,
Strength of recommendation: Strong,
Consensus level: 90.6%,
Comments: The recurrence of H. pylori after eradication can be reinfection or recrudescence. Reinfection is infection with a new strain of H. pylori, which is different from the strain previously eradicated. And recrudescence is the relapse of the original H. pylori strains temporarily suppressed by eradication therapy (69). Distinguishing between these two situations is quite important in clinical practice, as it relates to the selection of appropriate eradication regimens and to counseling to prevent reinfection. However, it is difficult to assess due to the requirement of culture and molecular typing of the strains. Two independent studies in Vietnam, conducted 15 years apart, showed a recurrence rate of H. pylori after 12 months of up to 23% (70, 71). A recent cohort study reported an H. pylori recurrence rate of 38.5% at 31-month follow-up (71).
Statement 24. Educating and raising public awareness about the potential sources and transmission routes of H. pylori can help reduce the risk of infection in the community.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 93.7%,
Comments: Most H. pylori infections occur in young children, whose mothers and direct caregivers are the most important sources of infection (61, 64, 72). Transmission of H. pylori to children appears to occur during meals. It is reported that the habit of feeding infants after chewing their food, which is very popular in rural areas in Vietnam, increased the risk of H. pylori infection (73). Sharing eating utensils favors oral-oral transmission, but carriage of H. pylori by chopsticks is probably very rare (74). The other main risk factors for H. pylori infection in children include living in crowded conditions and having a well as the source of home water (75, 76). In adults, H. pylori infection may be not associated with crowded living conditions (76). A recent systematic review reported that health professionals, individuals with soil-related occupations, and workers at institutions for the intellectually disabled showed a significantly higher prevalence of H. pylori infection than the general population (77).
Statement 25. In patients who have indications for upper gastrointestinal endoscopy and H. pylori biopsy-based tests, the assessment of the presence, severity, and extent of gastric precancerous lesions should always be considered.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 96.8%,
Comments: Precancerous gastric lesions are very frequent in Vietnamese patients presenting with dyspepsia (78, 79). Assessing the presence, severity, and extent of precancerous gastric lesions at the index endoscopy is crucial to stratify the risk of gastric cancer for an appropriate surveillance strategy. Image-enhanced endoscopy should be used as it provides a more accurate assessment of precancerous gastric lesions compared to white light endoscopy (80). For regions with limited resources, endoscopic assessment of gastric atrophy according to the Kimura–Takemoto classification can help identify subjects at high risk of gastric cancer who need to be followed up after H. pylori eradication (2, 80, 81). A recent study in Japan showed that the predictive value for gastric cancer development of this endoscopic classification was not inferior to well-validated histopathological classifications such as the operative link for gastritis assessment (OLGA) and operative link for gastric intestinal metaplasia (OLGIM) assessment (82).
Statement 26. All patients with severe and extensive gastric atrophy or gastric intestinal metaplasia or those with the incomplete subtype of gastric intestinal metaplasia should be followed up endoscopically after successful H. pylori eradication.
Evidence level: Moderate,
Strength of recommendation: Strong,
Consensus level: 100%,
Comments: The risk of gastric cancer development depends on the severity and extent of precancerous gastric lesions (2, 81–83). Gastric cancer may develop in some patients after successful eradication, especially in those who have already had extensive, severe precancerous gastric lesions or incomplete subtype of gastric intestinal metaplasia prior to eradication (2, 84). The time interval for endoscopically following up patients with precancerous lesions after eradication has been addressed in international guidelines, ranging from 1 to 3 years depending on other risk factors, such as a family history of gastric cancer, H. pylori infection status, and gastric intestinal metaplasia subtype (62, 85). Local evidence is limited, and further studies in Vietnamese are awaited.
Statement 27. All patients with gastric dysplasia detected at mapping biopsies should be endoscopically re-evaluated using image-enhanced endoscopes to establish appropriate surveillance and treatment.
