
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 20 October 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1014291
This article is part of the Research TopicArtificial Intelligence and its Applications in Urological Disease ManagementView all 6 articles
To compare the efficacy and safety of different interventions [including antimuscarinics, mirabegron, OnabotulinumtoxinA, sacral neuromodulation (SNM) and peripheral tibial nerve stimulation (PTNS)] for treating idiopathic overactive bladder (OAB). PubMed, Embase, Cochrane Library, and other sources were searched for randomized controlled trials (RCTs) comparing interventions for overactive bladder from 1 January 2000 to 19 April 2021. A systematic review and network meta-analysis were performed by two authors independently. Fifty-five RCTs involving 32,507 patients were included in this analysis. Overall, antimuscarinics, mirabegron, OnabotulinumtoxinA, sacral neuromodulation, and peripheral tibial nerve stimulation were more efficacious than placebo, and sacral neuromodulation showed the best effect for reducing micturition frequency, urgency episodes and urgency urinary incontinence episodes. OnabotulinumtoxinA was the best intervention for achieving reductions of 100 and ≥50% in the number of urinary incontinence episodes/day, and peripheral tibial nerve stimulation was the best intervention for reducing urinary incontinence episodes. Antimuscarinics, mirabegron and peripheral tibial nerve stimulation had a similar efficacy for reducing micturition frequency, urinary incontinence episodes and urgency urinary incontinence episodes. The results revealed that all interventions examined herein were efficacious for managing adult overactive bladder syndrome compared with placebo. Furthermore, sacral neuromodulation and OnabotulinumtoxinA were the most efficient treatments for overactive bladder.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=251966], identifier [CRD42021251966].
Overactive bladder (OAB) was defined in 2002 by the International Continence Society as a storage symptom characterized by “urgency, with or without urgency urinary incontinence, in the absence other obvious pathology” (1). The Epidemiology of Incontinence (EPIC) study suggested that the incidence rate of overactive bladder is high in both men and women (10.8 and 12.8%, respectively) (2, 3). Overactive bladder impairs health-related quality of life (HRQoL) and creates a significant economic burden. The cost of urinary incontinence is reported to account for approximately 2% of total health care costs in the United States each year (4). The American Urological Association (AUA) guidelines recommend behavioral therapy as the first-line treatment. The first-line behavioral therapy includes bladder training and pelvic floor muscle training (5). Seyda Toprak Celenay et al. found that pelvic floor muscle training could improve sexual dysfunction, sexual satisfaction of partners, urinary symptoms, and pelvic floor muscle strength in women with overactive bladder (6). If behavioral therapy does not work, oral antimuscarinics and mirabegron are recommended as second-line treatment. Con Kelleher et al. conducted a systematic review and revealed the efficacy and tolerability of different drug treatments for overactive bladder (7). Blayne Welk et al. found that antimuscarinics may increase the risk of mortality, and a single multicenter study showed that the mortality of β3 agonist users was 20% lower than that of antimuscarinics users (8). Mirabegron and vibegron, β3 receptor agonists, significantly improved the daily number of urgency episodes and micturition without anticholinergic adverse effects (7, 9, 10). Recently, more attention has been given to the combination treatment of antimuscarinics plus mirabegron (11, 12). Christian Gratzke et al. performed a randomized, double-blind, multicenter, phase 3 trial and proved that solifenacin succinate 5 mg plus mirabegron 50 mg was well tolerated by patients and led to greater improvements in symptoms (13). When patients are refractory to first- and second-line overactive bladder treatments, clinicians can provide third-line therapy, including OnabotulinumtoxinA, sacral neuromodulation (SNM) and peripheral tibial nerve stimulation (PTNS) (3, 14, 15). Sacral neuromodulation and peripheral tibial nerve stimulation are novel modalities and have been rapidly increasing over recent years (16, 17). Limin Liao et al. confirmed the effectiveness and safety of a novel sacral stimulation system that stimulates the sacral nerve for the treatment of overactive bladder (18). Abdullah Al-Danakh et al. suggested that peripheral tibial nerve stimulation was promising in terms of efficacy, safety, and high acceptance rate (16). This therapeutic modality was derived by balancing the potential benefits to the patient with the risks, including invasiveness of the treatment, the duration and severity of potential adverse events and the reversibility of potential adverse events. Although risks are difficult to assess, the benefits are easy to evaluate because of uniform outcome indicators. Sacral neuromodulation and peripheral tibial nerve stimulation are innovative treatments that have emerged in recent years (17, 19). There are many RCTs comparing the efficacy of those interventions for overactive bladder (13, 20), but most of them focus on drug therapy, such as antimuscarinics and Mirabegron, and very few examine sacral neuromodulation and peripheral tibial nerve stimulation, which leads to inadequate awareness among clinicians about the relative effectiveness of these two innovative treatments. Direct comparisons as well as network meta-analyses that included several of these interventions have been performed (21, 22), but no network meta-analysis has been conducted to evaluate all these treatments. Therefore, in this study, we intend to perform a systematic review and network meta-analysis to guide clinical practice by comparing the relative effectiveness of different interventions for the treatment of adults with overactive bladder.
