- 1Department of Breast Surgery, Affiliated Tumor Hospital of Xinjiang Medical University, Ürümqi, China
- 2Department of Breast Surgery, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 3Department of Immunology, Hainan Medical University, Haikou, China
- 4Key Laboratory of Molecular Biology, Hainan Medical University, Haikou, China
- 5Department of Biochemistry and Molecular Biology, Hainan Medical University, Haikou, China
- 6Biotechnology and Biochemistry Laboratory, Hainan Medical University, Haikou, China
Background: The usual treatment option for HER2 breast cancer is targeted therapy with trastuzumab. The common adverse effects of trastuzumab treatment are thrombocytopenia, however, acute thrombocytopenia is rare and its mechanism is still largely unknown.
Case presentation: We reported a patient who presented with acute thrombocytopenia on two consecutive occasions, and the predisposing factor was identified on the second occasion because of trastuzumab-only treatment. Routine blood results showed a dramatic increase in white blood cell count and neutrophil count after both trastuzumab treatments. Moreover, the complement reaction results suggested that the dramatic thrombocytopenia was probably due to platelet destruction after complement activation.
Conclusion: This case suggests that it would be useful to perform a platelet complement reaction test before trastuzumab treatment in patients with HER2 breast cancer.
Introduction
Breast cancer in women has surpassed lung cancer as the most common cancer, with an estimated 2.3 million new cases (11.7%) (1). Breast cancer is a heterogeneous disease with multiple subtypes, each of which (Luminal type, HER2 + type, triple-negative breast cancer) all have unique clinical, pathological, and molecular features (2, 3). HER2 is an epidermal growth factor receptor that is highly expressed in 20% of breast cancers (4). Anti-HER2 therapy has become the standard treatment for breast cancer patients with high HER2 expression, and a variety of anti-HER2 drugs, including trastuzumab, have achieved good clinical results (5).
Trastuzumab is 95% humanized and has low allergenicity (5). Common adverse reactions are infusion-related symptoms, including chills, fever, pain, vomiting, fatigue, etc., which mostly occur after the first medication (6). Thrombocytopenia is also induced by trastuzumab, a common grade 3 or higher adverse event (7).
Severe thrombocytopenia due to trastuzumab is rare, and only a small number of cases have been reported (8–10). The cause of severe thrombocytopenia with trastuzumab is unknown. Here, we report a case from our hospital and analyze the possible mechanism of trastuzumab-induced severe thrombocytopenia.
Case description
A 51-year-old woman, 55 kg, 155 cm, presented with a left breast tumor. The local skin has no redness, swelling, ulceration, or “orange peel-like” change, without pain, nipple bleeding, and nipple depression. The examination revealed a mass of about 3.0 cm × 2.0 cm, which was hard in texture, with poorly defined borders, a less smooth surface, and no tenderness. An enlarged lymph node of about 1.5 cm × 0.5 cm was found in the left axilla. The immunohistochemical pathology report suggested invasive unspecified carcinoma. E-cadherin (membrane +), ER (weak +, 5%), PR (weak +, 10%), CK5/6 (-), Her-2 (3 +), P120 (membrane +), Ki (+, 40%).
Medical history: No previous history of the disease. No family history of genetic predisposition. No preoperative history of blood transfusion. No psychosocial history.
Six cycles of neoadjuvant chemotherapy (docetaxel 120 mg, epirubicin 120 mg, and cyclophosphamide 950 mg) were performed in the TEC regimen. During chemotherapy, neutropenia occurred after IV chemotherapy, and it improved after treatment with recombinant human granulocyte-stimulating factor. The results of physical examination showed that the two breasts were symmetrical after chemotherapy, there was no obvious mass in both breasts, and there was no enlargement in axillary and supraclavicular lymph nodes. Dynamic re-examination of breast color Doppler ultrasound indicated that the mass was reduced. No platelet drop was observed during chemotherapy. Three months later, a modified radical left breast cancer was performed and intraoperative Lobaplatin 60 mg was administered to irrigate the wound. Five days after the modified radical mastectomy for breast cancer, the incision was well healed and the drainage tube was not removed from the operated area. The efficacy was evaluated as PR.
