
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 28 September 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1021941
This article is part of the Research Topic Reviews in: Ophthalmology 2022 View all 22 articles
 Po-Chin Kuo1
Po-Chin Kuo1 Jia-Horung Hung2,3*
Jia-Horung Hung2,3* Yu-Chen Su2
Yu-Chen Su2 Ching-Ju Fang4,5
Ching-Ju Fang4,5 Chaw-Ning Lee6,7
Chaw-Ning Lee6,7 Yi-Hsun Huang2
Yi-Hsun Huang2 Shih-Chieh Shao7,8*
Shih-Chieh Shao7,8* Edward Chia-Cheng Lai7
Edward Chia-Cheng Lai7Background: Phacoemulsification is an effective and widely performed technique in cataract surgery, but the comparative anatomical outcomes, including endothelial cell loss (ECL), central corneal thickness (CCT), and central macular thickness (CMT), between high-flow and low-flow phacoemulsification cataract surgery remain unclear.
Methods: This study followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. Random-effects models were applied to measure pooled mean differences (MD) with 95% confidence intervals (CI) of anatomical outcomes between high-flow and low-flow phacoemulsification cataract surgery. We judged overall certainty of evidence (CoE) based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
Results: We included six randomized controlled trials (RCTs) totaling 477 participants. The meta-analysis showed similar changes associated with these two surgery types in both ECL at postoperative days 2–14 (MD: −1.63%; 95% CI: −3.73 to 0.47%; CoE: very low), days 15–42 (MD: −0.65%; 95% CI −2.96 to 1.65%; CoE: very low) and day 43 to month 18 (MD: −0.35%; 95% CI: −1.48 to 0.78%; CoE: very low), and CCT at postoperative day 1 (MD: −16.37 μm; 95% CI: −56.91 to 24.17 μm; CoE: very low), days 2–14 (MD: −10.92 μm; 95% CI: −30.00 to 8.16 μm; CoE: very low) and days 15–42 (MD: −2.76 μm; 95% CI: −5.75 to 0.24 μm; CoE: low). By contrast, low-flow phacoemulsification showed less increase in CMT at postoperative days 15–42 (MD, −4.58 μm; 95% CI: −6.3 to −2.86 μm; CoE: low).
Conclusions: We found similar anatomical outcomes, except in CMT, for both high-flow and low-flow phacoemulsification cataract surgery. Future head-to-head RCTs on visual outcomes should confirm our findings.
Systematic review registration: PROSPERO, identifier: CRD42022297036.
Senile cataract, as the leading cause of blindness and the second most common cause of moderate and severe vision impairment according to the Global Burden of Disease, has a prevalence estimated at around 54.38% among populations over 60 years old (1). Visual impairment caused by cataract can be restored through timely cataract surgery. Phacoemulsification is an effective and widely performed technique for cataract surgery, with estimates exceeding 11,000 surgeries/million population in the US in 2011 (2). During phacoemulsification cataract surgery, irrigation fluid circulates through the eye intraoperatively to both maintain intraocular pressure and provide cooling in order to prevent ocular tissue damage while the lens nucleus is being emulsified through the use of ultrasound energy.
However, the optimal flow rate for phacoemulsification cataract surgery is not yet determined. High-flow settings, with aspiration flow rates ranging from 35 to 50 ml/min (3, 4) are generally considered to have higher efficiency. They are preferred by many surgeons for rock-hard dense cataract cases to improve the vacuum purchase of the hard nuclei, and to decrease phaco tip clogging (5), and also create sufficient space in the anterior segment for surgical manipulation, at the expense of risking surgical trauma caused by turbulence (3). Low-flow settings, often with aspiration flow rates lower than 25 ml/min (3), prioritize safety because they create a more stable intraoperative environment, theoretically decreasing the chance of intraoperative complications such as posterior capsule rupture. They are therefore as well recommended when dealing with cases with zonular insufficiency (6). However, they may require a longer surgical time, and limit surgeons to a relatively small space in the anterior segment (7). In clinical practice, ophthalmologists decide the fluidics settings based only on their own surgical experience.
Phacoemulsification by ultrasound energy is known to cause corneal endothelial cell loss (ECL) (8). In addition, phacoemulsification may also trigger inflammation, thus leading to macular edema. However, several randomized controlled trials (RCTs) with small sample sizes reported inconsistent surgical outcomes, regarding ECL, central corneal thickness (CCT) and central macular thickness (CMT), after high- or low-flow phacoemulsification cataract surgery (4, 9–13). To summarize the current evidence and better inform clinical decision-making, we conducted a systematic review and meta-analysis study on the surgical outcomes reported from RCTs comparing high-flow and low-flow phacoemulsification cataract surgery.