This network meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) extension statement for network meta-analysis (23). This study was registered with PROSPERO (CRD42021251966).
We included RCTs reported in English comparing anticholinergics, Mirabegron, OnabotulinumtoxinA, sacral neuromodulation, peripheral tibial nerve stimulation with each other or placebo in adults with overactive bladder. The inclusion criteria and exclusion criteria are summarized in Supplementary Table 1.
PubMed, Embase, Cochrane Library, and other sources were searched to find relevant articles published from 1 January 2000 to 19 April 2021. For an outcome in the same trial, only the data closest to the 12-week follow-up were extracted. The detailed search strategy is shown in Supplementary Table 2.
Two investigators (PL, YL) independently extracted data using a unified data extraction form, and differences were resolved by another reviewer (HG). We recorded the outcomes as close to 12 weeks as possible. The primary outcome was mean change in the frequency of micturition/day on the basis of European Medicines Agency and Food and Drug Administration guidelines for overactive bladder trials. Secondary outcomes were the proportions of patients achieving 100 and ≥50% reductions from baseline in urinary incontinence episodes/day, mean change in urgency episodes/day, mean change in urgency urinary incontinence episodes/day, and mean change in urinary incontinence episodes/day. The main characteristics of qualified trials, such as first author, publication year, methods, number of patients, inclusion and exclusion criteria, interventions and outcomes, were extracted to conduct further analysis. The risk of bias for each RCTs was assessed using the RoB (Cochrane risk-of-bias tool for randomized trials) tool, which evaluated the following aspects: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Two independent researchers (PL and YL) assessed the risk of bias of all included studies according to RoB. Disagreements were resolved by consulting a third researcher (HG). A risk of bias graph is shown in Supplementary Figure 1.
Pooled odds ratios (ORs) or weighted mean differences (WMDs) with 95% confidence intervals (CIs) were analyzed. Forest plots of pairwise meta-analyses were generated by Stata 16.0 (24). We assessed network connectivity using a network diagram (Stata, version 16.0). Network meta-analysis was conducted in a Bayesian framework within the GEMTC package in the R-Statistics (25) and the J.A.G.S. program as previously described (26). Consistent and inconsistent models were considered and compared using deviance information criteria (27) (Supplementary Table 3). If the difference between them was less than 5, a fixed effects model was selected. Otherwise, a random effects model was selected. We used an unrelated mean effects model to assess inconsistency by comparing the model fit and between-study variance (heterogeneity) estimate of the pairwise comparisons against the results of the consistency model. Network consistency was assessed by comparing direct evidence and indirect evidence for each comparison using a node-splitting technique (28, 29). The assessment of heterogeneity was performed by the R package “meta,” which is represented by I2 ranging between 0 and 100%. The ranking probabilities were also calculated using R, and the broken line graph was generated by GraphPad Prism. Sensitivity analysis was performed to test the robustness and reliability of the results by excluding studies deemed to have a high risk of bias.
The literature search identified 1,707 unique references, and 1,409 studies were excluded during screening (Figure 1). Of the 298 full-text articles assessed for eligibility, 243 were excluded. Overall, 55 studies (n = 32,507) were included in the network meta-analysis. Summaries of all included studies are shown in Supplementary Table 4.
The pairwise meta-analyses results are shown in Figure 2.
