
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 07 December 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1035201
Cryptococcal meningitis is a common fungal infection of the central nervous system with high mortality and disability rates. A prominent clinical manifestation is persistent and severe cranial hypertension, which is one of the most critical outcome determinants in patients with cryptococcal meningoencephalitis. Herein, we report and discuss a case of cryptococcal meningitis treated by an inadequate course of medical therapy and placement of a ventriculoperitoneal shunt in a patient who survived for more than 10 years.
Cryptococcal meningitis is the most common fungal infection of the central nervous system (CNS) and has a high mortality rate; it is mainly caused by Cryptococcus neoformans or Cryptococcus gattii infection (1, 2). Cryptococcal meningitis can occur in AIDS patients and other immunocompromised persons. An estimated 223,100 cases of cryptococcal meningitis occur worldwide each year, resulting in approximately 181,100 deaths annually (3). Early detection and management of cryptococcal infection, a rapid reduction in fungal burden, and control of intracranial pressure can reduce mortality, whereas suboptimal antifungal treatment and immunological dysregulation can lead to treatment failure (4). Intracranial hypertension is a serious complication of cryptococcal meningitis, and cerebral edema caused by a rapid increase in intracranial pressure is an important predictor of a poor prognosis in the early stage of the disease (4, 5). More than half of patients with cryptococcal meningitis have an intracranial pressure exceeding 250 mmH2O, and aggressive control of intracranial pressure significantly improves survival odds (6, 7). The mechanism of intracranial hypertension may be mechanical obstruction of the outflow by Cryptococci that block the passage of CSF across the arachnoid villi; similarly, cryptococcal capsular polysaccharides can accumulate in the arachnoid villi and subarachnoid spaces, impairing the CSF drainage system (6). Herein, we report a case of cryptococcal meningitis that was treated with only fluconazole for several days and eventual ventriculoperitoneal shunt placement; the patient was infected with Cryptococcus for a long time without thorough treatment.
A 34-year-old man who was previously healthy and who did not have a medical history or previous medical care was admitted to the hospital on 13 January 2011, due to intermittent fever for a month. The peak axillary temperature was approximately 38.5°C, and the patient reported progressively worsening headache, malaise, and decreased appetite and wasting, followed by bilateral lower limb malaise, walking instability, blurred vision and memory loss for 10 days prior to admission.
Initially, the patient was admitted to the Department of Infectious Diseases. On examination, the patient was thin, had lost 7.5 kg, exhibited diminished lower extremity muscle power in both legs (4/5), was positive for the Kernig sign, answered questions incoherently, had papilledema, and had poor memory. The results of other neurological and other system examinations were normal.
The patient was negative for HIV and exhibited normal liver function. A routine blood examination revealed a hemoglobin level of 86 g/L. The cerebrospinal fluid (CSF) was colorless and clear, the white blood cell (WBC) count was 69*106/L, mononuclear cells were 85%, the glucose level was 0.82 mmol/L, the chloride level was 116.0 mmol/L, and India ink staining was positive (Table 1). Despite the CSF pressure exceeding 330 mmH2O, brain edema and hydrocephalus were not observed on cranial CT (Figure 1). CSF culture was negative.