Trastuzumab targeted therapy was given for the first time 3 days after surgery. Pre-treatment routine blood tests showed normal. A total of 8 mg/kg trastuzumab was administered intravenously. Six hours after infusion, there was gingival bleeding, and subcutaneous ecchymosis in the operation area, and the red liquid was drawn out from the drainage tube. Routine blood tests showed a WBC count of 9.82 × 10^9/L, a PLT count of 2 × 10^9/L, and an NE count of 8.79 × 10^9/L (Figures 1A–C). Four coagulation tests showed normal. Meanwhile, a bone marrow puncture was performed to exclude the primary decrease in platelet. Fresh frozen plasma and platelets of the same type were transfused, and human immunoglobulin injection and recombinant human thrombopoietin were added. The patient gradually returned to normal.
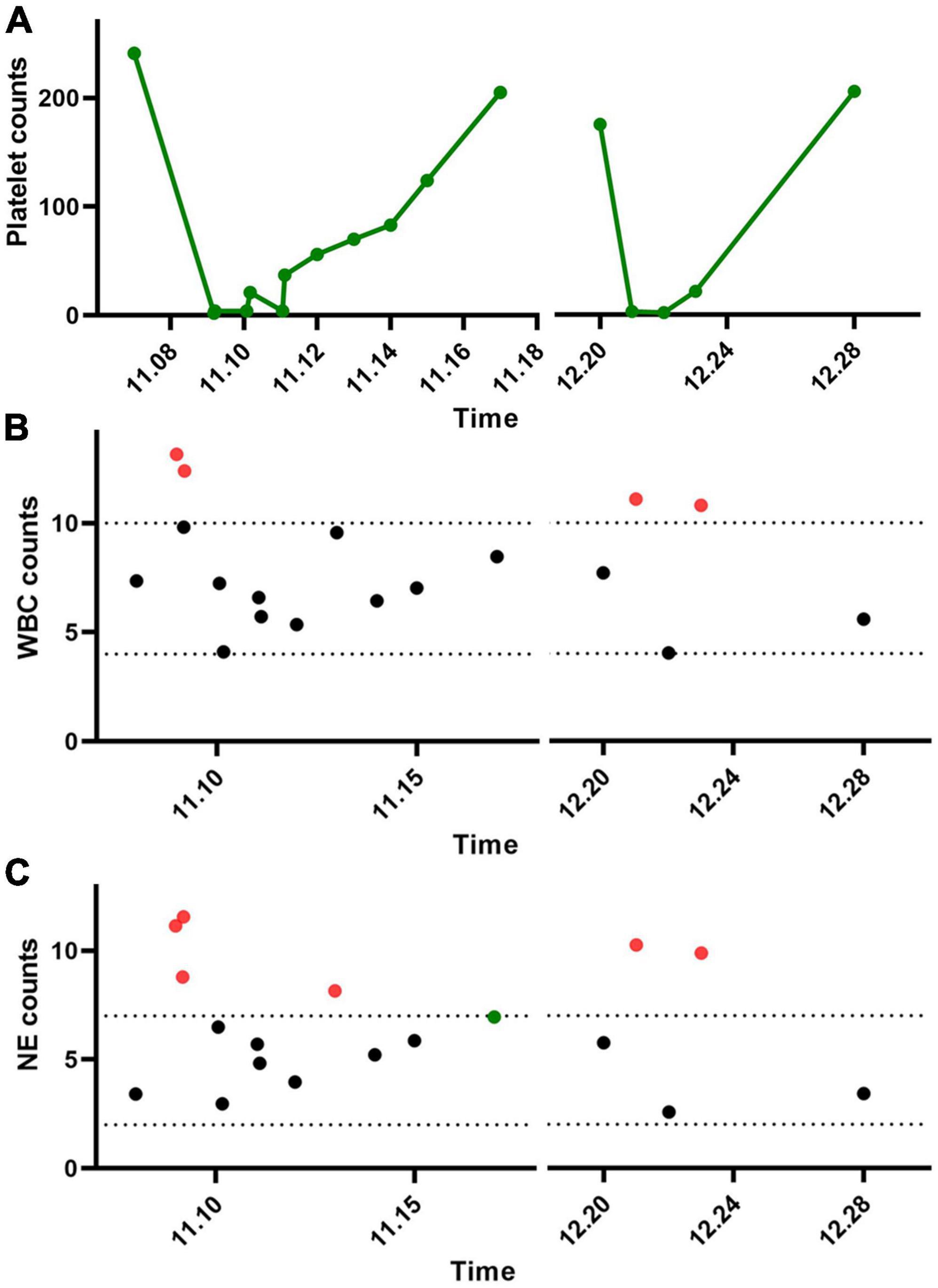
Figure 1. Changes in platelets and leukocytes after two trastuzumab treatments in the patient. (A) Platelets, (B) white blood cells, and (C) neutrophils.
