- 1Department of Pathology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2The Key Laboratory of Experimental Teratology, Ministry of Education, Department of Pathology, School of Basic Medical Sciences, Shandong University, Jinan, China
- 3Department of Pathology, Qilu Hospital, Shandong University, Jinan, China
Primary thyroid osteosarcoma is an extremely rare tumor which is associated with a poor prognosis. In this study, we describe an additional case. A 4.5 × 3.8 cm irregular heterogeneous nodule was examined in the left thyroid gland of a 72-year-old woman. Cytological smears and histopathological specimens showed typical features of osteosarcoma with a neoplastic lesion rich in spindle cells with occasional multinucleated cells and lace-like osteoid matrix. Negative immunoreaction with epithelial markers and positive immunoreaction with SATB2 and low Ki-67 labeling index suggested the diagnosis of osteosarcoma. Multiple KMT2C gene mutations determined by next-generation sequencing further confirmed the diagnosis.
Introduction
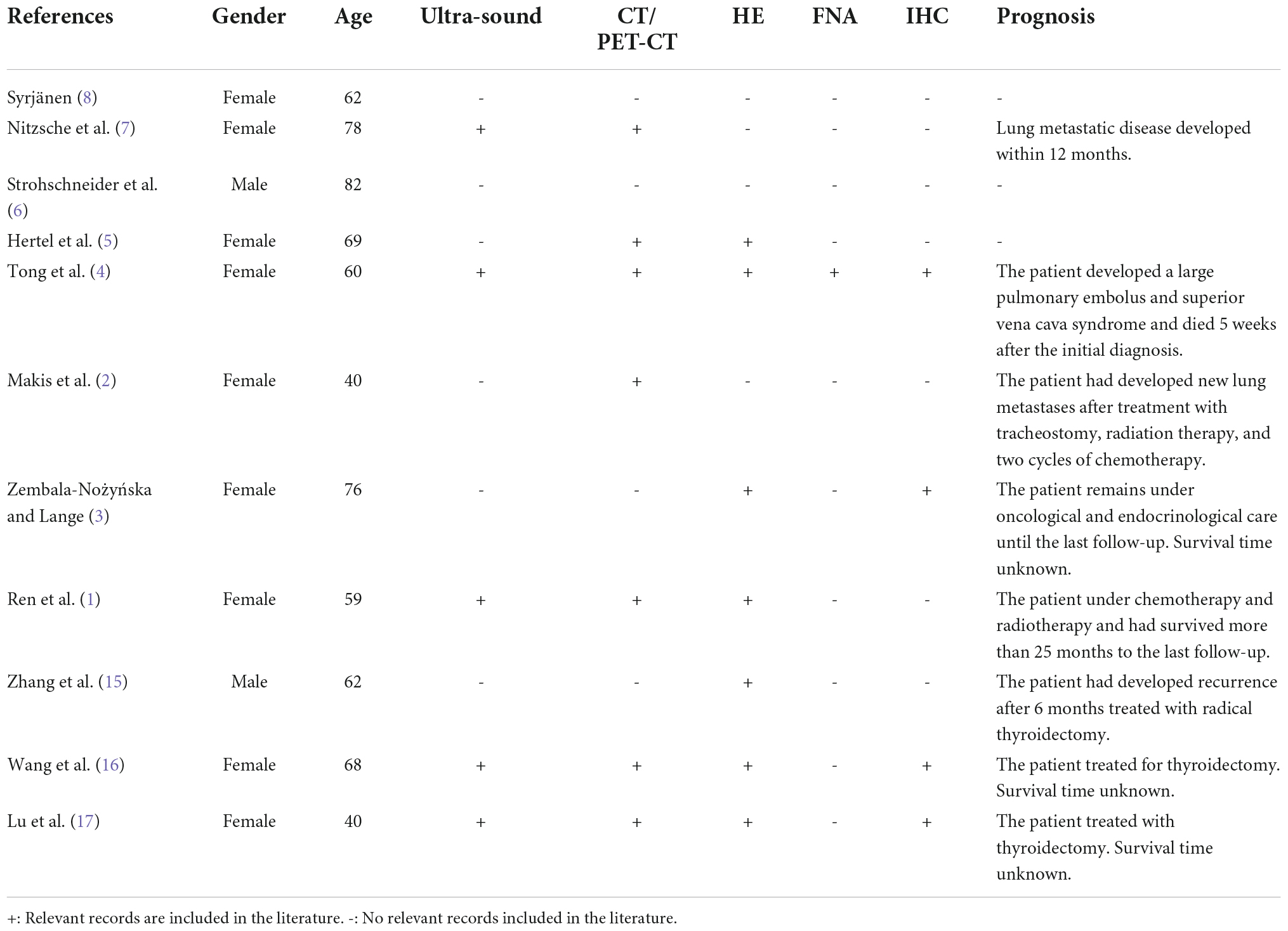
A 72-year-old woman presented with complaints of a painless rapidly growing mass in the neck. No family history of thyroid cancer was documented. Ultrasonic examination revealed a 4.5 × 3.8 cm irregular heterogeneous nodule in the left lobe with multiple patchy calcifications