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14). The review protocol was registered on PROSPERO (CRD42022297036) prior to conducting the review.
We searched for published RCTs via Medline, Embase, Cochrane Central Register of Controlled Trials and Scopus on July 8, 2022. The literature search was limited to human studies, with no language restrictions. The search strategy was developed by an experienced librarian (CJF), and the details are presented in Supplementary Table S1. To capture any unpublished studies, we also consulted the pharmaceutical and medical device companies associated with these cataract surgeries for additional studies.
We included RCTs comparing high-flow and low-flow fluidic settings for phacoemulsification. We recruited studies with patients randomly assigned to either high-flow or low-flow phacoemulsification. Fluidic settings of the phacoemulsification surgery were to be clearly stated.
Two review authors (PCK and JHH) independently selected eligible literatures based on the pre-specified inclusion criteria, including (1) Participants: cataract patients; (2) Intervention/Comparison: high-flow or low-flow phacoemulsification; (3) Primary study outcome: endothelial cell loss; (4) Study design: RCTs. We initially screened records by titles and abstracts to identify potential candidates, and then the review authors reviewed their full texts to select those for inclusion. Any discrepancy between the review authors was resolved by discussion with the third review author (SCS) before final decision.
Two review authors (PCK and JHH) independently extracted data including first author, publication year, country, study design, patient characteristics, interventions and comparators (surgical modes and parameter settings), and outcome measures from the included studies. In addition to the primary anatomic outcome of endothelial cell loss (ECL), we extracted other important anatomic parameters as secondary outcomes, including central corneal thickness (CCT) and central macular thickness (CMT). We investigated CCT because postoperative corneal thickness is known as a marker of endothelial damage after phacoemulsification (15). We also investigated CMT because phacoemulsification surgery commonly leads to postoperative macular edema (16), which may affect postoperative visual outcomes. Any discrepancy between the review authors was resolved by discussion.
Two review authors (PCK and JHH) independently assessed the risk of bias in the included studies with the Cochrane Risk of Bias tool 1.0 (17). The domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases, were categorized as high-, low- or unclear risk of bias. If an RCT did not report data of adverse effects, we rated it as having a high risk of selective reporting bias. We also judged there to be a high risk of other bias if a baseline imbalance was found between the intervention- and control groups after randomization. Any discrepancy between the review authors was resolved by discussion with the third review author (SCS) before final decision.
We conducted all meta-analyses using Review Manager version 5.3.4.1 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2020). Random-effects models were applied to measure the pooled mean differences (MD) and 95% confidence intervals (CI) of the study outcomes of interest comparing high-flow and low-flow phacoemulsification cataract surgery. We analyzed the study outcomes based on four follow-up time periods to evaluate the immediate (i.e., postoperative day 1), short-term (i.e., postoperative days 2–14), intermediate-term (i.e., postoperative days 15–42), and long-term (i.e., postoperative day 43 to month 18) comparative treatment effects between high-flow and low-flow phacoemulsification cataract surgery.
To achieve concordance between included studies, extracted outcome data from included studies were adjusted using reasonable statistical methods. For example, ECL was calculated as percentage change based on the formula: (postoperative ECL – preoperative ECL)/(preoperative ECL), while CCT and CMT were calculated as μm change based on the formula: (postoperative value – preoperative value). ECL was calculated as percentage change because absolute value data were not available in the studies from Baradaran-Rafii 2009 (11) and Schriefl 2014 (4). Where only the median and range of outcome measures were available, we estimated the mean and variance through the specific formula (18). We assessed statistical heterogeneity among included studies by I2 statistic, and considerable heterogeneity was defined as an I2 >50% (17). Publication bias was to be evaluated by funnel plots if over 10 studies were included for meta-analysis (17).
Two independent reviewers (PCK and JHH) assessed the overall certainty of evidence (CoE) for each study outcome based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (19). Any discrepancy between the review authors was resolved by discussion with the third review author (SCS) before final decision.
The study selection flowchart is presented in Supplementary Figure S1. We initially identified a total of 1,111 records through the systematic search, and after screening the study titles and abstracts, 12 potential articles (4, 9–13, 20–25) were evaluated for final eligibility. Of these potential records, we excluded two studies which were not designed as randomized controlled trials (23, 24), one study lacking comparisons between high-flow and low-flow phacoemulsification cataract surgery (25), and three studies without final reports (20–22). Ultimately, in this systematic review and meta-analysis, we only included six reports (4, 9–13), with a total of 477 participants from six RCTs.
