- Department of Nephrology, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
Acute oxalate nephropathy (AON), defined as the association between acute kidney injury (AKI) and the deposition of oxalate crystals in the renal parenchyma, is a rare complication of hyperoxaluria. We report a rare case of AON in an adult due to medicinal herbs intake leading to crystal-induced AKI. We recommend that a thorough medication history including the use of medicinal herbs, should be obtained for all patients with a rapid loss of kidney function, especially in the absence of known risk factors for AKI. The use of medicinal herbs with unknown oxalate contents would increase the risk of AON and should be avoided.
Introduction
Oxalate is originated endogenously from the metabolism of amino acids or exogenously from the intake of oxalate-rich foods (1). The kidney is the sole organ responsible for oxalate excretion (2). Excessive oxalate in the kidney causes the formation of insoluble calcium oxalate crystals (3). This, in turn, leads to a spectrum of kidney disorders, including nephrolithiasis, nephrocalcinosis, and acute oxalate nephropathy (AON) (4). AON can be observed with primary hyperoxaluria (PH) (5) and secondary hyperoxaluria (SH) (6), with the first being inborn errors of metabolism and the second a result of enteric hyperoxaluria or excessive oxalate intake.
Here, we describe a case of acute kidney injury (AKI) after excessive ingestion of Chinese medicinal herbs with previously normal renal function, with tubular deposition of oxalate crystals evident on renal biopsy.
Case
A 25-year-old male patient presented to the emergency room with recurrent episodes of lumbar pain, nausea, and vomiting in the preceding days. The patient was healthy and had no previous medical problems. A family history of urolithiasis was not noted. Laboratory workup revealed non-oliguric AKI with a serum creatinine of 237 μmol/L, elevated from a stable baseline of 84 μmol/L. Post-renal causes were excluded because the renal ultrasound did not show any signs of obstructive nephropathy or intra-renal or ureteral concrements. The patient was transferred to the nephrology department for further evaluation.
Except for the bilateral costovertebral angle and lower abdominal tenderness, the rest of the physical examination findings were unremarkable. Renal function was severely deteriorated with serum creatinine levels of 724 μmol/L and urea nitrogen level of 21.75 mmol/L. Electrolytes showed potassium of 4.86 mmol/L and sodium of 136 mmol/L. Laboratory findings demonstrated that parathyroid functions were within the normal range: calcium 2.41 mmol/L, phosphorus 1.43 mmol/L, and intact parathyroid hormone (PTH) 38.45 pg/ml (15–65 pg/ml). The liver function test results were unremarkable. Serological test revealed normal levels of immunoglobulin, complements and antinuclear antibodies. ANCA-antibodies and anti-GBM-antibodies were negative. His 24-h urine protein collection was normal (0.08 g/24 h), but the urine albumin-to-creatinine ratio was slightly elevated at 8.16 mg/mmol. Abdominal computed tomography (CT) were normal.
The calculated fractional excretion of sodium (FeNa) was 1.6% and renal function did not improve upon intravenous volume challenge. As such, pre-renal AKI is unlikely. Since the etiology of AKI remains unknown, a renal biopsy was performed, which showed features of severe acute tubular necrosis without infiltration of inflammatory cells. Abundant tubular calcium oxalate deposits were detected within the tubular lumen (Figure 1). Additional laboratory tests revealed elevated urinary oxalate excretion (1.11 mmol/24 h). Mutations in AGXT, GRHPR, and HOGA1 in PH were not detected (7) (Supplementary Appendix 1).