and strong echoes (TI-RADS: 4) without lymph node involvement (Figure 1A). PET-CT scan showed increased metabolism of FDG in the left thyroid lobe (maximum cross section: 4.5 × 4.2 cm; SUVmax: 17.2) with highly heterogeneous density and calcification foci. Multiple masses with high uptake of FDG were observed in the lung (maximum lung window: 4.0 × 2.2 cm; SUVmax: 21.0) but not in the other organs including bones, joints, and soft tissue (Figure 1B).
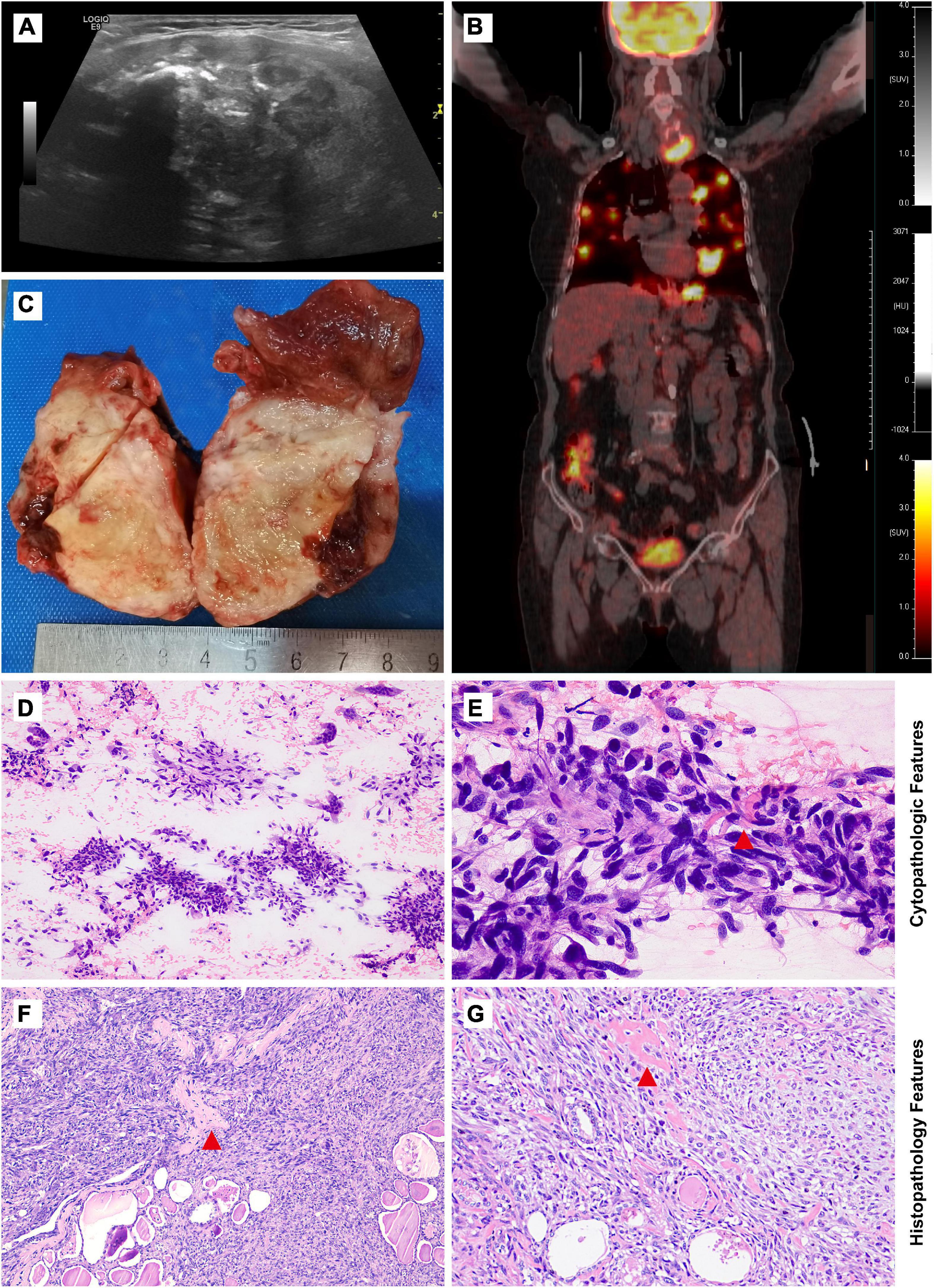
Figure 1. Imaging studies, macroscopic, cytological, and histological features of thyroid osteosarcoma. (A) Ultrasonic examination revealed an irregular heterogeneous nodule in the left lobe of the thyroid. (B) PET-CT scan showed increased metabolism of FDG in the nodle. (C) Grey-yellow nodule infiltrating normal thyroid tissue could be seen grossly. (D,E) Cytological features from low magnification in (D) and high magnification in (E). (F,G) Morphological features from low magnification in (F) and high magnification in (G). Red tangle: Pink lace-like osteoid matrix in cell smear and tissue section.
Case presentation
Cytopathologic and histopathological features
UG-FNA was performed. Cell smear specimen was investigated to be rich in atypical spindle tumor cells with a few multi-nucleated cells and mitosis. No necrosis or inflammation was observed in the background. A