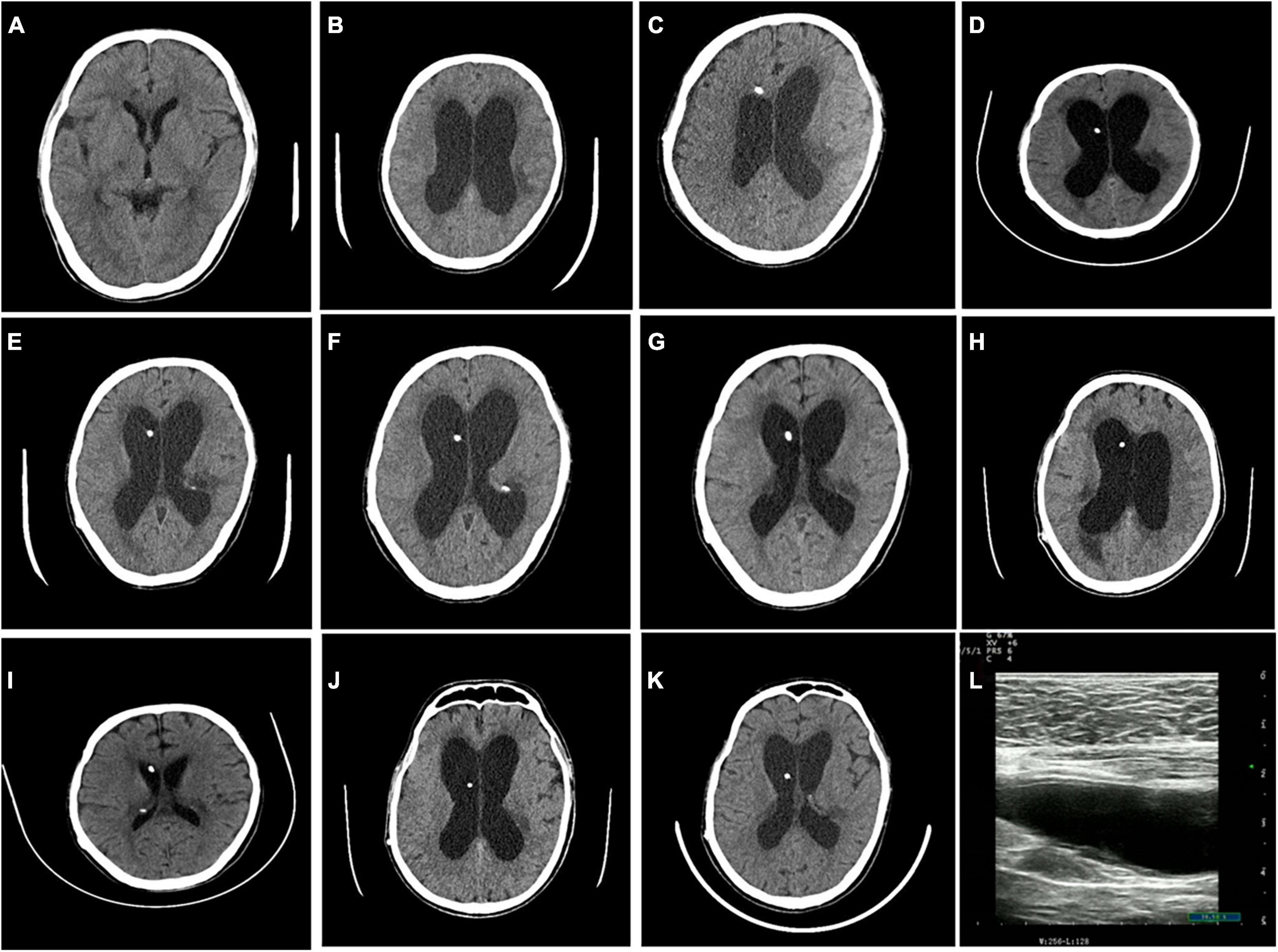
Figure 1. Cranial CT findings during the patient’s six hospitalizations. No hydrocephalus was observed during the first hospitalization (A). Hydrocephalus was visible before the operation during the second hospitalization (B), and hydrocephalus was relieved post-operatively (C). Hydrocephalus was visible before the operation during the third hospitalization (D), and hydrocephalus was relieved post-operatively (E). Hydrocephalus was visible before the operation during the fourth hospitalization (F), and hydrocephalus was relieved post-operatively (G). Hydrocephalus was visible before the operation during the fifth hospitalization (H), and hydrocephalus was relieved post-operatively (I). Hydrocephalus was visible before the operation during the sixth hospitalization (J), and hydrocephalus was relieved post-operatively (K). Abdominal ultrasonography before surgery suggested an intra-abdominal encapsulated effusion (L).
The patient’s family refused to accept the administration of amphotericin B liposomes or amphotericin B because of concerns about the side effects and financial costs of the drug. The patient was treated with intravenous fluconazole (400 mg/d), and cranial pressure was treated with intravenous mannitol and glycerol fructose. After the above treatments were administered, the patient’s body temperature normalized, and his neck stiffness was relieved. The symptoms of cranial hypertension, such as decreased visual acuity, papilledema, and weakness in both lower limbs, were significantly relieved. The patient’s family refused to continue treatment because of financial difficulties, and the patient was discharged on 24 January 2011.
After discharge, the patient did not follow the doctor’s advice regarding regular follow-ups and oral drug treatment. The patient self-treated at home with only intermittent oral herbal medicines of unknown compositions, and his condition did not improve. The patient was unable to perform daily physical activities due to remaining cognitive dysfunction and weakness in the lower limbs.
