- IRCCS – Fondazione Bietti, Rome, Italy
Purpose: The purpose of the present study was to measure in central serous chorioretinopathy (CSC) the salivary cortisol awake response (CAR) delta percentage (Δ%) variation, a distinct and robust indicator of cortisol rhythm during wakefulness, commonly proposed as a marker of hypothalamic-pituitary adrenal (HPA) axis activity, whose alteration is frequently associated with several adverse health outcomes.
Methods: In the present cross-sectional observational study, salivary CAR Δ% variation was assessed in 17 adult male subjects affected by acute naïve CSC and compared to 17 matched healthy controls. Choroid vasculature metrics were assessed in the study population by measuring the subfoveal choroidal thickness (FCT) and the choroidal vascularity index (CVI) by the imaging technique of enhanced-depth imaging spectral-domain optical coherence tomography (EDI-SD-OCT). Furthermore, flow signal void area features of the choriocapillaris were evaluated in the study population using OCT angiography (OCTA).
Results: Both the control and CSC groups showed a physiological cortisol increase that occurred during the first 30 min after awaking. However, CSC adult male patients showed remarkably blunted CAR Δ% variation in comparison with controls, which might reflect a CSC-related imbalance of HPA axis activity. Statistically significant correlations were shown by Pearson’s correlation test between salivary CAR Δ% and the selected choroidal and choriocapillaris imaging biomarkers (FCT, CVI, and flow signal void area) in the study population.
Conclusion: In conclusion, alterations of the CAR Δ% increase, associated with choroidal-retinal metrics, might provide a window into the physiopathology of acute CSC, suggesting a possible common factor to explain the association between stress and CSC.
Introduction
Central serous chorioretinopathy (CSC) is characterized by thickening of the choroid and abnormal choroidal circulation, which lead to impairment of the retinal pigment epithelium (1). CSC occurs most frequently in midlife and is more common in men than in women (2, 3). Among the several potential causes CSC-related, exposure to high levels of endogenous or exogenous glucocorticoids is considered one of the most important (4, 5). Furthermore, it has been shown that CSC patients have high daily cortisol levels, which reflect hypothalamus-pituitary-adrenal (HPA) axis hyperactivity (5–8).
The links between dysfunction of the HPA axis leading to alterations in diurnal salivary cortisol production and health consequences have already been studied for various medical conditions, including hypertension, burnout, emotional stress, nocturnal apnea, and eating behavior (9–16).
However, the mechanisms by which stress hormone overactivity and allostatic overload could lead to the choroidal hyperpermeability seen in CSC still warrant further investigation (5, 17–19).
A distinct and robust component, an indicator of HPA axis activity, is represented by the characteristic “rhythm within the rhythm,” under the control of the suprachiasmatic nucleus, the principal circadian clock of the brain, namely, the so-called cortisol awakening response (CAR), a post-wakening surge of cortisol that occurs approximately 30 min after awakening (20–24).
The CAR is a complex phenomenon that can be expressed by the calculation of the area under the curve with reference to ground (AUCG), representing the total level and/or duration of cortisol production at awakening and 30–60 min afterward (20). In contrast, in the present study, we calculated the changes in the increase in cortisol secretion 30 min after awakening, accurately representing a dynamic measure of the shape of the phenomenon (21, 25). Therefore, in this study, salivary CAR Δ% variation 30 min after awakening was calculated by using salivary samples collected at awakening and 30 min later (21, 26) in 17 adult male subjects affected by naïve acute CSC in comparison with 17 matched healthy controls.
The status of choroidal and choriocapillaris microvasculature was assessed qualitatively and quantitatively in the study population using new technologies such as optical coherence tomography (OCT) with the enhanced-depth imaging (EDI) technique, swept-source (SS) OCT, and OCT angiography (OCTA) (19, 27, 28). The choroidal vasculature metrics were assessed by measuring the subfoveal choroidal thickness (FCT) and the choroidal vascularity index (CVI), in healthy controls and CSC patients (29). Furthermore, to gather information on the choriocapillaris status, flow-signal void area features were also evaluated in the study population using OCTA (30).
Finally, we explored the interdependencies among the salivary CAR and all three selected markers from the CSC imaging features (FCT, CVI, and flow signal void area). Therefore, Pearson’s correlation test was used to show any correlations between variables.
Materials and methods
Study population
Approval for this study was obtained from the Central Ethics Committee for Lazio, Italy (protocol n° 4327/18 April 2018). In the a priori sample size calculation, it was estimated that at least 30 subjects (15 per group) were necessary to identify a difference of approximately 30% for the predictable changes in salivary cortisol production 30 min after awakening with α = 0.01, β = 0.2 and a statistical power of 80% (31).
Seventeen Caucasian male subjects consecutively attending the outpatient clinic of the Retina Medical Service at Bietti Foundation from 1 September 2018 to 15 December 2019 were enrolled. They were aged between 40 and 60 years and were newly diagnosed with an acute episode of unilateral idiopathic CSC, confirmed by fluorescein angiography and spectral-domain optical coherence tomography (SD-OCT) B-scan (Heidelberg Engineering, Heidelberg, Germany) (32–34). Furthermore, in order to exclude occult choroidal neovascularization, patients underwent to indocyanine green angiography (35).
Seventeen age-matched controls were recruited among Bietti Foundation employers/subjects accompanying patients to the visit without any ocular pathologies confirmed by the ophthalmological checkup and SD-OCT scan.
