- 1Department of Pulmonary and Critical Care Medicine, Xijing Hospital, Air Force Medical University, Xi’an, China
- 2Department of Clinical Laboratory, Xijing Hospital, Air Force Medical University, Xi’an, China
Background: Although asthma and chronic obstructive pulmonary disease (COPD) are two well-defined and distinct diseases, some patients present combined clinical features of both asthma and COPD, particularly in smokers and the elderly, a condition termed as asthma-COPD overlap (ACO). However, the definition of ACO is yet to be established and clinical guidelines to identify and manage ACO remain controversial. Therefore, in this study, inflammatory biomarkers were established to distinguish asthma, ACO, and COPD, and their relationship with the severity of patients’ symptoms and pulmonary function were explored.
Materials and methods: A total of 178 patients, diagnosed with asthma (n = 38), ACO (n = 44), and COPD (n = 96) between January 2021 to June 2022, were enrolled in this study. The patients’ pulmonary function was examined and routine blood samples were taken for the analysis of inflammatory indexes. Logistic regression analysis was used to establish inflammatory biomarkers for distinguishing asthma, ACO, and COPD; linear regression analysis was used to analyze the relationship between inflammatory indexes and symptom severity and pulmonary function.
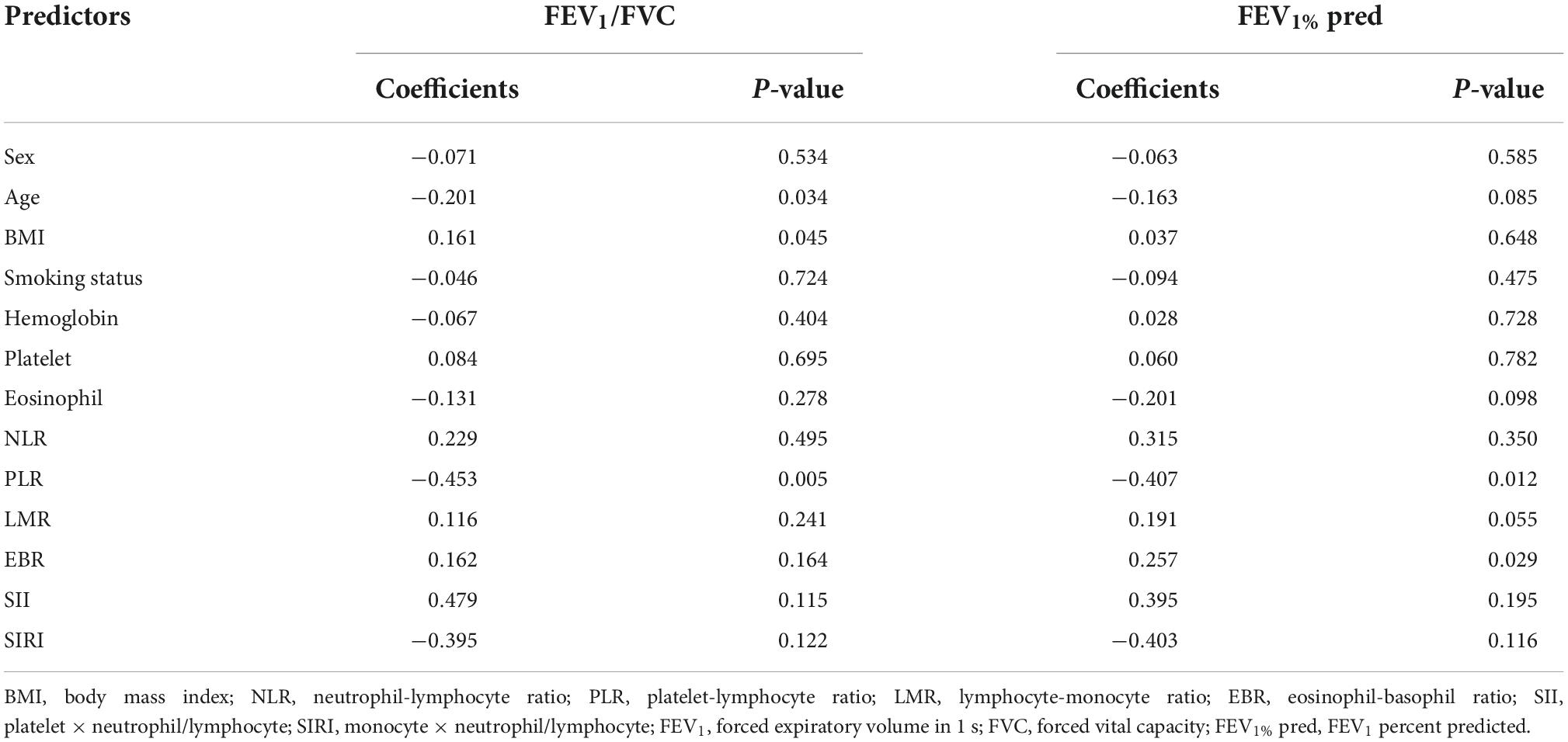
Result: The results showed that, compared with ACO, the higher the indexes of platelet, neutrophil-lymphocyte ratio (NLR) and eosinophil-basophil ratio (EBR), the more likely the possibility of asthma and COPD in patients, while the higher the eosinophils, the less likely the possibility of asthma and COPD. Hemoglobin and lymphocyte-monocyte ratio (LMR) were negatively correlated with the severity of patients’ symptoms, while platelet-lymphocyte ratio (PLR) was negatively correlated with forced expiratory volume in the 1 s/forced vital capacity (FEV1/FVC) and FEV1 percent predicted (% pred), and EBR was positively correlated with FEV1% pred.
Conclusion: Inflammatory indexes are biomarkers for distinguishing asthma, ACO, and COPD, which are of clinical significance in therapeutic strategies and prognosis evaluation.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are heterogeneous diseases characterized by chronic airway and/or lung diseases. Chronic respiratory diseases (CRD) were the third leading cause of death in the world, of which COPD was the most common cause of death, followed by asthma, and the number of deaths from COPD was eight times that of asthma (1, 2). Although the definitions of asthma and COPD are very clear (asthma is characterized by variable expiratory airflow limitation, and COPD is characterized by persistent airflow limitation), the definitions of the both are not mutually exclusive, which means that their diagnostic criteria may overlap (3). Therefore, it is difficult to completely distinguish asthma from COPD.