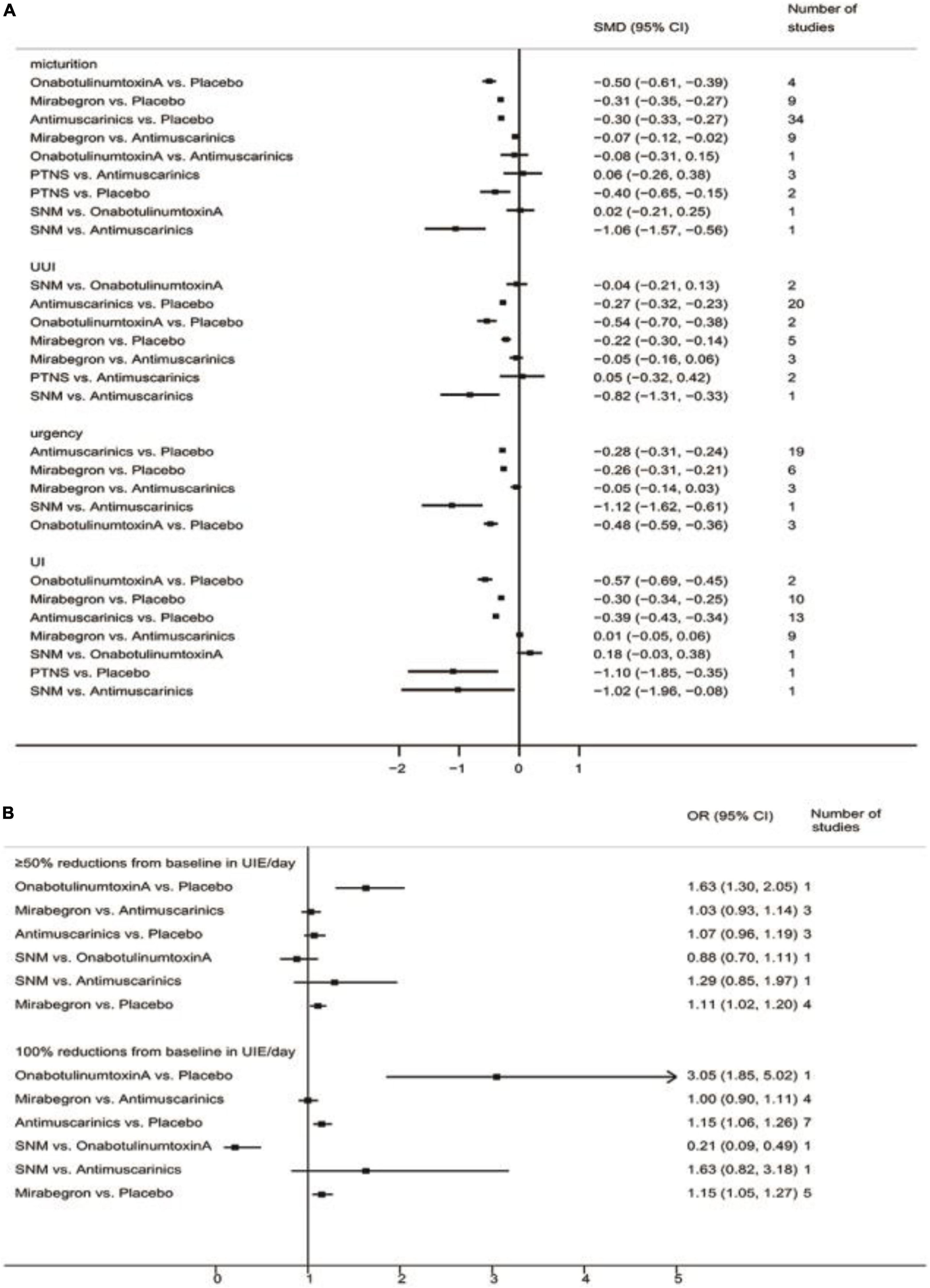
Figure 2. Pairwise meta-analyses result for different endpoints. (A) Pairwise meta-analyses result for reducing micturition frequency/day, urgency urinary incontinence episodes (UUIE)/day, urgency episodes/day, urinary incontinence episodes (UIE)/day; (B) Pairwise meta-analyses result for 100 and ≥50% reduction from baseline in urinary incontinence episodes (UIE)/day. SNM, sacral neuromodulation; PTNS, peripheral tibial nerve stimulation.
