- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Critical Care Medicine, Peking University Cancer Hospital and Institute, Beijing, China
Background: The purpose of this study was to clarify the incidence, risk factors, and clinical outcomes of septic acute kidney injury (AKI) in cancer patients with sepsis admitted to the intensive care unit (ICU).
Methods: A total of 356 cancer patients admitted to the ICU due to sepsis from January 2016 to October 2021 were analyzed retrospectively. According to the incidence of septic AKI, all patients were divided into the non-AKI group (n = 279) and the AKI group (n = 77). The clinical data after ICU admission were compared between the above two groups, and the risk factors and the clinical outcomes of septic AKI in the ICU were identified.
Results: The incidence of septic AKI in all patients was 21.6% (77/356). LASSO regression and logistic regression all showed that lactate, sequential organ failure assessment (SOFA) score and septic shock were closely related to the occurrence of septic AKI. In terms of clinical outcomes after ICU admission, the rate of mechanical ventilation (MV) and continuous renal replacement therapy (CRRT), MV time, hospitalization time and 28-day mortality in the ICU were significantly higher in the septic AKI group than in the non-septic AKI group. Among the three subgroups of septic AKI (AKI combined with septic shock, septic cardiac dysfunction or acute respiratory failure), the mortality of patients in the subgroup of AKI combined with septic shock was significantly higher than others. CRRT has no significant effect on the short-term outcome of these patients.
Conclusion: Lactate level, SOFA score and septic shock were closely related to the occurrence of septic AKI in the ICU. The clinical outcomes within 28 days after ICU admission of cancer patients with septic AKI were worse than those without septic AKI. The short-term outcome was worse in patients with septic AKI complicated with septic shock. CRRT does not have any significant effect on the short-term prognosis of cancer patients with septic AKI in the ICU.
Introduction
Acute kidney injury is considered as one of the serious comorbidities in critically ill patients. AKI may have higher short-term and long-term mortality, and the use of medical resources is considerably increased. AKI is characterized by a sudden decrease in glomerular filtration rate (GFR), resulting in the accumulation of nitrogenous waste and the inability to maintain the homeostasis of body fluids and electrolytes (1). Although there is not any clear causal relationship between AKI and chronic kidney disease (CKD), the AKI non-intervention group may increase the risk of CKD (2). Patients with AKI are the most likely to suffer from accelerated loss of renal function and progress to CKD than patients without AKI with all else being equal (3). CRRT is an effective treatment for AKI, but it does not reduce long-term mortality of AKI or the risk of CKD (4). Even if AKI patients return to normal kidney function after discharge from the hospital, there is still a risk of adverse kidney events for up to 10 years (5). In addition, a meta-analysis suggests that the duration of AKI is independently related to long-term mortality, cardiovascular events and the development incident CKD of stage 3 (6). Considering the above situation, AKI should be given full attention and early disposal.
The most common cause of AKI in critically ill patients is sepsis. Cohort studies indicate that the incidence of septic AKI ranges from 19 to 48%, while the mortality of patients with septic AKI fluctuates from 22 to 70% (7, 8). The pathophysiology of septic AKI is still not fully appreciated. Traditionally, it is believed that septic AKI is mainly caused by global renal ischemia and hypoperfusion, septic endotoxin-mediated cell damage, and renal tubular necrosis (9). However, other studies suggest that septic AKI is a bioenergy adaptive response of the body to microcirculation dysfunction and inflammation caused by sepsis, which has no significant correlation with the existence of systemic hypoperfusion or the severity of sepsis (10–12).
Because the immune system of cancer patients with sepsis cannot cope with the initial injury, pathogen invasion emerged on the basis of malignant cell transformation. Compared with non-cancer patients, cancer patients with sepsis had a 2.5-fold higher in-hospital mortality rate due to sepsis. Cancer patients with sepsis have a worse prognosis (13, 14). Therefore, there may be a great proportion of septic AKI in cancer patients with sepsis. This retrospective study aimed at the precise population of cancer patients with sepsis to clarify the incidence, risk factors and short-term clinical outcomes of septic AKI after ICU admission to guide clinical intervention and judge prognosis.
Materials and methods
Participants
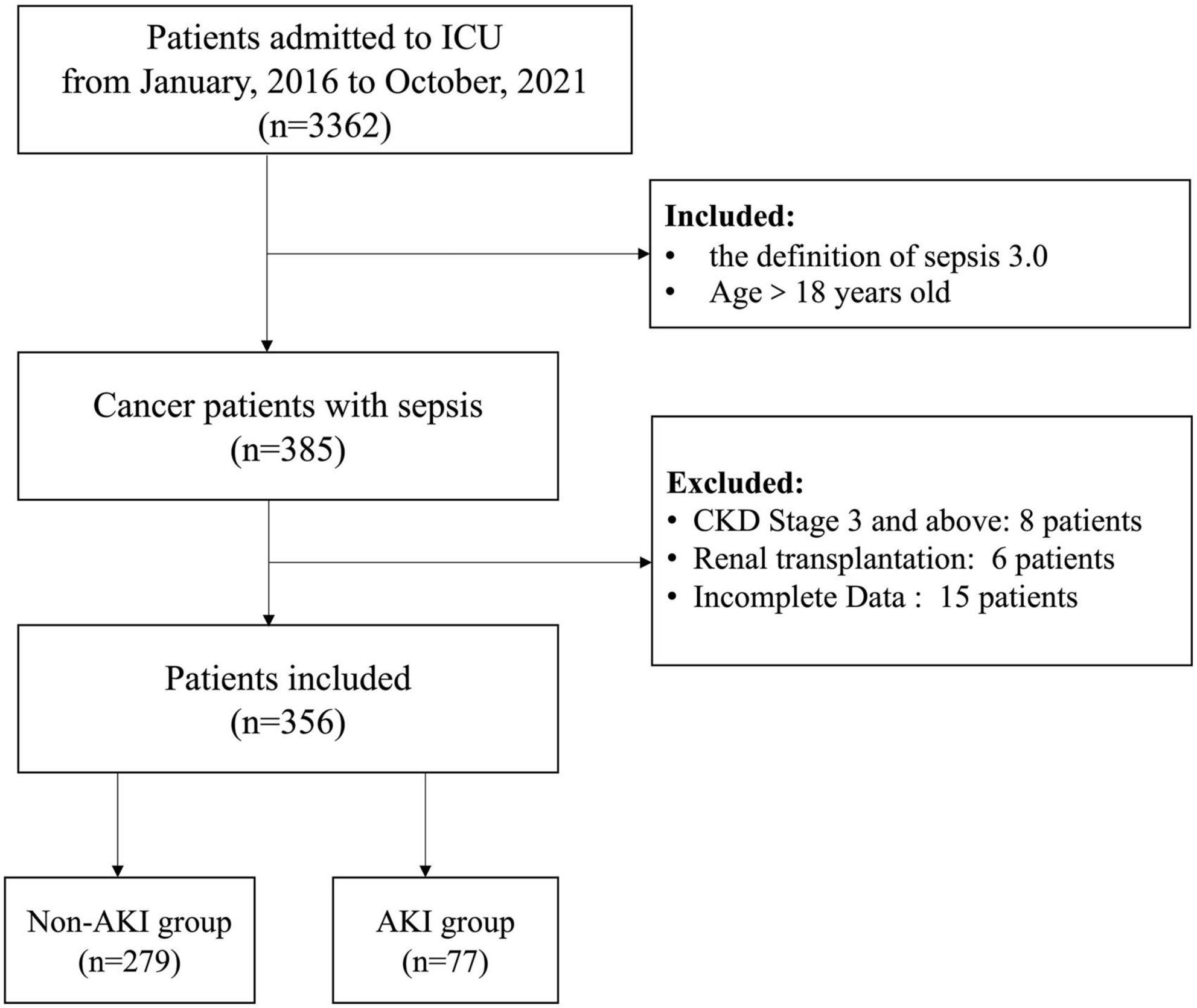
The study complies with the Declaration of Helsinki and was approved by the Ethics Committee of Peking University Cancer Hospital(ethics approval number 2020KT33), and all patients provided written informed consent for the treatment of sepsis and related scientific research purposes A total of 356 cancer patients with sepsis were retrospectively screened out of 3,362 patients admitted to the ICU in Peking University Cancer Hospital from January 2016 to October 2021, according to the inclusion criteria. Inclusion criteria: (1). Patients with sepsis aged >18 years; (2). Diagnosis satisfying the definition of sepsis 3.0. Exclusion criteria: (1). CKD stage 3 and above; (2). After kidney transplantation; (3). Incomplete clinical data. All the included patients were divided into the non-AKI group (n = 279) and the AKI group (n = 77) in terms of the occurrence of septic AKI (Figure 1).