The exclusion criteria were: chronic/recurrent CSC (duration of visual symptoms for more than 12 weeks), and the choroidal neovascularization. Furthermore, uveitis history, optic disk edema, choroidal infiltrates were also excluded. None of the study participants had received in the previous 12 months any drugs (steroidal anti-inflammatory, immunosuppressive drug, and vasoactive/psychoactive treatments) that could have potentially affected their cortisol level.
The routine laboratory tests were all normal.
The same population was previously studied for the CSC-related involvement of the autonomic nervous system (ANS) activity by measuring salivary α-Amylase production in different day times (19).
Spectral-domain optical coherence tomography scan protocol
The SD OCT images were acquired with Spectralis OCT (Heidelberg Engineering, Heidelberg Germany). The macular area was analyzed with 25 B raster scans centered on the fovea (the volume scan was 20° × 20°), and the EDI-OCT tool was activated to enhance the visualization of the choroid-sclera boundary. The value of the subfoveal choroidal thickness (FCT) was measured at the scan passing through the fovea, considered as the vertical space between the outer margin of the RPE and the choroid-sclera boundary. To estimate the interrater and intrarater observer changeability for the FCT measurement, all images were examined by two senior graders (ophthalmologists: EC and FS), and the intraclass correlation coefficients (ICCs) were computed for statistical analyses. Values of ICC < 3.5% were considered poor agreement. In the present study, the ICC was 0.87 for intrarater agreement and 0.89 for interrater agreement.
For the choroidal analysis, the CVI was calculated as follows (29): an EDI-OCT foveal scan was examined by using ImageJ software version 1.50 (National Institutes of Health, Bethesda, MD, USA), as previously described (36, 37). To ascertain the total choroidal area (TCA), a polygon tool was used to define the total subfoveal scan and added to the region of interest (ROI) manager. The Niblack automatic local threshold was used to binarize the image, which was previously converted into an 8-bit image and then transformed into red, green, and blue images. To compute the luminal choroidal area (LCA), the color threshold tool was used to measure the dark pixels, which were consequently added up with the ROI manager; white pixels were considered the stromal choroidal area (SCA), and the ratio between LCA and TCA was used to calculate the CVI (37).
Spectral-domain optical coherence tomography angiography scan protocol
To study the choriocapillaris (CC), the study eye of each patient underwent SS-OCTA imaging with the PLEX Elite 9000 tool, and a 3 × 3 mm scan pattern centered on the fovea was used. Images showing detachment of the pigmented epithelium were not included in the study.
In the analysis, the total flow signal void area of the CC was considered. This area shows the total area of the CC vascular failures as a ratio of each examined area (38, 39). Additionally, superficial capillary plexus (SCP) and CC face OCTA, automatically provided by SS-OCTA, were evaluated using ImageJ software version 1.50 (National Institutes of Health, Bethesda, MD, USA). The SCP portion was segmented between the internal limiting membrane and the inner plexiform layer. The CC slab of 15 mm was considered starting from 16 mm below the RPE/Bruch’s membrane complex. All images were evaluated to exclude segmentation errors before processing.
The “Max Entropy” threshold was used when opening the enface SCP image to show only the greater superficial retinal vessels, which might cause shadowing and artifacts (27). The Phansalkar method was used to dichotomize the CC images, which were processed with the analyze particles tool to gather information on the total flow signal void area (40). Then, these two images were combined to exclude possibly confounding artifacts because of shadowing or projection, as previously shown (27, 38, 39). The images were processed with the “Analyze Particles” tool to quantify the total flow signal voids.
Experimental procedure
Briefly, written informed consent was obtained from all participants. Furthermore, clinical and demographic characteristics were collected, and all subjects were taught how to collect their saliva at home using the Salivette sampling device (Sarstedt, Germany). They were asked to avoid eating, coffee, teeth brushing and any physical effort 30 min before each saliva collection (24, 41, 42).
Home diurnal saliva collection was planned on the sampling day upon waking (always between 07:00 and 08:00 h), 30 and 60 min after awakening. The day after, subjects returned the samples to the clinic, and the presence of CSC at the time of salivary collection was confirmed by a further SD-OCT scan. All participants were asked to text both available coauthors (FP, FS) at the planned collection time.
Saliva collection and biomarker assay
As previously described in details saliva was collected using the Salivette sampling device (Sarstedt, Germany) (24, 41). Biomarker assays was obtained by commercially available kits (Demeditec-Diagnostic, Kiel, Germany) with the inter-assay coefficient of variation <10%, and the intra-assay coefficient of variation <7% (minimum detectable concentration of 0.5 ng/ml) (24, 41).
Statistics
The statistical analyses and data visualization were performed by the SigmaPlot-11 software package (SxST.it, Italy). All quantitative variables were reported in the results as the mean and SE, unless otherwise specified. For each subject, raw biomarker data were used to estimate the Δ% increase in salivary CAR assessed by applying the formula CAR Δ% variation = [(cortisol level 30 min after awakening-awake cortisol level)/awake cortisol level] × 100.
The Kolmogorov–Smirnov test was applied prior to statistical analyses to check the normality of the distribution and the homogeneity of variance. Student’s t-test and the Mann–Whitney U test were applied for group comparisons. Pearson’s correlation test was used to examine the relationships between variables. The statistical significance was set at P < 0.05 (43).