8 years later, the patient showed an obvious decline in cognition, an unstable walking gait, incontinence, nausea, frequent vomiting, blurred vision, and no fever for 20 days. Head computed tomography (CT) suggested hydrocephalus (Figure 1), and the patient was subsequently admitted to the Department of Neurosurgery for ventriculoperitoneal shunt placement on 22 February 2019. The CSF was colorless and clear, with a protein concentration of 1.5 g/L, a WBC count of 8*106/L, a glucose level of 0.26 mmol/l, and a chloride level of 117.30 mmol/L (Table 1). The CSF pressure exceeded 330 mmH2O before the operation (Table 1). CSF India ink staining and CSF culture were negative. The CSF results remained suggestive of intracranial infection. The patient’s symptoms improved significantly after surgery, so this patient refused further examination and treatment.
Due to recurrent mechanical shunt obstruction, the patient underwent ventriculoperitoneal shunt tube explorations in the neurosurgery department on 09 January 2020; 26 March 2020; and 29 September 2020 (Figure 1). The patient underwent shunt tube exploration in the neurosurgery department again on 29 November 2020, due to hydrocephalus caused by mechanical shunt obstruction (Figure 1). Abdominal ultrasonography before the operation suggested an intra-abdominal encapsulated effusion with a size of approximately 80 mm * 20 mm * 31 mm; the boundary was clear, and there was no blood-flow signal. CSF examination revealed a protein concentration of 4.45 g/L, a WBC count of 138*106/L, a glucose level of 0.95 mmol/L, and a chloride level of 114.50 mmol/L (Table 1). CSF India ink staining and CSF culture were negative, but the amount of C. neoformans capsular polysaccharide was 13.91 ng/ml in serum (lateral flow assay, normal range: 0–0.5 ng/ml). The results of metagenomic next-generation sequencing (mNGS) to detect pathogenic microbial DNA in the CSF revealed 12 C. neoformans-specific sequences; the procedure has been described elsewhere (8). The patient was then transferred to the neurology ward, and antifungal treatment was again recommended, but the patient’s family refused due to the excessive financial burden and possible serious side effects. Here, we present a detailed clinical timeline with the course of disease development (Figure 2).
The patient received a diagnosis of cryptococcal meningitis during the first hospitalization based on the symptoms of fever and cranial hypertension and positive CSF India ink staining, despite a negative CSF culture. The patient required several subsequent hospitalizations due to hydrocephalus and mechanical shunt obstruction, during which CSF protein concentrations, cell counts, glucose levels, and chloride level changes were suggestive of intracranial infection. C. neoformans capsular polysaccharide testing confirmed the presence of a large number of capsular polysaccharide antigens. Additionally, CSF mNGS detected C. neoformans-specific sequences, and no other secondary infections were identified. In China, C. neoformans is the most common pathogen of Cryptococcus infection (9), and this patient’s CSF mNGS results suggested that the pathogen was C. neoformans.
Current treatment guidelines recommend either amphotericin B plus flucytosine, amphotericin B liposomes plus flucytosine or fluconazole plus flucytosine during the induction phase for the treatment of cryptococcal meningoencephalitis in otherwise healthy people for at least 2 weeks (5, 10). Interestingly, the patient was treated with fluconazole for less than 2 weeks without consolidation and maintenance therapy but still survived for a long time. One reason for this is suggested by the fact that CSF culture results, which are an important indicator of the efficacy of induction-phase treatment, were consistently negative in this patient. Although fluconazole monotherapy can achieve good therapeutic effects, studies have shown that mortality in patients receiving this treatment regimen is more than 55% (11, 12), while the mortality rate in patients receiving the international standard induction treatment of amphotericin B plus flucytosine is 24.2% (13).
Although altered mental status, high CSF pressure, and positive India ink staining were considered risk factors for death due to cryptococcal meningitis in this patient, timely diagnosis and treatment may have contributed to this patient’s long-term survival (4, 14, 15).
In general, more than 50% of patients with cryptococcal meningitis have CSF pressures exceeding 250 mmH2O, and the risk of death can be reduced by controlling CSF pressure (7, 16). This patient was hospitalized several times for symptoms of cranial hypertension, including nausea, frequent vomiting, walking gait instability, incontinence, and blurred vision. The patient refused continuous antifungal therapy and had poor medical adherence, so a ventriculoperitoneal shunt was placed due to persistent cryptococcal infection. Studies have shown that ventriculoperitoneal shunt placement is a viable option in the presence of severe hydrocephalus with persistent cryptococcal infection (17, 18). The patient, despite benefiting from the alleviation of symptoms associated with persistent cranial hypertension by ventriculoperitoneal shunt placement, which may be one of the reasons for long-term survival, was hospitalized several times because of repeated mechanical shunt obstruction. According to a previous report, a high level of CSF polysaccharides may increase the risk of mechanical shunt obstruction (19).