Table 1 summarizes the study, participant and surgery characteristics, outcomes, and main findings of the included RCTs. These RCTs recruited participants from Iran, Sweden, India and Austria. Briefly, all RCTs included participants undergoing phacoemulsification for senile cataract with the mean age ranging from 53 to 74 years old. In two RCTs (12, 13) phacoemulsification was performed by longitudinal ultrasound, in two RCTs (9, 10) it was by torsional ultrasound, in one RCT (11) by transversal ultrasound, and the last RCT (4) did not specify the mode of ultrasound used. The flow rate ranged from 35 to 45 cc/min in the high-flow group, and from 20 to 25 cc/min in the low-flow group. The vacuum pressure ranged from 400 to 650 mmHg in the high-flow group, and from 200 to 400 mmHg in the low-flow group.
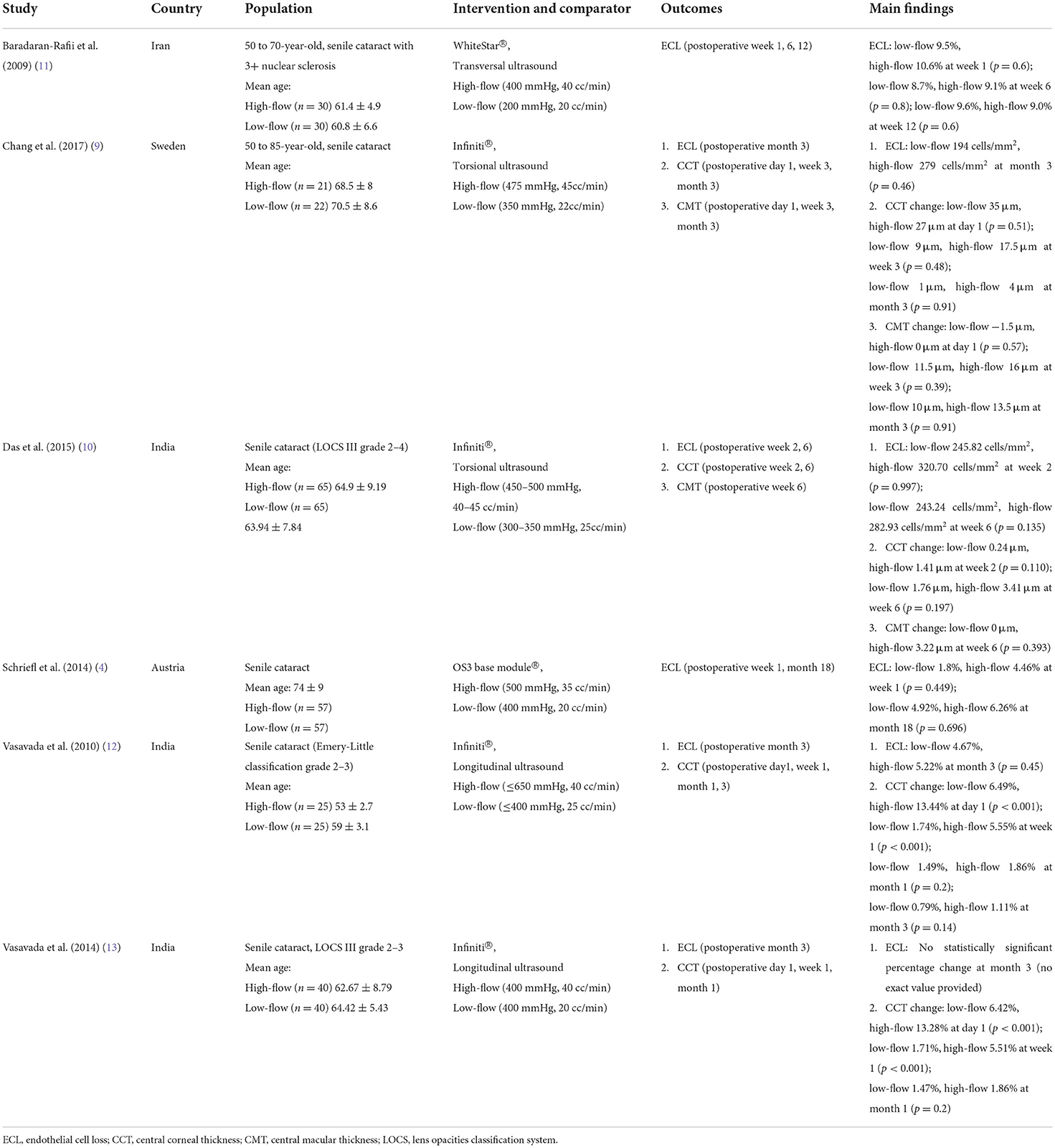
Table 1. Characteristics of comparative studies regarding high-low and low-flow aspiration flow settings of patients who underwent phacoemulsification.