The diagnosis of septic AKI: (1). Clinical judgment of AKI has a positive correlation with sepsis; (2). AKI refers to the definition and diagnostic criteria from kidney disease improving global outcomes (KIDGO) in 2012.
Data collection
Demographic characteristics and baseline data [including sex, age, body mass index (BMI), cancer types, cancer treatment, chronic diseases], infection site and etiological data, some laboratory test results within 24 h after ICU admission, the first SOFA scores, and other complications related to sepsis, including septic shock, septic cardiac dysfunction (SCD), and acute respiratory failure (ARF), were collected from all the included patients. The short-term clinical outcomes of all patients in the ICU were recorded, including mechanical ventilation (MV), continuous renal replacement therapy (CRRT), MV time, length of stay in the ICU and 28-day mortality in the ICU.
Statistics
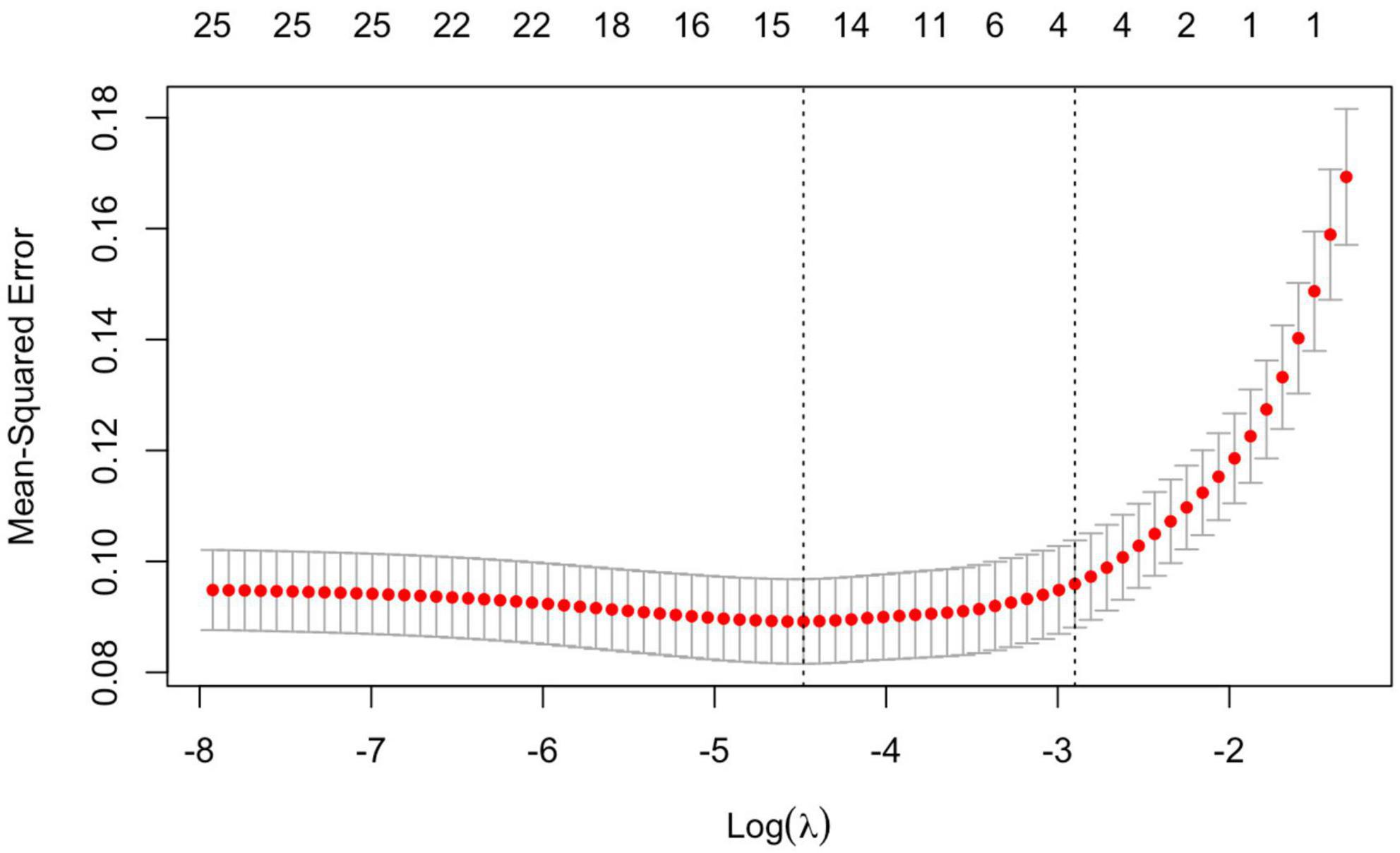
SPSS 26.0 (Armonk, NY: IBM) and R language (version 4.1.2, involving software packages such as “survival,” “surviviner,” “glmnet,” “pROC”) were used for statistical analysis. Continuous variables with normal distributions were expressed as means ± SD; otherwise, they were expressed as medians (IRQ). Categorical data were expressed as numbers (proportions). categorical variables were reported as frequency or percentage (%). Continuous variables with a normal distribution were compared by unpaired independent t test, continuous variables with a skewed distribution were compared by the Mann–Whitney U test, and the classified data were compared using the χ2 test or Fisher’s exact probability method. Logistic regression and LASSO regression were utilized to compare and screen out the significant risk factors of septic AKI. The number of septic AKI related variables of non-zero parameters was controlled by adjusting the Lambda(λ) value in the LASSO regression. 1se (the dashed line on the right side) was taken as a reference, the method of ten-fold cross-validation was utilized to obtain the minimum number of variables for the optimal model. The Kaplan–Meier method was used to analyze the short-term clinical prognosis of patients with septic AKI. The ROC curve was used to determine the predictive value of relevant risk factors for septic acute renal injury. Bivariate correlation analysis is applied to the comparison of critical variables. For all the above tests, a two-tailed P < 0.05 was regarded as statistically significant.
Results
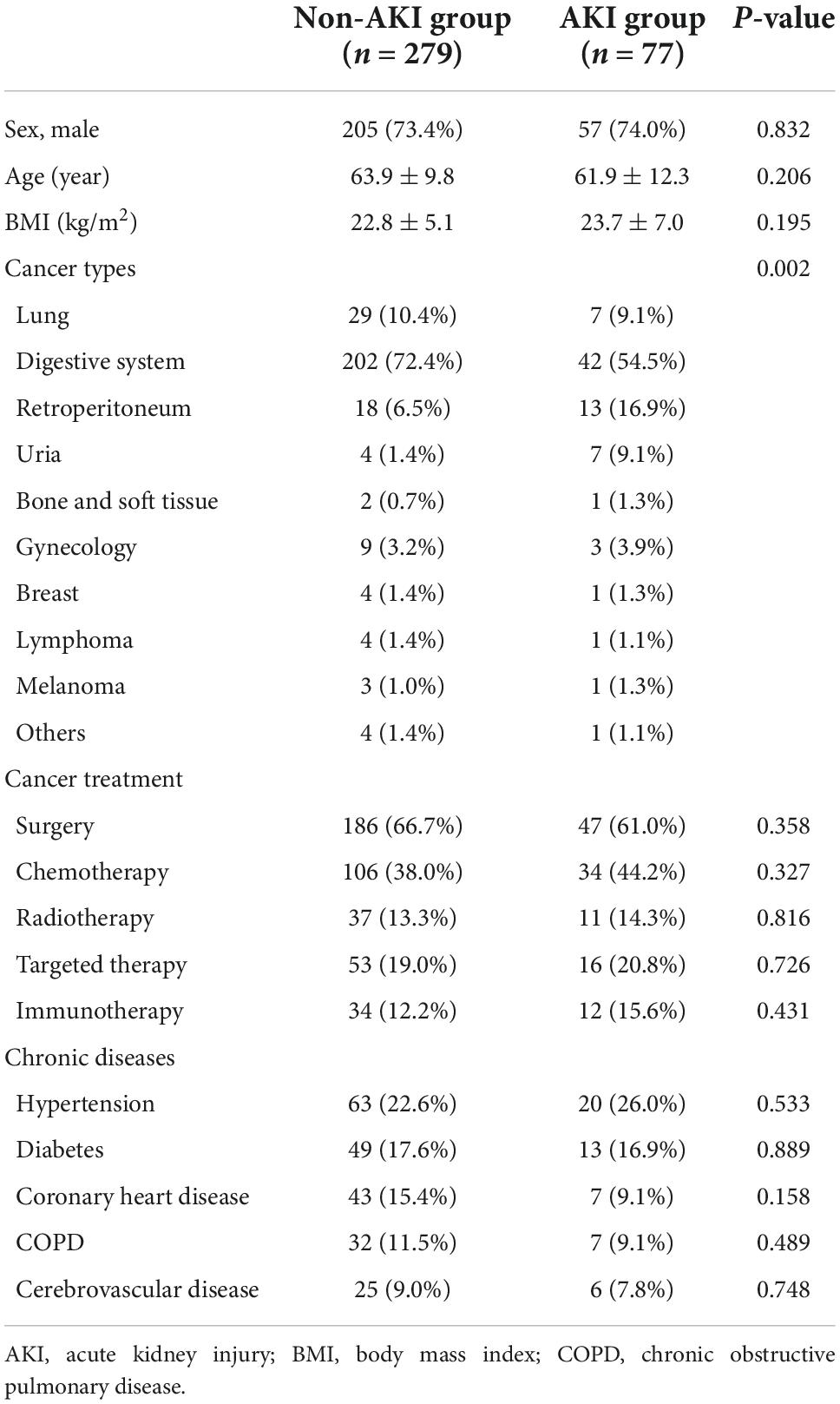
1. Occurrence of sepsis-related AKI among different cancer types: After being regrouped, septic patients with retroperitoneal cancers and urinary cancers were more likely to suffer from septic AKI (P = 0.002) (Table 1).