The results suggested that antimuscarinics, mirabegron, OnabotulinumtoxinA, and peripheral tibial nerve stimulation were more efficacious than placebo in reducing micturition frequency. Patients receiving antimuscarinics and mirabegron showed no significant difference in reducing micturition frequency. Other direct comparisons were of limited significance due to the insufficient number of studies.
The results suggested that antimuscarinics, mirabegron, and OnabotulinumtoxinA were more efficacious than placebo in reducing urgency urinary incontinence episodes. Patients receiving antimuscarinics and mirabegron showed no significant difference in reducing urgency urinary incontinence episodes. Other direct comparisons were of limited significance due to the insufficient number of studies.
The results suggested that antimuscarinics, mirabegron, and OnabotulinumtoxinA were more efficacious than placebo in reducing urgency episodes. Patients receiving antimuscarinics and mirabegron showed no significant difference in reducing urgency episodes. Other direct comparisons were of limited significance due to the insufficient number of studies.
The results suggested that antimuscarinics, mirabegron, and OnabotulinumtoxinA were more efficacious than placebo in reducing urinary incontinence episodes. Patients receiving antimuscarinics and mirabegron showed no significant difference in reducing urinary incontinence episodes. Other direct comparisons were of limited significance due to the insufficient number of studies.
The results suggested that mirabegron was more efficacious than placebo in achieving ≥50% reductions from baseline in urinary incontinence episodes/day. There was no difference in patients receiving antimuscarinics and mirabegron, antimuscarinics and placebo. Other direct comparisons were of limited significance due to the insufficient number of studies.
The results suggested that antimuscarinics and mirabegron were more efficacious than placebo in achieving 100% reductions from baseline in urinary incontinence episodes/day. There was no difference in patients receiving antimuscarinics and mirabegron. Other direct comparisons were of limited significance due to the insufficient number of studies.