Results
Demographic and clinical characteristics across the study population
All enrolled subjects were Caucasian men. Table 1 reports the demographic and clinical data of patients (n = 17) compared with those of the controls (n = 17). The control and CSC groups showed no differences in age or educational level. Furthermore, the two groups did not show statistically significant differences in body mass index or basal cardiovascular parameters. There were no smokers in the two groups, and alcohol consumption limited to meals was moderate, with less than 1 drink per day (44).
Choroidal and choriocapillaris spectral-domain optical coherence tomography angiography image metrics in the study population
The comparison of imaging biomarkers between the study eye and control eye shows that eyes with CSC presented significantly higher FCT (control: 254 ± 13.1 SD: 53.9; CSC: 394 ± 23.3 SD: 96.03; Mann–Whitney U test for continuous non-normally distributed variables: U = 29,000, T = 182,000, P < 0.001) and CVI values (control: 62.26 ± 0.5 SD: 2.28; CSC: 76.45 ± 0.8 SD: 3.45; Mann–Whitney U test for continuous non-normally distributed variables: U = 47,000, T = 200,000, P < 0.001) than healthy control eyes. The evaluation of OCTA images of the choriocapillaris revealed that the prevalence of the flow signal void area was higher in the CSC group than in the control group (control: 31.92 ± 1.88 SD: 7.77; CSC: 36.96 ± 1.60 SD: 6.60; Mann–Whitney U test for continuous non-normally distributed variables: U = 92,000, T = 245,000).
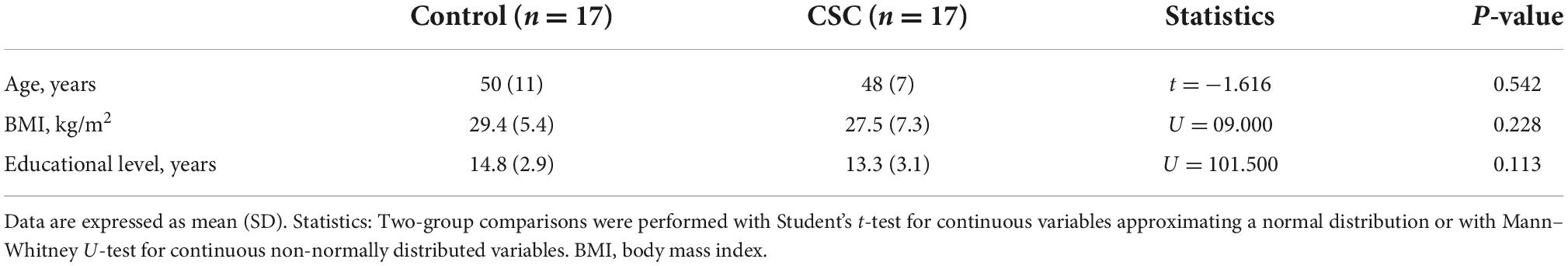