It Is well known that capsular polysaccharides may still be present for an indeterminate period of time in patients who have recovered from C. neoformans infection, so a positive capsular polysaccharide test does not serve as strong evidence of active cryptococcal infection, especially when initial capsular polysaccharide levels are not known. However, the detection of high concentrations of capsular polysaccharides can often reflect the corresponding cryptococcal load (6). Little is known about how long the genetic material of pathogens can remain in the CSF of rehabilitated patients, so active cryptococcal infection could not be unequivocally diagnosed according to the CSF mNGS results in this patient, but it is extremely unlikely that cryptococcal genetic material from a previous infection could still be detected more than 10 years later. Additionally, a previous study suggested that when a C. neoformans-specific sequence read number was ≥2 on mNGS, a positive diagnosis of cryptococcal meningitis could be made, as the sensitivity and specificity were 76.92 and 99.52% (20), respectively. CSF glucose and chloride level changes in this patient also strongly suggested the presence of this specific intracranial infection.
Previous studies have reported that more than 65% of patients with cryptococcal meningitis in China are immunocompetent (21). However, recent findings have revealed that some underlying immunogenetic functional defects may exist in these immunocompetent individuals (22, 23). This patient could have been characterized in the past as immunocompetent but did not have the particular test results needed to clarify the presence of some level of immunodeficiency.
To the best of our knowledge, the longest duration of infection following shunt placement was more than two decades (24), but persistent, long-term cryptococcal meningitis has rarely been reported. The length of time that the patient received antifungal therapy and the remaining symptoms after discharge implied that this patient’s cryptococcal meningitis was probably never completely cured. It did not resemble recurrent cryptococcal infection; rather, it is likely that this patient experienced persistent cryptococcal infection. To our knowledge, this is the first case of persistent infection with long-term survival. The reason for this may be due to a somewhat delicate immune response balance between the fungus and the host (4). This immune response may not be sufficient to completely clear the fungus, but it may be sufficient to prevent the fungus from causing extensive injury to the host.
In conclusion, in patients with cryptococcal infection who have limiting conditions that preclude the use of internationally standard treatment options, active intracranial pressure management, such as dehydrating drugs, lumbar puncture, or ventriculoperitoneal shunt placement, may effectively improve clinical symptoms and prolong survival time.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the report of a single clinical case here in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was supported by the Hainan Provincial Natural Science Foundation of China (No. 819QN348) and the Innovation Platform for Academicians of Hainan Province.
We thank Hugobiotech Co., Ltd., Beijing, China, for sharing their mNGS procedure and providing the sequencing data for this case.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Maziarz E, Perfect J. Cryptococcosis. Infect Dis Clin North Am. (2016) 30:179–206. doi: 10.1016/j.idc.2015.10.006
2. May R, Stone N, Wiesner D, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. (2016) 14:106–17. doi: 10.1038/nrmicro.2015.6
3. Rajasingham R, Smith R, Park B, Jarvis J, Govender N, Chiller T, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. (2017) 17:873–81. doi: 10.1016/S1473-3099(17)30243-8
4. Stott K, Loyse A, Jarvis J, Alufandika M, Harrison T, Mwandumba H, et al. Cryptococcal meningoencephalitis: time for action. Lancet Infect Dis. (2021) 21:e259–71. doi: 10.1016/S1473-3099(20)30771-4
5. Perfect J, Dismukes W, Dromer F, Goldman D, Graybill J, Hamill R, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. (2010) 50:291–322. doi: 10.1086/649858
