- 1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Respiratory and Critical Care Medicine, Liangping People’s Hospital, Chongqing, China
- 3Department of Critical Care Medicine, Chongqing University Three Gorges Hospital, Chongqing, China
- 4Department of Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Parvimonas micra is an anaerobic Gram-positive coccus frequently found in the oral cavity and gastrointestinal tract, but rarely in the lung. Therefore, pneumonia caused by P. micra is also rare. Although there are some reports of P. micra related pneumonia due to aspiration or blood-borne infection with definite remote infection source, there are no reported cases of hematogenous P. micra pneumonia in healthy adults lacking a remote source of infection. Herein, we described the intact disease of P. micra-related pneumonia mimicking hematogenous Staphylococcus aureus pneumonia in terms of chest imagery and diagnosed via metagenomic next-generation sequencing (mNGS). Interestingly, there was no clear remote pathogenic source identified in the patient. Microbiome analysis revealed dysbiosis of the oral flora possibly related to poor oral hygiene and a long history of smoking. The patient was treated with moxifloxacin for 3 months. Ultimately, computed tomography (CT) of the chest showed total resolution of the lung lesion. Clinicians need to update the etiology of community-acquired pneumonia. When antibiotic therapy is not effective, pathogen examination becomes very important. New methods of pathogen detection such as mNGS should be employed to this end. For the treatment of P. micra pneumonia, no standardized course of treatment was reported. Imaging absorption of lung infections may provide a more objective guidance for the duration of antibiotics in P. micra pneumonia.
Introduction
Parvimonas micra, previously known as Peptostreptococcus micros and Micromonas micros, is commonly found on human skin, in oral cavity, and among gastrointestinal flora (1). It is an anaerobic Gram-positive coccus with a diameter of 0.3–0.7 μm. This opportunistic pathogen is frequently isolated from infected root canals of teeth with chronic apical periodontitis, whereas remote infections are rare (2). A few sporadic cases of remote infection in patients with underlying diseases or a recent oral operation have been reported (3–6).
Because it is anaerobic, P. micra is not a dominant bacterium in aerobic environments, including the lung. Although there are some reports of P. micra pneumonia due to aspiration, hematogenous P. micra pneumonia is rare (7–9). Moreover, to the best of our knowledge, there are no reports of P. micra-related pneumonia induced by bloodstream infection without a definitive, remote pathogenic source.
The gold standard method of diagnosing P. micra infection is microbiological examination. However, it is difficult to detect using traditional cultural methods (10). Recently developed non-culture methods, such as metagenomic next-generation sequencing (mNGS), provide an alternative for the identification of unknown pathogens (11). As a new tool, mNGS is any of several high-throughput sequencing methods whereby billions of nucleic acid fragments can be simultaneously and independently sequenced. It can be used to precisely and rapidly identify potential pathogens regardless of pathogen type (bacterium, virus, fungus, parasite, and so on) (12). The mNGS is a promising method for diagnosing sophisticated infections, especially severe pneumonias require stay in the intensive care units (13, 14).
Herein, we describe P. micra pneumonia mimicking hematogenous Staphylococcus aureus pneumonia in terms of chest imagery and diagnosed via mNGS in pleural effusion. Interestingly, there was no definitive, remote pathogenic source identified in the patient. A cautious approach to diagnosis is necessary for rare cases such as this to avoid misdiagnosis, and clinical knowledge of P. micra-related pneumonia should be updated.