One month later, the patient returned to the hospital for targeted therapy. Before treatment, routine blood tests were normal, platelet count was normal, and the four coagulation tests were normal. No other medication was given. A total of 6 mg/kg trastuzumab was administered intravenously. Gum bleeding with scattered petechiae and petechiae under the skin reappeared at 6 h. The routine blood test showed that the WBC count was 11.10 × 10^9/L, HGB count was 122 g/L, PLT count was 3 × 10^9/L, and NE count was 10.26 × 10^9/L (Figures 1A–C). The four coagulation tests showed normal. He improved after symptomatic treatment with human immunoglobulin, blood transfusion, dexamethasone, and recombinant human platelet thrombopoietin.
Since sharp increases in leukocytes and neutrophils in patients, it is hypothesized that the complement reaction is the main cause of the dramatic decrease in platelets. Therefore, the extracted platelets were subjected to a complement hemolysis assay (Figure 2). It was shown that a large number of platelets from the patient were destroyed after the addition of trastuzumab and complement. In contrast, platelets in other HER2 breast cancer patients maintained normal morphology.
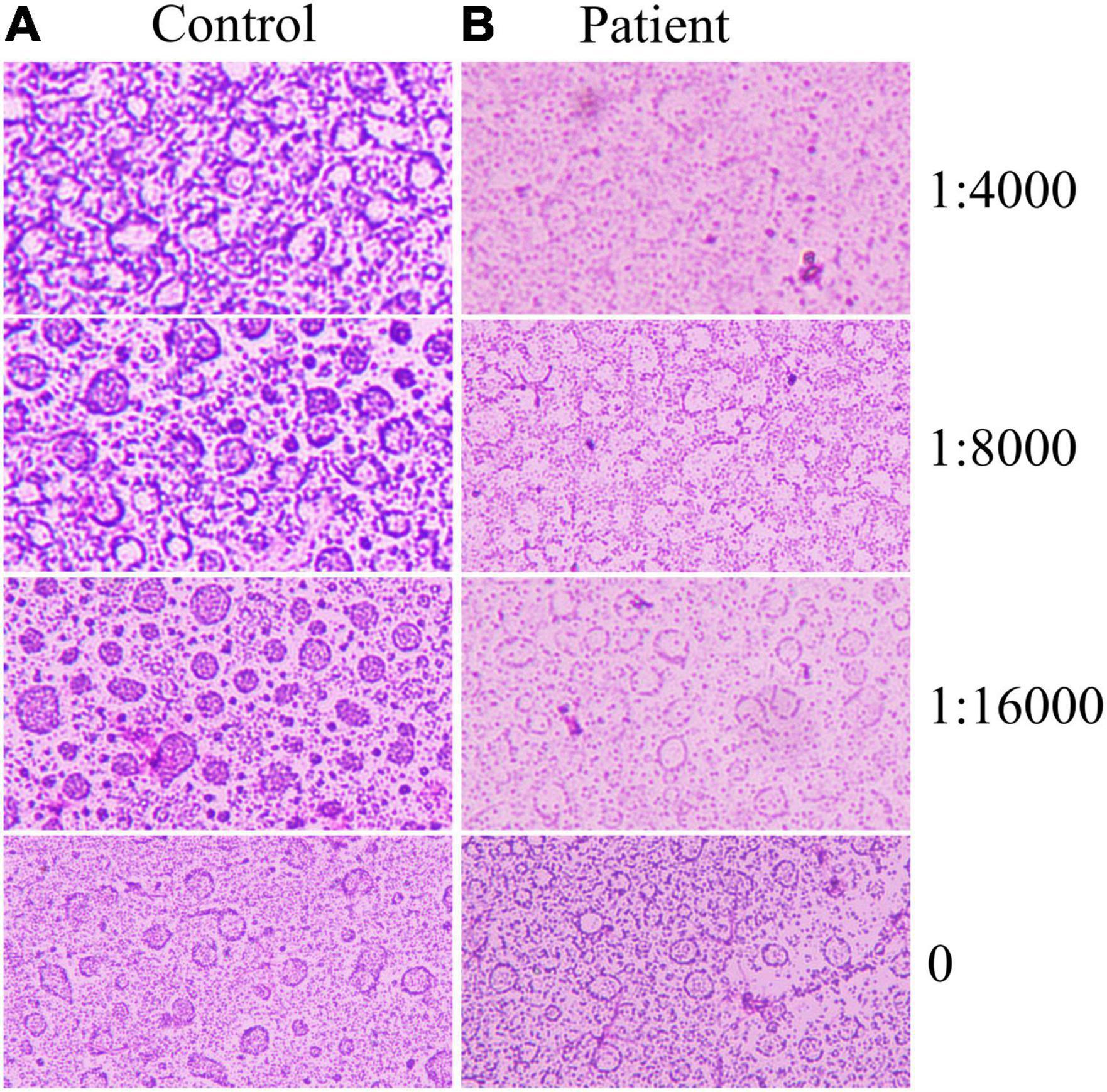
Figure 2. Platelet complement reaction. Trastuzumab was diluted 16,000, 8,000, and 4,000 times, and different samples of platelets reacted with biological complement. (A) Trastuzumab-treated controls without acute hemolysis. (B) Trastuzumab in patients with acute hemolysis.
The patient was rechecked every 3 months after discharge, and the blood routine, liver and kidney function, and tumor markers were normal. No further decline in platelets was detected. The patient’s compliance was good During the follow-up with no adverse or unexpected conditions.
Discussion
Multiple causes can lead to thrombocytopenia, such as bacterial and viral infections, liver disease, kidney disease, alcohol abuse, pregnancy, and the use of medications (11–15). Pharmacogenetic thrombocytopenia is a sharp drop in platelet count that occurs in patients during the use of drugs. Epidemiological studies have shown that the annual incidence of pharmacogenetic thrombocytopenia is 10 cases/1 million individuals (16). Patients with severe thrombocytopenia are at increased risk of microtraumatic bleeding and spontaneous bleeding, which endanger health. Drug-induced reduction in platelet count is mainly through two pathways: inhibition of platelet production, including inhibition of differentiation and maturation of megakaryocytes and mature shedding of platelets; induction of specific antibody production, which destroys platelets through an immune response (17). The case report demonstrated a patient with HER2 high expression breast cancer who developed symptoms of acute thrombocytopenic purpura after the use of trastuzumab.