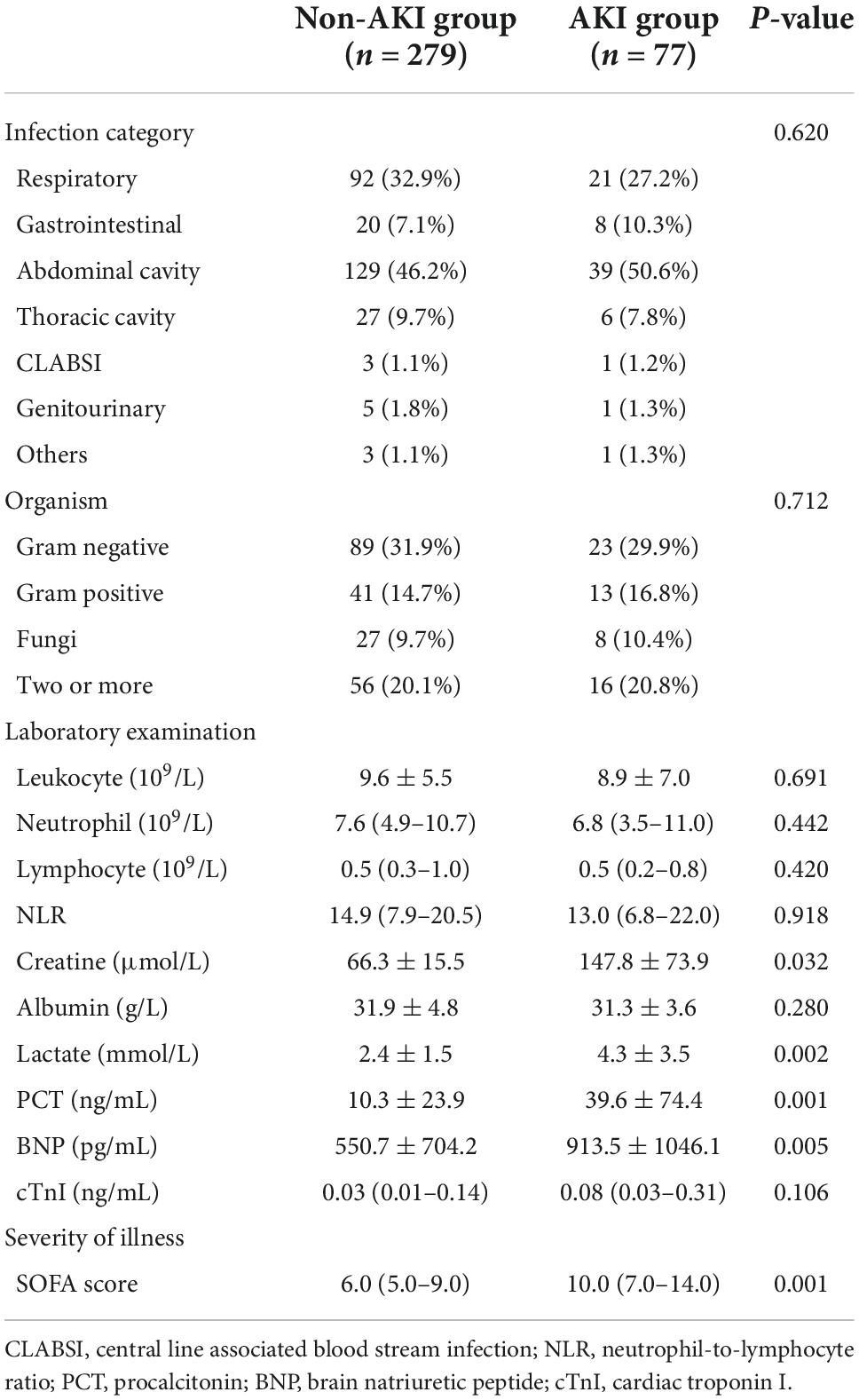
2. Comparison of variables with statistical differences between the two groups of patients: There were significant differences in creatinine, lactate, procalcitonin (PCT), brain natriuretic peptide (BNP), and SOFA scores after ICU admission between the septic AKI group and the non-septic AKI group (P = 0.032, P = 0.002, P = 0.001, P = 0.005, P = 0.001) (Table 2).
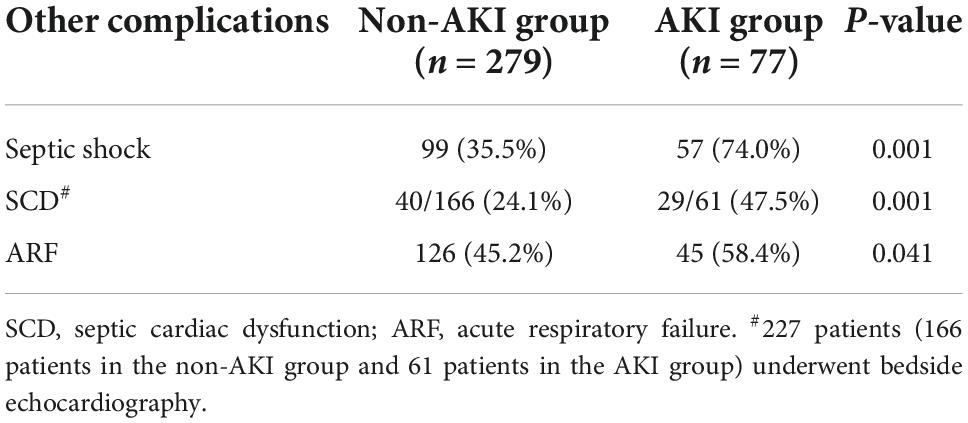
3. Comparison of sepsis-related complications between the two groups: Sepsis-related complications (septic shock, SCD and ARF) were more likely to occur in the septic AKI group than in the non-septic AKI group (P = 0.001, P = 0.001, P = 0.041) (Table 3).