The overall risk of bias assessment is presented in Supplementary Figure S2, and the authors' detailed judgements for each domain of the risk of bias tools are presented in Supplementary Table S2. We considered most of the included RCTs (5/6) to have performance bias, since the surgeons were not blinded to the intervention (4, 9, 10, 12, 13). In addition, we found 5 RCTs may have suffered from selection bias (4, 9–12) and detection bias (4, 9, 11). Finally, we judged two RCTs as having other bias (12, 13), because, despite having a similar study population source but different sample sizes and surgical parameters, they reported exactly the same outcome data for preoperative and postoperative CCT.
We found five RCTs reporting the ECL changes after high-flow or low-flow phacoemulsification cataract surgery (4, 9–12). The meta-analysis showed no significant differences in ECL at postoperative days 2–14 (three RCTs; 304 participants; MD: −1.63%; 95% CI: −3.73 to 0.47%; I2 = 0%) (4, 10, 11) (Figure 1A), at days 15–42 (2 RCTs; 190 participants; MD: −0.65%; 95% CI: −2.96 to 1.65%; I2 = 0%) (10, 11) (Figure 1B), and at day 43 to month 18 (four RCTs; 267 participants; MD: −0.35%; 95% CI: −1.48 to 0.78%; I2 = 0%) (4, 9, 11, 12) (Figure 1C). However, the included RCTs lacked data regarding the differences in ECL between these two surgery types at postoperative day 1.
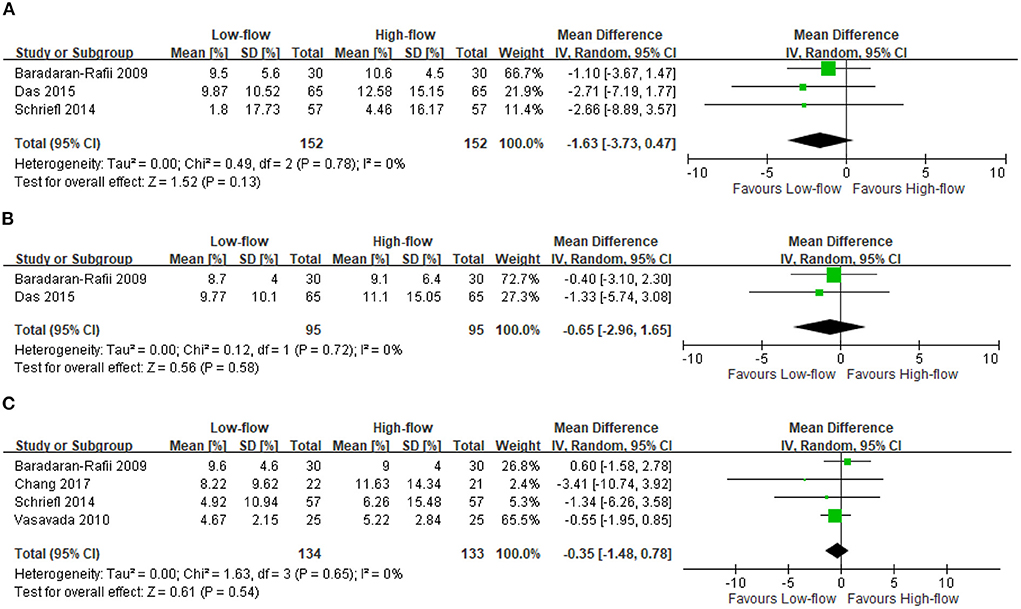
Figure 1. Endothelial cell loss (ECL). (A) Postoperative days 2–14 [%]. (B) Postoperative days 15–42 [%]. (C) Postoperative day 43 - month 18 [%].
We found three RCTs reporting the CCT changes after high-flow or low-flow phacoemulsification cataract surgery (9, 10, 13). The meta-analysis showed no significant differences in CCT changes at postoperative day 1 (two RCTs; 123 participants; MD: −16.37 μm; 95% CI: −56.91 to 24.17 μm; I2 = 99%) (9, 13) (Figure 2A), at days 2–14 (two RCTs; 210 participants; MD: −10.92 μm; 95% CI: −30 to 8.16 μm; I2 = 92%) (10, 13) (Figure 2B) and at days 15–42 (three RCTs; 253 participants; MD: −2.76 μm; 95% CI: −5.75 to 0.24 μm; I2 = 0%) (9, 10, 13) (Figure 2C). However, the included RCTs lacked data regarding the differences in CCT changes between these two surgery types at postoperative day 43 to month 18.