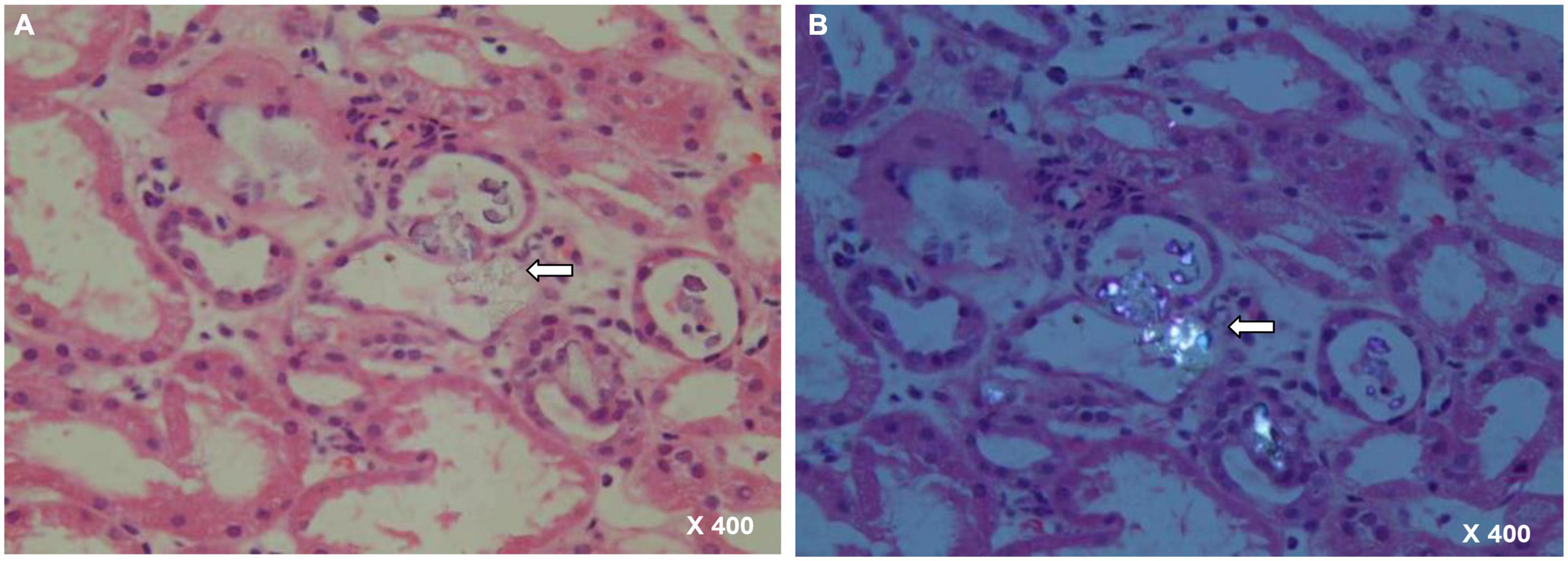
Figure 1. Renal biopsy specimen with arrow showing a couple of calcium oxalate deposits within renal tubules. (A) HE staining, 400× magnification. (B) Polarized light, 400× magnification.
Upon further questioning, the patient reported an ingestion of approximately 30 g (dry weight) of a mixture of several herbs (Panax notoginseng, Clematis chinensis Osbeck, Szechwan Lovage Rhizome, borneol, safflower, and Eupolyphaga sinensis) per day in the last 4 weeks before admission for ankle sprain. This traditional remedy is in powder form and mixed with water. He said that the ingestion of such remedies was a customary practice in his hometown. Consumption of vitamin C or ethylene glycol was ruled out.
Elevated urine oxalate levels and renal biopsy led to the diagnosis of oxalate nephropathy, which is the most likely cause of AKI. Given the lack of other explanations, hyperoxaluria was believed to be due to excessive intake of oxalate-rich herbs.