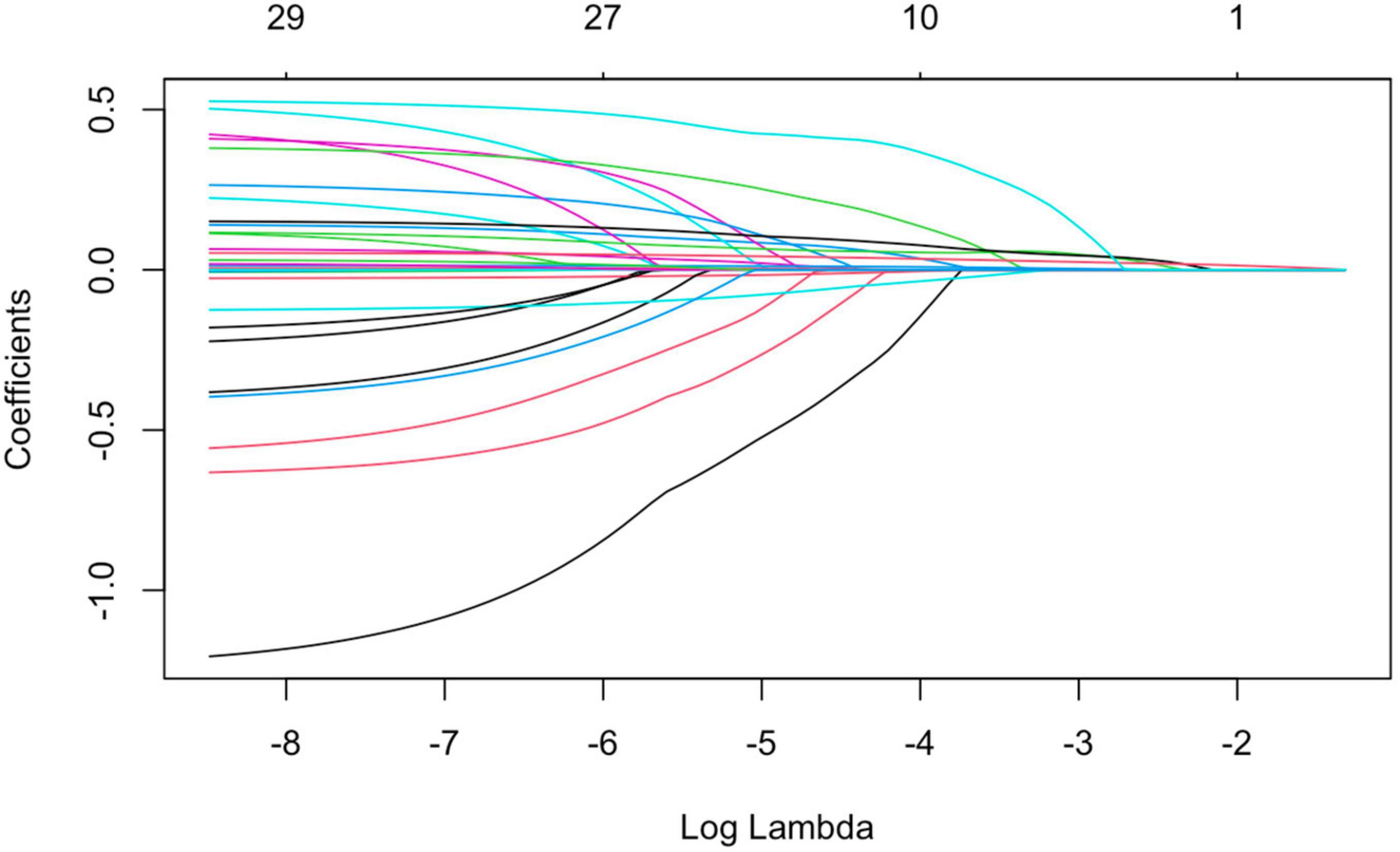
4. LASSO regression was used to screen the important risk factors of septic AKI: All the variables in Tables 1–3 were screened with LASSO regression for avoiding overfitting the data in order to improve accuracy (Figures 2, 3). These important variables including lactate, SOFA score, septic shock, and PCT were strongly associated with septic AKI.
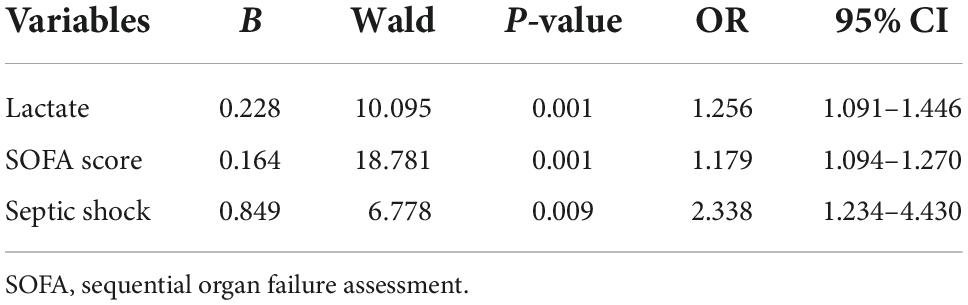
5. Independent risk factors of septic AKI were screened out by multivariate analysis of Logistic regression: Lactate, SOFA score and septic shock (variables from Tables 1, 3) were closely related to septic AKI, and these three variables were independent risk factors for septic AKI (P = 0.001, P = 0.001, P = 0.009) (Table 4).
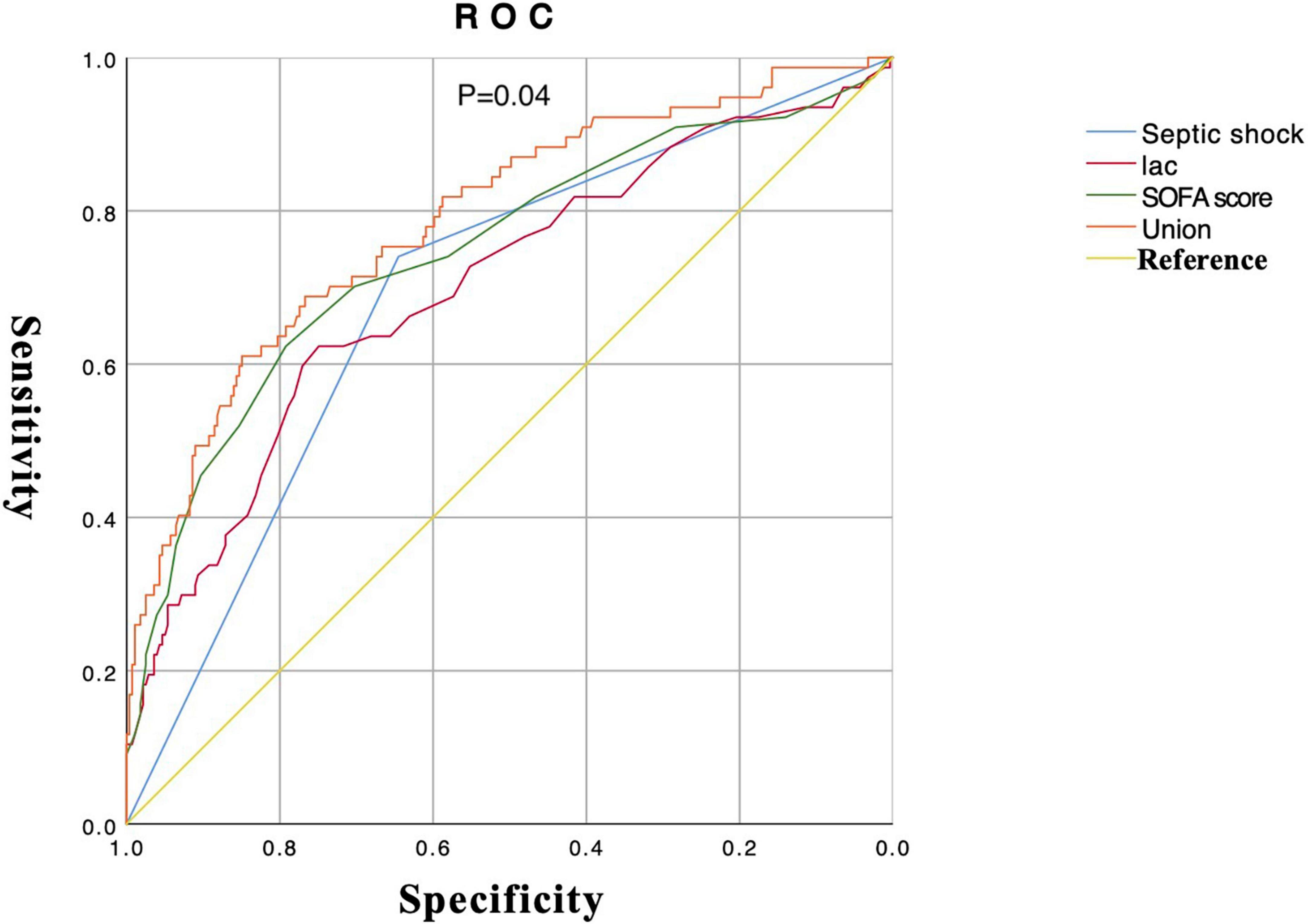
6. The drawing of ROC about important variables that affect the occurrence of septic AKI: Lactate, SOFA score, and septic shock were screened out with the intersection of Wayne diagram adopted from the combination of Lasso regression and logistic regression. The union ROC (lactate combined with SOFA score and septic shock) showed that the performance in predicting septic AKI (AUC 0.79, 95% CI 0.73–0.85) is better than the predictive performance of each variable (septic shock, AUC 0.69, 95% CI 0.63–0.76; lactate, AUC 0.70, 95% CI 0.63–0.77; SOFA sore, AUC 0.74, 95% CI 0.67–0.81) (P = 0.04) (Figure 4). Bivariate correlation analysis of these three variables showed that there was a positive correlation between septic shock and lactate (P < 0.001, r = 0.330), a positive correlation between septic shock and SOFA score (P < 0.001, r = 0.413), and a positive correlation between lactate and SOFA score (P < 0.001, r = 0.378).