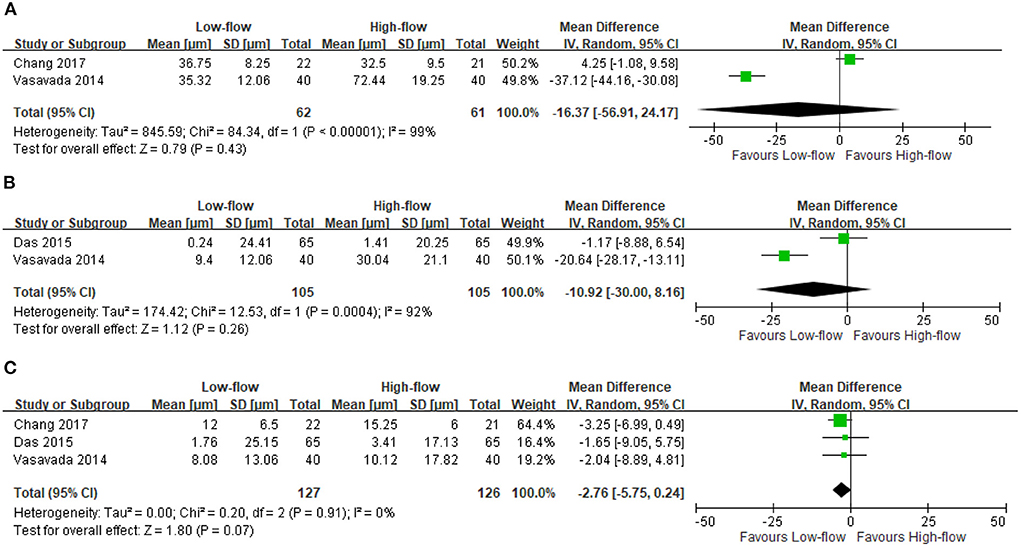
Figure 2. Change in central corneal thickness (CCT). (A) Postoperative day 1 [μm]. (B) Postoperative days 2–14 [μm]. (C) Postoperative days 15–42 [μm].
We found two RCTs reporting the CMT changes after high-flow or low-flow phacoemulsification cataract surgery (9, 10). The meta-analysis showed less increase in CMT after low-flow phacoemulsification at postoperative days 15–42 (two RCTs; 173 participants; MD: −4.58 μm; 95% CI: −6.3 to −2.86 μm; I2 = 0%) (9, 10) (Figure 3). However, the included RCTs lacked data regarding the differences in CMT changes between these two surgery types at postoperative days 1–14, and day 43 to month 18.
Table 2 summarizes the main findings and certainty of evidence for each pooled outcome estimate. All outcome measures were downgraded due to risk of bias and imprecision. The overall certainty of evidence ranged from very low to low.
This systematic review and meta-analysis of 6 RCTs with 477 participants mostly with senile cataract found that, compared to low-flow phacoemulsification cataract surgery, high-flow phacoemulsification cataract surgery led to a greater increase in postoperative CMT, while no differences were observed in postoperative ECL and CCT. Since the overall CoE for these comparisons was judged as low to very low, whether the conclusions can be fully applied in clinical decisions is uncertain.
The results of our analysis showed that high-flow fluidic settings triggered greater increase in postoperative CMT. Pseudophakic cystoid macular edema (PCME), with a post-phacoemulsification incidence ranging from 0.1% to 2.35%, is one of the complications after cataract surgery, and may lead to long-term visual deterioration that is difficult to treat. We included an analysis of the impact of different fluidic parameters on postoperative CMT, since abundant research has demonstrated that cystoid macular edema may occur even after uncomplicated phacoemulsification procedures (26, 27). Possible reasons for this are as follows: Higher vacuum level is often set together with higher aspiration flow rate to achieve the intended efficiency (28). Consequently, high-flow fluidics are associated with postocclusion surge and thus increase risk of posterior capsule rupture due to anterior chamber shallowing (29). Postocclusion surge brought about by high-flow, high-vacuum fluidics leads to greater intraoperative maximum IOP and greater IOP fluctuation (13), which may induce oxidative stress and damage the blood-retinal barrier, subsequently giving rise to macular edema (30). Also, IOP fluctuations may carry the risk of unstable orbital blood flow and oxygen supply, causing oxidative stress and further resulting in cystoid macular edema after phacoemulsification (31). Although our study showed that low-flow fluidic settings resulted in less increase in postoperative CMT, it is not known whether this is clinically significant. We reviewed previous studies to help find correlations. Bamahfouz A. has reported that CMT changes correlate with best-corrected vision changes in the first month after phacoemulsification cataract surgery (32). Greater macular thickness is also reported to be related to worse mesopic visual acuity (33). This raised our concerns about a greater increase in postoperative CMT implying the development of PCME. By optimizing the fluidic settings, we hope we can reduce the risk of PCME, thus relieving the treatment burden for the elderly after cataract surgery.