pink lace-like osteoid matrix suggested the diagnosis of osteosarcoma (Figures 1D,E, Supplementary Figure 2). A total thyroidectomy was performed with a gray or yellow section cutting plane (Figure 1C). Morphologically, the tumor is composed of infiltrative diffuse spindle cells, sparse multi-nucleated giant cells, and an osteoid matrix, which indicated differentiation toward osteosarcoma (Figures 1F,G). No tumor thrombi and necrosis were identified.
Immunohistochemical features
The tumor cells were positive for SATB2, vimentin, and negative for cytokeratin, calcitonin, chromogranin A, synaptophysin, TTF-1, TG, S100, ALK, CD1α, CD34, and CD99 (Figure 2, Supplementary Figure 1). The Ki-67 labeling index ranges between 10 and 15%, which is unusual in anaplastic thyroid carcinoma (ATC) (nearly always higher than 30%).
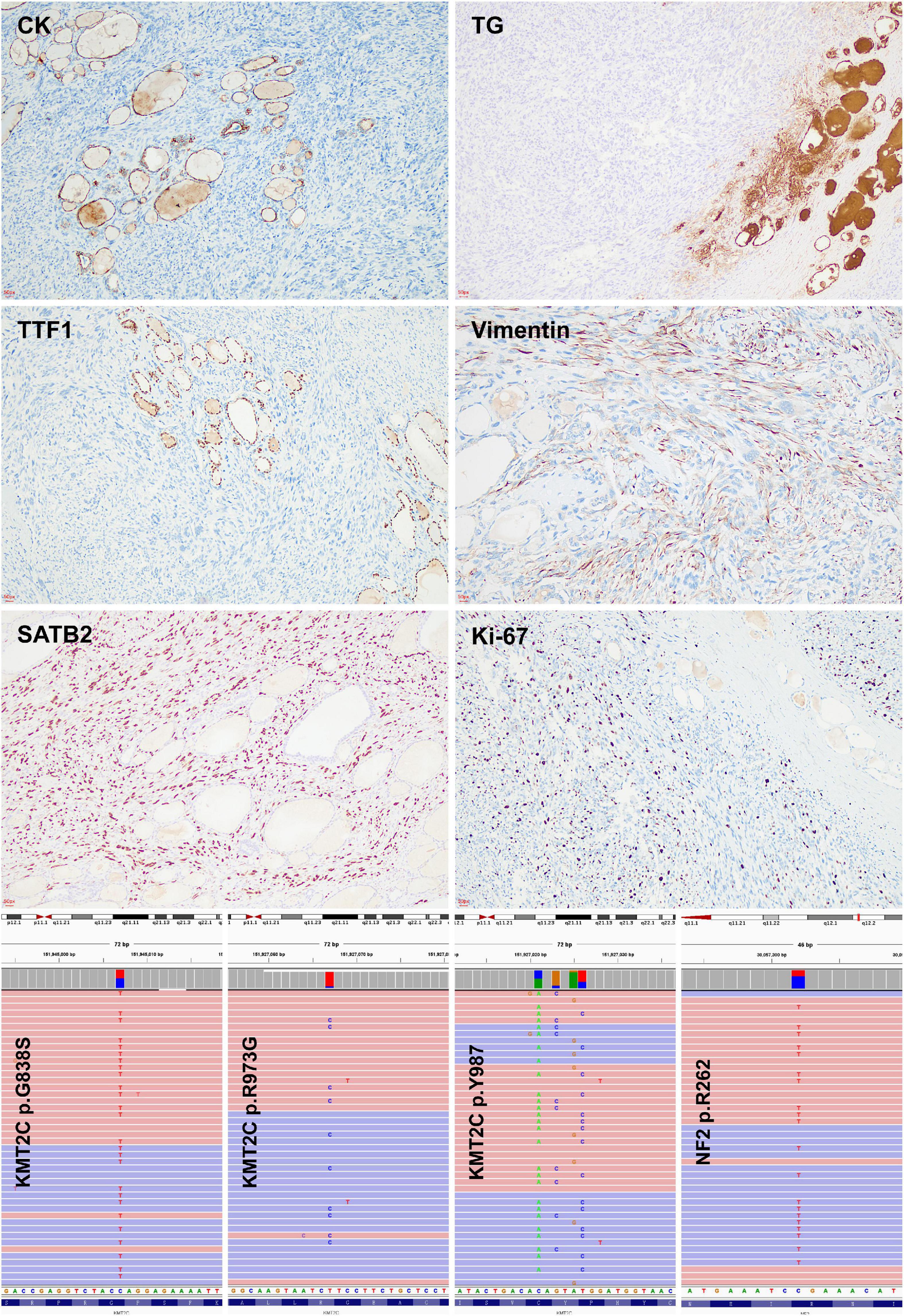
Figure 2. Immunohistochemical and molecular features of thyroid osteosarcoma. Negative immunoreaction with cytokeratin, thyroglobulin, and TTF-1 indicated the non-thyroid follicular origination of the tumour. Positive immunoreaction with SATB-2 and low ki67 labeling index suggested the diagnosis of osteosarcoma. KMT2C mutations were detected by targeted next generation sequencing.
Molecular feature