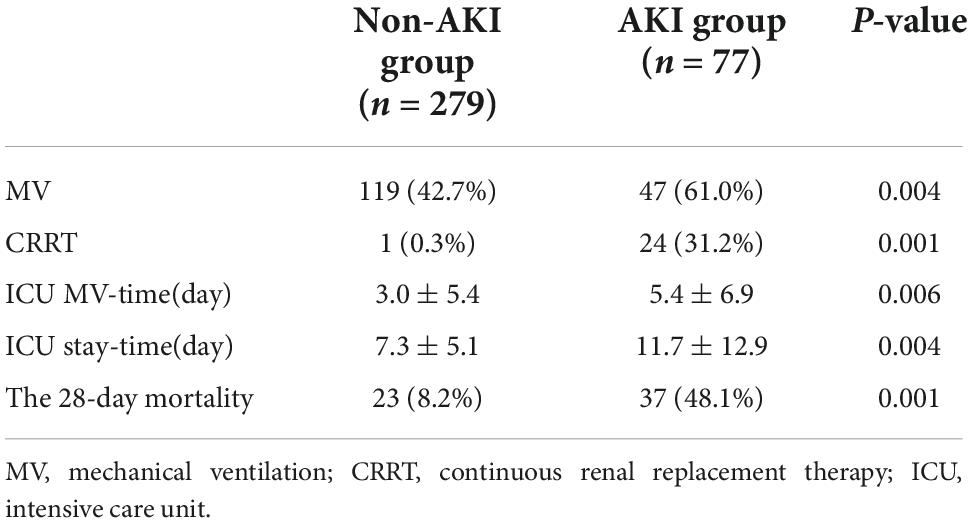
7. Comparison of the difference on the short-term clinical outcome between two groups: In terms of short-term clinical outcomes, patients with septic AKI had higher rates of MV and CRRT, longer durations of MV-time and ICU stay-time, and higher 28-day mortality in the ICU (P = 0.004, P = 0.001, P = 0.006, P = 0.004, P = 0.001) (Table 5).
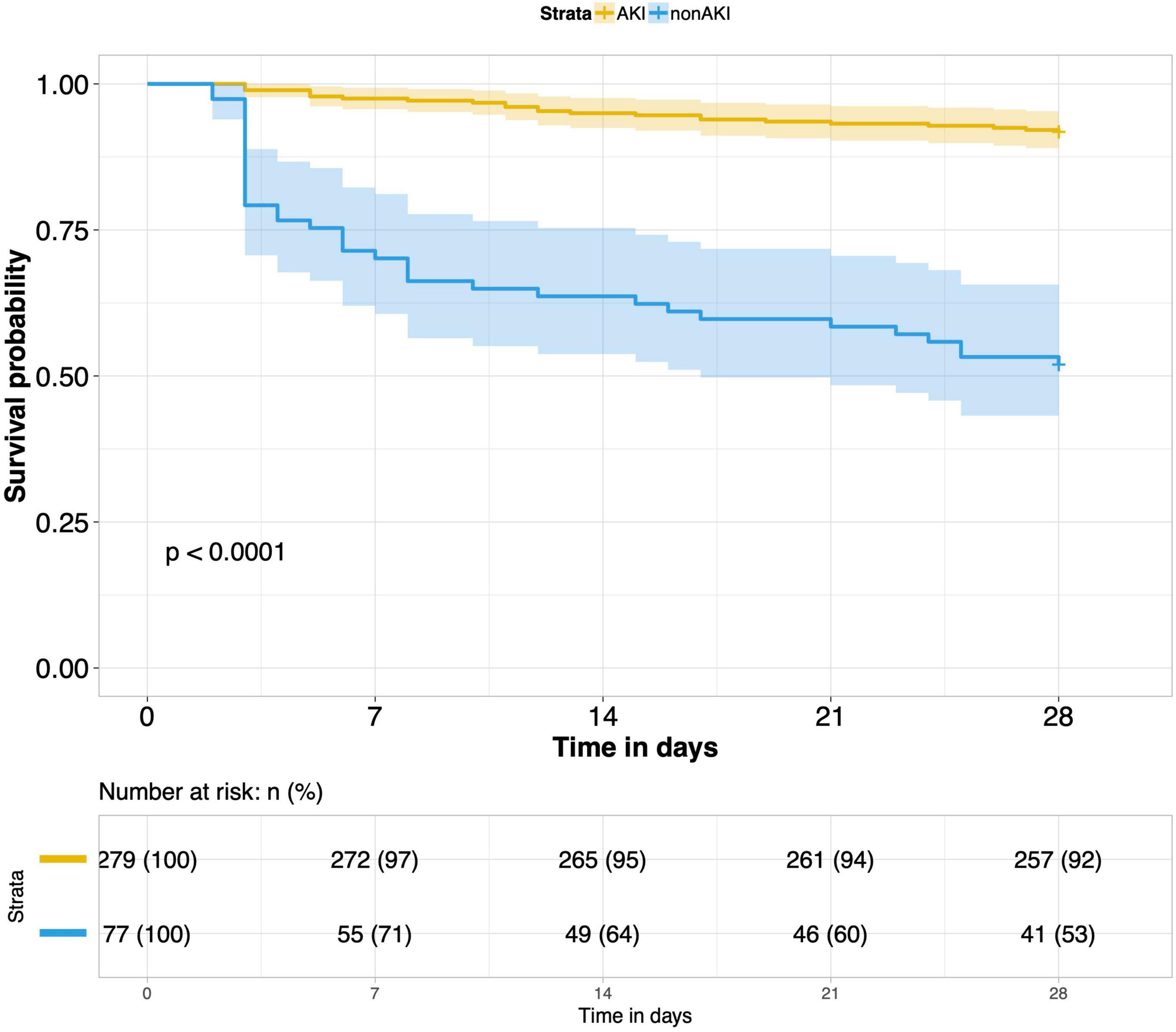
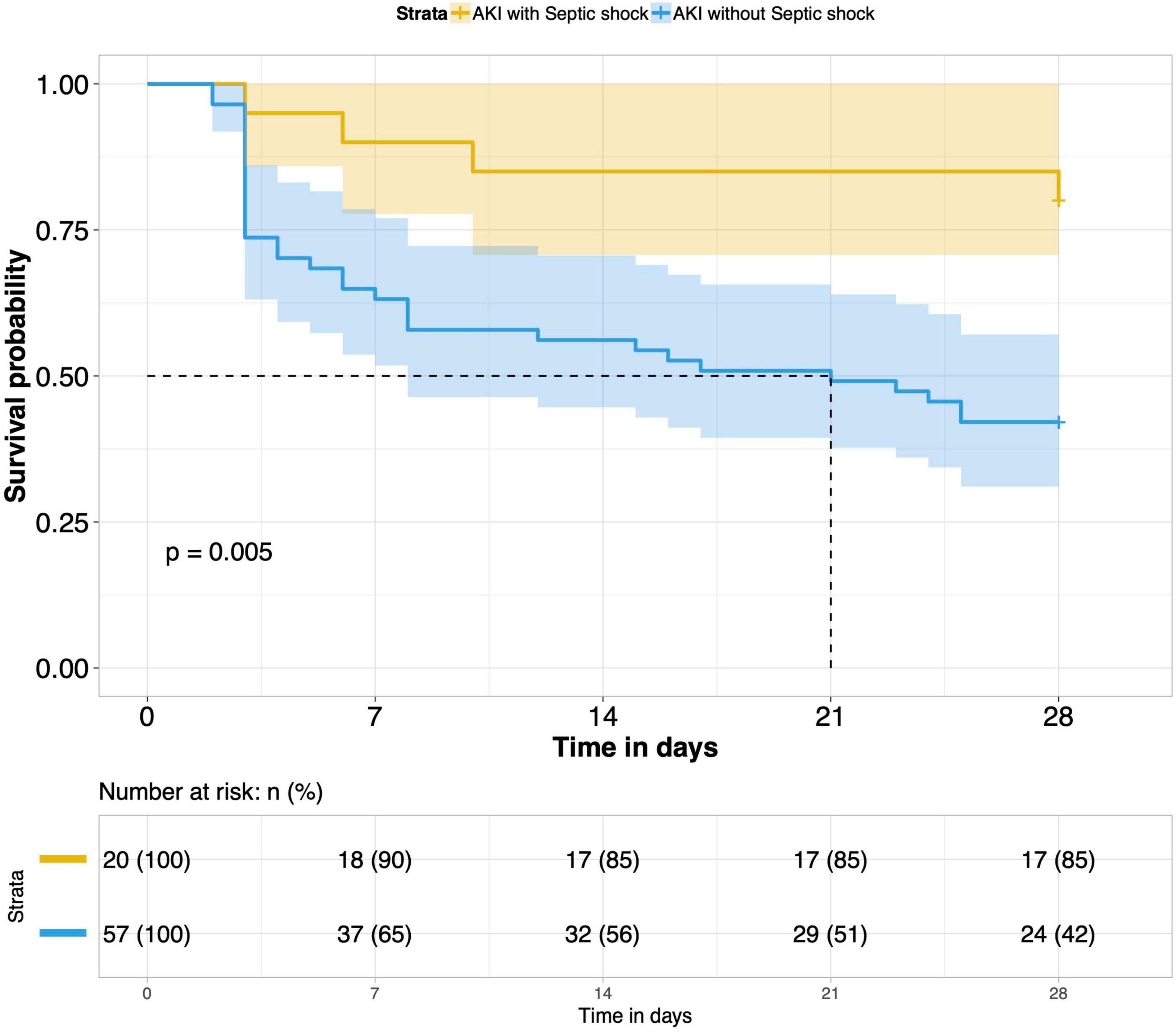
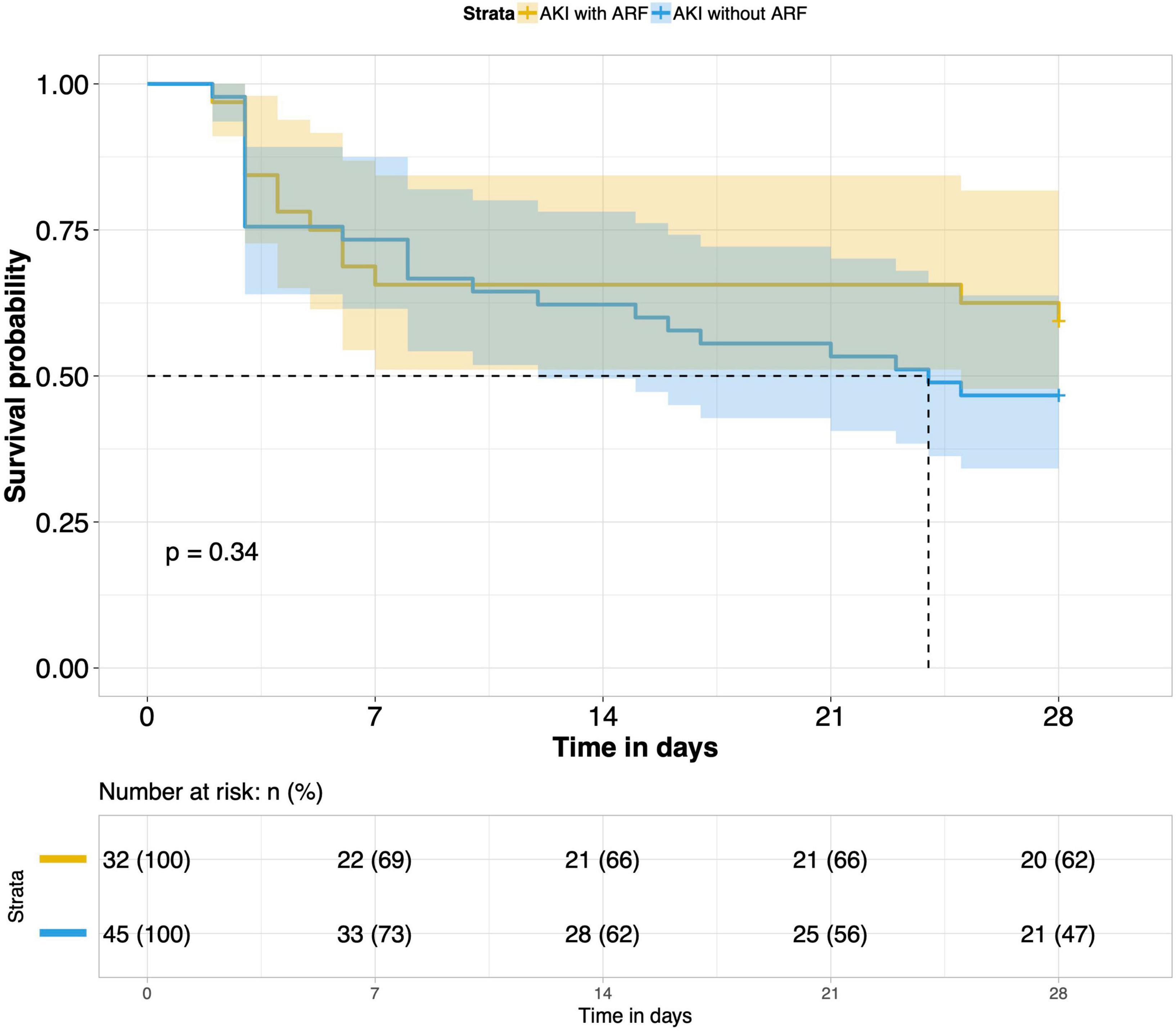
8. Comparison of 28-day survival rates in the two groups and in multiple subgroups of sepsis AKI: The 28-day survival rate of patients with septic AKI was