Trastuzumab is a monoclonal antibody that blocks the growth of cancer cells by attaching to HER2 and preventing the binding of human epidermal growth factor to HER2 (18). Trastuzumab is mainly used clinically for metastatic breast cancer with overexpression of HER2 (18). Trastuzumab has been shown to inhibit the proliferation of HER2-overexpressing tumor cells both in vitro and in animal studies (19, 20). Additionally, trastuzumab is a potential mediator of antibody-dependent cell-mediated cytotoxicity (21, 22). In the present case report, trastuzumab was the drug of choice because the patient’s pathological findings showed a high expression of HER2.
Studies show that trastuzumab adverse reactions are mostly mild thrombocytopenia (23, 24), and the incidence of thrombocytopenia in treated patients is about 25 to 31%, with an incidence of grade ≥3 of about 2–15% (25). The risk of thrombocytopenia is higher in the Asian population, up to 52.5–69.8%, and the incidence of grade ≥3 is about 29.8–45% (25). Studies have shown that trastuzumab generally has the lowest platelet count on day 8 of dosing (26). At present, a few cases of acute thrombocytopenia induced by trastuzumab have been reported. The youngest patient was 29 years old and half of the patients were over 50 years old (27). Seven patients developed acute thrombocytopenia on the first dose of trastuzumab, most of which occurred within 24 h (9). A patient developed acute thrombocytopenia about 10 h after the first dose of trastuzumab (9). The rest of the patients developed symptoms at 1–10 days (10, 28, 29). Trastuzumab-induced acute thrombocytopenia resulted in petechiae, nosebleeds, dental bleeding, and uterine bleeding (10). The present case report demonstrated a patient with HER2-positive breast cancer who developed acute thrombocytopenia at about 6 h on the first dose of trastuzumab. Multiple drugs were administered concurrently with this treatment and it is not certain that the symptoms were induced by trastuzumab. The second treatment, with only trastuzumab, reappeared with acute thrombocytopenia at about 6 h, thus confirming that the symptoms were due to trastuzumab treatment. In this case, the patient had two consecutive episodes of thrombocytopenic purpura, the first of which could not be determined to be due to trastuzumab induction because of interference from other drugs. The second time only trastuzumab was used. Therefore, this case report clarifies that trastuzumab can induce thrombocytopenic purpura in some patients.