The characteristics of asthma and COPD can coexist in a given patient, who are diagnosed with asthma-COPD overlap (ACO), especially in smokers and the elderly (4). However, so far, there are few studies on ACO because it is excluded from most clinical studies on COPD and asthma (5, 6). Moreover, there is no universally recognized specific definition of ACO (4). In epidemiological investigations, the reported prevalence of ACO ranged from 9 to 55% of those with either diagnosis, which reflects the different diagnostic criteria used by different researchers (5). A large number of studies have found that the prognosis of patients with ACO is worse than those with asthma or COPD alone (7). In summary, because the diagnostic criteria of asthma and COPD may overlap, and the definition of ACO is ambiguous, clinicians need to subjectively combine the clinical characteristics of patients in addition to the objective indicators (e.g., computed tomography and pulmonary function test) for diagnosis (4). Therefore, it is urgent to find objective biomarkers to understand the pathological characteristics of asthma, ACO, and COPD, so as to assist clinical differential diagnosis, guide clinical treatment and evaluate prognosis (8).
Asthma and COPD are two common CRD of chronic airway inflammatory (9). Asthma is predominantly airway reactive inflammation mediated by T-helper (Th) two cells and type 2 innate lymphoid cells (ILC2), while COPD is considered a Th1-mediated inflammatory process (4). Neutrophil infiltration becomes evident in severe cases of asthma and COPD (10). Some of these complex inflammatory mechanisms may be common to both asthma and COPD, which means that clinical features of asthma and COPD may overlap (11). For example, eosinophil is not only the characteristic diagnostic biomarker for asthma (12), but also closely related to COPD exacerbations (13); neutrophil-lymphocyte ratio (NLR) is associated with the exacerbation of asthma and COPD (14–16). Although the pathogenesis of ACO is not well established (17), some scholars have proposed that systemic inflammation may be a contributing factor to the increased morbidity associated with the ACO (18). Meanwhile, it was found that ACO was a heterogeneous disease, and the classical diagnostic categories could not fully explain the great complexity of the potential inflammatory process that ultimately determined the treatment response (19–21). Consequently, it is in great clinical interest to analyze the inflammatory index in hemogram for establishing biomarkers of asthma, ACO, and COPD.
The purposes of this study are: (1) to compare the inflammatory indexes of asthma, ACO, and COPD, in order to understand their pathological characteristics; (2) to explore biomarkers that can distinguish three groups of CRD, in order to assist clinical differential diagnosis; and (3) to analyze the relationship between inflammatory indexes and the severity of patients’ symptoms and pulmonary function, in order to guide clinical treatment and evaluate the prognosis of the disease.
Materials and methods
Study design
This study was a single-center, retrospective observational study. In this study, participants diagnosed with asthma (22), ACO (22), and COPD (23) were included according to the diagnosis of clinicians. The participants’ baseline data were collected, their condition was evaluated, and pulmonary function was assessed. Blood samples were taken for complete blood counts (CBC) analysis in order to form new inflammatory indexes through hemogram combination. The levels of inflammatory indexes were compared among the three groups, the biomarkers that could distinguish the three groups of diseases were explored, and the relationship between inflammatory indexes and symptom severity and pulmonary function was analyzed.
Setting and participants
In this study, patients with stable CRD diagnosed for the first time were recruited from Xijing Hospital of Air Force Medical University from January 2021 to June 2022. They were divided into asthma, ACO, and COPD according to physician diagnosis and spirometry. Individuals were excluded if they: (1) were hospitalized; (2) had used systemic corticosteroids and/or antibiotics in the past month; (3) had pneumonia, tuberculosis, and other respiratory diseases; (4) had malignant tumors, autoimmune diseases, systemic infectious diseases, and important organ dysfunction; and (5) had other reasons that are not suitable for the study.
Measurements
Pulmonary function test (PFT): PFT was conducted with spirometer (MasterScreen PFT system, Jaeger, Hoechberg, Germany) following the American Thoracic Society/European Respiratory Society (ATS/ERS) standards (24). Participants also inhaled 400 μg Salbutamol for bronchodilation test.
Routine blood test: 2–5 ml of peripheral venous blood was drawn into ethylenediamine tetraacetic acid (EDTA) anticoagulant blood tubes according to the standard procedure of venipuncture (25). All samples were analyzed for CBC.
Statistical analysis
The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The results are described as median interquartile range (IQR). The differences between more than two groups were analyzed by Kruskal–Wallis test according to the distribution of data points. Logistic regression analysis was used to predict the biomarkers that could distinguish the three diseases. Linear regression analysis was used to analyze the relationship between inflammatory indexes and symptom severity and pulmonary function. Statistical significance was accepted if the P-value was <0.05.