Evidence level: Low,
Strength of recommendation: Strong,
Consensus level: 87.5%,
Comments: Approximately 2.5% of cases with low-grade gastric dysplasia were identified based on mapping biopsies without any endoscopically suspected findings. Some may present with gastric dysplasia in multiple biopsy sites in the same patient (86). Image-enhanced endoscopy is recommended for endoscopic re-evaluation of these patients due to its better ability to identify dysplastic lesions compared to white light endoscopy (62, 85). Dysplastic lesions that are endoscopically detected should be resected for accurate histopathology (62, 85). In cases where dysplastic lesions are not endoscopically detected, patients should be re-evaluated endoscopically after 6 months (for high-grade gastric dysplasia) or 12 months (for low-grade gastric dysplasia) (62, 85).
H. pylori is a common infection in Vietnam with a rapidly increasing antibiotic resistance rate. Gastric cancer is one of the most common malignancies in the country and can occur even after successful H. pylori eradication. This consensus provides recommendations on managing H. pylori infection and the post-eradicated follow-up strategies in Vietnam. Important clinical issues that require additional local evidence for future recommendations include effective third-line regimens, reinfection prevention methods, and specific post-eradicated follow-up plans for patients with gastric precancerous lesions. We hope that this consensus will be a helpful tool to guide clinical practice in Vietnam and promote international research collaborations.
DQ drafted all the statements and prepared the supporting literature and further revisions were done by BM, LT, MT, LD, KTV, and KVV. BM, LT, MT, LD, KTV, and DQ edited the revised statements. DQ drafted, critically revised, and submitted the manuscript. All authors approved the final version of the manuscript.
We want to thank Ms. Cao Thi Hoa, Dr. Luu Ngoc Mai, and Dr. Nguyen Thi Nha Doan for their help during the consensus development.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1065045/full#supplementary-material
1. Hooi J, Lai W, Ng W, Suen M, Underwood F, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
2. Sugano K, Tack J, Kuipers E, Graham D, El-Omar E, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. (2015) 64:1353–67. doi: 10.1136/gutjnl-2015-309252
3. Quach D, Vilaichone R, Vu K, Yamaoka Y, Sugano K, Mahachai V. Helicobacter pylori infection and related gastrointestinal diseases in southeast asian countries: An expert opinion survey. Asian Pac J Cancer Prev. (2018) 19:3565–9. doi: 10.31557/APJCP.2018.19.12.3565
4. Khien V, Thang D, Hai T, Duat N, Khanh P, Ha D, et al. Management of antibiotic-resistant Helicobacter pylori infection: Perspectives from Vietnam. Gut and Liver. (2019) 13:483–97. doi: 10.5009/gnl18137
5. Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
6. Wroblewski L, Peek R Jr., Wilson K. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. (2010) 23:713–39. doi: 10.1128/CMR.00011-10
7. Hoang T, Bengtsson C, Phung D, Sörberg M, Granström M. Seroprevalence of Helicobacter pylori Infection in Urban and Rural Vietnam. Clin Vaccine Immunol. (2005) 12:81–5. doi: 10.1128/CDLI.12.1.81-85.2005
8. Malfertheiner P, Megraud F, O’Morain C, Gisbert J, Kuipers E, Axon A, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. (2017) 66:6–30. doi: 10.1136/gutjnl-2016-312288
9. Mahachai V, Vilaichone R, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Maneerattanaporn M, et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. (2018) 33:37–56. doi: 10.1111/jgh.13911