The mechanism of trastuzumab-associated thrombocytopenia has not been clarified. Studies have shown that it may be related to the endocytosis of trastuzumab in megakaryocytes (30). The intracellular release of trastuzumab after endocytosis affects the differentiation of megakaryocytes, ultimately leading to the impairment of megakaryocyte maturation and platelet generation (27). Neutrophils are important members of white blood cells and they can rapidly participate in early innate immune responses due to their accumulation in bone marrow reservoirs (31). After vascular injury and endothelial activation, P-selectin on the platelet surface interacts with P-selectin protein-ligand 1 expressed by neutrophils to activate neutrophils and improve their phagocytosis, lethality, and clearance rate (32, 33). Neutrophils interacting with platelets can phagocytose platelets and also promote the generation of neutrophil extracellular traps (NETs) (34). NETs provide a scaffold for thrombosis and promote thrombosis and coagulation (35). In the present case, the patient’s white blood cells and neutrophil levels increased dramatically during the initial phase of trastuzumab-induced thrombocytopenia. After the remission of symptoms, white blood cell and neutrophil levels returned to normal. Therefore, it is speculated that the dramatic increase in neutrophils may be associated with a sharp decrease in platelets, the mechanism of which deserves further investigation.
The complement system is an important part of innate immunity and consists of more than 40 activating proteins, regulators, and receptors on vascular cells (36). Activation of the complement system mediates the immune response and inflammatory reaction (37). Antigen-antibody complexes can activate complement, and the activated complement components can combine with antigen-antibody complexes, resulting in a series of immunological reactions, such as cytotoxic/bacteriolytic reactions and hemolytic reactions (36). In this case report, a complement reaction assay was performed with the patient’s consent. The results showed that the platelets of control patients (HER +, Treated with trastuzumab) did not decrease after the addition of trastuzumab. On the contrary, the platelets of the patient in this case decreased dramatically after the addition of low concentrations of trastuzumab. Since trastuzumab is a HER2-specific antibody, this result indirectly reflects that the patient’s platelets are enriched in HER2. Therefore, this case highlights that the acute thrombocytopenia may be due to the trastuzumab-HER2-mediated complement reaction.
Conclusion
This case report is the first to elucidate the risk of trastuzumab, a specific antibody to HER2 protein, to induce a complement response leading to acute thrombocytopenia. Therefore, the results of the case indicate that a simple platelet complement reaction is an option for analyzing the applicability of trastuzumab before HER2-positive breast cancer patients receive treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Hainan Medical College (2022kyl153). The patients/participants provided their written informed consent to participate in this study.
Author contributions
GC contributed to the funding acquisition and investigation. GD contributed to the methodology, project administration, resources, and writing – original draft and editing. HL and PF contributed to the software. JL and LD contributed to the conceptualization and formal analysis. PF contributed to the writing – original drafts and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kamal AM, Sakorikar T, Pal UM, Pandya HJ. Engineering approaches for breast cancer diagnosis: a review. IEEE Rev Biomed Eng. (2022) 1–21. doi: 10.1109/RBME.2022.3181700 [Epub ahead of print].