The primary network including all studies (regardless of RoB) for all outcomes is presented in Figure 3.
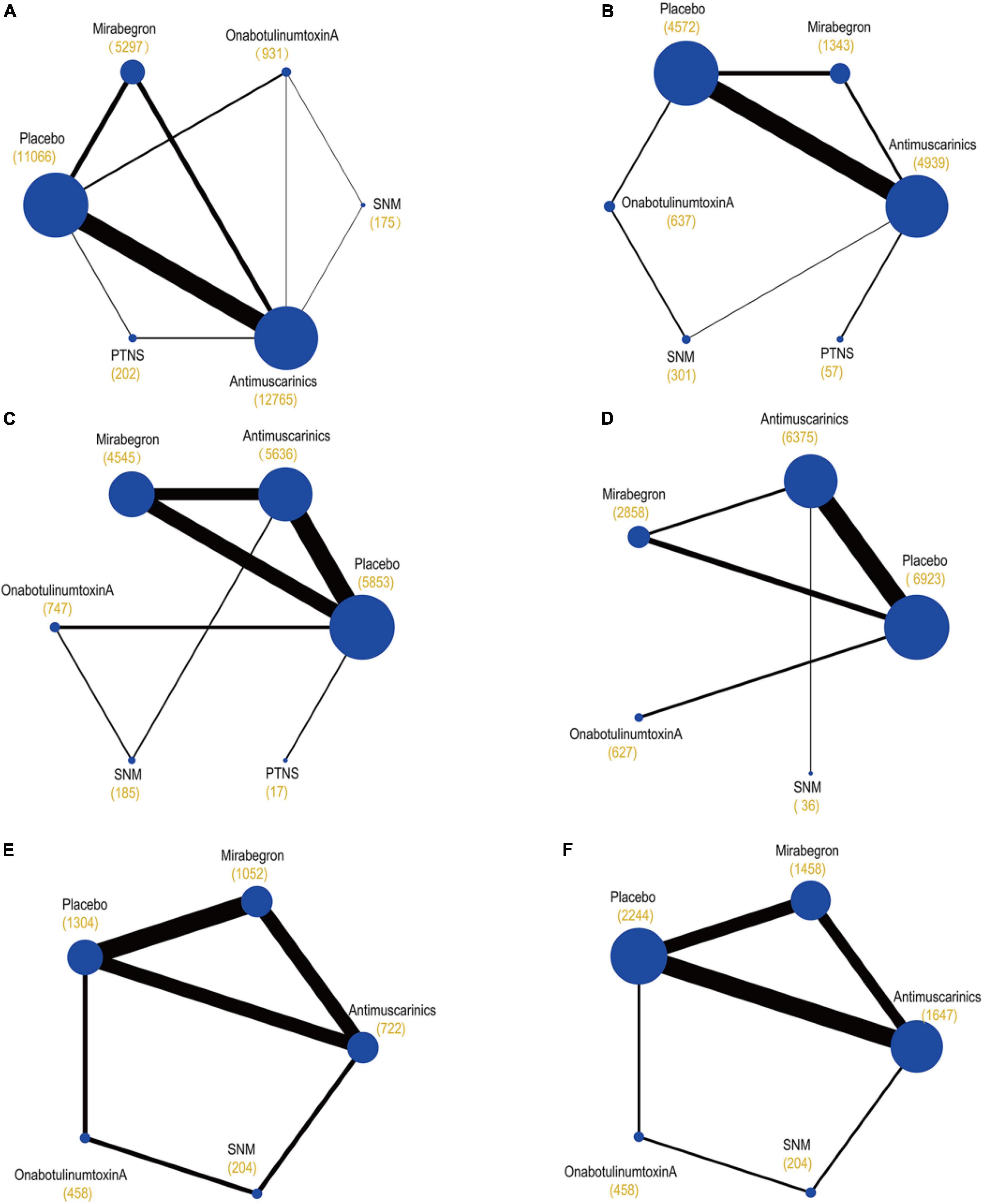
Figure 3. Network plots of (A) mean change in the frequency of micturition/day; (B) mean change in urgency urinary incontinence episodes (UUIE)/day; (C) mean change in urinary incontinence episodes (UIE)/day; (D) mean change in urgency/day; (E) ≥50% reductions from baseline in urinary incontinence episodes (UIE)/day; (F) 100% reductions from baseline in urinary incontinence episodes (UIE)/day. These plots were made by Stata 16.0. Each circular node represents a type of treatment. Each line shows a type of head-to-head comparison. Node size and line thickness are weighted according to the number of studies evaluating each treatment and direct comparison, respectively. The total number of participants receiving a treatment is shown in brackets. SNM, sacral neuromodulation; PTNS, peripheral tibial nerve stimulation.
The network meta-analysis (Figure 4) indicated that treatment with OnabotulinumtoxinA or sacral neuromodulation resulted in obviously greater mean reductions in micturition frequency, urinary incontinence episodes, urgency episodes, and urgency urinary incontinence episodes compared with all of the other interventions included in the network, and the efficacy of antimuscarinics, mirabegron and peripheral tibial nerve stimulation were similar and were better than that of placebo. In addition, the results suggested that patients receiving OnabotulinumtoxinA had the highest odds of achieving reductions of 100% and ≥50% in the number of urinary incontinence episodes/day [odds ratios relative to placebo: 5.92 (95% CI 3.53–10.29) and 3.57 (95% CI 2.56–5.00)]. Additionally, antimuscarinics or mirabegron was superior to placebo in 100 and ≥50% reductions from baseline in urinary incontinence episodes/day, respectively.