Case presentation
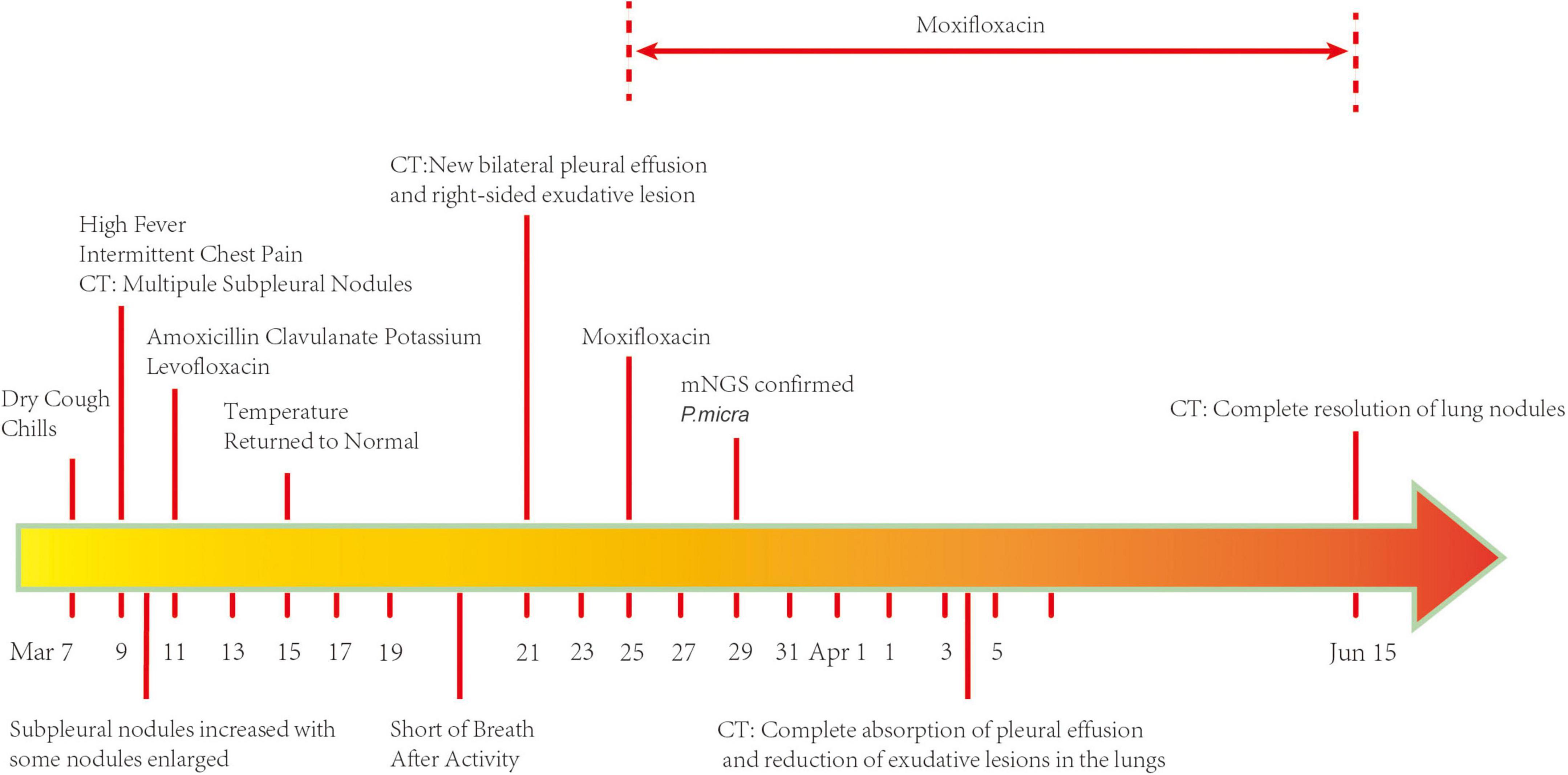
A 74-year-old man was admitted to the Department of Respiratory and Critical Care Medicine of the First Affiliated Hospital of Chongqing Medical University on 25 March 2022 with a complaint of dry cough, fever, and intermittent chest pain for 17 days and shortness of breath after activity for 4 days. He denied night sweats and weight loss. There was no history of hemoptysis, recent contact with tuberculosis or irritant gas, or any other underlying health conditions, except for 20-year smoking history. When the symptoms first occurred, the patient underwent a series of computed tomography (CT) scans. At that time, he was treated with amoxicillin clavulanate potassium for 4 days and with levofloxacin for 6 days (Figure 1). His chest pain was significantly relieved and his temperature returned to normal. However, the patient continued to cough intermittently and experience shortness of breath after activity. As shown in Figure 2, consecutive CT images showed an increasing number of nodules and exudative inflammatory lesions with a newly emerged pleural effusion. Physical examination indicated normal vital signs (Body temperature: 36.2°C, Pulse rate: 70/min, Respiratory rate: 20/min, Blood pressure: 146/74 mmHg) but asymmetric breath sounds without rales or wheezing. Blood gas analysis indicated a Pondus Hydrogenii (PH) of 7.48, a Partial Pressure of Carbon Dioxide (PCO2) of 37 mmHg, Oxygen Permeance (PO2) of 73 mmHg, Bicarbonate (HCO3–) of 27.6 mmol/L, and oxygen saturation (SO2) of 96%. His leukocyte levels were normal, but his erythrocyte sedimentation rate was elevated, at 61 mm/h.