A targeted next-generation sequencing using a 128-gene panel disclosed pathogenic mutations of KMT2C (G838S, Y987*, R973G) and NF2 R262* (Figure 2), but not BRAF, RAS, RET, TP53, and TERT promoter alterations (Supplementary Table 1).
Treatment and patient outcome
A total thyroidectomy followed by four courses of first-line chemotherapy regimen of cisplatin and doxorubicin (AP regimen). The patient died of pulmonary metastasis 11 months after surgery.
Discussion
Osteosarcoma occurs most commonly in the long bones of teenagers and young adults. The extraosseous form of osteosarcoma accounts for less than 5% of all osteosarcomas, especially in the malignant non-epithelial neoplasms of the thyroid and has been reported in only several cases (1–10). Clinically, primary osteosarcoma of the thyroid presents as an aggressive, rapidly growing thyroid mass with a poor prognosis (4). The diagnosis of this rare entity has always been a challenge, for which an overall evaluation must be performed. Here, we report primary osteosarcoma of the thyroid with clinicopathological, cytopathologic, immunohistochemical, and molecular characteristics. Although morphological features have been discussed in the previous studies (1–4), there have been no reports on molecular characteristics of thyroid malignancy with osteoid differentiation. This report is helpful for a better understanding of primary thyroid osteosarcoma and provides the basis for clinical precision therapies.