6. Graybill J, Sobel J, Saag M, Van Der Horst C, Powderly W, Cloud G, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID mycoses study group and AIDS cooperative treatment groups. Clin Infect Dis. (2000) 30:47–54. doi: 10.1086/313603
7. Rolfes M, Hullsiek K, Rhein J, Nabeta H, Taseera K, Schutz C, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. (2014) 59:1607–14. doi: 10.1093/cid/ciu596
8. Yu L, Zhang Y, Zhou J, Zhang Y, Qi X, Bai K, et al. Metagenomic next-generation sequencing of cell-free and whole-cell DNA in diagnosing central nervous system infections. Front Cell Infect Microbiol. (2022) 12:951703. doi: 10.3389/fcimb.2022.951703
9. Fan X, Xiao M, Chen S, Kong F, Dou H, Wang H, et al. Predominance of Cryptococcus neoformans var. grubii multilocus sequence type 5 and emergence of isolates with non-wild-type minimum inhibitory concentrations to fluconazole: a multi-centre study in China. Clin Microbiol Infect. (2016) 22:887.e1–9. doi: 10.1016/j.cmi.2016.07.008
10. Izumikawa K, Kakeya H, Sakai F, Shibuya K, Sugita T, Takazono T, et al. Executive summary of JSMM clinical practice guidelines for diagnosis and treatment of cryptococcosis 2019. Med Mycol J. (2020) 61:61–89. doi: 10.3314/mmj.20.001
11. Gaskell K, Rothe C, Gnanadurai R, Goodson P, Jassi C, Heyderman R, et al. A prospective study of mortality from cryptococcal meningitis following treatment induction with 1200 mg oral fluconazole in Blantyre, Malawi. PLoS One. (2014) 9:e110285. doi: 10.1371/journal.pone.0110285
12. Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. (2008) 47:1556–61. doi: 10.1086/593194
13. Molloy S, Kanyama C, Heyderman R, Loyse A, Kouanfack C, Chanda D, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. (2018) 378:1004–17. doi: 10.1056/NEJMoa1710922
14. Lee Y, Wang J, Sun H, Chen Y. Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect. (2011) 44:338–45. doi: 10.1016/j.jmii.2010.08.011
15. Zuger A, Louie E, Holzman R, Simberkoff M, Rahal J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. (1986) 104:234–40. doi: 10.7326/0003-4819-104-2-234
16. Kambugu A, Meya D, Rhein J, O’Brien M, Janoff E, Ronald A, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. (2008) 46:1694–701. doi: 10.1086/587667
17. Liu L, Zhang R, Tang Y, Lu H. The use of ventriculoperitoneal shunts for uncontrollable intracranial hypertension in patients with HIV-associated cryptococcal meningitis with or without hydrocephalus. Biosci Trends. (2014) 8:327–32. doi: 10.5582/bst.2014.01070
18. Corti M, Priarone M, Negroni R, Gilardi L, Castrelo J, Arechayala A, et al. Ventriculoperitoneal shunts for treating increased intracranial pressure in cryptococcal meningitis with or without ventriculomegaly. Rev Soc Bras Med Trop. (2014) 47:524–7. doi: 10.1590/0037-8682-0176-2013
19. Cherian J, Atmar R, Gopinath S. Shunting in cryptococcal meningitis. J Neurosurg. (2016) 125:177–86. doi: 10.3171/2015.4.JNS15255
20. Xing X, Zhang J, Ma Y, He M, Yao G, Wang W, et al. Metagenomic next-generation sequencing for diagnosis of infectious encephalitis and meningitis: a large, prospective case series of 213 patients. Front Cell Infect Microbiol. (2020) 10:88. doi: 10.3389/fcimb.2020.00088
21. Zhu L, Wu J, Xu B, Ou X, Zhang Q, Weng X. Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997-2007. Med Mycol. (2010) 48:570–9. doi: 10.3109/13693780903437876
22. Ou X, Wu J, Zhu L, Guan M, Xu B, Hu X, et al. Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. J Infect Dis. (2011) 203:1686–91. doi: 10.1093/infdis/jir152
23. Hu X, Wu J, Zhu L, Wang X, Xu B, Wang R, et al. Association of Fcγ receptor IIB polymorphism with cryptococcal meningitis in HIV-uninfected Chinese patients. PLoS One. (2012) 7:e42439. doi: 10.1371/journal.pone.0042439
Keywords: cryptococcal meningitis, hydrocephalus, ventriculoperitoneal shunt, CSF pressure, standardized medical therapy
Citation: Hu S, Liu T, Huang S and Liang H (2022) Management of long-term cryptococcal meningitis neoformans in a surviving patient: A case report. Front. Med. 9:1035201. doi: 10.3389/fmed.2022.1035201
Received: 02 September 2022; Accepted: 21 November 2022;
Published: 07 December 2022.
Edited by:
Michal Adam Olszewski, University of Michigan, United StatesReviewed by:
Stephen Vreden, Academic Hospital Paramaribo, SurinameCopyright © 2022 Hu, Liu, Huang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liang, MTg2ODk1MzM5MjNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.