Results
Baseline characteristics
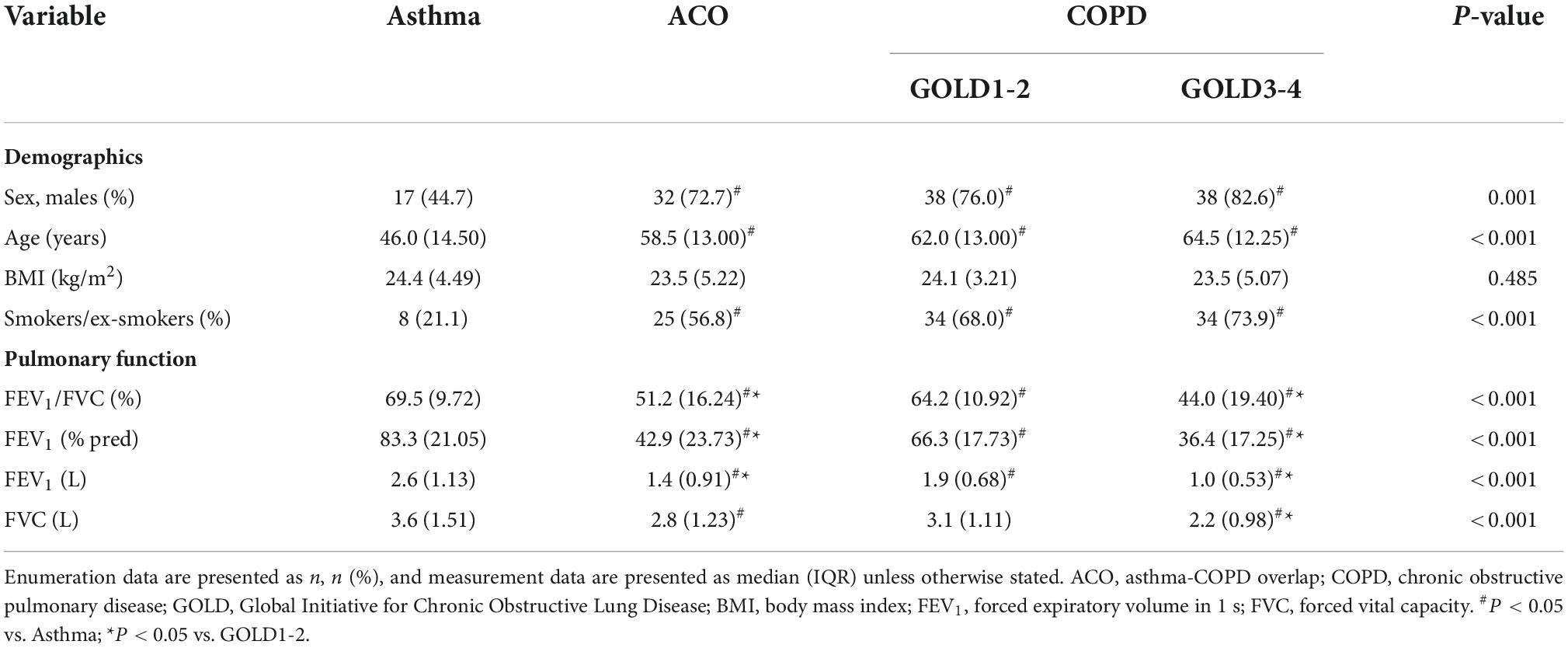
A total of 178 patients who met the criteria were included in this study, including asthma (n = 38), ACO (n = 44), and COPD (n = 96). Statistical analysis showed significant differences in sex, age, smoking status, and pulmonary function between patients with asthma and patients with ACO and COPD, but there were no significant differences between ACO and COPD patients. Since the wide range of pulmonary function in patients with COPD, they were divided it into GOLD1-2 (n = 50) and GOLD3-4 (n = 46) for inter-group comparison in this study. The baseline characteristics of these patients are shown in Table 1. We found that there were more male patients, elderly people and smokers with ACO and COPD than with asthma, but there was no significant difference between ACO and COPD, which reflects the clinical characteristics of COPD (prevalent among smokers, the elderly and men). At the same time, it was found that the pulmonary function of ACO and COPD was worse than asthma, and ACO and GOLD3-4 were worse than GOLD1-2, while the difference between ACO and GOLD3-4 was not statistically significant. This shows that the airflow limitation is not serious or even completely normal in patients with stable asthma, while it is more serious in patients with ACO.
Differences in inflammatory index levels among asthma, asthma-COPD overlap, and chronic obstructive pulmonary disease
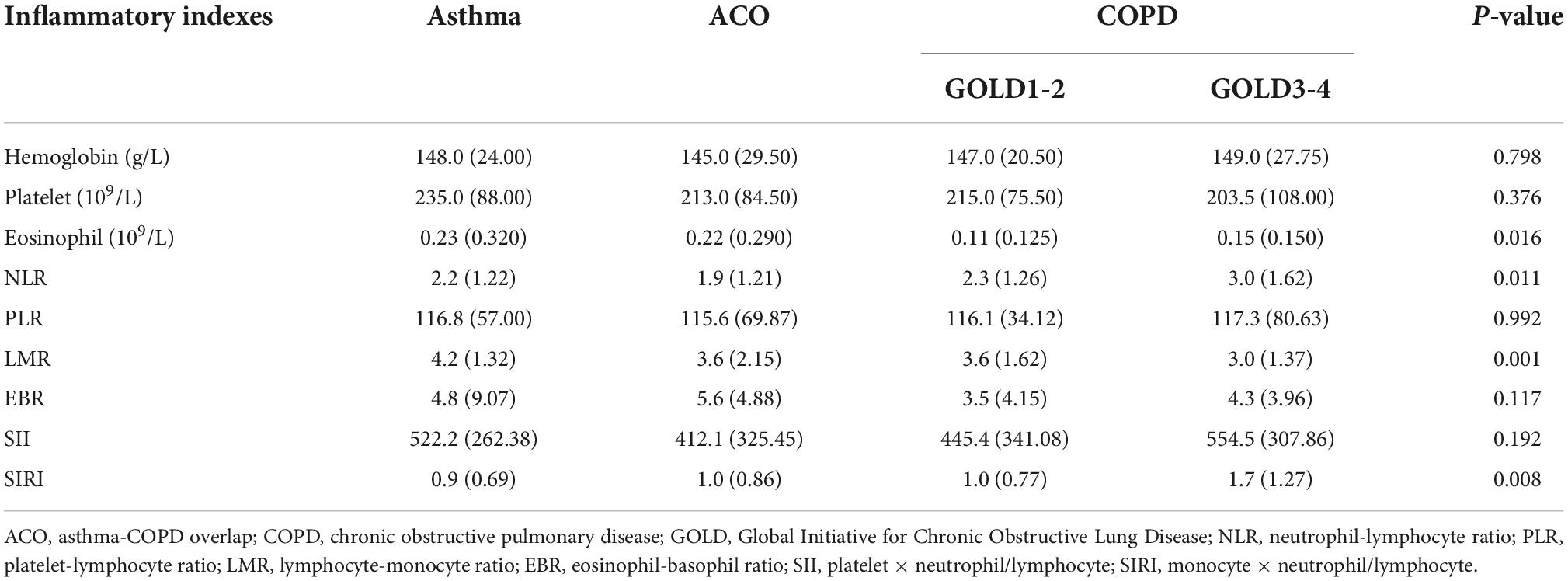
There were statistically significant differences in eosinophil, NLR, lymphocyte-monocyte ratio (LMR), and SIRI between patients with asthma, ACO, and COPD (Table 2). Patients with COPD have lower eosinophil and LMR, higher NLR, and SIRI than patients with asthma and ACO. Moreover, eosinophil, NLR and SIRI in GOLD3-4 are higher than in GOLD1-2, while LMR is lower than in GOLD1-2. There was no significant difference in these inflammatory indexes between asthma and ACO. The differences between groups are shown in Figure 1.