Our results showed no differences in postoperative ECL and CCT between patients operated on with high-flow or low-flow fluidic settings. For the purpose of assessing postoperative outcomes regarding the corneal structure, previous studies have demonstrated the importance of documenting corneal thickness and endothelial cell status (34, 35); hence, our study investigated both postoperative ECL and CCT changes in the two groups under different fluidics settings. We hypothesized that the advantages of low-flow fluidic settings, namely less turbulence and less damage to corneal endothelial cells, may be offset by longer surgical time and higher cumulative dissipated energy (11). It is also possible that the effect on CCT change might be transient and reversible, based on clinical findings wherein corneal edema is usually noted on postoperative day 1 and gradually resolves over the course of weeks. Therefore, our result suggested that the selection of high-flow or low-flow fluidic settings not necessarily be based on preoperative corneal parameters, including endothelial cell density or CCT.
To evaluate the overall CoE of the study outcomes from the present meta-analysis, we have applied the GRADE system for ECL, CCT and CMT. We judged the CoE of these outcomes as low to very low, possibly because of the serious risk of bias or result inconsistency among included studies and the wide confidence intervals of pooled result estimates. First, most included RCTs were vulnerable to performance bias (i.e., non-blinded designs), whereby this is unavoidable with surgical interventions like phacoemulsification cataract surgery. Detection bias was another common source of bias in the included studies. Since our study outcomes, including ECL, CCT and CMT changes, were obtained as objective values from instrument examinations in ophthalmology clinics, we considered such detection bias may not have seriously affected the result estimates. Second, significant statistical heterogeneity was observed in the CCT comparisons at postoperative days 1–14. Christakis et al. and previous studies have reported that torsional ultrasound phacoemulsification, compared to other modes of ultrasound, caused less chatter and less corneal edema postoperatively (36, 37). Hence, given that different modes of phacoemulsification, such as longitudinal and torsional ultrasound, were used in the included RCTs, the effect sizes of these RCTs were inconsistent. Third, we found wide confidence intervals around the ECL, CCT and CMT outcomes because relatively few patients were included in the meta-analysis. To reach more definite conclusions about the comparative anatomic effects of high-flow and low-flow phacoemulsification cataract surgery, future, updated systematic reviews integrating large-scale head-to-head comparisons are suggested.
This present study summarized the current evidence from RCTs, comparing anatomic effects between high-flow and low-flow phacoemulsification cataract surgery. However, we must acknowledge several limitations to the present study. First, CoE for each study outcome was judged suboptimal, and therefore we should interpret the study findings carefully. Also, we only evaluated the anatomic effects of the phacoemulsification cataract surgery, while future studies should investigate if these findings could be translated into functional outcomes, such as best-corrected visual acuity. Moreover, the present study only included participants who received phacoemulsification surgery for senile cataract from RCTs. Whether our findings could be applied to those receiving phacoemulsification surgery for solely refractive purposes, namely clear lens exchange, should be further investigated. Finally, our included RCTs mostly used the gravity-based infusion system, instead of newer technology, such as active fluidic system [e.g., Centurion system (Alcon)®], for phacoemulsification cataract surgery.
In conclusion, the low to very low CoE from the meta-analysis of the RCTs notwithstanding, high-flow phacoemulsification cataract surgery results in greater increase in postoperative CMT, but shows no difference in postoperative ECL and CCT, compared to low-flow phacoemulsification cataract surgery. Updated systematic reviews integrating future, large-scale, head-to-head comparisons of anatomic outcomes between these two phacoemulsification cataract surgery types are suggested.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Conception, design, and data collection: P-CK and J-HH. Data analysis and interpretation: P-CK, J-HH, and S-CS. Preparation of the manuscript: P-CK, J-HH, Y-CS, and S-CS. Obtained funding: J-HH. All authors read and approved the final manuscript.