significantly lower than that of patients with non-septic AKI within 28 days after ICU admission (P < 0.001) (Figure 5). In the three subgroups of septic AKI (septic AKI combined with septic shock, septic cardiac dysfunction or acute respiratory failure), the 28-day survival rate of septic AKI combined with septic shock decreased significantly (P = 0.005) (Figure 6); However, there was no significant difference in the other two subgroups of patients (P = 0.07, P = 0.34) (Figures 7, 8).
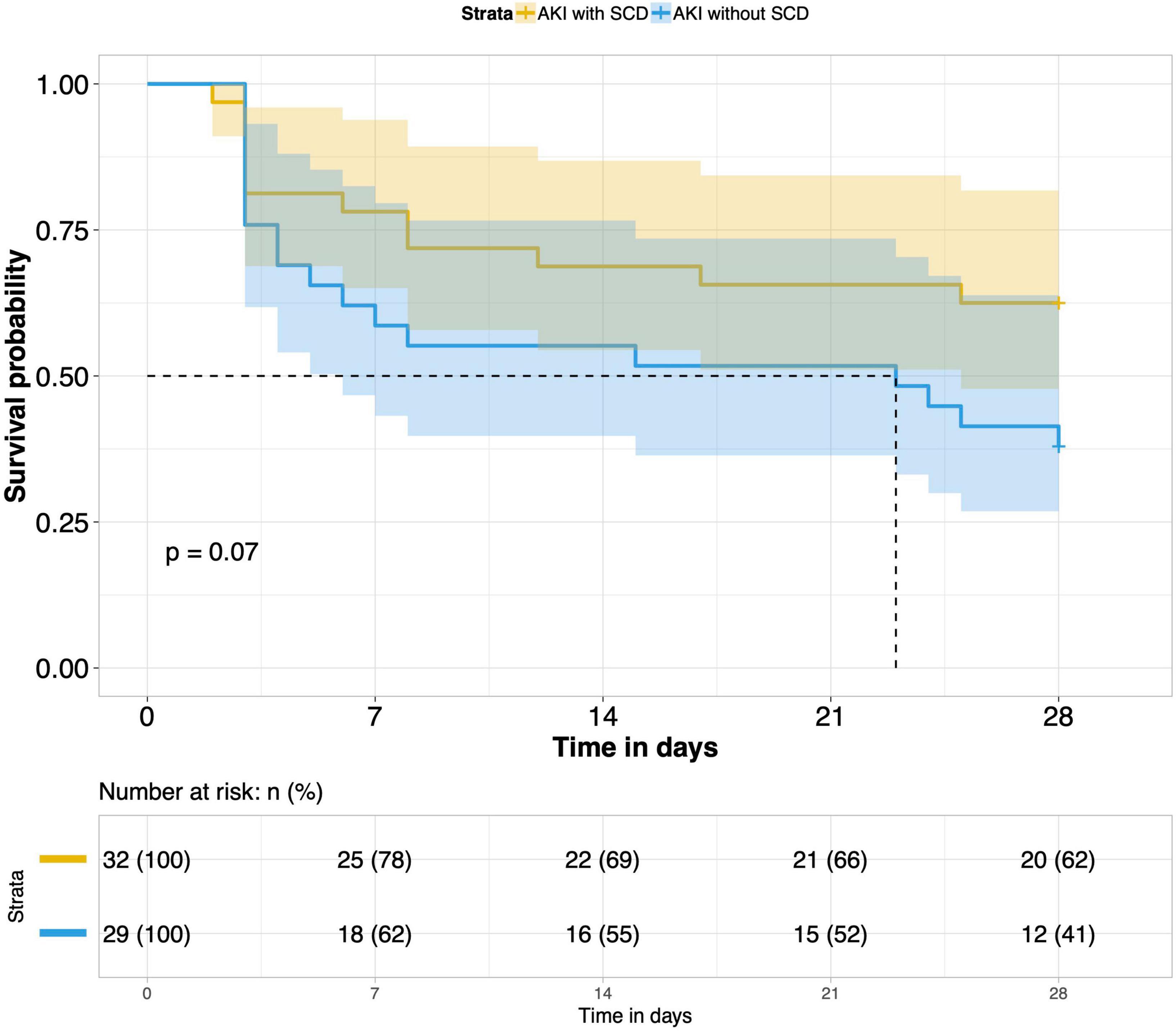
Figure 7. Survival analysis of AKI with SCD group and AKI without SCD group (61 of 77 patients underwent bedside echocardiography).