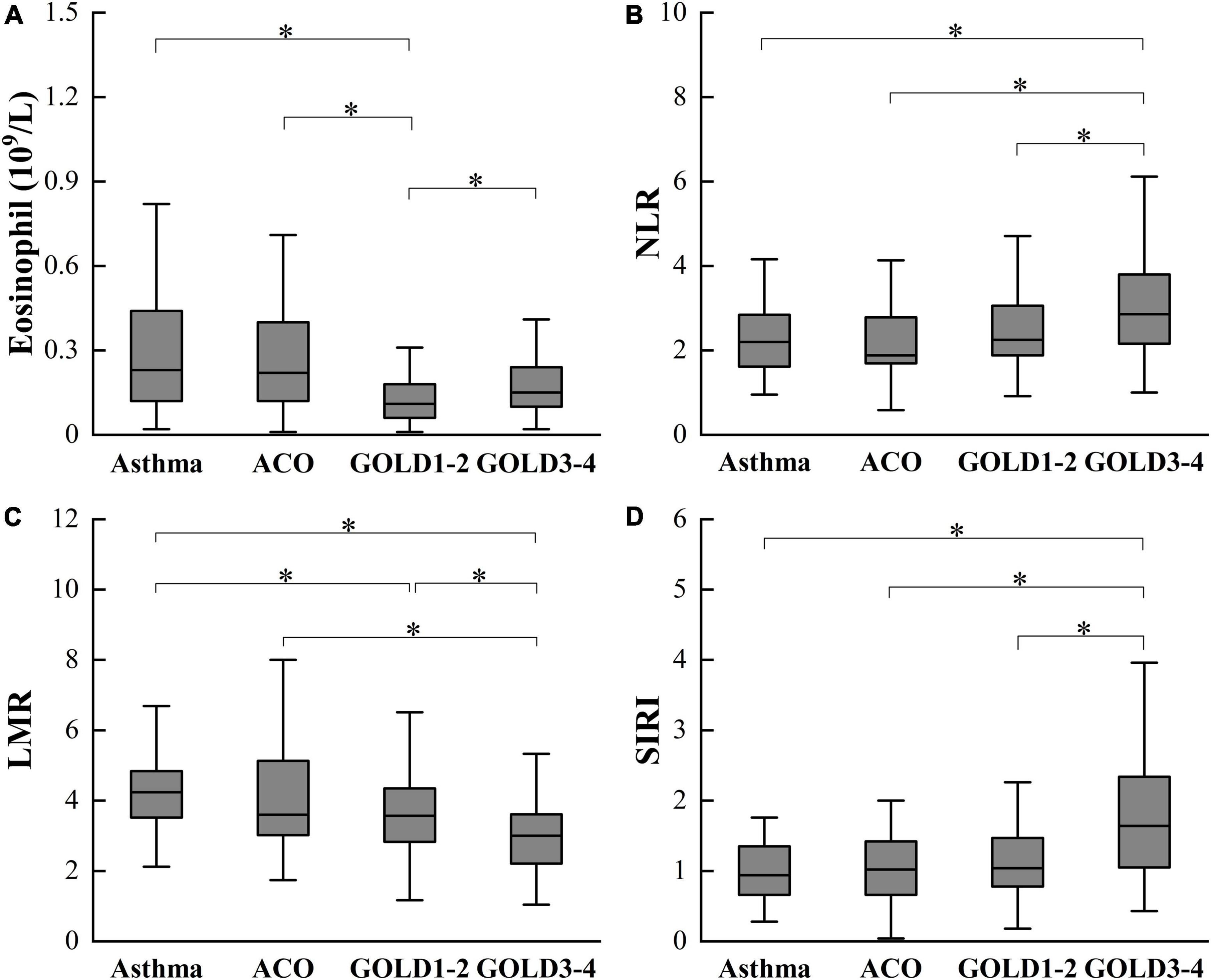
Figure 1. Differences in eosinophil (A), NLR (B), LMR (C), and SIRI (D) among asthma, ACO and COPD. ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; NLR, neutrophil-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; SIRI, monocyte × neutrophil/lymphocyte. *P < 0.05.
To distinguish the inflammatory indexes of asthma, asthma-COPD overlap, and chronic obstructive pulmonary disease
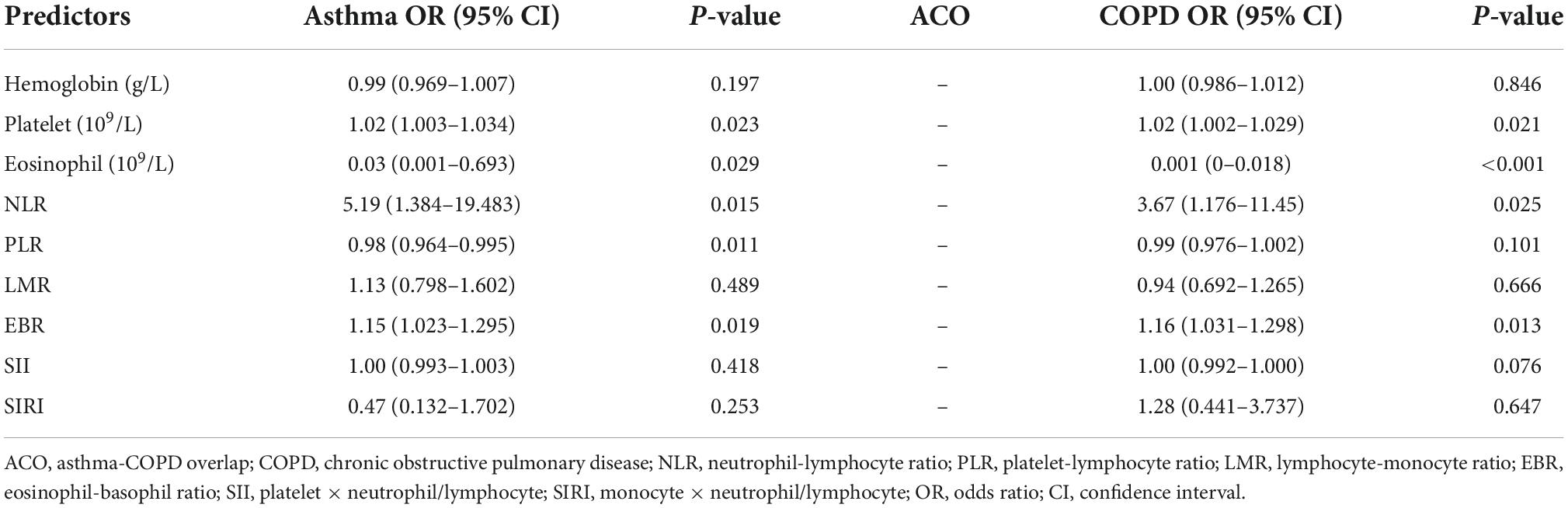
This study used multinomial logistic regression to analyze the differences in inflammatory levels among patients with asthma, ACO, and COPD (Table 3). We found that with ACO as a reference, the higher the indexes of platelet, NLR, and eosinophil-basophil ratio (EBR), the more likely the patient will develop asthma, whereas the higher the indexes of eosinophil and platelet-lymphocyte ratio (PLR), the less likely the patient will develop asthma. In addition, compared to ACO, the higher the indexes of platelet, NLR, and EBR, the greater the possibility of COPD occurred, while the higher the index of eosinophil, the less likely COPD occurred.