10. Yeomans N, Lanas A, Talley N, Thomson A, Daneshjoo R, Eriksson B, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. (2005) 22:795–801. doi: 10.1111/j.1365-2036.2005.02649.x
11. Ng J, Yeomans N. Helicobacter pylori infection and the risk of upper gastrointestinal bleeding in low dose aspirin users: systematic review and meta-analysis. Med J Aust. (2018) 209:306–11. doi: 10.5694/mja17.01274
12. Chan F, Ching J, Suen B, Tse Y, Wu J, Sung J. Effects of Helicobacter pylori infection on long-term risk of peptic ulcer bleeding in low-dose aspirin users. Gastroenterology. (2013) 144:528–35. doi: 10.1053/j.gastro.2012.12.038
13. Nguyen T, Uchida T, Tsukamoto Y, Trinh D, Ta L, Mai B, et al. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. (2010) 10:114. doi: 10.1186/1471-230X-10-114
14. Moayyedi P, Wason C, Peacock R, Walan A, Bardhan K, Axon A, et al. Changing patterns of Helicobacter pylori gastritis in long-standing acid suppression. Helicobacter. (2008) 5:206–14. doi: 10.1046/j.1523-5378.2000.00032.x
15. Yuan W, Li Y, Yang K, Ma B, Guan Q, Wang D, et al. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. (2010) 45:665–76. doi: 10.3109/00365521003663670
16. Kim B, Kim H, Jang H, Kim J. Helicobacter pylori eradication in idiopathic thrombocytopenic purpura: A meta-analysis of randomized trials. Gastroenterol Res Pract. (2018) 2018:6090878. doi: 10.1155/2018/6090878
17. Lee Y, Chiang T, Chou C, Tu Y, Liao W, Wu M, et al. Association Between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. (2016) 150:1113–24. doi: 10.1053/j.gastro.2016.01.028
18. Liou J, Malfertheiner P, Lee Y, Sheu B, Sugano K, Cheng H, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. (2020) 69:2093–112. doi: 10.1136/gutjnl-2020-322368
19. Bai Y, Li Z, Zou D, Wu R, Yao Y, Jin Z, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102 665 patients from 1996 to 2006. Gut. (2010) 59:722–8. doi: 10.1136/gut.2009.192401
20. Quach D, Ha D, Hiyama T. The endoscopic and clinicopathological characteristics of early-onset gastric cancer in vietnamese patients. Asian Pac J Cancer Prev. (2018) 19:1883–6.
21. Miwa H, Ghoshal U, Fock K, Gonlachanvit S, Gwee K, Ang T, et al. Asian consensus report on functional dyspepsia. J Gastroenterol Hepatol. (2012) 27:626–41. doi: 10.1111/j.1440-1746.2011.07037.x
22. Quach D, Tran L, Tran T, Tran V, Le N, Hiyama T, et al. Age cutoff and yield of prompt esophagogastroduodenoscopy to detect malignancy in vietnamese with upper gastrointestinal symptoms: An endoscopic database review of 472,744 patients from 2014 to 2019. Canad J Gastroenterol Hepatol. (2021) 2021:1184848. doi: 10.1155/2021/1184848
23. Chung H. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol. (2010) 16:256–63. doi: 10.3748/wjg.v16.i2.256
24. Quach D. Evaluation of a local rapid urease test for Helicobacter pylori diagnosis in comparison with Pyloritek. J Gastroenterol Hepatol. (2006) 21(Suppl.6):A391.
25. Quach D, Tran M. The agreement between 14C breath test and local rapid urease test in assessing Helicobacter pylori status after eradication. Vietnamese J Gastroenterol. (2008) 3:659–63.
26. Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: Invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. (2007) 21:299–313. doi: 10.1016/j.bpg.2006.11.002
27. Ferwana M. Accuracy of urea breath test in Helicobacter pylori infection: Meta-analysis. World J Gastroenterol. (2015) 21:1305–14. doi: 10.3748/wjg.v21.i4.1305
28. Tran T, Quach D, Ly K. The agreement among 13c- urea breath test, a local rapid urease test and a serological test in h. Pylori diagnosis. Hochiminh City J Med. (2009) 13(Suppl 1):18–23.
29. Nguyen V. Evaluation of the accuracy of the stool antigen test (HPSA) for the diagnosis of Helicobacter pylori. J Pract Med (Vietnamese). (2011) 764:43–5.