Figure 1 shows representative images of the CSC and healthy eyes, displaying hyporeflective flow signal void areas and the CVI.
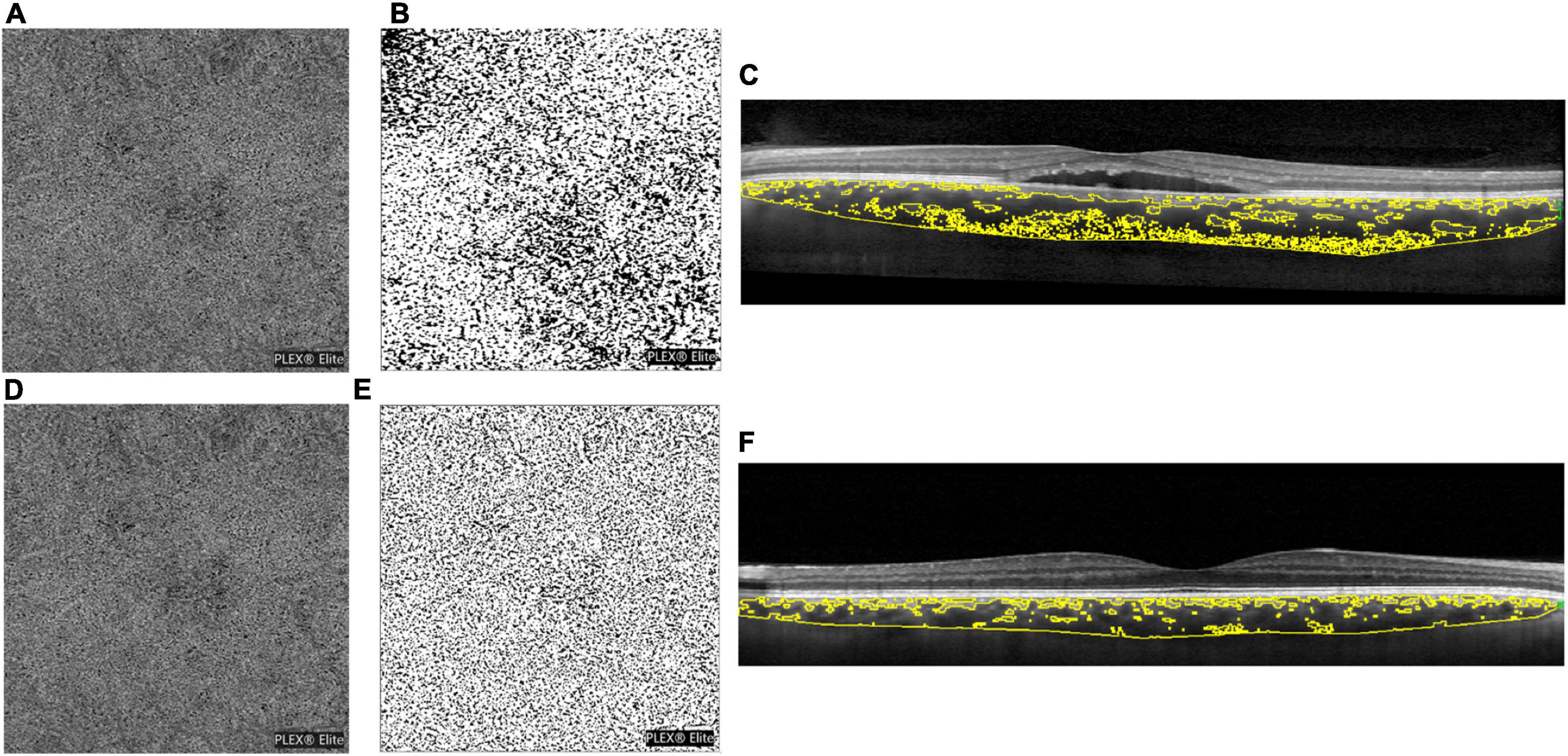
Figure 1. (A) Optical coherence tomography angiography (OCTA) scan of a central serous chorioretinopathy (CSC) eye after choriocapillaris segmentation and exportation from the PLEX Elite 900 device, showing a hyporeflective flow signal void area. (B) The choriocapillaris images binarized with the Phansalkar method for the quantification of flow signal voids by using ImageJ (public domain software). (C) Enhanced depth optical coherence tomography images highlighting the choroidal vascularity index (CVI). Image binarization was performed with the Niblack autolocal threshold with ImageJ (public domain software) to calculate the total, luminal, and stromal choroidal areas [total choroidal area (TCA), luminal choroidal area (LCA), and stromal choroidal areas (SCA)], and the CVI was calculated as the ratio between LCA and TCA. (D–F) Images in the healthy eye.
Salivary cortisol awake response delta percentage variation in the study population
Figure 2 shows the CAR Δ% change reported as the percentage variation of salivary cortisol measured in both groups 30 min after awakening.
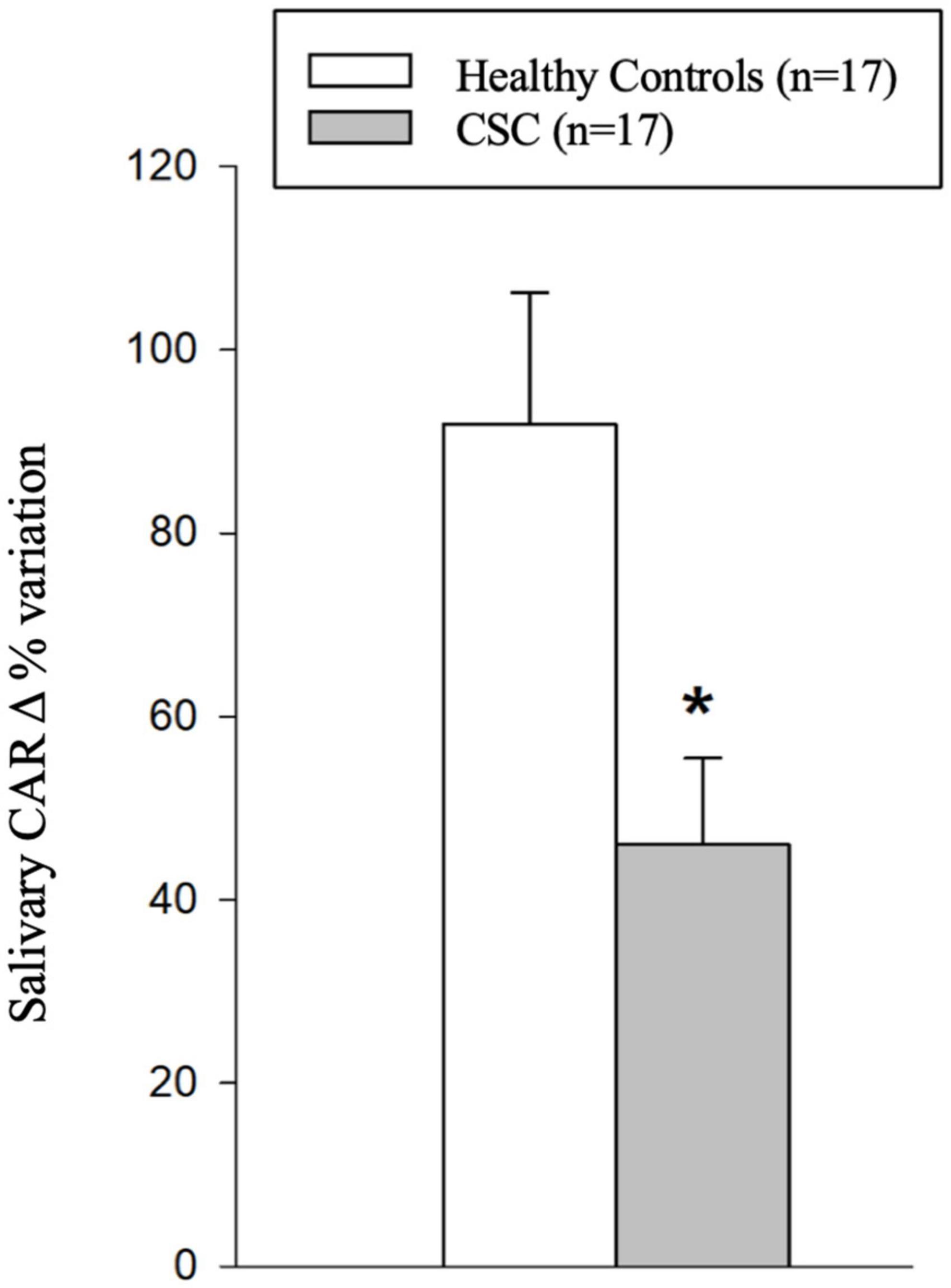
Figure 2. Histograms representing the salivary cortisol awake response (CAR) delta percentage (Δ%) variation in the study population. Statistics: * Student’s t-test for continuous normally distributed variables: t = 2.925; p = 0.006 vs. controls.