Relationship between symptom and inflammatory indexes
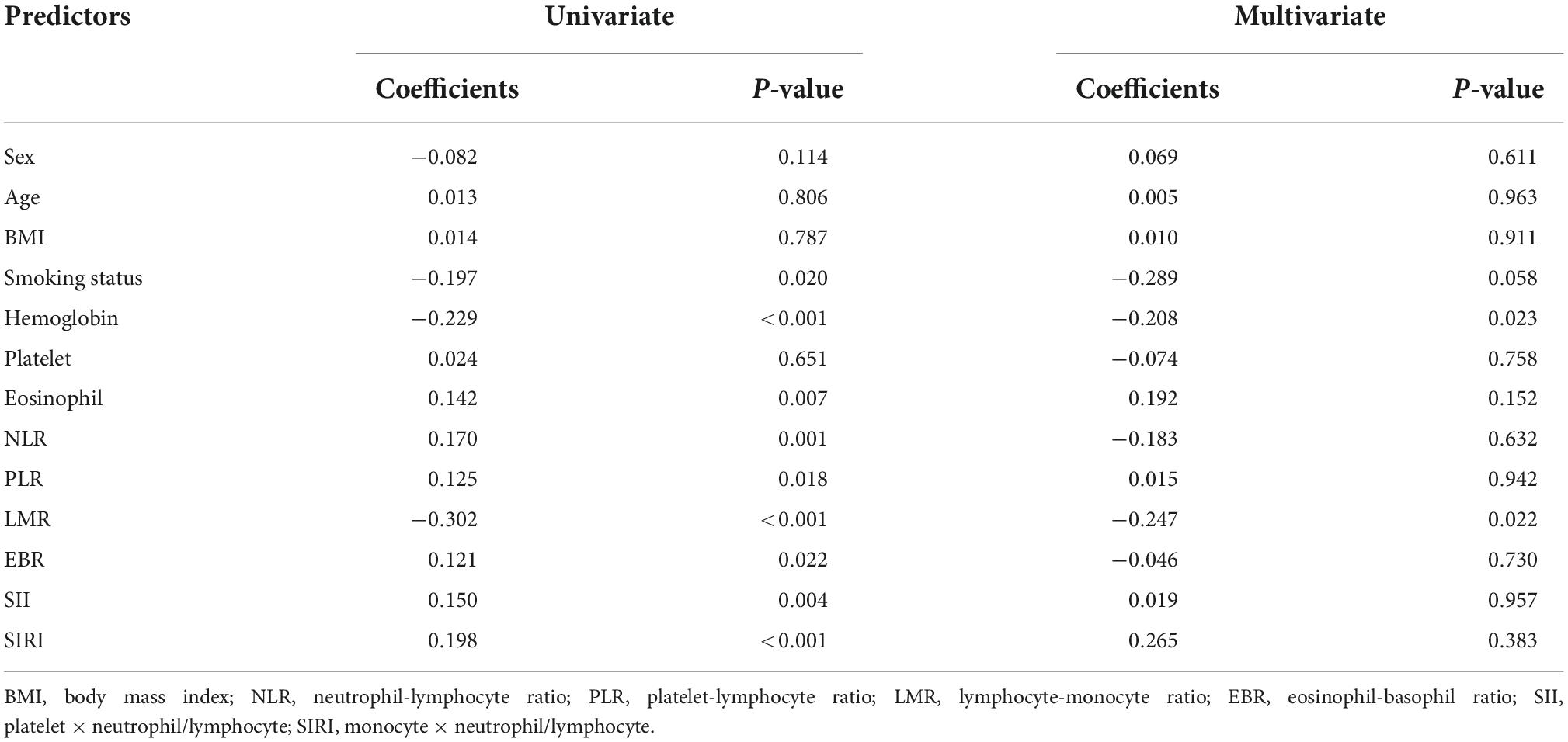
Since the symptoms of asthma patients are not obvious in the stable period, we used the COPD assessment test (CAT) score to evaluate the symptoms of ACO and COPD patients. Through the correlation analysis of CAT score and inflammatory indexes, it was found that hemoglobin and LMR were negatively correlated with the severity of patients’ symptoms (Table 4). In other words, the lower the value of hemoglobin and LMR, the more serious the patient’s symptoms.
Relationship between pulmonary function and inflammatory indexes
The relationship between inflammatory indexes and pulmonary function was analyzed by multiple linear regression (Table 5). We found that PLR was negatively correlated with FEV1/FVC and FEV1% pred, respectively; age was negatively correlated with FEV1/FVC; body mass index (BMI) was positively correlated with FEV1/FVC; and EBR was positively correlated with FEV1% pred.
Discussion
Chronic respiratory disease is a heterogeneous group of disorders characterized by airway inflammation and airway obstruction, in which asthma and COPD are the most common disease entities (20). Although clinicians can easily recognize typical COPD and asthma, it is difficult to completely identify the undefined ACO, which affects the follow-up treatment of patients (6). Therefore, there is an urgent need for establishing biomarkers that can help distinguish CRD (8). Our study focuses on the comprehensive evaluation of easily accessible hemogram indexes, especially ratio indexes, which avoids the influence of individual differences and shows greater stability (16).