30. Nguyen T, Bengtsson C, Nguyen G, Granström M. Evaluation of a novel monoclonal-based antigen-in-stool enzyme immunoassay (Premier Platinum HpSA PLUS) for diagnosis of Helicobacter pylori infection in vietnamese children. Helicobacter. (2008) 13:269–73. doi: 10.1111/j.1523-5378.2008.00598.x
31. Bravo L, Realpe L, Campo C, Mera R, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter Pylori infection. Am J Gastroenterol. (1999) 94:2380–3. doi: 10.1111/j.1572-0241.1999.01361.x
32. Gatta L, Vakil N, Ricci C, Osborn J, Tampieri A, Perna F, et al. Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter Pylori infection. Am J Gastroenterol. (2004) 99:823–9. doi: 10.1111/j.1572-0241.2004.30162.x
33. Gisbert J, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: A systematic review and meta-analysis. Am J Gastroenterol. (2006) 101:848–63. doi: 10.1111/j.1572-0241.2006.00528.x
34. Hiyama T, Quach D, Le Q, Ho L, Vu N, Shimamoto F, et al. Rate of unintended Helicobacter pylori eradication in the vietnamese. Helicobacter. (2015) 20:156–7. doi: 10.1111/hel.12210
35. Sánchez-Delgado J, Gené E, Suárez D, García-Iglesias P, Brullet E, Gallach M, et al. Has H. pylori prevalence in bleeding peptic ulcer been underestimated? A meta-regression. Am J Gastroenterol. (2011) 106:398–405. doi: 10.1038/ajg.2011.2
36. Quach D, Luu M, Hiyama T, To T, Bui Q, Tran T, et al. Early diagnosis of Helicobacter pylori infection in vietnamese patients with acute peptic ulcer bleeding: A prospective study. Gastroenterol Res Pract. (2017) 2017:3845067. doi: 10.1155/2017/3845067
37. Katelaris P, Hunt R, Bazzoli F. World Gastroenterology Organisation Global Guidelines: Helicobacter pylori. (2021). Available online at: https://www.worldgastroenterology.org/guidelines/helicobacter-pylori/helicobacter-pylori-english (accessed January 5, 2022).
38. Tran V, Ha T, Le P, Nguyen V, Phan T, Paglietti B. Helicobacter pylori 23S rRNA gene mutations associated with clarithromycin resistance in chronic gastritis in Vietnam. J Infect Dev Ctries. (2018) 12:526–32. doi: 10.3855/jidc.10000
39. Tran T, Nguyen A, Quach D, Pham D, Cao N, Nguyen U, et al. Emergence of amoxicillin resistance and identification of novel mutations of the pbp1a gene in Helicobacter pylori in Vietnam. BMC Microbiol. (2021) 22:41. doi: 10.1186/s12866-022-02463-8
40. Van Thieu H, Duc N, Nghi B, Van Bach N, Khoi H, Tien V, et al. Antimicrobial resistance and the successful eradication of Helicobacter pylori-Induced Gastroduodenal Ulcers in Vietnamese Children. Med Arch. (2021) 75:112–5. doi: 10.5455/medarh.2021.75.112-115
41. Phan T, Santona A, Tran V, Tran T, Le V, Cappuccinelli P, et al. High rate of levofloxacin resistance in a background of clarithromycin- and metronidazole-resistant Helicobacter pylori in Vietnam. Int J Antimicrob Agents. (2015) 45:244–8. doi: 10.1016/j.ijantimicag.2014.10.019
42. Dang N, Ha T, Nguyen S, Le N, Nguyen T, Nguyen T, et al. High rates of clarithromycin and levofloxacin resistance of Helicobacter pylori in patients with chronic gastritis in the south east area of Vietnam. J Global Antimicrob Resist. (2020) 22:620–4. doi: 10.1016/j.jgar.2020.06.007
43. Graham D, Lew G, Malaty H, Evans D, Evans D Jr., Klein P, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. (1992) 102:493–6. doi: 10.1016/0016-5085(92)90095-G
44. Wermeille J, Cunningham M, Dederding J, Girard L, Baumann R, Zelger G, et al. Failure of Helicobacter pylori eradication: is poor compliance the main cause? Gastroenterol Clin Biol. (2002) 26:216–9.