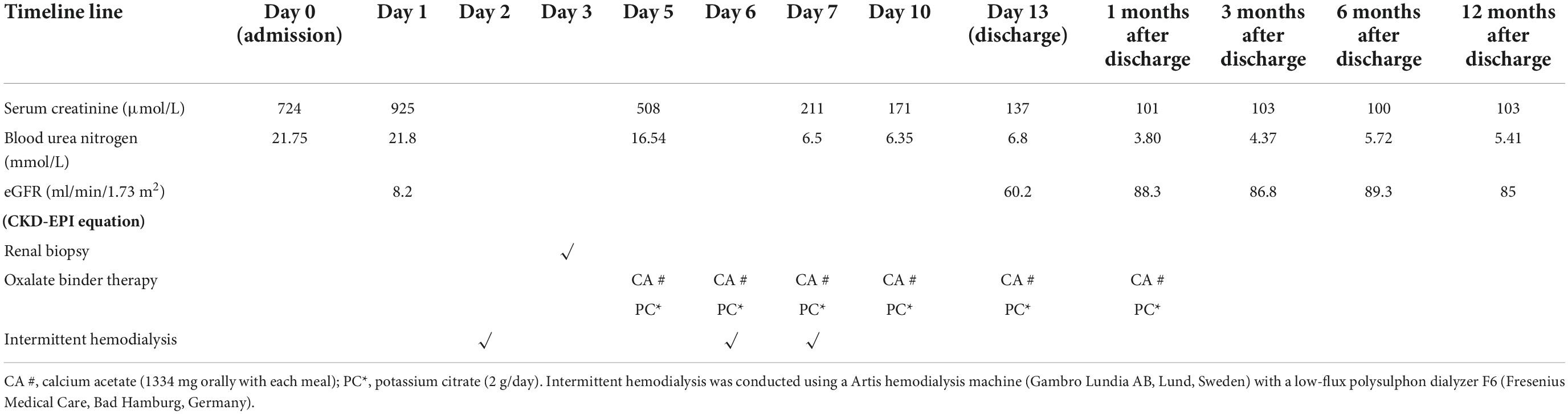
Due to uremic symptoms, emergent hemodialysis via a temporary catheter was started before renal biopsy. Totally, three standard hemodialysis were conducted on an Artis hemodialysis machine (Gambro Lundia AB, Lund, Sweden) with a low-flux polysulphon dialyzer F6 (Fresenius Medical Care, Bad Hamburg, Germany). Serum creatinine peaked at 211 μmol/L before plateauing with cessation of dialysis. Once the diagnosis is established, the patient was advised to avoid a high oxalate diet, drink plenty of water (>3 L/1.73 m2) and consume calcium acetate (1,334 mg orally with each meal), and potassium citrate (2 g/day, orally). By the day of discharge, renal function had partially recovered, and creatinine levels had decreased to 137 μmol/L. His creatinine level improved and plateaued at 103 μmol/L at 12 months follow-up. Table 1 describes the renal function tests of the patient.
Discussion
Acute oxalate nephropathy, defined as the association between AKI and the deposition of oxalate crystals in the renal parenchyma, is a rare complication of hyperoxaluria. In the present case, renal biopsy under polarized light revealed massive oxalate crystals in the tubular lumen. This highlights the importance of histopathological confirmations of AON.
Acute oxalate nephropathy can occur due to PH or SH (5, 6). PHs comprise a group of three distinct genetic disorders of glyoxylate metabolism, characterized by endogenous oxalate overproduction (7). Patients with PH typically develop recurrent renal lithiasis and progressive nephrocalcinosis (8). Our patient had no personal history of lithiasis and the genetic testing results were normal. Thus, the clinical picture in our patient was not consistent with a diagnosis of PH.
Secondary hyperoxaluria is more common. The leading cause of SH is enteric hyperoxaluria (9), which is usually a consequence of fat malabsorption (10). In enteric hyperoxaluria, fat malabsorption leads to increased binding of calcium to free fatty acids, resulting in more soluble oxalates in the intestinal lumen, which are subsequently absorbed (11). Enteric hyperoxaluria is often present in patients with inflammatory bowel disease, celiac disease, short bowel syndrome, chronic pancreatitis, or bariatric surgery (12–16). However, our patient showed no clinical symptoms of fat malabsorption.