The raw data reveal the expected physiologically significant presence of CAR in the first half-hour after waking up in both the control (salivary cortisol at awakening: 3.6 ± 0.23 SD: 0.97 ng/ml; 30 min after awakening: 6.39 ± 0.34 SD: 1.42 ng/ml; Student’s t-test: t = −6.789 p < 0.001 vs. awakening; 60 min after awakening: 4.17 ± 0.25 SD: 1.06 ng/ml; ns vs. awakening) and CSC (salivary cortisol at awakening: 6.26 ± 0.53 SD: 2.179 ng/ml; 30 min after awakening: 8.81 ± 0.63 SD: 2.614 ng/ml; Mann–Whitney rank-sum test: T = −207.000 p = 0.002 vs. awakening; 60 min after awakening: 6.25 ± 0.58 SD: 2.40 ng/ml; ns vs. awakening). However, as depicted in Figure 2, data transformation in Δ% points out that subjects with CSC show, 30 min after awakening, a significantly lower increase in CAR Δ% variation (+46.0 ± 9.40; SD: 38.88) in comparison with controls (+79.8 ± 6.63; SD: 27.33; Student’s t-test for continuous normally distributed variables: t = 2.925; p = 0.006).
Relationships between salivary cortisol awake response delta percentage variation and choroid-retinal metrics in the study population
Figure 3 depicts scatterplots showing the statistically significant relationships between salivary CAR Δ% variation and FCT (Figure 3A), CVI (Figure 3B), and the flow signal void area (Figure 3C) in the study population.
Figure 3. Scatterplots showing relationships between salivary cortisol awake response (CAR) delta percentage (Δ%) variation and the selected biomarkers of central serous chorioretinopathy (CSC) imaging in the study population: subfoveal choroidal thickness (FCT) (A), choroidal vascularity index (CVI) (B), and total flow signal void areas (C) in the study population. Continuous lines represent the best-fit linear regression; long dashed lines represent confidence bands; dotted lines represent prediction bands.
Discussion
In the present study, EDI-OCT scans followed by SS-OCTA showed that CSC subjects had increased FCT and CVI, as well as a higher distribution of total flow signal void areas in comparison with the matched controls, confirming and providing additional evidence that CSC may be caused by increased hydrostatic pressure in the choroid (45).
Hypothalamic-pituitary adrenal axis activity, assessed by measuring the trend of salivary cortisol production over the first hour after awakening, showed that the CAR Δ% variation was physiologically elevated 30 min after awaking in both the control and CSC groups. However, the CSC patients showed a remarkably blunted CAR Δ% variation in comparison with the controls, which might reflect CSC-related dysregulation of HPA axis activity. A key result of the present study is that a statistically significant relationship was selectively found between the salivary CAR Δ% variation and FCT, CVI, and flow signal void area in the study participants.
Overall, the present study confirms that CSC involves a generalized overproduction of salivary cortisol compared controls at awakening and 30 and 60 min later (6–8). Hypercortisolism has been related with hypertension and/or obesity (46, 47). However, the confounding effects of these comorbidities were avoided since the subjects enrolled in the present study were not obese (BMI < 30 kg/m2) or hypertensive (47).
Cortisol awake response is a useful index of the major neuroendocrine stress response system, and it was found to be altered among people with post-traumatic stress, fatigue symptoms, burnout, or exhaustion (26, 48). Furthermore, altered CAR has been described in psychiatric disorders (49) and several stress-related diseases (50–52). CAR was not detectable in the OSA population, (52) while it was restored after 3 and 6 months of continuous positive airway pressure (CPAP) therapy (53).
The data reported here are part of a larger study that previously showed that in acute CSC, the production of cortisol and the scores on the Daily Hassles and Stress Scale were higher in CSC subjects than in controls (5), resulting in allostatic overload and leading to erratic neuroendocrine responses (54). Thus, CSC subjects might be experiencing a pathogenetic process with a high level of stress hormone production in association with elevated subjective stress perceptions induced by daily hassles.
As a whole, by measuring the magnitude of the CAR, we observed in the present study an imbalance in the functional chronobiology of the stress system in CSC patients at awakening, while diurnal rhythmicity was preserved (8). We believe that this should be included among the CSC stress-related features (5, 18, 19, 55). In fact, the magnitude of the morning production of salivary cortisol followed a distinct trend in the CSC group during the first half hour of waking up with a lower CAR Δ% variation in comparison with healthy subjects.