45. O’Connor J, Taneike I, O’Morain C. Improving compliance with Helicobacter pylori eradication therapy: When and how? Therap Adv Gastroenterol. (2009) 2:273–9. doi: 10.1177/1756283X09337342
46. Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. (2006) 119:217–24. doi: 10.1016/j.amjmed.2005.10.003
47. Ozeki K, Asano M, Furuta T, Ojima T. Relationship between primary eradication of Helicobacter pylori and drinking habits in women: collaborative research between a pharmacy and a clinic. Epidemiol Infect. (2019) 147:e292. doi: 10.1017/S0950268819001730
48. Baena J, Lopez C, Hidalgo A, Rams F, Jimenez S, Garcia M, et al. Relation between alcohol consumption and the success of Helicobacter pylori eradication therapy using omeprazole, clarithromycin and amoxicillin for 1 week. Eur J Gastroenterol Hepatol. (2002) 14:291–6. doi: 10.1097/00042737-200203000-00014
49. Mergenhagen K, Wattengel B, Skelly M, Clark C, Russo T. Fact versus Fiction: A review of the evidence behind alcohol and antibiotic interactions. Antimicrob Agents Chemother. (2020) 64:e2167–2119. doi: 10.1128/AAC.02167-19
50. Labenz J. Current role of acid suppressants in Helicobacter pylori eradication therapy. Best Pract Res Clin Gastroenterol. (2001) 15:413–31. doi: 10.1053/bega.2001.0188
51. McNicholl A, Linares P, Nyssen O, Calvet X, Gisbert J. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. (2012) 36:414–25. doi: 10.1111/j.1365-2036.2012.05211.x
52. Xin Y, Manson J, Govan L, Harbour R, Bennison J, Watson E, et al. Pharmacological regimens for eradication of Helicobacter pylori: An overview of systematic reviews and network meta-analysis. BMC Gastroenterol. (2016) 16:80. doi: 10.1186/s12876-016-0491-7
53. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors—comparison of effects on intragastric pH. Eur J Clin Pharmacol. (2008) 65:19–31. doi: 10.1007/s00228-008-0576-5
54. El Rouby N, Lima J, Johnson J. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Exp Opin Drug Metab Toxicol. (2018) 14:447–60. doi: 10.1080/17425255.2018.1461835
55. Zhu Y, Zhang Y, Wang T, Zhao J, Zhao Z, Zhu J, et al. High dose PPI-amoxicillin dual therapy for the treatment of Helicobacter pylori infection: a systematic review with meta-analysis. Ther Adv Gastroenterol. (2020) 13:1756284820937115. doi: 10.1177/1756284820937115
56. Lyu Q, Pu Q, Zhong X, Zhang J. Efficacy and safety of vonoprazan-based versus proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: A meta-analysis of randomized clinical trials. BioMed Res Int. (2019) 2019:9781212. doi: 10.1155/2019/9781212
57. Shinozaki S, Kobayashi Y, Osawa H, Sakamoto H, Hayashi Y, Lefor Alan K, et al. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: Systematic review and meta-analysis. Digestion. (2021) 102:319–25. doi: 10.1159/000504939
58. Fischbach L, Evans E. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. (2007) 26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x
59. Dore M, Lu H, Graham D. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. (2016) 65:870–8. doi: 10.1136/gutjnl-2015-311019
60. Bui H, Le T, Luong A, Do T. Application of antimicrobial susceptibility testing and Cyp2c19 Polymorphism In Helicobacter pylori eradication in patients after treatment failure. Hochiminh City J Med. (2017) 21:120–9.