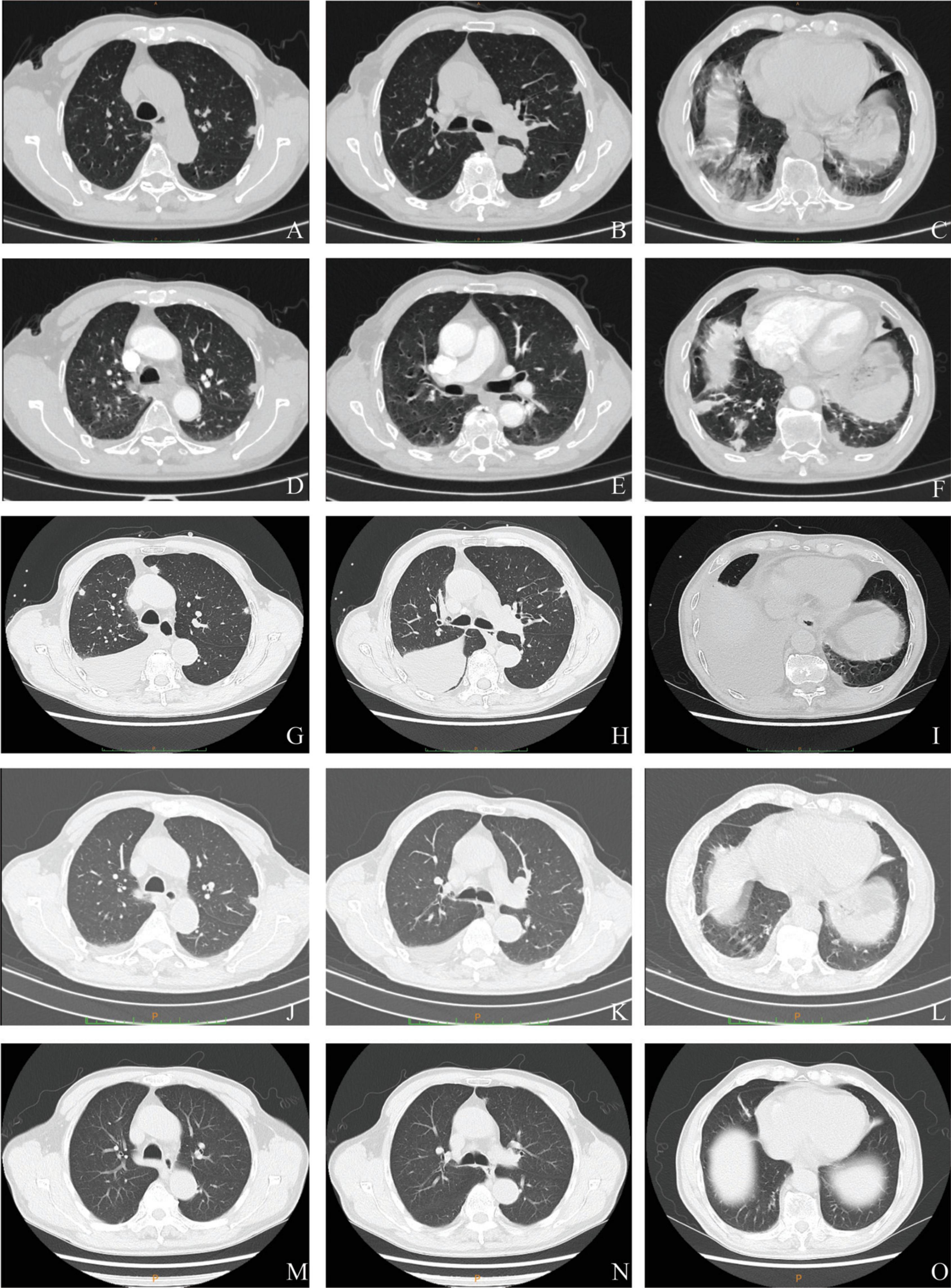
Figure 2. Serial chest computed tomography (CT) scans of the patient with Parvimonas micra pneumonia. (A–C) The initial CT scan on 9 March 2022 (2 days after symptom onset) shows subpleural nodules in both lungs, some nodules with ground glass appearance, and a few exudative inflammatory lesions in both lower lungs. (D–F) A CT on the next day (10 March 2022) showed more nodules and exudative lesions in both lungs and some enlarged nodules. (G–I) A follow-up scan (21 March 2022) after treatment with amoxicillin clavulanate potassium and levofloxacin showed multiple subpleural nodules with newly emerged bilateral pleural effusion and an exudative lesion in the right lung. (J–L) Another CT (4 April 2022) showed complete absorption of the pleural effusion and a reduced exudative lesion. (M–O) Another CT after moxifloxacin treatment for 3 months (15 June 2022) exhibited an almost normal chest image.