Our study analyzed the inflammatory indexes of asthma, ACO, and COPD, and found that the higher the indexes of platelet, NLR, and EBR, the greater the risk of asthma and COPD compared with ACO. That may be related to the pathological mechanism of the disease, including the inflammatory process and the increase of inflammation in the aggravation period. Some researchers have found that in addition to playing a critical role in hemostasis and thrombosis, platelets are also considered immune cells participated in various immune related processes (26). In patients with allergic asthma, platelet-specific derived mediators increased significantly in serum, indicating platelet activation (27). Meanwhile, platelets are recruited and located in the lung tissue, and participate in the pathogenesis and pulmonary inflammatory response of allergic asthma by interacting with dendritic cells, eosinophils, and neutrophils (28–30). Moreover, hypoxemia can promote platelet activation, and further activation occurs during acute exacerbation, which is a spiral process (16). Based on the above research, we also confirmed that PLR was negatively correlated with pulmonary function. In other words, platelet not only affect the occurrence and development of the disease, but also affect patients’ symptoms and pulmonary function.
The reason why high eosinophil is less likely the possibility of asthma and COPD compared with ACO, may be that the exacerbation frequency of ACO is higher than that of asthma or COPD alone (3, 31). A large number of studies have shown that eosinophil is closely related to the exacerbation of COPD and asthma (32–34). Compared with patients without eosinophilia, those with eosinophilia (≥300 cells/μl) had a higher frequency of readmission for acute exacerbation of COPD (AECOPD) during the 1-year follow-up period. And the incidence of AECOPD increased progressively with increasing blood eosinophil counts among stable patients with COPD (35). It was also found that high eosinophil count was an independent risk factor for two or more asthma exacerbations or any asthma emergency department visit or hospitalization (36). In a word, eosinophil is strongly associated with high-frequency exacerbation, while ACO exacerbations are more frequent than asthma or COPD alone. Therefore, high eosinophil is less likely to develop asthma and COPD compared with ACO. Because there are few studies on the inflammatory mechanism of ACO, we suspect that high platelet, NLR, and EBR are associated with the high risk of asthma and COPD with ACO as a reference, which may be related to the better response of ACO to pharmacological therapies, not least inhaled corticosteroids (4).
We also found that hemoglobin was negatively correlated with the severity of patients’ symptoms. Although it is traditionally considered that COPD is associated with polycythemia, a large number of studies have shown that anemia in COPD is more prevalent than expected (37). Anemia of chronic disease is a systemic inflammation driven by immunity, which is now recognized as a feature of COPD. Anemia in COPD may aggravate dyspnea and limit exercise tolerance, and increase the risk of mortality and exacerbation (38–40). Consequently, the lower the hemoglobin of patients, the more serious the symptoms.
In addition, our study confirmed that EBR was positively correlated with FEV1% pred. Although there is a line of evidence to show that eosinophil is related to exacerbation of patients (41), it is found that patients with persistent eosinophilia have significantly higher FEV1% pred and less dyspnea symptoms (42), which is consistent with our results. For this interesting phenomenon, the specific mechanism is still unclear and controversial. It is speculated that although eosinophil is related to exacerbations, patients with high eosinophil are sensitive to inhaled corticosteroid (ICS) (43), thus have a better response to therapy resulting in quicker recovery (13). Secondly, it was found that lower eosinophilic COPD patients had higher neutrophil counts. Neutrophilia is known to be a marker of bacterial infection, which is a common cause of exacerbations. The exacerbations of COPD caused by bacterial infection are associated with longer hospital stay (34). Although EBR is an eosinophil-related index, we believe that EBR tends to predict the prognosis of the disease, while eosinophil is related to the acute exacerbation of patients according to the results.
In summary, our study has established inflammatory biomarker to distinguish CRD by using easily accessible blood routine. These biomarkers can assist clinicians to accurately identify patients, thereby to maximize the effects of the personalized treatment (11). There are extremely differences in evidence-based treatment between asthma and COPD: because frequent use of ICS can increase the risk of pneumonia (44), it is recommended to use long-acting β2-agonist (LABA) and/or long-acting muscarinic antagonists (LAMA) alone (without ICS) as the initial treatment for COPD; but contraindicated for asthma and ACO due to the risk of serious deterioration and death (45). In addition, because the prognosis of patients with ACO is significantly worse, some guidelines recommend add-on therapy of LAMA to the basis treatment of ICS and LABA (i.e., triple therapy) (46). Therefore, whether we can accurately identify CRD is closely related to the follow-up personalized treatment (47).
Our study also has limitations. Firstly, this study was a single-center retrospective clinical study, which reflects the characteristics of specific populations and has regional limitations. Secondly, the number of samples in the study was limited. With the increase of the sample size, the sensitivity and specificity of the research results may also increase. At the same time, large samples can be divided into subgroups (according to acute exacerbation frequency, with/without ICS, et al.) to better understand CRD in many aspects. Thirdly, since there is no universally recognized definition of ACO currently, our diagnosis was based on the comprehensively assessment of clinical characteristics as well as the result of pulmonary function. Therefore, there may be some differences in the results of different diagnostic criteria. Finally, this study was designed as a cross-sectional study without long-term follow-up of patients, so it was difficult to evaluate the relationship between inflammatory indexes and patient outcomes. Despite these many limitations, we believe that our study shows that inflammatory indexes have clinical application value to a certain extent.
Although our research shows that inflammatory biomarkers can distinguish CRD, the clinical application value is still in the exploration stage. We mainly differentiate CRD according to diagnostic criteria and some clinical indicators that can reflect the characteristics of the disease (e.g., fractional exhaled nitric oxide, total immunoglobulin E, allergy tests, et al.) (48–50). Inflammatory biomarkers are just another auxiliary means for the hard-to-distinguish CRD.
Conclusion
The results of this study show that patients with CRD can be distinguished by inflammatory indexes. In addition, it was also found that different inflammatory indexes have different effects. Platelet, NLR, and EBR can distinguish asthma, ACO, and COPD; hemoglobin and LMR were correlated with the severity of patients’ symptoms; PLR and EBR were related to pulmonary function. In clinical application, we can use different indexes to achieve different purposes, providing a new direction for accurate identification and individualized treatment in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Xijing Hospital of Air Force Medical University (No. KY20212201-C-1). The patients/participants provided their written informed consent to participate in this study.