This study was supported by grants from National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-11102004) to J-HH, and from the Ministry of Science and Technology (MOST 110-2314-B-006-086-MY3) to J-HH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1021941/full#supplementary-material
1. Hashemi H, Pakzad R, Yekta A, Aghamirsalim M, Pakbin M, Ramin S, et al. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye. (2020) 34:1357–70. doi: 10.1038/s41433-020-0806-3
2. Wang W, Yan W, Fotis K, Prasad NM, Lansingh VC, Taylor HR, et al. Cataract surgical rate and socioeconomics: a global study. Invest Ophthalmol Vis Sci. (2016) 57:5872–81. doi: 10.1167/iovs.16-19894
3. Verges C, Cazal J, Lavin C. Surgical strategies in patients with cataract and glaucoma. Curr Opin Ophthalmol. (2005) 16:44–52. doi: 10.1097/00055735-200502000-00008
4. Schriefl SM, Stifter E, Menapace R. Impact of low versus high fluidic settings on the efficacy and safety of phacoemulsification. Acta Ophthalmol. (2014) 92:e454–7. doi: 10.1111/aos.12200
5. Foster GJL, Allen QB, Ayres BD, Devgan U, Hoffman RS, Khandelwal SS, et al. Phacoemulsification of the rock-hard dense nuclear cataract: options and recommendations. J Cataract Refract Surg. (2018) 44:905–16. doi: 10.1016/j.jcrs.2018.03.038
6. Praveen MR, Vasavada AR, Singh R. Phacoemulsification in subluxated cataract. Indian J Ophthalmol. (2003) 51:147–54.
7. Corydon L, Krag S, Thim K. One-handed phacoemulsification with low settings. J Cataract Refract Surg. (1997) 23:1143–8. doi: 10.1016/S0886-3350(97)80306-9
8. Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. (1996) 22:1079–84. doi: 10.1016/S0886-3350(96)80121-0
9. Chang A, Fridberg A, Kugelberg M. Comparison of phacoemulsification cataract surgery with low versus standard fluidic settings and the impact on postoperative parameters. Eur J Ophthalmol. (2017) 27:39–44. doi: 10.5301/ejo.5000813
10. Das S, Nanaiah SG, Kummelil MK, Nagappa S, Shetty R, Shetty BK. Effect of fluidics on corneal endothelial cell density, central corneal thickness, and central macular thickness after phacoemulsification with torsional ultrasound. Ind J Ophthalmol. (2015) 63:641–4. doi: 10.4103/0301-4738.169786
11. Baradaran-Rafii A, Rahmati-Kamel M, Eslani M, Kiavash V, Karimian F. Effect of hydrodynamic parameters on corneal endothelial cell loss after phacoemulsification. J Cataract Refract Surg. (2009) 35:732–7. doi: 10.1016/j.jcrs.2008.12.017
12. Vasavada AR, Praveen MR, Vasavada VA, Vasavada VA, Raj SM, Asnani PK, et al. Impact of high and low aspiration parameters on postoperative outcomes of phacoemulsification: randomized clinical trial. J Cataract Refract Surg. (2010) 36:588–93. doi: 10.1016/j.jcrs.2009.11.009
13. Vasavada V, Raj SM, Praveen MR, Vasavada AR, Henderson BA, Asnani PK. Real-time dynamic intraocular pressure fluctuations during microcoaxial phacoemulsification using different aspiration flow rates and their impact on early postoperative outcomes: a randomized clinical trial. J Refract Surg. (2014) 30:534–40. doi: 10.3928/1081597X-20140711-06
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Perone JM, Boiche M, Lhuillier L, Ameloot F, Premy S, Jeancolas AL, et al. Correlation between postoperative central corneal thickness and endothelial damage after cataract surgery by phacoemulsification. Cornea. (2018) 37:587–90. doi: 10.1097/ICO.0000000000001502
16. Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. (2016) 123:316–23. doi: 10.1016/j.ophtha.2015.10.001
17. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration. (2011).
18. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
19. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
20. Morya AK. Analysis of Different Parameter in Various Methods of Phacoemulsification Technique for Cataract Surgery. Geneva: International Clinical Trials Registry Platform (ICTRP). (2020).
21. Chang A. Comparison of Postoperative Parameters in Cataract Surgery with Two Different Fluidic Settings. Geneva: International Clinical Trials Registry Platform (ICTRP). (2016). doi: 10.1186/ISRCTN11821734
22. Rahmati-Kamel M, Baradaran-Rafii A, Kiavash V. Corneal endothelial cell loss after phacoemulsification in relation to hydrodynamic parameters: low-vacuum vs. high-vacuum techniques. Am Acad Ophthalmol. (2008) 186. Available online at: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00746043/full
23. Khan MS, Sharjeel M, Ghaffar MT, Bokhari MH, Anwar F, Nazir MI. Effect of phacoemulsification power on corneal endothelial cell count and corneal thickness in age related cataract surgery. Pak J Med Health Sci. (2020) 14:1261–4.