The ANS dysregulation was previously highlighted in CSC subjects showing an overall higher diurnal production of salivary a-AMY, representing a sympathetic “drive” playing a role in the stress-induced pathophysiology of CSC (19, 55).
As previously shown, the aforementioned anomalies affecting salivary cortisol production at awakening are consistent with HPA axis imbalance in CSC, very likely in association with elevated subjective stress perceptions induced by daily hassles (5). For this purpose, McEwen’s allostatic load model for stress (56) originally speculated that a flatter pattern of cortisol secretion produced in response to physio-pathological challenges reflects an alteration of HPA activity that protects the stress system from dysfunction (57).
A weakness of the study is the absence of follow up. However, a prolonged decline in vision may contribute to altered HPA axis activity; thus, careful follow-up of patients should be taken into consideration in future studies to expand the understanding of the relationship between stress-related psycho-neuroendocrine imbalance and CSC. Furthermore, future research will be necessary to confirm the present results in women and elderly subjects, and also to consider the day-to-day variability not measured in the present protocol (58, 59).
Stress hormones have been linked to health and wellbeing. There are, however, a few studies on how the imbalance of the HPA axis, in the absence of particularly stressful stimuli during everyday life, might represent a marker of a functional disease. With this regard, in clinical practice, an example occurs in Cushing’s syndrome, which is diagnosed by measuring an elevation in late-night salivary cortisol (nadir) (60). The present study suggests the possibility that measuring salivary CAR patterns could be a specific and sensitive method for the determination of HPA axis imbalance in individuals with CSC (61).
As a whole, we believe that further investigation will ascertain the mechanism by which the dysregulation of the HPA axis leads to CSC, and altered functional chronobiology during wakefulness should be included among some of the stress-related CSC features (19) somehow linked to the choroidal hyperpermeability seen in CSCs. Future mechanistic studies are clearly needed, as corroborated by the fact that the salivary CAR correlates with choroidal and choriocapillaris features.
Finally, a follow-up of the patients already affected and then healed from the CSC will clarify whether the healing process is associated with a normalization of the CAR. If so, these patients might have a lower risk for recurrence, providing ophthalmologists with a new subclinical indicator of the disease and a possible therapeutic target (62).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by obtained from the Central Ethics Committee for Lazio, Italy (protocol no. 4327/18 April 2018). The patients/participants provided their written informed consent to participate in this study.
Author contributions
FS, FP, and MP: conceptualization, project administration, and writing—review and editing. FS, FP, EC, and MP: data curation, investigation, methodology, and validation. MP: resources. FS and FP: software and writing—original draft. FP: supervision. All authors have read and approved the final manuscript.
Funding
The research for this manuscript was financially supported by the Italian Ministry of Health and Fondazione Roma. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
MP reports personal fees from Allergan (I), Bayer (I), and Novartis (I) outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Manayath GJ, Ranjan R, Shah VS, Karandikar SS, Saravanan VR, Narendran V. Central serous chorioretinopathy: current update on pathophysiology and multimodal imaging. Oman J. Ophthalmol. (2018) 11:103–12. doi: 10.4103/ojo.OJO_75_2017
2. Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. (2008) 115:169–73. doi: 10.1016/j.ophtha.2007.02.032
3. Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. (2015) 48:82–118. doi: 10.1016/j.preteyeres.2015.05.003
4. Nicholson BP, Atchison E, Idris AA, Bakri SJ. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Surv Ophthalmol. (2018) 63:1–8.
5. Scarinci F, Ghiciuc CM, Patacchioli FR, Palmery M, Parravano M. Investigating the hypothesis of stress system dysregulation as a risk factor for central serous chorioretinopathy: a literature mini-review. Curr Eye Res. (2019) 44:583–9. doi: 10.1080/02713683.2019.1565891
6. Agarwal A, Garg M, Dixit Godara R. Evaluation and correlation of stress scores with blood pressure, endogenous cortisol levels, and homocysteine levels in patients with central serous chorioretinopathy and comparison with age matched controls. Indian J Ophthalmol. (2016) 64:803–5. doi: 10.4103/0301-4738.195591
7. Scarinci F, Patacchioli FR, Palmery M, Pasquali V, Costanzo E, Ghiciuc CM, et al. Diurnal trajectories of salivary cortisol and α-amylase and psychological profiles in patients with central serous chorioretinopathy. Chronobiol Int. (2020) 37:510–9. doi: 10.1080/07420528.2019.1702553