61. Dao L, Dao H, Nguyen H, Vu V, Tran A, Dat V, et al. Helicobacter pylori infection and eradication outcomes among Vietnamese patients in the same households: Findings from a non-randomized study. PLoS One. (2021) 16:e0260454. doi: 10.1371/journal.pone.0260454
62. Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. (2019) 51:365–88. doi: 10.1055/a-0859-1883
63. Stolte M, Seitter V, Müller H. Improvement in the quality of the endoscopic/bioptic diagnosis of gastric ulcers between 1990 and 1997 - an analysis of 1,658 patients. Z für Gastroenterol. (2001) 39:349–55. doi: 10.1055/s-2001-13709
64. Nguyen V, Nguyen G, Phung D, Okrainec K, Raymond J, Dupond C, et al. Intra-familial transmission of Helicobacter pylori infection in children of households with multiple generations in vietnam. Eur J Epidemiol. (2006) 21:459–63. doi: 10.1007/s10654-006-9016-y
65. Binh T, Tuan V, Dung H, Tung P, Tri T, Thuan N, et al. Molecular epidemiology of Helicobacter pylori infection in a minor ethnic group of vietnam: A multiethnic, population-based study. Int J Mol Sci. (2018) 19:708. doi: 10.3390/ijms19030708
66. Parsonnet J. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. (1999) 282:2240–5. doi: 10.1001/jama.282.23.2240
67. Thomas J, Gibson G, Darboe M, Weaver L, Dale A. Isolation of Helicobacter pylori from human faeces. Lancet. (1992) 340:1194–5. doi: 10.1016/0140-6736(92)92894-L
68. Nishikawa J, Kawai H, Takahashi A, Seki T, Yoshikawa N, Akita Y, et al. Seroprevalence of immunoglobulin G antibodies against Helicobacter pylori among endoscopy personnel in Japan. Gastrointest Endosc. (1998) 48:237–43. doi: 10.1016/S0016-5107(98)70184-1
69. Cameron E, Bell G, Baldwin L, Powell K, Williams S. Long-term study of re-infection following successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. (2006) 23:1355–8. doi: 10.1111/j.1365-2036.2006.02899.x
70. Wheeldon T, Hoang T, Phung D, Bjorkman A, Granstrom M, Sorberg M. Long-term follow-up of Helicobacter pylori eradication therapy in Vietnam: reinfection and clinical outcome. Aliment Pharmacol Ther. (2005) 21:1047–53. doi: 10.1111/j.1365-2036.2005.02408.x
71. Do N, Nguyen T, Nguyen T, Nguyen D. Re-infection and recurrence of H. pylori bacteria in duodenal ulcer after successful eradication at E Central Hospital. J Clin Med Pharmacy. (2019) 14:24–9.
72. Yucel O. Prevention of Helicobacter pylori infection in childhood. World J Gastroenterol. (2014) 20:10348–54. doi: 10.3748/wjg.v20.i30.10348
73. Albenque M, Tall F, Dabis F, Megraud F. Epidemiological study of Helicobactor pylori transmission from mother to child in Africa. Rev Esp Enf Dig. (1990) 78(suppl 1):48.