24. Kunishige T, Takahashi H. Effects of combinations of ophthalmic viscosurgical devices and suction flow rates on the corneal endothelial cell damage incurred during phacoemulsification. J Ophthalmol. (2020) 2020:2159363. doi: 10.1155/2020/2159363
25. Wang Y, Xia Y, Zeng M, Liu X, Luo L, Chen B, et al. Torsional ultrasound efficiency under different vacuum levels in different degrees of nuclear cataract. J Cataract Refract Surg. (2009) 35:1941–5. doi: 10.1016/j.jcrs.2009.05.055
26. Mentes J, Erakgun T, Afrashi F, Kerci G. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica. (2003) 217:408–12. doi: 10.1159/000073070
27. Vukicevic M, Gin T, Al-Qureshi S. Prevalence of optical coherence tomography-diagnosed postoperative cystoid macular oedema in patients following uncomplicated phaco-emulsification cataract surgery. Clin Exp Ophthalmol. (2012) 40:282–7. doi: 10.1111/j.1442-9071.2011.02638.x
28. Shi DS, Jensen JD, Kramer GD, Zaugg B, Stagg BC, Pettey JH, et al. Comparison of vacuum and aspiration on phacoemulsification efficiency and chatter using a monitored forced infusion system. Am J Ophthalmol. (2016) 169:162–67. doi: 10.1016/j.ajo.2016.06.030
29. Ting DSJ, Rees J, Ng JY, Allen D, Steel DHW. Effect of high-vacuum setting on phacoemulsification efficiency. J Cataract Refract Surg. (2017) 43:1135–39. doi: 10.1016/j.jcrs.2017.09.001
30. Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. (2004) 30:752–60. doi: 10.1016/S0886-3350(03)00582-0
31. Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. (2013) 34:1270–8. doi: 10.1093/eurheartj/eht023
32. Bamahfouz A. Correlation of central macular thickness and the best-corrected visual acuity in three months after cataract surgery by phacoemulsification and with intraocular lens implantation. Cureus. (2021) 13:e13856. doi: 10.7759/cureus.13856
33. Puell MC, Pérez-Carrasco MJ, Palomo Alvarez C. Macular thickness and mesopic visual acuity in healthy older subjects. Curr Eye Res. (2019) 44:82–8. doi: 10.1080/02713683.2018.1522648
34. Lundberg B, Jonsson M, Behndig A. Postoperative corneal swelling correlates strongly to corneal endothelial cell loss after phacoemulsification cataract surgery. Am J Ophthalmol. (2005) 139:1035–41. doi: 10.1016/j.ajo.2004.12.080
35. Bamdad S, Bolkheir A, Sedaghat MR, Motamed M. Changes in corneal thickness and corneal endothelial cell density after phacoemulsification cataract surgery: a double-blind randomized trial. Electron Physician. (2018) 10:6616–23. doi: 10.19082/6616
36. Christakis PG, Braga-Mele RM. Intraoperative performance and postoperative outcome comparison of longitudinal, torsional, and transversal phacoemulsification machines. J Cataract Refract Surg. (2012) 38:234–41. doi: 10.1016/j.jcrs.2011.08.035
Keywords: phacoemulsification, cataract surgery, fluidics, endothelial cell loss, central corneal thickness, central macular thickness, systematic review, meta-analysis
Citation: Kuo P-C, Hung J-H, Su Y-C, Fang C-J, Lee C-N, Huang Y-H, Shao S-C and Lai EC-C (2022) Comparative anatomical outcomes of high-flow vs. low-flow phacoemulsification cataract surgery: A systematic review and meta-analysis. Front. Med. 9:1021941. doi: 10.3389/fmed.2022.1021941
Received: 17 August 2022; Accepted: 12 September 2022;
Published: 28 September 2022.
Edited by:
Georgios Panos, Nottingham University Hospitals NHS Trust, United KingdomReviewed by:
Mehran Zarei-Ghanavati, Tehran University of Medical Sciences, IranCopyright © 2022 Kuo, Hung, Su, Fang, Lee, Huang, Shao and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-Horung Hung, aHVuZ2poQG1haWwubmNrdS5lZHUudHc=; Shih-Chieh Shao, c2NzaGFvQGNnbWgub3JnLnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.