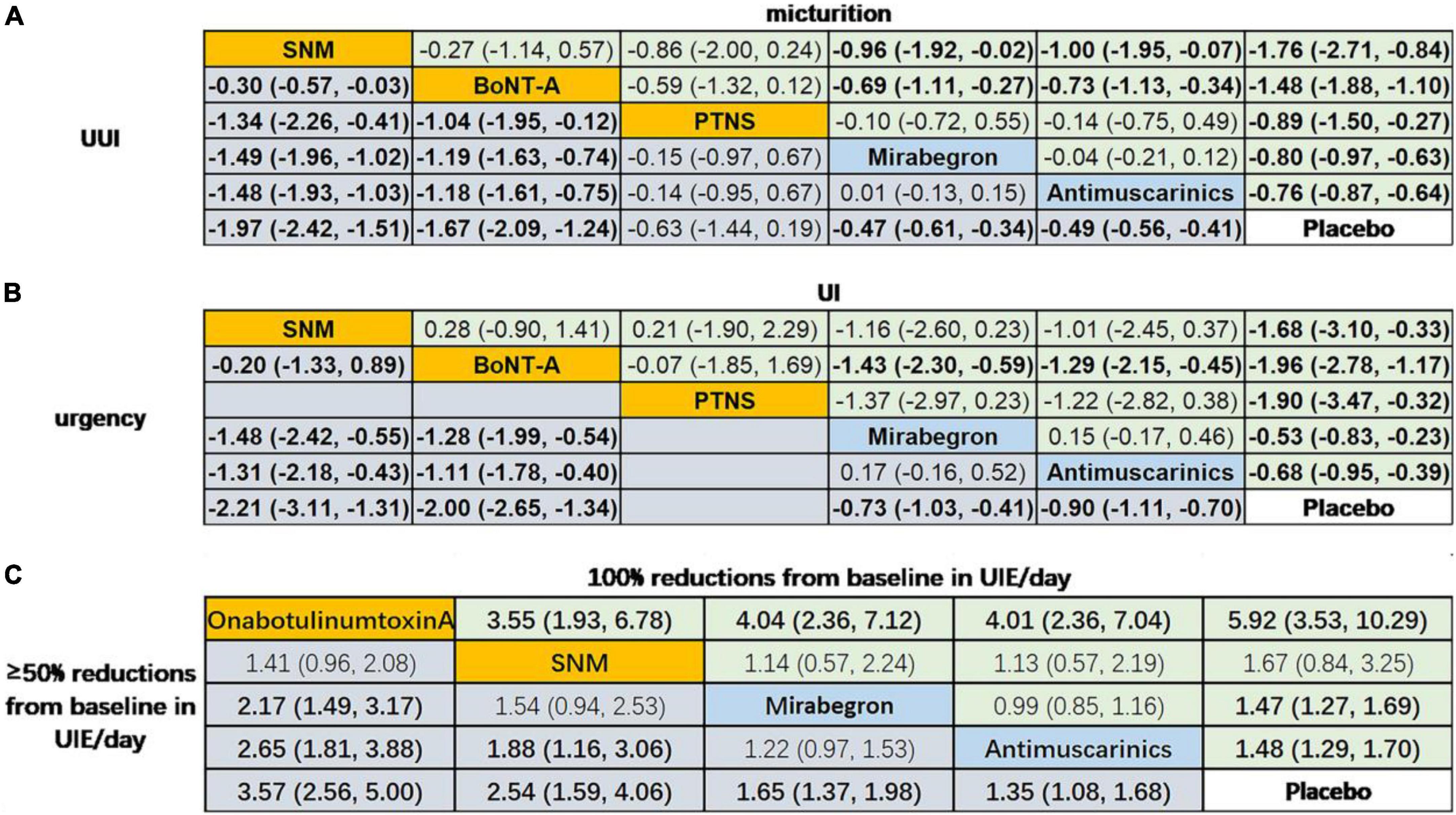
Figure 4. Network meta-analysis on the outcomes of interests. Data in (A) and (B) are SMD (95% CI) for the comparison of row-defining treatment versus column-defining treatment. SMD less than 0 favors upper-row treatment. Data in (C) are OR (95% CI) for the comparison of row-defining treatment versus column-defining treatment. OR more than 1 favors upper-row treatment. Significant results are highlighted in bold; second line treatment are highlighted in blue; third line treatment are highlighted in yellow. SNM, sacral neuromodulation; PTNS, peripheral tibial nerve stimulation.
The Bayesian ranking probabilities of comparable treatments in different populations are shown in Figure 5.