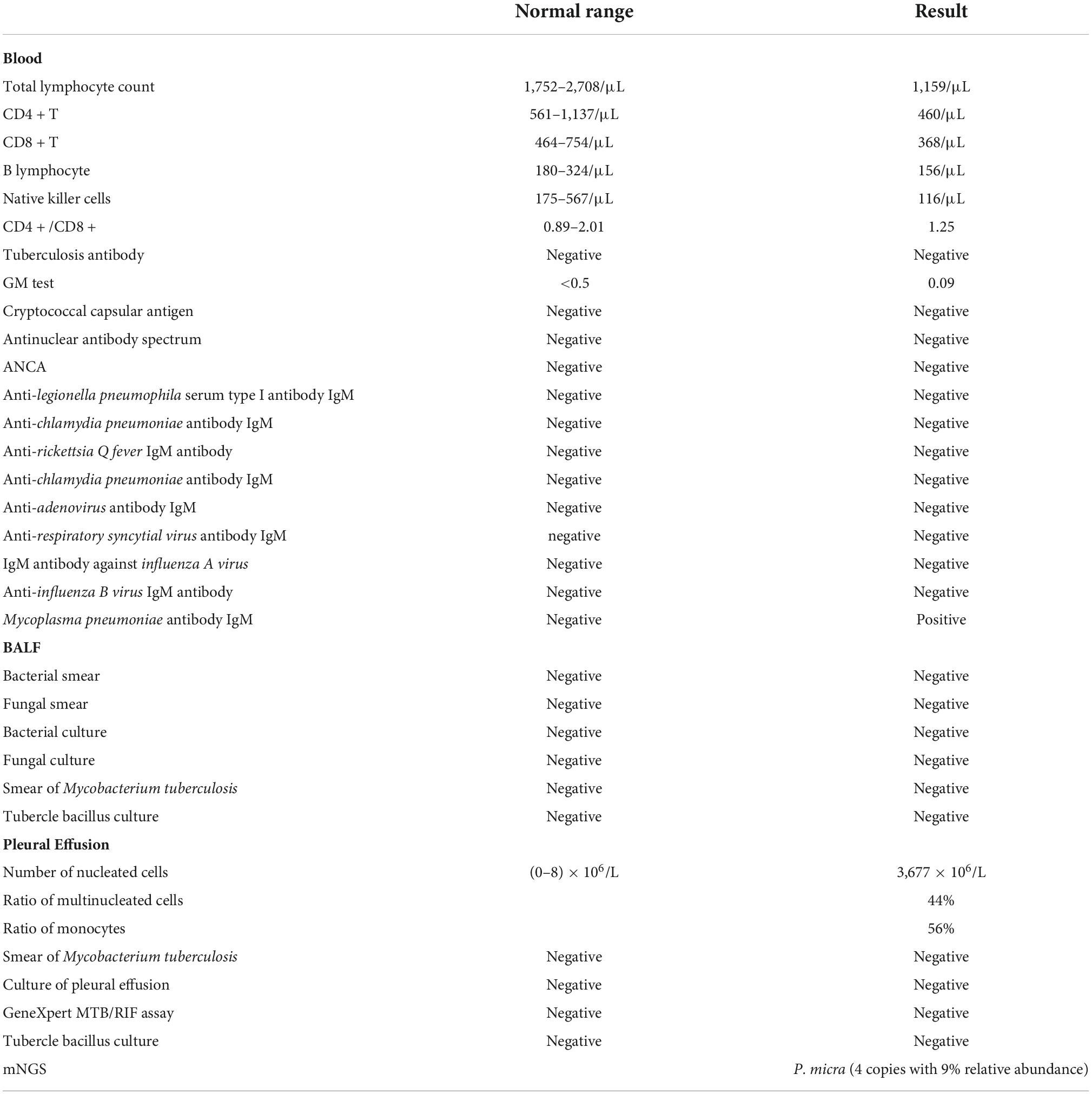
According to his clinical symptoms and the dynamic changes observed in chest CT, we highly suspected an infectious lung disease. Because of the non-specific clinical manifestations, pathogenetic examination was very important for this patient. Therefore, we collected microbiological samples and performed routine bacteria culture. The GeneXpert MTB/RIF assay and mNGS were performed on 29 March 2022, 4 days after admission, in an attempt to identify pathogenic bacteria. Meanwhile, his antibiotic treatment was switched to moxifloxacin to better fight against Gram-positive bacteria and covered the antibacterial spectrum of anaerobic bacteria. As shown in Table 1, the laboratory tests, such as Galactomannan Antigen Testing (GM), β-D-glucan fungal antigen test (G test), and tests for cryptococcal pod antigen, were negative; however, the patient was positive for mycoplasma antibody (IgM). The pleural effusion was exudate consisting of 44% leukocyte. Samples of it were sent for routine bacteria culture and were analyzed using the GeneXpert MTB/RIF assay and mNGS. BALF samples were also subjected to routine bacteria culture and GeneXpert MTB/RIF assay. All samples returned negative in all of the tests except mNGS, which identified four specific reads of P. micra with 9% relative abundance from pleural effusion samples.