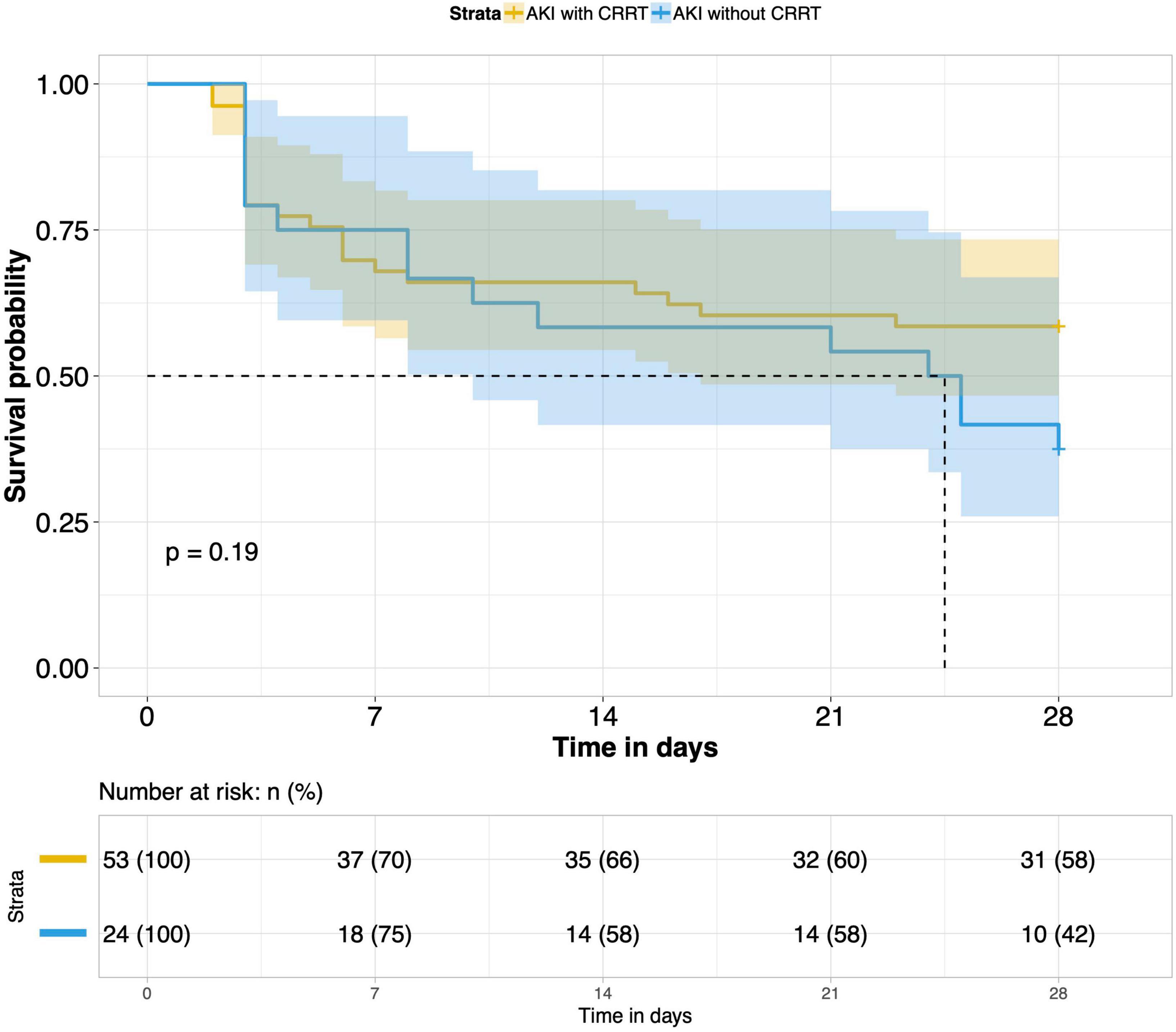
9. Effects of CRRT treatment on the short-term prognosis of septic AKI patients: According to whether CRRT was performed in the ICU, patients with septic AKI were divided into the CRRT group and the non-CRRT group. There was not any significant difference in the 28-day outcome of the two groups. CRRT had no meaningful effect on the short-term prognosis of septic AKI patients (P = 0.19) (Figure 9).
Discussion
Septic AKI is a life-threatening complication characterized by an abrupt deterioration in renal function, manifested as elevated serum creatinine levels, oliguria, or both. It closely relates to infection or sepsis. Septic AKI is one of the earliest focal manifestations in patients with sepsis. Current estimates suggest that septic AKI affects 10–67% of patients with sepsis (8, 15). However, more than two-thirds of patients with septic shock may be complicated with septic AKI (16). For unexplained AKI, the possibility of sepsis should be examined first. Cancer patients are more likely to suffer from sepsis and have a significantly higher mortality rate due to sepsis than non-cancer patients (14). Our study aimed to understand the related factors of septic AKI in cancer patients with sepsis and is used as a basis for the prevention, treatment and renal function recovery of septic AKI for this population.
We found that there may be a definite relationship between septic AKI and cancer type. Regroup analysis showed that sepsis patients with retroperitoneal and urinary tumors were more vulnerable to septic AKI. For the two types of cancer patients, we analyzed the reasons. The mechanism of retroperitoneal and urinary tumors with septic acute kidney injury may include the following: Firstly, the tumor has oppressed or invaded the urinary system, causing local obstruction or postrenal obstruction, resulting in impaired renal function. Secondly, most patients with retroperitoneal and urinary tumors have undergone surgery, and there is a risk of low organ perfusion during the operation. Some patients may undergo single nephrectomy, and patients may be complicated with abdominal infection, paralytic intestinal obstruction, intra-abdominal hypertension after surgery (17). In addition, tumor-related thrombotic microvascular disease and septic coagulation dysfunction may affect the kidneys, resulting in acute kidney damage caused by renal microvascular thrombosis with endothelial swelling and microvascular obstruction (18). All of the above related factors may significantly increase the probability of septic AKI in these cancer patients. However, it was not found that the two types of cancers were closely related to the occurrence of septic AKI in the septic patients with the two types of cancers were included in the multivariate analysis.
Our study also concluded that lactate, SOFA score and septic shock were closely related to the occurrence of septic AKI with LASSO regression and Logistic regression. Serum lactate levels in the septic AKI group were significantly higher than those in the non-septic AKI group. The serum lactate level is a sensitive but non-specific indicator of metabolic stress (19). As a product of anaerobic glycolysis, lactate is markedly elevated in settings of hypoxia, stress, and critical illness (20). Most studies have demonstrated that high levels of lactate are significantly positively correlated with sepsis mortality, and the higher the lactate level is, the worse the prognosis of sepsis (21, 22). Hyperlactatemia is a significant manifestation of increasing tissue anaerobic metabolism in patients with sepsis. It is regarded as a sensitive marker of systemic or local organ tissue hypoperfusion (23). Based on the above studies, it is reasonable to believe that elevated lactate levels can predict renal hypoperfusion, which may eventually progress to AKI. SOFA score in the septic AKI group was also significantly higher than that in the non-septic AKI group. The SOFA score is a key component of the third edition of the definition of sepsis. Clinical diagnosis of infection and SOFA ≥ 2 points can be considered as the definition of sepsis (24). The higher the SOFA score, the more severe organ dysfunction due to sepsis. In our study, the differences in SOFA score between the two groups were consistent with the short-term prognosis, which suggested that the higher the SOFA score, the more severe the illness and the worse the prognosis. Studies have demonstrated that there is a good correlation between the SOFA score and lactate level. The higher the SOFA score is, the higher the lactate level in serum, both of which are signals of increased organ dysfunction and suggest the need for urgent medical intervention (25). We also found that the proportion of patients with septic shock in the septic AKI group was considerably higher than that in the non-septic AKI group. This indicated that septic shock was closely related to the occurrence of septic AKI, which was an independent risk factor for septic AKI. Septic shock leads to systemic hypotension and hypoperfusion of multiple organs, including kidney hypoperfusion. In addition, studies have shown that septic shock may lead to dysfunction of the renal vascular bed, leading to a dramatic decrease in GFR and the development of septic AKI (26). Finally, we carried out a bivariate correlation analysis of these three variables, which showed a significant positive correlation among these variables (P < 0.001). This result shows that septic shock may have higher levels of blood lactate, and both of which are positively correlated with the severity of the disease, that is, SOFA score.
In our study, rates of MV and CRRT for the septic AKI group were significantly higher than those of the non-septic AKI group, and the MV time and the ICU stay time were also significantly prolonged. There was a major difference in the 28-day mortality between the above two groups. The 28-day mortality was also significantly increased when septic AKI was combined with septic shock. We compared the effect of CRRT on the prognosis of patients with septic AKI. CRRT cannot prolong the short-term survival time of patients with septic AKI, so CRRT did not improve the short-term prognosis of septic AKI. These conclusions are in agreement with most studies (27, 28).