Author contributions
XT, SQ, and HM participated in the study design. LY, XZ, and XX tested the samples. LL, YZ, and XG contributed to the data collection. SW provided administrative support. HM conducted data analysis and wrote the manuscript. XT and SQ revised the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82070029) and the Air Force Medical University Program (21FYFH02 and 2020JSTS10).
Acknowledgments
We thank all the patients who participated in this study for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. (2017) 5:691–706. doi: 10.1016/s2213-2600(17)30293-x
2. Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Vos T, Abrams EM, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. (2020) 8:585–96. doi: 10.1016/s2213-2600(20)30105-3
3. Odimba U, Senthilselvan A, Farrell J, Gao Z. Current knowledge of asthma-COPD overlap (ACO) genetic risk factors, characteristics, and prognosis. COPD. (2021) 18:585–95. doi: 10.1080/15412555.2021.1980870
4. Mekov E, Nunez A, Sin DD, Ichinose M, Rhee CK, Maselli DJ, et al. Update on asthma-COPD overlap (ACO): a narrative review. Int J Chron Obstruct Pulmon Dis. (2021) 16:1783–99. doi: 10.2147/copd.S312560
5. Jo YS, Hwang YI, Yoo KH, Lee MG, Jung KS, Shin KC, et al. Racial differences in prevalence and clinical characteristics of asthma-chronic obstructive pulmonary disease overlap. Front Med. (2021) 8:780438. doi: 10.3389/fmed.2021.780438
6. Milne S, Mannino D, Sin DD. Asthma-COPD overlap and chronic airflow obstruction: definitions, management, and unanswered questions. J Allergy Clin Immunol Pract. (2020) 8:483–95. doi: 10.1016/j.jaip.2019.10.044
7. Gayle AV, Minelli C, Quint JK. Respiratory-related death in individuals with incident asthma and COPD: a competing risk analysis. BMC Pulm Med. (2022) 22:28. doi: 10.1186/s12890-022-01823-4
8. Pérez de Llano L, Miravitlles M, Golpe R, Alvarez-Gutierrez FJ, Cisneros C, Almonacid C. A proposed approach to Chronic Airway Disease (CAD) using therapeutic goals and treatable traits: a look to the future. Int J Chron Obstruct Pulmon Dis. (2020) 15:2091–100. doi: 10.2147/copd.S263430
9. Karakioulaki M, Papakonstantinou E, Goulas A, Stolz D. The role of atopy in COPD and asthma. Front Med. (2021) 8:674742. doi: 10.3389/fmed.2021.674742
10. Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol. (2004) 27:35–43. doi: 10.1385/criai:27:1:035
11. Hizawa N, Fukunaga K, Sugiura H, Nakano Y, Kato M, Sugiyama Y, et al. A prospective cohort study to assess obstructive respiratory disease phenotypes and endotypes in Japan: the TRAIT study design. Int J Chron Obstruct Pulmon Dis. (2021) 16:1813–22. doi: 10.2147/copd.S308327
12. Tanaka A, Sato H, Akimoto K, Matsunaga T, Sagara H. Spontaneous sputum discriminates inflammatory phenotypes in patients with asthma. Ann Allergy Asthma Immunol. (2021) 126:54–60.e1. doi: 10.1016/j.anai.2020.06.017
13. Ko FWS, Chan KP, Ngai J, Ng SS, Yip WH, Ip A, et al. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology. (2020) 25:259–66. doi: 10.1111/resp.13660
14. Zhu XM, Zhou LN, Li QQ, Pan RL, Zhang J, Cui YB. Combined score of C-reactive protein level and neutrophil-to-lymphocyte ratio: A novel marker in distinguishing children with exacerbated asthma. Int J Immunopathol Pharmacol. (2021) 35:20587384211040641. doi: 10.1177/20587384211040641
15. Huang WJ, Huang GT, Zhan QM, Chen JL, Luo WT, Wu LH, et al. The neutrophil to lymphocyte ratio as a novel predictor of asthma and its exacerbation: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2020) 24:11719–28. doi: 10.26355/eurrev_202011_23819
16. Liu X, Ge H, Feng X, Hang J, Zhang F, Jin X, et al. The combination of hemogram indexes to predict exacerbation in stable chronic obstructive pulmonary disease. Front Med. (2020) 7:572435. doi: 10.3389/fmed.2020.572435
17. Maselli DJ, Hanania NA. Management of asthma COPD overlap. Ann Allergy Asthma Immunol. (2019) 123:335–44. doi: 10.1016/j.anai.2019.07.021
18. Fu JJ, McDonald VM, Gibson PG, Simpson JL. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res. (2014) 6:316–24. doi: 10.4168/aair.2014.6.4.316
19. Kolsum U, Ravi A, Hitchen P, Maddi S, Southworth T, Singh D. Clinical characteristics of eosinophilic COPD versus COPD patients with a history of asthma. Respir Res. (2017) 18:73. doi: 10.1186/s12931-017-0559-0
20. Pérez-de-Llano L, Cosio BG, Grp CS. Asthma-COPD overlap is not a homogeneous disorder: further supporting data. Respir Res. (2017) 18:183. doi: 10.1186/s12931-017-0667-x
21. Morissette M, Godbout K, Cote A, Boulet LP. Asthma COPD overlap: Insights into cellular and molecular mechanisms. Mol Aspects Med. (2022) 85:101021. doi: 10.1016/j.mam.2021.101021
22. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2022 update. Milwaukee, WI: Global Initiative for Asthma (2022).
23. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Waltham, MA: UpToDate (2022).