For the literature review, PubMed and CNKI were searched using the terms: Thyroid Osteosarcoma. The search covered publications in the past century. Only studies describing primary tumors were considered and 11 full-length articles were identified. Only gender/age and final diagnosis were described in some cases, whereas thorough information regarding these cases is limited. Availability of detailed information regarding the reviewed cases is presented in Table 1. The cases consisted of 9 females and 2 males (1–10). The age distribution ranges from 40 to 82 years, which is an older group considering the conventional osteosarcoma. This tumor is characterized by fast growth with pressure-related symptoms of dyspnea and is highly aggressive with invading the surrounding tissue (3). Radical thyroidectomy was performed, combined with postoperative local radiotherapy and/or systemic chemotherapy, for the highly malignant and local recurrence rate. And it is prone to hematogenous metastasis to the lungs, but rarely to cervical lymph nodes. Most patients die of superior vena cava syndrome caused by pulmonary metastasis and extensive invasion of large vessels in the neck. It has been diagnosed in only two cases with fine needle aspiration biopsy and immunohistochemical analysis, but there is no information with molecular characteristics (4, 8). For patients, timely diagnosis and immediate postoperative radiotherapy and chemotherapy can prolong survival time.
Study images showed no lesions in the bones and joints, which supported the thyroid to be the primary site of the tumor in the present case. SATB2 is an emerging immunohistochemical marker of osteoblastic differentiation (Supplementary Figure 2), which is used frequently in the diagnosis of osteosarcoma. The differential diagnosis includes ATC, medullary thyroid carcinoma (MTC), and mesenchymal tumors, in which osteoid and/or cartilage tissue differentiation is occasionally found. ATC could be ruled out morphologically and immunohistochemically of the relatively low Ki-67 labeling index and negative cytokeratin. Negative immunoreaction with neuroendocrine markers, calcitonin, and TTF-1 was helpful to rule out MTC. No expression of S100, CD1α, ALK, CD34, and CD99 was helpful in excluding follicular dendritic cell sarcoma, inflammatory myofibroblastic tumor, angiosarcoma, and synovial sarcoma.
KMT2C encodes histone methyltransferase, which regulates gene transcription by modifying chromatin structure. It is essential for embryonic development and cell proliferation. KMT2C mutations were observed in 87% of osteosarcoma and may be related to the carcinogenesis of osteosarcoma, among which R973G somatic mutation was the most common variant (11). Mutated KMT2C can modify its cooperation with estrogen receptors and then affect bone remodeling and matrix production (11–13). In the current case, four variants of KMT2C mutations were detected, including R973G, and no alteration of BRAF, RAS, and RET genes was found, which indicated that it is not a follicular cell-derived thyroid carcinoma but osteosarcoma (14). KMT2C mutation should be related to the tumorigenesis of thyroid osteosarcoma.
In summary, we demonstrated KMT2C mutation in thyroid osteosarcoma for the first time. Given the wide morphologic spectrum of osteosarcoma and the propensity to mimic other spindle cell neoplasms, osteosarcoma should be considered in the differential diagnosis of poorly differentiated and anaplastic thyroid malignancies with osteoid differentiation. The osteoid matrix and SATB2 are useful characteristics to confirm the diagnosis. The finding of KMT2C mutation in thyroid osteosarcoma suggested the possibility of same driver mutation in extraosseous and intraosseous osteosarcomas, which may have important targeted therapy implications.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of the School of Basic Medical Science of Shandong University (protocol code ECSBMSSDU2019-1-024). Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this study.