3. Luo M, Chen H, Deng H, Jin Y, Wang G, Zhang K, et al. Postmastectomy radiotherapy after neoadjuvant chemotherapy in cT1-2N+ Breast cancer patients: a single center experience and review of current literature. Front Oncol. (2022) 12:881047. doi: 10.3389/fonc.2022.881047
4. Gao C, Li H, Zhou C, Liu C, Zhuang J, Liu L, et al. Survival-associated metabolic genes and risk scoring system in HER2-positive breast cancer. Front Endocrinol. (2022) 13:813306. doi: 10.3389/fendo.2022.813306
5. Smolarz B, Nowak AZ, Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (review of literature). Cancers. (2022) 14:2569. doi: 10.3390/cancers14102569
6. Cargnin S, Shin JI, Genazzani AA, Nottegar A, Terrazzino S. Comparative efficacy and safety of trastuzumab biosimilars to the reference drug: a systematic review and meta-analysis of randomized clinical trials. Cancer Chemother Pharmacol. (2020) 86:577–88. doi: 10.1007/s00280-020-04156-3
7. Ciruelos E, Villagrasa P, Pascual T, Oliveira M, Pernas S, Pare L, et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA Trial. Clin Cancer Res. (2020) 26:5820–9. doi: 10.1158/1078-0432.CCR-20-0844
8. Wang X, Zhu X, Zou J, Zhang X, Kong X, Nie J. Severe thrombocytopenia induced by trastuzumab rechallenge: a case report and literature review. J Clin Pharm Ther. (2021) 46:1173–7. doi: 10.1111/jcpt.13406
9. Parikh O, Neave F, Palmieri C. Severe thrombocytopenia induced by a single infusion of trastuzumab. Clin Breast Cancer. (2008) 8:285–6. doi: 10.3816/CBC.2008.n.034
10. Mantzourani M, Gogas H, Katsandris A, Meletis J. Severe thrombocytopenia related to trastuzumab infusion. Med Sci Monit. (2011) 17:CS85–7. doi: 10.12659/msm.881838
11. Suzuki T, Sato Y, Sano K, Arashiro T, Katano H, Nakajima N, et al. Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J Clin Invest. (2020) 130:799–812. doi: 10.1172/JCI129171
12. Arora P, Belwal S, Uniyal B, Saxena S. Plasmapheresis in a case of acute kidney injury with severe hemolysis and thrombocytopenia due to hematotoxic (Russell’s viper) snake bite. Saudi J Kidney Dis Transpl. (2020) 31:276–80. doi: 10.4103/1319-2442.279953
13. Zhang YY, Liu DX, Fan WB, Yu JH, Liu L, Du WJ, et al. Hazardous alcohol consumption and aging synergistically increase the risk of death in patients with severe fever with thrombocytopenia syndrome. Ticks Tick Borne Dis. (2020) 11:101505. doi: 10.1016/j.ttbdis.2020.101505
14. Perez Botero J, Reese JA, George JN, McIntosh JJ. Severe thrombocytopenia and microangiopathic hemolytic anemia in pregnancy: a guide for the consulting hematologist. Am J Hematol. (2021) 96:1655–65. doi: 10.1002/ajh.26328
15. Song R, Chen Z, Li W. Severe fever with thrombocytopenia syndrome (SFTS) treated with a novel antiviral medication, favipiravir (T-705). Infection. (2020) 48:295–8. doi: 10.1007/s15010-019-01364-9
16. Wang Y, Song Z, Xu X, Wei X, Yuan H, Liang H, et al. Clinical symptoms associated with fatality of severe fever with thrombocytopenia syndrome: a systematic review and meta-analysis. Acta Trop. (2022) 232:106481. doi: 10.1016/j.actatropica.2022.106481
17. Takayama-Ito M, Saijo M. Antiviral drugs against severe fever with thrombocytopenia syndrome virus infection. Front Microbiol. (2020) 11:150. doi: 10.3389/fmicb.2020.00150
18. Xu H, Zhang H, Guo W, Zhong X, Sun J, Zhang T, et al. Safety and efficacy profile of trastuzumab deruxtecan in solid cancer: pooled reanalysis based on clinical trials. BMC Cancer. (2022) 22:923. doi: 10.1186/s12885-022-10015-6
19. Park S, Park JM, Park M, Ko D, Kim S, Seo J, et al. beta-Escin overcomes trastuzumab resistance in HER2-positive breast cancer by targeting cancer stem-like features. Cancer Cell Int. (2022) 22:289. doi: 10.1186/s12935-022-02713-9