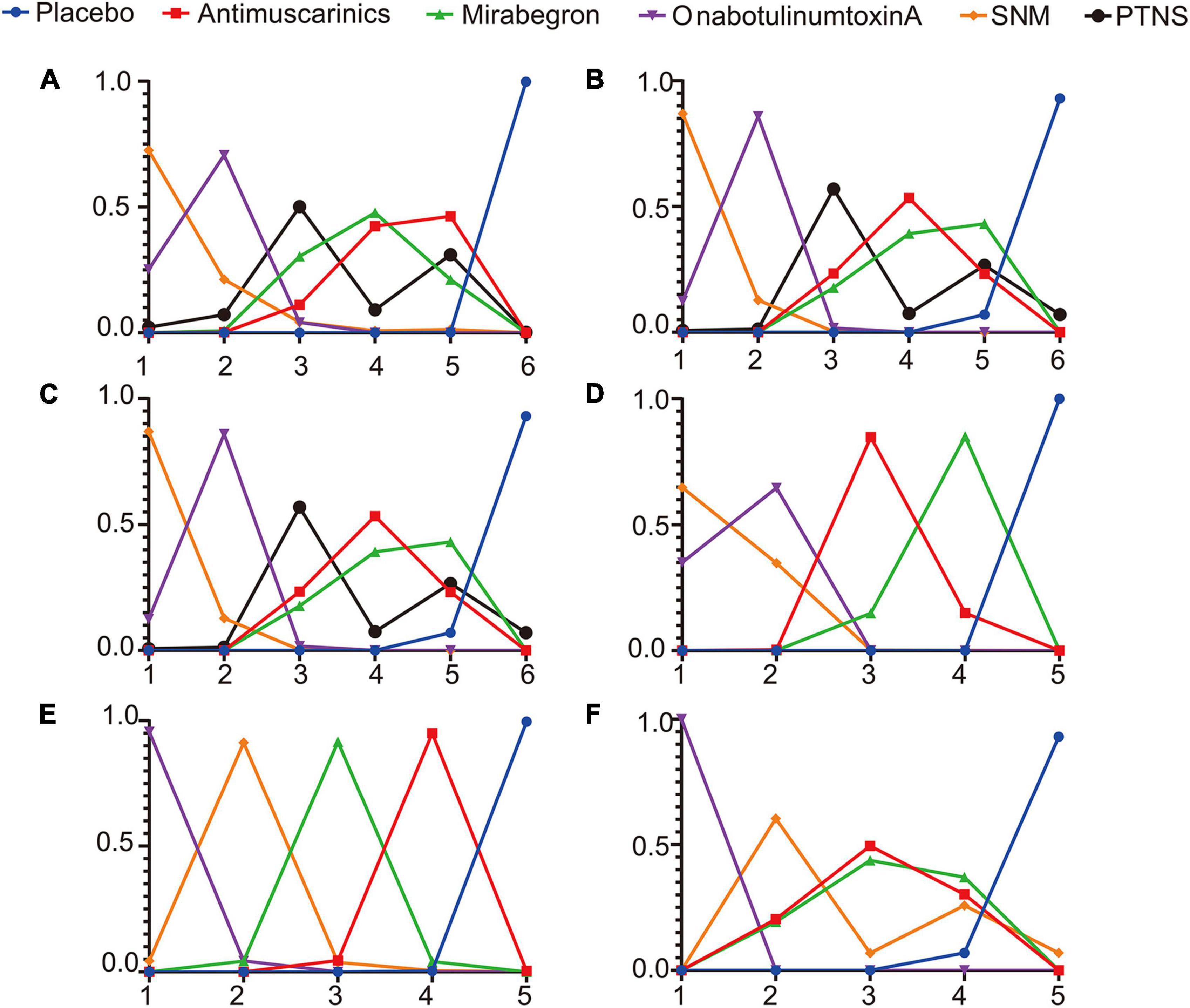
Figure 5. Rank probabilities of (A) mean change in the frequency of micturition/day; (B) mean change in urgency urinary incontinence episodes (UUIE)/day; (C) mean change in urinary incontinence episodes (UIE)/day; (D) mean change in urgency/day; (E) ≥50% reductions from baseline in urinary incontinence episodes (UIE)/day; (F) 100% reductions from baseline in urinary incontinence episodes (UIE)/day. The X-axis represents the ranking, and the Y-axis represents the possibility of the ranking. These plots were made by GraphPad Prism 8. SNM, sacral neuromodulation; PTNS, peripheral tibial nerve stimulation.