To search for the source of infection, enhanced CT of the oropharyngeal and abdomen as well as cardiac ultrasound were conducted. A medical history inquiring about toothache, periodontitis, and oral invasive operations was conducted, and the patient denied underlying oral diseases. No abscesses were detected in any CT image, and cardiac ultrasound revealed a lack of bacterial emboli. However, an examination of the oral cavity indicated that most tooth crowns of this patient were missing, while the roots remained. Oropharyngeal CT also suggested multiple root remnants with partial alveolar bone resorption. Further inquiries revealed that the patient wore dentures for an extended period of time; these were cleaned and soaked in normal tap water that was not changed every day. Based on the poor oral hygiene and colonization of the oral cavity with P. micra, we intended to conduct mNGS to analyze oral and lung pathogens. However, because of the high cost of mNGS, we had to analyze the oral and lung microbiome by 16s rRNA gene sequencing. Interestingly, the results identified Parvimonas not only in the oral cavity but also in the BALF (Figure 3). Compared to the oral flora of healthy men (15), the patient’s α diversity of the oral cavity was significantly reduced (Figure 3). Based on these factors, we speculated that the oral cavity could have been the potential infection source. Moxifloxacin treatment was continued. Fortunately, a follow-up chest CT on 4 April 2022 showed complete absorption of pleural fluid and significant resolution of the exudative lesions (Figure 2). Given that the adjusted treatment was effective, moxifloxacin was continued for 3 more months. Finally, a chest CT on 15 June 2022 showed almost total resolution of the lung lesion (Figure 2).
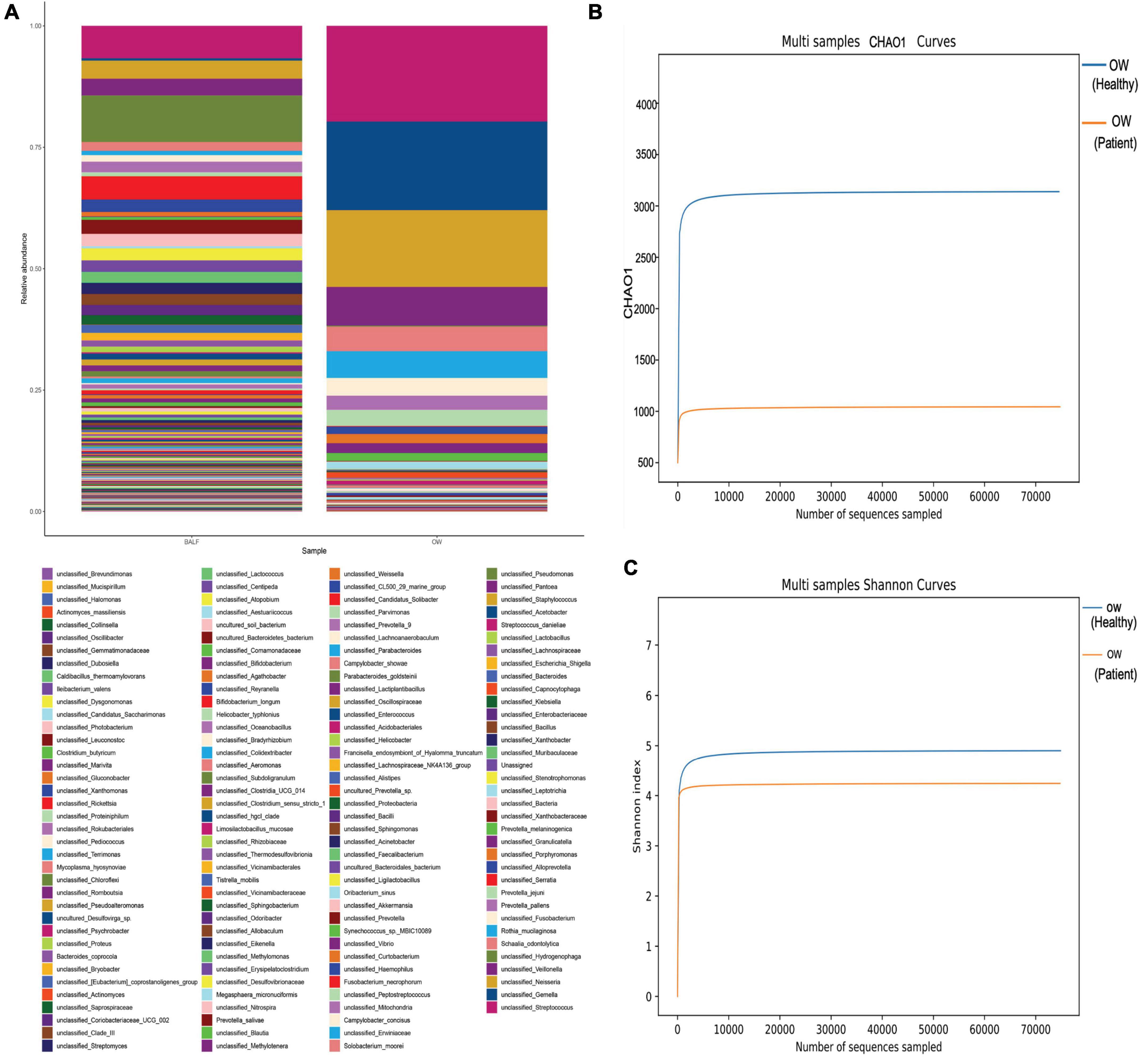
Figure 3. Comparison of the oral and lung microbiome (heatmap). (A) No significant differences were found in P. micra between oral wash and BALF samples (0.11% in BALF vs. 0.13% in oral wash). (B,C) The α diversity of the oral microbiome comparing the patient to a healthy man. Both the Chao1 and Shannon indexes were lower in the patient (Chao1: 986 vs. 3,079, Shannon index: 4.244 vs. 4.829).