20. Bloom MJ, Song PN, Virostko J, Yankeelov TE, Sorace AG. Quantifying the effects of combination trastuzumab and radiation therapy in human epidermal growth factor receptor 2-positive breast cancer. Cancers. (2022) 14:4234. doi: 10.3390/cancers14174234
21. Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. (2013) 11:307. doi: 10.1186/1479-5876-11-307
22. Collins DM, O’donovan N, McGowan PM, O’Sullivan F, Duffy MJ, Crown J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. (2012) 23:1788–95. doi: 10.1093/annonc/mdr484
23. Tang SC, Capra CL, Ajebo GH, Meza-Junco J, Mairs S, Craft BS, et al. Systemic toxicities of trastuzumab-emtansine predict tumor response in HER2+ metastatic breast cancer. Int J Cancer. (2021) 149:909–16. doi: 10.1002/ijc.33597
24. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. (2020) 38:1887–96. doi: 10.1200/JCO.19.02318
25. Bender BC, Schaedeli-Stark F, Koch R, Joshi A, Chu YW, Rugo H, et al. A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol. (2012) 70:591–601. doi: 10.1007/s00280-012-1934-7
26. Liu Z, Wang C, Chen X, Zhu J, Sun X, Xia Q, et al. Pathological response and predictive role of tumour-infiltrating lymphocytes in HER2-positive early breast cancer treated with neoadjuvant pyrotinib plus trastuzumab and chemotherapy (Panphila): a multicentre phase 2 trial. Eur J Cancer. (2022) 165:157–68. doi: 10.1016/j.ejca.2022.01.022
27. Zeng XQ, Jiang SS, Peng YY, Liu MF, Ye CS, Dong JY. Trastuzumab-induced severe thrombocytopenia: a case report and literature review. Chin Med Sci J. (2020) 35:377–82. doi: 10.24920/003799
28. Takano Y, Furune S, Miyai Y, Morita S, Inoue M, Shimokata T, et al. Drug-induced thrombocytopenia associated with trastuzumab in a patient with HER2-positive recurrent gastric cancer. Int Cancer Conf J. (2022) 11:67–70. doi: 10.1007/s13691-021-00520-z
29. Zhou Q, Dong J, Jiang X, Pan Y, Han X. Trastuzumab-induced thrombocytopenia after eight cycles of trastuzumab treatment. Open Med. (2020) 15:659–62. doi: 10.1515/med-2020-0201
30. Dieras V, Bachelot T. The success story of trastuzumab emtansine, a targeted therapy in HER2-positive breast cancer. Target Oncol. (2014) 9:111–22. doi: 10.1007/s11523-013-0287-4
31. Kumar V, Sharma A. Neutrophils: cinderella of innate immune system. Int Immunopharmacol. (2010) 10:1325–34. doi: 10.1016/j.intimp.2010.08.012
32. Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, et al. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood. (2009) 113:5254–65. doi: 10.1182/blood-2008-09-180794
33. Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. (1993) 74:541–54. doi: 10.1016/0092-8674(93)80055-j
34. Zucoloto AZ, Jenne CN. Platelet-neutrophil interplay: insights into neutrophil extracellular trap (NET)-driven coagulation in infection. Front Cardiovasc Med. (2019) 6:85. doi: 10.3389/fcvm.2019.00085
35. Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. (2012) 32:1777–83. doi: 10.1161/ATVBAHA.111.242859
36. Rus H, Cudrici C, Niculescu F. The role of the complement system in innate immunity. Immunol Res. (2005) 33:103–12. doi: 10.1385/IR:33:2:103
Keywords: breast cancer, HER2, trastuzumab, dramatic thrombocytopenia, complement response
Citation: Chen G, Ou J, Liu J, Liao H, Ding L, Fan P and Du G (2022) Acute thrombocytopenia induced by trastuzumab due to complement reaction: A case report. Front. Med. 9:1037493. doi: 10.3389/fmed.2022.1037493
Received: 05 September 2022; Accepted: 22 November 2022;
Published: 06 December 2022.
Edited by:
Mohammad Safiqul Islam, Noakhali Science and Technology University, BangladeshReviewed by:
Md. Siddiqul Islam, Southeast University, BangladeshZiyi Fu, Nanjing Medical University, China
Copyright © 2022 Chen, Ou, Liu, Liao, Ding, Fan and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guankui Du, ZHVndWFua3VpQDE2My5jb20=; Pingming Fan, ZnBtaGFpbmFuQDE2My5jb20=
 Guoping Chen1,2†
Guoping Chen1,2† Pingming Fan
Pingming Fan Guankui Du
Guankui Du