24. Graham BL, Steenbruggen I, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, et al. Standardization of Spirometry 2019 Update. an official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
25. Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S, Kleidon T, et al. Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs. (2021) 44:S1–224. doi: 10.1097/nan.0000000000000396
26. Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. (2018) 122:337–51. doi: 10.1161/circresaha.117.310795
27. Idzko M, Pitchford S, Page C. Role of platelets in allergic airway inflammation. J Allergy Clin Immunol. (2015) 135:1416–23. doi: 10.1016/j.jaci.2015.04.028
28. Yue M, Hu MJ, Fu FD, Ruan HF, Wu CL. Emerging roles of platelets in allergic asthma. Front Immunol. (2022) 13:846055. doi: 10.3389/fimmu.2022.846055
29. Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin, and eosinophil beta(1)-integrin activation in asthma. Am J Respir Crit Care Med. (2012) 185:498–507. doi: 10.1164/rccm.201109-1712OC
30. Tian J, Zhu TY, Liu J, Guo ZH, Cao XT. Platelets promote allergic asthma through the expression of CD154. Cell Mol Immunol. (2015) 12:700–7. doi: 10.1038/cmi.2014.111
31. Hiles SA, Gibson PG, McDonald VM. Disease burden of eosinophilic airway disease: comparing severe asthma, COPD and asthma-COPD overlap. Respirology. (2021) 26:52–61. doi: 10.1111/resp.13841
32. Toledo-Pons N, van Boven JFM, Muncunill J, Millan A, Roman-Rodriguez M, Lopez-Andrade B, et al. Impact of blood eosinophil variability in asthma: a real-life population study. Ann Am Thorac Soc. (2022) 19:407–14. doi: 10.1513/AnnalsATS.202103-409OC
33. Kang HS, Kim SK, Kim YH, Kim JW, Lee SH, Yoon HK, et al. The association between eosinophilic exacerbation and eosinophilic levels in stable COPD. BMC Pulm Med. (2021) 21:74. doi: 10.1186/s12890-021-01443-4
34. Wu HX, Zhuo KQ, Cheng DY. Peripheral blood eosinophil as a biomarker in outcomes of acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2019) 14:3003–15. doi: 10.2147/copd.S226783
35. Hasegawa K, Camargo CA. Prevalence of blood eosinophilia in hospitalized patients with acute exacerbation of COPD. Respirology. (2016) 21:761–4. doi: 10.1111/resp.12724
36. Zeiger RS, Schatz M, Dalal AA, Chen W, Sadikova E, Suruki RY, et al. Blood eosinophil count and outcomes in severe uncontrolled asthma: a prospective study. J Allergy Clin Immunol Pract. (2017) 5:144–53.e8. doi: 10.1016/j.jaip.2016.07.015
37. Similowski T, Agusti A, MacNee W, Schonhofer B. The potential impact of anaemia of chronic disease in COPD. Eur Respir J. (2006) 27:390–6. doi: 10.1183/09031936.06.00143704
38. Koç Ç, S̨ahin F. What are the most effective factors in determining future exacerbations, morbidity weight, and mortality in patients with COPD attack? Medicina. (2022) 58:163. doi: 10.3390/medicina58020163
39. Balasubramanian A, Henderson RJ, Putcha N, Fawzy A, Raju S, Hansel NN, et al. Haemoglobin as a biomarker for clinical outcomes in chronic obstructive pulmonary disease. ERJ Open Res. (2021) 7:00068–2021. doi: 10.1183/23120541.00068-2021
40. Park SC, Kim YS, Kang YA, Park EC, Shin CS, Kim DW, et al. Hemoglobin and mortality in patients with COPD: a nationwide population-based cohort study. Int J Chron Obstruct Pulmon Dis. (2018) 13:1599–605. doi: 10.2147/copd.S159249
41. Greulich T, Tuffers J, Mager S, Eder A, Maxheim M, Alter P, et al. High eosinophil blood counts are associated with a shorter length of hospital stay in exacerbated COPD patients – a retrospective analysis. Respir Res. (2020) 21:106. doi: 10.1186/s12931-020-01365-5
42. Xiong W, Xu M, Zhao Y, Wu X, Pudasaini B, Liu JM. Can we predict the prognosis of COPD with a routine blood test? Int J Chron Obstruct Pulmon Dis. (2017) 12:615–25. doi: 10.2147/copd.S124041
43. Citgez E, van der Palen J, van der Valk P, Kerstjens HAM, Brusse-Keizer M. Stability in eosinophil categorisation during subsequent severe exacerbations of COPD. BMJ Open Respir Res. (2021) 8:e000960. doi: 10.1136/bmjresp-2021-000960
44. Vedel-Krogh S, Nordestgaard BG, Lange P, Vestbo J, Nielsen SF. Blood eosinophil count and risk of pneumonia hospitalisations in individuals with COPD. Eur Respir J. (2018) 51:1800120. doi: 10.1183/13993003.00120-2018
45. Romem A, Rokach A, Bohadana A, Babai P, Arish N, Azulai H, et al. Identification of asthma-COPD overlap, asthma, and chronic obstructive pulmonary disease phenotypes in patients with airway obstruction: influence on treatment approach. Respiration. (2020) 99:35–42. doi: 10.1159/000503328
46. Ishiura Y, Fujimura M, Ohkura N, Hara J, Nakahama K, Sawai Y, et al. Tiotropium add-on and treatable traits in asthma-COPD overlap: a real-world pilot study. J Asthma Allergy. (2022) 15:703–12. doi: 10.2147/jaa.S360260
47. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. (2016) 47:410–9. doi: 10.1183/13993003.01359-2015
48. Kobayashi S, Hanagama M, Yamanda S, Ishida M, Yanai M. Inflammatory biomarkers in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. (2016) 11:2117–23. doi: 10.2147/copd.S113647
49. Peng J, Wang M, Wu Y, Shen Y, Chen L. Clinical indicators for asthma-COPD overlap: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. (2022) 17:2567–75. doi: 10.2147/copd.S374079
Keywords: asthma, asthma-COPD overlap, chronic obstructive pulmonary disease, inflammatory index, biomarker, routine blood test, pulmonary function
Citation: Ma H, Yang L, Liu L, Zhou Y, Guo X, Wu S, Zhang X, Xu X, Ti X and Qu S (2022) Using inflammatory index to distinguish asthma, asthma-COPD overlap and COPD: A retrospective observational study. Front. Med. 9:1045503. doi: 10.3389/fmed.2022.1045503
Received: 15 September 2022; Accepted: 03 November 2022;
Published: 17 November 2022.
Edited by:
Paula Tejera, Harvard University, United StatesReviewed by:
Serghei Covantsev, S.P. Botkin Clinical Hospital, RussiaRamcés Falfán-Valencia, Instituto Nacional de Enfermedades Respiratorias (INER), Mexico
Copyright © 2022 Ma, Yang, Liu, Zhou, Guo, Wu, Zhang, Xu, Ti and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyu Ti, dGl4aW55dUBmbW11LmVkdS5jbg==; Shuoyao Qu, cXN5MTI5QGZtbXUuZWR1LmNu
†These authors have contributed equally to this work
 Haiman Ma
Haiman Ma Liu Yang2†
Liu Yang2† Xiaoxiao Zhang
Xiaoxiao Zhang