Discussion
Parvimonas micra is part of the normal microbiome of the oral cavity, gastrointestinal tract, and skin. However, it can be a pathogen in patients with impaired immune function or with underlying disease. Cases of P. micra infection of the hip joint, pericardium, cervix, liver, and brain have been reported (4, 16–18). However, because of the aerobic environment of the lung, this pathogen is not common in the lung and it rarely causes pneumonia. Tsyshi et al. (19) reported a case of hematogenous lung abscess caused by P. micra in an 85-year-old male with diabetes. In that case, remote sites of infection were detected: apical periodontitis and an infratemporal fossa abscess.
Here, we report a case of P. micra pneumonia mimicking hematogenous Staphylococcus aureus pneumonia in terms of chest imagery without a definitive remote site of infection. There was no direct evidence of blood-borne infection, but we still highly suspected hematogenous infection based on the chest imagery. According to microbiome analyses of the oral cavity and lung by 16s rRNA gene sequencing, the patient’s poor oral hygiene was suspected to be related to the infection. However, as mentioned above, P. micra is a normal component of the oral and intestinal flora in healthy people. The patient in our case denied any underlying disease, except for a long history of heavy smoking. It has been reported that smoking tobacco disrupts immune homeostasis, resulting in a variety of illnesses (20). It also affects oral microbiota composition (21). The level of P. micra and other bacteria in the oral cavity is higher in smokers than in non-smokers (8). We also noted poor hygiene with suspected periodontitis and decreased α diversity in the oral microbiome of our patient, accompanied by similar copies of P. micra between oral wash and BALF samples. Therefore, we speculate that our patient’s P. micra pneumonia may have been induced by dysbiosis of his oral microflora and tobacco smoking.
Common hematogenous lung abscesses always present with high fever, cough with or without sputum, and multiple small subpleural nodules with dynamic change in CT scans. According to a previous systematic review (22), the common pathogens of hematogenous lung abscesses are methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA). Isolation of P. micra as the causative pathogen of a hematogenous lung abscess is rare. Little is known about the clinical characteristics of P. micra hematogenous lung infections. In our case, the patient showed the much similar manifestations to hematogenous lung infection. Because there are no known specific symptoms of hematogenous P. micra pneumonia, it was extremely critical to conduct a microbiological examination. Unfortunately, however, it is difficult to detect this microbe using conventional culture methods. Hence, we used the mNGS technique, which has a wide detection range and does not need to specify the suspected causative microorganism in advance. In addition, comparing to conventional culture methods, the results are obtained very quickly (2–3 days vs. 5–7 days for conventional methods) and the positive rate of identified pathogens is much higher (95 vs. 54%) (23).
Mycoplasma pneumoniae IgM is an early antibody that appears after infection, and it usually appears only after 4–5 days and lasts 1–3 months or more. A positive IgM for Mycoplasma pneumoniae may indicate current infection with Mycoplasma pneumoniae or it may not, as some patients may show prolonged IgM seropositivity caused by prior Mycoplasma pneumoniae infection (24). So, in our case, the patient was thought to be a false positive Mycoplasma pneumoniae IgM and diagnosed with P. micra detection in pleural effusion with mNGS in our case. Normal pleural effusion fluid is sterile, and it can be identified as pathogenic if bacteria are detected in it. Therefore, we confirmed that P. micra was the pathogen causing the pulmonary infection in our patient rather than Mycoplasma pneumoniae.
Although P. micra is typically susceptible to antibiotics such as penicillin, clindamycin, metronidazole, and imipenem, drug-resistant strains may also exist (9, 25). In this case, amoxicillin clavulanate potassium and levofloxacin were administered successively. Unfortunately, the clinical symptoms of the patient remained poorly controlled and the lesions in the lung became exacerbated. So we highly suspected that the patient suffered from drug-resistant strains, even P. micra was theoretical sensitivity to penicillin (26). Hence, if possible, we recommend acquiring drug sensitivity test along with the pathogenic cultures or drug-resistant gene test, which may provide us with the necessary information to develop precise treatments.