Author contributions
ZL: conceptualization, methodology, software, formal analysis, investigation, resources, project administration, and funding acquisition. XW: writing—original draft preparation and visualization. ZL and BH: validation and supervision. ZL and PS: data curation. XW and QW: writing—review and editing. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 81972500), the Natural Science Foundation of Shandong Province, China (Grant No. ZR2019MH024), and clinical research funds from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (ZL 2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1030888/full#supplementary-material
References
1. Ren BX, Liu XL, Liu DS, Jing GW, Han S, Xiong L, et al. [Chronic lymphocytic thyroiditis accompanied with primary osteosarcoma of thyroid : a case report and literature review]. Zhonghua Zhong Liu Za Zhi. (2018) 40:967–8. doi: 10.3760/cma.j.issn.0253-3766.2018.12.014
2. Makis W, Novales-Diaz JA, Hickeson M. Primary thyroid osteosarcoma: staging and evaluation of response to therapy with F-18 Fdg Pet-Ct. Clin Nucl Med. (2010) 35:517–20. doi: 10.1097/RLU.0b013e3181e05f30
3. Zembala-Nożyńska E, Lange D. Primary osteosarcoma of the thyroid gland – a case report. Contemp Oncol. (2013) 17:97–9. doi: 10.5114/wo.2013.33783
4. Tong GX, Hamele-Bena D, Liu JC, Horst B, Remotti F. Fine-needle aspiration biopsy of primary osteosarcoma of the thyroid: report of a case and review of the literature. Diagn Cytopathol. (2008) 36:589–94. doi: 10.1002/dc.20840
5. Hertel V, Basten O, Bockmühl U. [Extraosseous osteosarcoma of the thyroid gland]. Laryngorhinootologie. (2006) 85:913–6. doi: 10.1055/s-2006-925096
6. Strohschneider T, Wirth W, Holzwarth M, Timm D. [Primary Osteosarcoma of the Thyroid Gland]. Zentralbl Chir. (1999) 124:840–2.
7. Nitzsche EU, Seeger LL, Klosa B, Freudenberg N, Moser EA. Primary osteosarcoma of the thyroid gland. J Nucl Med. (1992) 33:1399–401.
8. Syrjänen KJ. Fine needle aspiration cytology of the thyroid osteosarcoma. report of a case. J Cancer Res Clin Oncol. (1980) 96:319–23. doi: 10.1007/bf00408105
9. Syrjänen KJ. An osteogenic sarcoma of the thyroid gland (report of a case and survey of the literature). Neoplasma. (1979) 26:623–8.
10. Lang E, De Nunno R. [A case of primary osteogenetic sarcoma of the thyroid gland. (Clinico-statistical contribution)]. Minerva Chir. (1963) 18:141–4.
11. Chiappetta C, Mancini M, Lessi F, Aretini P, De Gregorio V, Puggioni C, et al. Whole-exome analysis in osteosarcoma to identify a personalized therapy. Oncotarget. (2017) 8:80416–28. doi: 10.18632/oncotarget.19010
12. Chiappetta C, Carletti R, Della Rocca C, Di Cristofano C. Kmt2c modulates migration and invasion processes in osteosarcoma cell lines. Pathol Res Pract. (2019) 215:152534. doi: 10.1016/j.prp.2019.152534
13. Faundes V, Newman WG, Bernardini L, Canham N, Clayton-Smith J, Dallapiccola B, et al. Histone lysine methylases and demethylases in the landscape of human developmental disorders. Am J Hum Genet. (2018) 102:175–87. doi: 10.1016/j.ajhg.2017.11.013
14. Asa SL, Baloch ZW, De Krijger R,R, Erickson LA, Ghossein RA, Hyrcza MD, et al. Who Classification of Tumours Series, Endocrine and Neuroendocrine Tumours. 5th ed. Lyon: International Agency for Research on Cancer (2022).
16. Wang W, Wang Y, Hao Z. Primary osteosarcoma of thyroid: A case report. Chin J Clin Oncol. (2015) 42:372. doi: 10.3969/j.issn.1000-8179.20142080. [in Chinese]
Keywords: thyroid, osteosarcoma, SATB2, KMT2C, mutation
Citation: Wang X, Wang Q, Su P, Chen C, Han B and Liu Z (2022) KMT2C mutation is a diagnostic molecular marker for primary thyroid osteosarcoma: A case report and literature review. Front. Med. 9:1030888. doi: 10.3389/fmed.2022.1030888
Received: 29 August 2022; Accepted: 19 October 2022;
Published: 08 November 2022.
Edited by:
Weiren Luo, The Second Affiliated Hospital of Southern University of Science and Technology, ChinaReviewed by:
Kennichi Kakudo, Izumi City General Hospital, JapanBerrin Çetinarslan, Kocaeli University, Turkey
Copyright © 2022 Wang, Wang, Su, Chen, Han and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyan Liu, emhpeWFubGl1QHNoc211LmVkdS5jbg==
†ORCID: Qianqian Wang, orcid.org/0000-0003-2291-8595
 Xinpei Wang
Xinpei Wang Qianqian Wang
Qianqian Wang Peng Su
Peng Su Chunyan Chen
Chunyan Chen Bo Han
Bo Han Zhiyan Liu
Zhiyan Liu