Our study on septic AKI is of definite clinical significance. Firstly, the group of this study focused on cancer patients with sepsis, and we found that septic cancer patients of retroperitoneal and urinary tumors were more likely to have septic AKI. The group studied and this conclusion are not common in previous studies. Secondly, we screened out three variables with the intersection of Wayne diagram adopted from the combination of Lasso regression and logistic regression. The prediction of the combined ROC based on the three variables for the occurrence of septic AKI has good performance. Later, it can be modeled and verified after increasing the sample size. If the predictive ability of the model is reliable, it can be adopted in clinical application to judge the prognosis of septic AKI at an early stage. Finally, we understand that if cancer patients with sepsis have septic AKI at the same time, the short-term outcome will be poor, and CRRT cannot effectively improve the prognosis. The above aspects are helpful for us to understand the risk factors of septic AKI in cancer patients with sepsis, which play a good reference role in the diagnosis, treatment, and prognosis of septic AKI in cancer patients.
However, our study has its limitations. Firstly, this study was a retrospective study, and our data were taken from single-center studies, so the incidence and severity of septic AKI may be biased. Secondly, for all patients with septic AKI, we focused on the short-term outcomes within 28 days after ICU admission and lacked 90-day or longer follow-up data on cancer patients with sepsis. The lack of awareness of the long-term survival and physical and mental health of patients with sepsis is also something that needs to be improved in future research. In addition, in view of the small number of CRRT treatments, a total of 24 cases, we did not conduct a subgroup analysis, but only to explore the overall prognostic differences. If patients with septic AKI were graded to different subgroups according to the KIDGO criteria, and the prognostic value of CRRT in each subgroup is compared, different results may be obtained, which also represents one of the limitations of this study.
Conclusion
Lactate level, SOFA score and septic shock were closely related to the occurrence of septic AKI in the ICU. The clinical outcomes within 28 days after ICU admission of cancer patients with septic AKI were worse than those without septic AKI. The short-term outcome was worse in patients with septic AKI complicated with septic shock. CRRT does not have any significant effect on the short-term prognosis of cancer patients with septic AKI in the ICU. This study was a preliminary exploration of the incidence, influencing factors and clinical outcomes of septic AKI in cancer patients with sepsis, which has certain guiding significance for the diagnosis, treatment and prognosis of septic AKI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors for a reasonable purpose, without undue reservation.
Ethics statement
The study was approved by the Ethics Committee of Peking University Cancer Hospital and all patients provided written informed consent for the treatment of sepsis and related scientific research purposes.
Author contributions
YY and JD designed, analyzed, and drafted the manuscript. XC and RC collected and interpreted the patients’ data. HW administered and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Elise for English language revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ICU, intensive care unit; AKI, acute kidney injury; SOFA, sequential organ failure assessment; MV, mechanical ventilation; CRRT, continuous renal replacement therapy; CKD, chronic kidney disease; GFR, glomerular filtration rate; KIDGO, kidney disease improving global outcomes; SCD, septic cardiac dysfunction; ARF, acute respiratory failure; PCT, procalcitonin; cTnI, cardiac troponin I; BNP, brain natriuretic peptide.
References
1. Hoste E, Kellum J, Selby N, Zarbock A, Palevsky P, Bagshaw S, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. (2018) 14:607–25. doi: 10.1038/s41581-018-0052-0
2. Rifkin D, Coca S, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. (2012) 23:979–84. doi: 10.1681/ASN.2011121185
3. Hsu C. Yes, AKI truly leads to CKD. J Am Soc Nephrol. (2012) 23:967–9. doi: 10.1681/ASN.2012030222
4. Christiansen S, Christensen S, Pedersen L, Gammelager H, Layton J, Brookhart M, et al. Timing of renal replacement therapy and long-term risk of chronic kidney disease and death in intensive care patients with acute kidney injury. Crit Care. (2017) 21:326. doi: 10.1186/s13054-017-1903-y
5. Sawhney S, Marks A, Fluck N, Levin A, McLernon D, Prescott G, et al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. (2017) 92:440–52. doi: 10.1016/j.kint.2017.02.019
6. Mehta S, Chauhan K, Patel A, Patel S, Pinotti R, Nadkarni G, et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. (2018) 19:91. doi: 10.1186/s12882-018-0876-7
7. Bouchard J, Acharya A, Cerda J, Maccariello E, Madarasu R, Tolwani A, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. (2015) 10:1324–31. doi: 10.2215/CJN.04360514
8. Bagshaw S, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. (2008) 12:R47. doi: 10.1186/cc6863
9. Schrier R, Wang W. Acute renal failure and sepsis. N Engl J Med. (2004) 351:159–69. doi: 10.1056/NEJMra032401
10. Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care. (2009) 15:503–8. doi: 10.1097/MCC.0b013e328332f6cf
11. Zarbock A, Gomez H, Kellum J. Sepsis-induced acute kidney injury revisited. Pathophysiology, prevention and future therapies. Curr Opin Crit Care. (2014) 20:588–95. doi: 10.1097/MCC.0000000000000153
12. Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. (2014) 41:3–11. doi: 10.1097/SHK.0000000000000052
13. Torres V, Azevedo L, Silva U, Caruso P, Torelly A, Silva E, et al. Sepsis-associated outcomes in critically ill patients with malignancies. Ann Am Thoracic Soc. (2015) 12:1185–92. doi: 10.1513/AnnalsATS.201501-046OC
14. Hensley M, Donnelly J, Carlton E, Prescott H. Epidemiology and outcomes of cancer-related versus non-cancer-related sepsis hospitalizations. Crit Care Med. (2019) 47:1310–6. doi: 10.1097/CCM.0000000000003896
15. White L, Hassoun H, Bihorac A, Moore L, Sailors R, McKinley B, et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. (2013) 75:432–8. doi: 10.1097/TA.0b013e31829de6cd
16. Kellum J, Chawla L, Keener C, Singbartl K, Palevsky P, Pike F, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. (2016) 193:281–7. doi: 10.1164/rccm.201505-0995OC
17. Demarchi A, de Almeida C, Ponce D, e Castro M, Danaga A, Yamaguti F, et al. Intra-abdominal pressure as a predictor of acute kidney injury in postoperative abdominal surgery. Ren Fail. (2014) 36:557–61. doi: 10.3109/0886022X.2013.876353
18. Kwaan H, Gordon L. Thrombotic microangiopathy in the cancer patient. Acta Haematol. (2001) 106:52–6. doi: 10.1159/000046589
19. Kraut J, Madias N. Lactic acidosis. N Engl J Med. (2014) 371:2309–19. doi: 10.1056/NEJMra1309483
21. Singer A, Taylor M, Domingo A, Ghazipura S, Khorasonchi A, Thode H Jr., et al. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Med. (2014) 21:853–7. doi: 10.1111/acem.12444
22. Nichol A, Egi M, Pettila V, Bellomo R, French C, Hart G, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. (2010) 14:R25. doi: 10.1186/cc8888
23. Jones A, Puskarich M. Sepsis-induced tissue hypoperfusion. Crit Care Clin. (2009) 25:769–79. doi: 10.1016/j.ccc.2009.06.003
24. Singer M, Deutschman C, Seymour C, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
25. Jansen T, van Bommel J, Woodward R, Mulder P, Bakker J. Association between blood lactate levels, sequential organ failure assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. (2009) 37:2369–74. doi: 10.1097/CCM.0b013e3181a0f919
26. Langenberg C, Wan L, Egi M, May C, Bellomo R. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med. (2007) 33:1614–8. doi: 10.1007/s00134-007-0734-8
27. Romagnoli S, Ricci Z, Ronco C. CRRT for sepsis-induced acute kidney injury. Curr Opin Crit Care. (2018) 24:483–92. doi: 10.1097/MCC.0000000000000544
Keywords: cancer, sepsis, acute kidney injury, risk factor, outcome
Citation: Yang Y, Dong J, Chen X, Chen R and Wang H (2022) Incidence, risk factors and clinical outcomes of septic acute renal injury in cancer patients with sepsis admitted to the ICU: A retrospective study. Front. Med. 9:1015735. doi: 10.3389/fmed.2022.1015735
Received: 10 August 2022; Accepted: 28 November 2022;
Published: 14 December 2022.
Edited by:
Marcos Ferreira Minicucci, São Paulo State University, BrazilReviewed by:
Qiuyang Li, The First Medical Center of Chinese PLA General Hospital, ChinaMeili Duan, Affiliated Beijing Friendship Hospital, Capital Medical University, China
Copyright © 2022 Yang, Dong, Chen, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhi Wang, d2FuZ2h6NThAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yong Yang
Yong Yang Jun Dong†
Jun Dong†