The previous studies have suggested that the durations of antibiotic therapy for P. micra pneumonia are highly variable (8) and there is little evidence of hematogenous P. micra pneumonia. Watanabe et al. (7) reported a case of P. micra-related hematogenous lung abscess in which chest CT showed that the lung lesions resolved after 1 month of antibiotic treatment with ampicillin sulbactam. Ubukata et al. (19) reported a similar case where antibiotics were continuously administered for 3 months. Similarly, we administered moxifloxacin for 3 months in our patient. Considering the large variation in antibiotic duration to treat P. micra pneumonia, we recommend using lung imaging instead to guide the antibiotic regimen.
There were some limitations to this study. Due to the usage of antibiotics prior to admission and the relatively high cost of mNGS in the clinical setting, blood samples were not used for mNGS tests. In addition, the patient’s oral and pulmonary flora were not analyzed after treatment, and thus any differences pre- and post-treatment with moxifloxacin could not be determined.
Conclusion
In summary, we report a case of P. micra pneumonia in a patient without any apparent underlying diseases and no remote site of infection. Clinicians need to update the etiology of community-acquired pneumonia, especially in smoking patients with poor oral hygiene. When antibiotic therapy is ineffective, early initiation of mNGS is critical for the rapid screening of rare pathogens such as P. micra. If possible, we recommend choosing a reasonable antibiotic based on drug sensitivity tests of P. micra to avoid possible drug resistance. Finally, lung infection absorption as determined via imaging may provide more objective guidance of treatment than the duration of antibiotic treatment.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CW, XHH, and XH were involved in the patient’s clinical treatment. YF and LP contributed to the diagnosis. RG analyzed the pathological and CT images. YF integrated all information and wrote the manuscript. LP and RG provided critical guidance and revisions for YF throughout the writing process. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Basic Research and Frontier Exploration Project of Chongqing Yuzhong District Science & Technology Committee (under Grant No: 20180139) and Medical Talents Program of Chongqing for Young and Middle-aged.
Acknowledgments
We thank the patient for providing permission to share his information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ryan PM, Shin CP. Native joint infections caused by Parvimonas micra. Anaerobe. (2021) 71:102412. doi: 10.1016/j.anaerobe.2021.102412
2. Marinković J, Marković T, Brkić S, Radunović M, Soldatović I, Ćirić A, et al. Microbiological analysis of primary infected root canals with symptomatic and asymptomatic apical periodontitis of young permanent teeth. Balk J Dent Med. (2020) 24:170–7.
3. Watanabe T, Hara Y, Yoshimi Y, Fujita Y, Yokoe M, Noguchi Y. Clinical characteristics of bloodstream infection by Parvimonas micra: retrospective case series and literature review. BMC Infect Dis. (2020) 20:578. doi: 10.1186/s12879-020-05305-y
4. Cesta N, Foroghi Biland L, Neri B, Mossa M, Campogiani L, Caldara F, et al. Multiple hepatic and brain abscesses caused by Parvimonas micra: a case report and literature review. Anaerobe. (2021) 69:102366. doi: 10.1016/j.anaerobe.2021.102366
5. Randall D, Jee Y, Vanood A, Mayo D. Atypical presentation of periprosthetic joint infection after total knee arthroplasty due to Parvimonas micra. Arthroplast Today. (2020) 6:901–5. doi: 10.1016/j.artd.2020.09.021
6. Prieto R, Callejas-Diaz A, Hassan R, de Vargas AP, Lopez-Pajaro LF. Parvimonas micra: a potential causative pathogen to consider when diagnosing odontogenic brain abscesses. Surg Neurol Int. (2020) 11:140. doi: 10.25259/SNI_20_2020
7. Watanabe T, Yokoe M, Noguchi Y. Septic pulmonary embolism associated with periodontal disease: a case report and literature review. BMC Infect Dis. (2019) 19:74. doi: 10.1186/s12879-019-3710-3
8. Zhang Y, Song P, Zhang R, Yao Y, Shen L, Ma Q, et al. Clinical characteristics of chronic lung abscess associated with Parvimonas micra diagnosed using metagenomic next-generation sequencing. Infect Drug Resist. (2021) 14:1191–8. doi: 10.2147/IDR.S304569