Increased intake of dietary oxalate or oxalate precursor (such as ethylene glycol or vitamin C) can also contribute to AON (17–20). Prior reports have described patients with oxalate nephropathy due to hyperoxaluria after ingesting oxalate-rich food (17, 18). Dietary sources rich in oxalate include nuts, plums, chocolate, beetroot, strawberries, and spinach (21). Our patient occasionally consumed these foods. In addition, he denied the use of ethylene glycol products or consumption of vitamin C. Therefore, it is unlikely that the dietary oxalate or oxalate precursor was the culprit.
Traditional herbal medicines are naturally occurring, plant or animal-derived substances that are not processed to treat diseases according to local practices. In a study by Huang et al. (22), the total oxalate content of 22 herbs ranged from 165 to 3,204 mg/100 g, which was much higher than that of daily foods such as various flours and nuts. Different medicinal herbs, even those from the same family, contain significantly different amounts of oxalate (22). Oxalate nephropathy has been reported following the consumption of medicinal herbs, such as rhubarb and star fruit (23, 24). In the present case, the absence of evidence for any other cause of AON incriminates the herbal mixture as the culprit. There are no previous case reports in the literature describing oxalate nephropathy in association with any of the involved herbs in our patient. The concentration of oxalate in the same herb may be quite different in different place of origin. Different parts of the same herb, even those from the same place of origin, contain significantly different amounts of oxalate. Due to the lack of detailed information, the concentration of oxalate in each herb was not tested in our study. Thus, it is not known which component of the mixture is responsible for AON. Even so, the findings from our case, at least to some extent, indicate that the use of medicinal herbs with unknown oxalate contents may increase the risk of AON.
We report a rare case of AON in an adult due to medicinal herbs intake leading to crystal-induced AKI. We recommend that a thorough medication history including the use of medicinal herbs, should be obtained for all patients with a rapid loss of kidney function, especially in the absence of known risk factors for AKI. The use of medicinal herbs with unknown oxalate contents would increase the risk of AON and should be avoided.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZZ and JL reviewed the medical literature and clinically managed the patients and manuscript. LW reviewed the relevant histopathology and prepared the figure and manuscript. All authors contributed to the conception, drafting, and final approval of the submitted work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1063681/full#supplementary-material
References
1. Thompson CS, Weinman EJ. The significance of oxalate in renal failure. Am J Kidney Dis. (1984) 4:97–100. doi: 10.1016/s0272-6386(84)80055-4
2. Elder TD, Wyngaarden JB. The biosynthesis and turnover of oxalate in normal and hyperoxaluric subjects. J Clin Invest. (1960) 39:1337–44. doi: 10.1172/JCI104151
3. Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol. (2007) 18:2198–204. doi: 10.1681/ASN.2007020219
4. Glew RH, Sun Y, Horowitz BL, Konstantinov KN, Barry M, Fair JR, et al. Nephropathy in dietary hyperoxaluria: a potentially preventable acute or chronic kidney disease. World J Nephrol. (2014) 3:122–42. doi: 10.5527/wjn.v3.i4.122
6. Demoulin N, Aydin S, Gillion V, Morelle J, Jadoul M. Pathophysiology and management of hyperoxaluria and oxalate nephropathy: a review. Am J Kidney Dis. (2022) 79:717–27. doi: 10.1053/j.ajkd.2021.07.018
7. Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, et al. Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol. (2015) 26:2559–70. doi: 10.1681/ASN.2014070698
8. Sas DJ, Enders FT, Mehta RA, Tang X, Zhao F, Seide BM, et al. Clinical features of genetically confirmed patients with primary hyperoxaluria identified by clinical indication versus familial screening. Kidney Int. (2020) 97:786–92. doi: 10.1016/j.kint.2019.11.023
9. Lumlertgul N, Siribamrungwong M, Jaber BL. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. (2018) 3:1363–72. doi: 10.1016/j.ekir.2018.07.020
10. Asplin JR. The management of patients with enteric hyperoxaluria. Urolithiasis. (2016) 44:33–43. doi: 10.1007/s00240-015-0846-5
11. Siener R, Petzold J, Bitterlich N, Alteheld B, Metzner C. Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology. (2013) 81:17–24.
12. Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, et al. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatr Nephrol. (2012) 27:1103–9. doi: 10.1007/s00467-012-2126-8
13. Saccomani MD, Pizzini C, Piacentini GL, Boner AL, Peroni DG. Analysis of urinary parameters as risk factors for nephrolithiasis in children with celiac disease. J Urol. (2012) 188:566–70. doi: 10.1016/j.juro.2012.04.019
14. Ceulemans LJ, Nijs Y, Nuytens F, De Hertogh G, Claes K, Bammens B, et al. Combined kidney and intestinal transplantation in patients with enteric hyperoxaluria secondary to short bowel syndrome. Am J Transplant. (2013) 13:1910–4. doi: 10.1111/ajt.12305
15. Demoulin N, Issa Z, Crott R, Morelle J, Danse E, Wallemacq P, et al. Enteric hyperoxaluria in chronic pancreatitis. Medicine (Baltimore). (2017) 96:e6758. doi: 10.1097/MD.0000000000006758
16. Whitson JM, Stackhouse GB, Stoller ML. Hyperoxaluria after modern bariatric surgery: case series and literature review. Int Urol Nephrol. (2010) 42:369–74. doi: 10.1007/s11255-009-9602-5
17. Clark B, Baqdunes MW, Kunkel GM. Diet-induced oxalate nephropathy. BMJ Case Rep. (2019) 12:e231284. doi: 10.1136/bcr-2019-231284
18. Makkapati S, D’Agati VD, Balsam L. Green smoothie cleanse” causing acute oxalate nephropathy. Am J Kidney Dis. (2018) 71:281–6. doi: 10.1053/j.ajkd.2017.08.002
19. Monet C, Richard E, Missonnier S, Rebouissoux L, Llanas B, Harambat J. Secondary hyperoxaluria and nephrocalcinosis due to ethylene glycol poisoning. Arch Pediatr. (2013) 20:863–6. doi: 10.1016/j.arcped.2013.05.011
20. Fijen L, Weijmer M. Acute oxalate nephropathy due to high vitamin C doses and exocrine pancreatic insufficiency. BMJ Case Rep. (2019) 12:e231504. doi: 10.1136/bcr-2019-231504
21. Noonan SC, Savage GP. Oxalate content of foods and its effect on humans. Asia Pac J Clin Nutr. (1999) 8:64–74.
22. Huang J, Huang C, Liebman M. Oxalate contents of commonly used Chinese medicinal herbs. J Tradit Chin Med. (2015) 35:594–9. doi: 10.1016/s0254-6272(15)30145-x
23. Albersmeyer M, Hilge R, Schröttle A, Weiss M, Sitter T, Vielhauer V. Acute kidney injury after ingestion of rhubarb: secondary oxalate nephropathy in a patient with type 1 diabetes. BMC Nephrol. (2012) 13:141. doi: 10.1186/1471-2369-13-141
Keywords: acute kidney injury, acute oxalate nephropathy, medicinal herbs, hyperoxaluria, renal biopsy
Citation: Wang L, Zhu Z and Li J (2022) Case report: Acute oxalate nephropathy due to traditional medicinal herbs. Front. Med. 9:1063681. doi: 10.3389/fmed.2022.1063681
Received: 07 October 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Sree Bhushan Raju, Nizam’s Institute of Medical Sciences, IndiaReviewed by:
Faizan Ahmed Ansari, Ansari Hospital, IndiaRavi Tej Madipalli, Nizam’s Institute of Medical Sciences, India
Copyright © 2022 Wang, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangtao Li, bGlqaWFuZ3Rhb3N1eWFuQDEyNi5jb20=
 Lirui Wang
Lirui Wang Zhuxian Zhu
Zhuxian Zhu Jiangtao Li
Jiangtao Li