8. Scarinci F, Patacchioli FR, Parravano M. Exploring the biopsychosocial pathways shared by obstructive sleep apnea (OSA) and central serous chorioretinopathy (CSC): a literature overview. J Clin Med. (2021) 10:1521. doi: 10.3390/jcm10071521
9. Vedhara K, Miles J, Bennett P, Plummer S, Tallon D, Brooks E, et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol Psychol. (2003) 62:89–96. doi: 10.1016/s0301-0511(02)00128-x
10. Cohen L, Cole SW, Sood AK, Prinsloo S, Kirschbaum C, Arevalo JM, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of Inflammatory signaling. PLoS One. (2012) 7:e42324. doi: 10.1371/journal.pone.0042324
11. Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. (2007) 133:25–45. doi: 10.1037/0033-2909.133.1.25
12. Newman E, O’Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. (2007) 32:125–32. doi: 10.1016/j.psyneuen.2006.11.006
13. Sonnenschein M, Sorbi MJ, van Doornen LJ, Schaufeli WB, Maas CJ. Electronic diary evidence on energy erosion in clinical burnout. J Occup Health Psychol. (2007) 12:402–13. doi: 10.1037/1076-8998.12.4.402
14. Wirtz P, von Känel R, Emini L, Ruedisueli K, Groessbauer S, Maercker A, et al. Evidence for altered hypothalamus–pituitary–adrenal axis functioning in systemic hypertension: blunted cortisol response to awakening and lower negative feedback sensitivity. Psychoneuroendocrinology. (2007) 32:430–6. doi: 10.1016/j.psyneuen.2007.02.006
15. Bellingrath S, Weigl T, Kudielka BM. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort–reward-imbalance. Biol Psychol. (2008) 78:104–13. doi: 10.1016/j.biopsycho.2008.01.006
16. Torres-Harding S, Sorenson M, Jason L, Maher K, Fletcher MA, Reynolds N, et al. The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. J Appl Biobehav Res. (2008) 13:157–80. doi: 10.1111/j.1751-9861.2008.00033.x
18. Kumar M, van Dijk EHC, Raman R, Mehta P, Boon CJF, Goud A, et al. Stress and vision-related quality of life in acute and chronic central serous chorioretinopathy. BMC Ophthalmol. (2020) 20:90. doi: 10.1186/s12886-020-01361-9
19. Scarinci F, Patacchioli FR, Costanzo E, Parravano M. Relationship of choroidal vasculature and choriocapillaris flow with alterations of salivary α-amylase patterns in central serous chorioretinopathy. Invest Ophthalmol Vis Sci. (2021) 62:19. doi: 10.1167/iovs.62.15.19
20. Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening:a reliable biological marker for the assessment of adrenocortical activity. Life Sci. (1997) 61:2539–49. doi: 10.1016/S0024-3205(97)01008-4)
21. Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. (2004) 7:29–37. doi: 10.1080/10253890410001667205
22. Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. (2007) 32:358–66. doi: 10.1016/j.psyneuen.2007.01.008
23. Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. (2009) 72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014
24. Ghiciuc CM, Dima-Cozma CL, Pasquali V, Renzi P, Simeoni S, Lupusoru CE, et al. Awakening responses and diurnal fluctuations of salivary cortisol, DHEA-S and α-amylase in healthy male subjects. Neuroendocrinol Lett. (2011) 32:475–80.
25. Koudela-Hamila S, Santangelo PS, Ebner-Priemer UW, Schlotz W. Under which circumstances does academic workload lead to stress? Explaining intraindividual differences by using the cortisol-awakening response as a moderator. J Psychophysiol. (2022) 36:188–97. doi: 10.1027/0269-8803/a000293
26. Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. (2004) 29:516–28. doi: 10.1016/s0306-4530(03)00072-6
27. Borrelli E, Mastropasqua R, Senatore A, Palmieri M, Toto L, Sadda SR, et al. Impact of choriocapillaris flow on multifocal electroretinography in intermediate age-related macular degeneration eyes. Invest Ophthalmol Vis Sci. (2018) 59:AMD25–30. doi: 10.1167/iovs.18-23943
28. Parravano M, Costanzo E, Borrelli E, Sacconi R, Virgili G, Sadda SR, et al. Appearance of cysts and capillary non perfusion areas in diabetic macular edema using two different OCTA devices. Sci Rep. (2020) 10:800. doi: 10.1038/s41598-020-57680-w
29. Iovino C, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Govetto A, et al. Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med. (2020) 9:595. doi: 10.3390/jcm9020595
30. Matet A, Daruich A, Hardy S, Behar-Cohen F. Patterns of choriocapillaris flow signal voids in central serous chorioretinopathy: an optical coherence tomography angiography study. Retina. (2019) 39:2178–88. doi: 10.1097/IAE.0000000000002271
32. Tittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. (1999) 128:63–8.
33. Spaide RF, Campeas L, Haas A. Central serous chorioretinopathy in younger and older adults. Ophthalmology. (1996) 103:2070–80. doi: 10.1016/S0161-6420(96)30386-2
34. Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, et al. Indocyanine green video angiography of older patients with central serous chorioretinopathy. Retina. (1996) 16:203–13. doi: 10.1136/bjo.2009.162651
35. Regillo CD, Benson WE, Maguire JI, Annesley WH Jr. Indocyanine green angiography and occult choroidal neovascularization. Ophthalmology. (1994) 101:280–8.
36. Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. (2016) 36:1646–51. doi: 10.1097/IAE.0000000000001040
37. Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. (2014) 55:3893–9. doi: 10.1167/iovs.14-14447
38. Borrelli E, Uji A, Toto L, Viggiano P, Evangelista F, Mastropasqua R. In Vivo mapping of the choriocapillaris in healthy eyes: a widefield swept-source OCT angiography study. Ophthalmol Retina. (2019) 3:979–84. doi: 10.1016/j.oret.2019.05.026
39. Di Antonio L, Viggiano P, Ferro G, Toto L, D’Aloisio R, Porreca A, et al. Retinal vascular metrics difference by comparison of two image acquisition modes using a novel OCT angiography prototype. PLoS One. (2020) 15:e0243074. doi: 10.1371/journal.pone.0243074
40. Chu Z, Cheng Y, Zhang Q, Zhou H, Dai Y, Shi Y, et al. Quantification of choriocapillaris with Phansalkar local thresholding: pitfalls to avoid. Am J Ophthalmol. (2020) 213:161–76. doi: 10.1016/j.ajo.2020.02.003
41. Simeoni S, Biselli R, D’Amelio R, Rocca B, Lattanzio S, Mucci L, et al. Stress-induced salivary cortisol secretion during hypobaric-hypoxia challenge and in vivo urinary thromboxane production in healthy male subjects. Stress. (2011) 14:282–9. doi: 10.3109/10253890.2010.545458
42. Hackney AC, Viru A. Research methodology: endocrinologic measurements in exercise science and sports medicine. J Athl Train. (2008) 43:631–9. doi: 10.4085/1062-6050-43.6.631
44. Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. (2007) 116:1306–17.
45. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. (2009) 29:1469–73. doi: 10.1097/IAE.0b013e3181be0a83
46. Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. (1998) 83:1853–9. doi: 10.1210/jcem.83.6.4843
47. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. Guidelines for the management of arterial hypertension:the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). Eur Heart J. (2007) 28:1462–536.
48. Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. (2009) 80:265–78.
49. Delle Chiaie R, Trabucchi G, Girardi N, Marini I, Pannese R, Vergnani L, et al. Group psychoeducation normalizes cortisol awakening response in stabilized bipolar patients under pharmacological maintenance treatment. Psychother Psychosom. (2013) 82:264–6. doi: 10.1159/000348609
51. Cozma S, Ghiciuc CM, Damian L, Pasquali V, Saponaro A, Lupusoru EC, et al. Distinct activation of the sympathetic adreno-medullar system and hypothalamus pituitary adrenal axis following the caloric vestibular test in healthy subjects. PLoS One. (2018) 13:e0193963. doi: 10.1371/journal.pone.0193963
52. Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. (2011) 73:114–26.
53. Ghiciuc CM, Dima Cozma LC, Bercea RM, Lupusoru CE, Mihaescu T, Szalontay A, et al. Restoring the salivary cortisol awakening response through nasal continuous positive airway pressure therapy in obstructive sleep apnea. Chronobiol Int. (2013) 30:1024–31. doi: 10.3109/07420528.2013.795155
54. Fava GA. Subclinical symptoms in mood disorders: pathophysiological and therapeutic implications. Psychol Med. (1999) 29:47–61.
55. Abdelhakim AH, Ledesma-Gil G, Yannuzzi LA. Salivary alpha amylase levels may correlate with central serous chorioretinopathy activity. Retina. (2021) 41:2007–8. doi: 10.1097/IAE.0000000000003265
56. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. (2003) 43:2–15. doi: 10.1016/S0018-506X(02)00024-7
57. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. (2000) 21:55–89. doi: 10.1210/edrv.21.1.0389
58. Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. (2007) 32:80–6. doi: 10.1016/j.psyneuen.2006.10.005
59. Bazzazi N, Ahmadpanah M, Akbarzadeh S, Seif Rabiei MA, Holsboer-Trachsler E, Brand S. In patients suffering from idiopathic central serous chorioretinopathy, anxiety scores are higher than in healthy controls, but do not vary according to sex or repeated central serous chorioretinopathy. Neuropsychiatr Dis Treat. (2015) 11:1131–6.
60. Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for cushing’s syndrome. J Clin Endocrinol Metab. (1998) 83:2681–6. doi: 10.1210/jcem.83.8.4936
61. van Haalen FM, van Dijk E, Dekkers O, Bizino MB, Dijkman G, Biermasz NR, et al. Cushing’s syndrome and hypothalamic-pituitary-adrenal axis hyperactivity in chronic central serous chorioretinopathy. Front Endocrinol. (2018) 9:39. doi: 10.3389/fendo.2018.00039
Keywords: central serous chorioretinopathy (CSC), salivary cortisol awake response, choroidal vascularity index (CVI), subfoveal choroidal thickness, flow signal void area, optical coherence tomography (OCT), optical coherence tomography angiography (OCTA)
Citation: Scarinci F, Patacchioli FR, Costanzo E and Parravano M (2022) Cortisol awake response imbalance as an indicator of acute central serous chorioretinopathy: Relationship with choriocapillaris and choroidal features. Front. Med. 9:1030352. doi: 10.3389/fmed.2022.1030352
Received: 28 August 2022; Accepted: 15 November 2022;
Published: 30 November 2022.
Edited by:
Vincenzo Scorcia, University of Magna Graecia, ItalyReviewed by:
Marco Pellegrini, Policlinico S. Orsola-Malpighi, ItalyAdriano Carnevali, University of Magna Graecia, Italy
Copyright © 2022 Scarinci, Patacchioli, Costanzo and Parravano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Scarinci, ZmFiaW9zY2FyaW5jaUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Fabio Scarinci, orcid.org/0000-0001-5444-4377; Francesca Romana Patacchioli, orcid.org/0000-0001-9672-2603; Eliana Costanzo, orcid.org/0000-0002-9916-8895; Mariacristina Parravano, orcid.org/0000-0002-2223-7311
 Fabio Scarinci
Fabio Scarinci Francesca Romana Patacchioli
Francesca Romana Patacchioli Eliana Costanzo‡
Eliana Costanzo‡ Mariacristina Parravano
Mariacristina Parravano