74. Leung W, Sung J, Ling T, Siu K, Cheng A. Does the use of chopsticks for eating transmit Helicobacter pylori? Lancet. (1997) 350:31. doi: 10.1016/S0140-6736(05)66240-X
75. Aguemon B, Struelens M, Massougbodji A, Ouendo E. Prevalence and risk-factors for Helicobacter pylori infection in urban and rural Beninese populations. Clin Microbiol Infect. (2005) 11:611–7. doi: 10.1111/j.1469-0691.2005.01189.x
76. Krueger W, Hilborn E, Converse R, Wade T. Environmental risk factors associated with Helicobacter pylori seroprevalence in the United States: a cross-sectional analysis of NHANES data. Epidemiol Infect. (2015) 143:2520–31. doi: 10.1017/S0950268814003938
77. Kheyre H, Morais S, Ferro A, Costa A, Norton P, Lunet N, et al. The occupational risk of Helicobacter pylori infection: a systematic review. Int Arch Occupat Environ Health. (2018) 91:657–74. doi: 10.1007/s00420-018-1315-6
78. Cu P, Huyen N, Luan T, Hung N, Hop T. Helicobacter Pylori and precancerous gastric lesions. Dig Endosc. (2001) 12:221–4. doi: 10.1046/j.1443-1661.2000.0048a.x
79. Quach D, Le H, Hiyama T, Nguyen O, Nguyen T, Uemura N. Relationship between endoscopic and histologic gastric atrophy and intestinal metaplasia. Helicobacter. (2013) 18:151–7. doi: 10.1111/hel.12027
80. Chiu P, Uedo N, Singh R, Gotoda T, Ng E, Yao K, et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut. (2019) 68:186–97. doi: 10.1136/gutjnl-2018-317111
81. Quach D, Hiyama T. Assessment of endoscopic gastric atrophy according to the kimura-takemoto classification and its potential application in daily practice. Clin Endosc. (2019) 52:321–7. doi: 10.5946/ce.2019.072
82. Kawamura M, Uedo N, Koike T, Kanesaka T, Hatta W, Ogata Y, et al. Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Dig Endosc. (2021) 34:508–16. doi: 10.1111/den.14114
83. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med. (2001) 345:784–9. doi: 10.1056/NEJMoa001999
84. Shichijo S, Uedo N, Michida T. Detection of Early Gastric Cancer after Helicobacter pylori Eradication. Digestion. (2022) 103:54–61.
85. Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, et al. British society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. (2019) 68:1545–75. doi: 10.1136/gutjnl-2018-318126
Keywords: consensus, guidelines, Helicobacter pylori, Vietnam, diagnosis, eradication, management
Citation: Quach DT, Mai BH, Tran MK, Dao LV, Tran HV, Vu KT, Vu KV, Pham HT-T, Bui HH, Ho DD-Q, Trinh DT, Nguyen VT, Duong TH, Tran TT-K, Nguyen HT-V, Nguyen TT, Nguyen TD, Nguyen LC, Dao HV, Thai KD, Phan NT, Le LT, Vo CH-M, Ho PT, Nguyen TL, Le QD, Le NV, Phan HQ, Nguyen BC, Tran TT, Tran TV and Ta L (2023) Vietnam Association of Gastroenterology (VNAGE) consensus on the management of Helicobacter pylori infection. Front. Med. 9:1065045. doi: 10.3389/fmed.2022.1065045
Received: 09 October 2022; Accepted: 21 December 2022;
Published: 12 January 2023.
Edited by:
Yeong Yeh Lee, Universiti Sains Malaysia (USM), MalaysiaReviewed by:
Byung-Wook Kim, Catholic University of Korea, Republic of KoreaCopyright © 2023 Quach, Mai, Tran, Dao, Tran, Vu, Vu, Pham, Bui, Ho, Trinh, Nguyen, Duong, Tran, Nguyen, Nguyen, Nguyen, Nguyen, Dao, Thai, Phan, Le, Vo, Ho, Nguyen, Le, Le, Phan, Nguyen, Tran, Tran and Ta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duc Trong Quach,  ZHJxdWFjaHRkQGdtYWlsLmNvbQ==; orcid.org/0000-0003-0141-921X; Bang Hong Mai,
ZHJxdWFjaHRkQGdtYWlsLmNvbQ==; orcid.org/0000-0003-0141-921X; Bang Hong Mai,  YmFuZ21oQGJlbmh2aWVuMTA4LnZu
YmFuZ21oQGJlbmh2aWVuMTA4LnZu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.