9. Miyazaki M, Asaka T, Takemoto M, Nakano T. Severe sepsis caused by Parvimonas micra identified using 16S ribosomal RNA gene sequencing following patient death. IDCases. (2020) 19:e00687. doi: 10.1016/j.idcr.2019.e00687
10. Yu Q, Sun L, Xu Z, Fan L, Du Y. Severe pneumonia caused by Parvimonas micra: a case report. BMC Infect Dis. (2021) 21:364. doi: 10.1186/s12879-021-06058-y
11. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
12. Schlaberg R, Chiu CY, Miller S, Procop GW, Weinstock G, Professional Practice Committee and Committee on Laboratory Practices of the American Society for Microbiology, et al. Validation of metagenomic next-generation sequencing tests for Universal Pathogen Detection. Arch Pathol Lab Med. (2017) 141:776–86. doi: 10.5858/arpa.2016-0539-RA
13. Langelier C, Kalantar KL, Moazed F, Wilson MR, Crawford ED, Deiss T, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U.S.A. (2018) 115:E12353–62. doi: 10.1073/pnas.1809700115
14. Long Y, Zhang Y, Gong Y, Sun R, Su L, Lin X, et al. Diagnosis of sepsis with cell-free DNA by next-generation sequencing technology in ICU patients. Arch Med Res. (2016) 47:365–71. doi: 10.1016/j.arcmed.2016.08.004
15. Chen B, Wang Z, Wang J, Su X, Yang J, Zhang Q, et al. The oral microbiome profile and biomarker in Chinese type 2 diabetes mellitus patients. Endocrine. (2020) 68:564–72. doi: 10.1007/s12020-020-02269-6
16. Ryan PM, Morrey BF. Parvimonas micra causing native hip joint septic arthritis. Proc Bayl Univ Med Cent. (2021) 34:486–8. doi: 10.1080/08998280.2021.1906827
17. Morinaga H, Kato K, Hisagi M, Tanaka H. Purulent pericarditis-induced intracardiac perforation and infective endocarditis due to Parvimonas micra: a case report. Eur Heart J Case Rep. (2021) 5:ytaa528. doi: 10.1093/ehjcr/ytaa528
18. Fujiwara E, Tsuda T, Wada K, Waki S, Hanada Y, Nishimura H. A rare case of cervical abscess caused by Parvimonas micra. SAGE Open Med Case Rep. (2021) 9:2050313X211024505. doi: 10.1177/2050313X211024505
19. Ubukata S, Jingu D, Yajima T, Shoji M, Takahashi H. A case of septic pulmonary embolism due to Peptostreptococcus micros with multiple infection of the head and neck. Kansenshogaku Zasshi. (2013) 87:761–6. doi: 10.11150/kansenshogakuzasshi.87.761
20. Jiang C, Chen Q, Xie M. Smoking increases the risk of infectious diseases: a narrative review. Tob Induc Dis. (2020) 18:60. doi: 10.18332/tid/123845
21. Lee YH, Chung SW, Auh QS, Hong SJ, Lee YA, Jung J, et al. Progress in oral microbiome related to oral and systemic diseases: an update. Diagnostics. (2021) 11:1283. doi: 10.3390/diagnostics11071283
22. Ye R, Zhao L, Wang C, Wu X, Yan H. Clinical characteristics of septic pulmonary embolism in adults: a systematic review. Respir Med. (2014) 108:1–8. doi: 10.1016/j.rmed.2013.10.012
23. Qian YY, Wang HY, Zhou Y, Zhang HC, Zhu YM, Zhou X, et al. Improving pulmonary infection diagnosis with metagenomic next generation sequencing. Front Cell Infect Microbiol. (2020) 10:567615. doi: 10.3389/fcimb.2020.567615
24. Jeon HE, Kang HM, Yang EA, Han HY, Han SB, Rhim JW, et al. Early confirmation of Mycoplasma pneumoniae infection by two short-term serologic IgM examination. Diagnostics. (2021) 11:353. doi: 10.3390/diagnostics11020353
25. Rams TE, Sautter JD, van Winkelhoff AJ. Antibiotic resistance of human periodontal pathogen Parvimonas micra over 10 years. Antibiotics. (2020) 9:709. doi: 10.3390/antibiotics9100709
Keywords: P. micra pneumonia, dysbiosis, oral flora, poor oral hygiene, new etiology
Citation: Feng Y, Wu C, Huang X, Huang X, Peng L and Guo R (2022) Case report: Successful management of Parvimonas micra pneumonia mimicking hematogenous Staphylococcus aureus pneumonia. Front. Med. 9:1017074. doi: 10.3389/fmed.2022.1017074
Received: 11 August 2022; Accepted: 30 September 2022;
Published: 28 October 2022.
Edited by:
Hui Chen, Brigham and Women’s Hospital and Harvard Medical School, United StatesReviewed by:
Yue Zheng, Dana-Farber/Brigham and Women’s Cancer Center, United StatesSahar Rostamian, Brigham and Women’s Hospital and Harvard Medical School, United States
Xiangxiang Hu, University of North Carolina at Chapel Hill, United States
Xun Li, University of Maryland, Baltimore, United States
Copyright © 2022 Feng, Wu, Huang, Huang, Peng and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Peng, cGxpMTIyOEAxNjMuY29t; Rui Guo, Z3VvLnJ1aS5jcW11QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Yanmei Feng
Yanmei Feng Chunxia Wu1†
Chunxia Wu1† Li Peng
Li Peng Rui Guo
Rui Guo