Overall, sacral neuromodulation was most likely to be ranked first for reducing micturition frequency, urgency episodes and urgency urinary incontinence episodes, and placebo ranked lowest. The ranking results for urinary frequency reduction and urgency urinary incontinence episodes reduction were as follows: sacral neuromodulation ranked first, OnabotulinumtoxinA ranked second, peripheral tibial nerve stimulation ranked third, one of Mirabegron and Antimuscarinics ranked fourth and the other ranked fifth, and placebo ranked sixth. The ranking results for urgency episodes reduction were as follows: sacral neuromodulation ranked first, OnabotulinumtoxinA ranked second, Antimuscarinics ranked third, Mirabegron ranked fourth, and placebo ranked fifth. The ranking results for urinary incontinence episode reduction were as follows: sacral neuromodulation, OnabotulinumtoxinA and peripheral tibial nerve stimulation were the top three in no particular order, antimuscarinics ranked fourth, Mirabegron ranked fifth, and placebo ranked sixth. The ranking results for achieving reductions of 100 and ≥50% in the number of urinary incontinence episodes/day were as follows: OnabotulinumtoxinA ranked first, sacral neuromodulation ranked second, and placebo ranked lowest.
The fit of the consistency model in all comparisons was similar to or better than the fit of the inconsistency model (Supplementary Table 3). Node splitting analysis was performed to evaluate consistencies by comparing differences between the direct and indirect evidence, and the results are shown in Supplementary Table 5, which showed no significant differences in most comparisons except for placebo vs. OnabotulinumtoxinA, Anticholinergics vs. sacral neuromodulation, OnabotulinumtoxinA vs. sacral neuromodulation in the comparison of micturition, urinary incontinence episodes and 100% reductions from baseline in urinary incontinence episodes/day.
The results of the sensitivity analysis (Supplementary Figures 2 and 3) excluding 12 studies considered to have a high RoB showed little impact on the results of the network meta-analysis. The main changes in the sensitivity analysis are summarized in Supplementary Table 6.
In the absence of a direct head-to-head comparison of all second-line and third-line treatments recommended by AUA for adult overactive bladder symptoms, the present detailed systemic review and network meta-analysis is the first review to compare the relative effectiveness of all those interventions. Six efficacy outcomes were assessed, with each network including between 7 and 52 studies.
The results from pairwise meta-analysis revealed that all five interventions were more efficacious than placebo with regard to the outcomes included in this study. The results from the network meta-analysis indicated that OnabotulinumtoxinA and sacral neuromodulation were more efficacious than all of the other interventions in reducing micturition frequency, urinary incontinence episodes, urgency episodes and urgency urinary incontinence episodes. The efficacy of antimuscarinics, mirabegron and peripheral tibial nerve stimulation was similar. In terms of reductions of 100 and ≥50% in the number of urinary incontinence episodes/day, OnabotulinumtoxinA had the best effect.
However, this study only focuses on short-term efficacy, and the long-term results also need further discussion. Christian Gratzke et al. conducted a randomized, double-blind, multicenter, phase 3 trial to explore the long-term potential of solifenacin and mirabegron (13). They proved that patients receiving solifenacin or mirabegron showed significant improvement in reducing urinary incontinence episodes and micturition frequency. In addition, more than 40% of the patients had treatment emergent adverse events (TEAEs) after 12 months of follow-up, although the majority of the treatment emergent adverse events were mild or moderate in severity. Daisuke Kato et al. found that only 6.3% of patients experienced treatment-emergent adverse events after 1 year of mirabegron treatment. The most common treatment-emergent adverse events of antimuscarinics were dry mouth and constipation (30), which seriously affected patient compliance. Mirabegron caused an increase in blood pressure that was positively correlated with the duration of the drug (20, 31). Therefore, mirabegron is not recommended in patients with severe uncontrolled hypertension or end-stage renal disease. David Eldred-Evans et al. conducted a review and revealed that repeated OnabotulinumtoxinA injections were safe and efficacious in patients with overactive bladder (32). OnabotulinumtoxinA can cause urinary tract infections and retention of urine (22, 33), sometimes requiring catheterization to empty the bladder. Sacral neuromodulation has been shown to have long-term efficacy and subsequently exposed treatment-emergent adverse events. Bilal Kaaki et al. performed a retrospective single-institution study and proved that the success rate of sacral neuromodulation is 75% with a complication rate of 14.5% after a median follow-up of 32 months (34). Sam Tilborghs et al. conducted a systematic review of sacral neuromodulation for the treatment of overactive bladder and found that sacral neuromodulation has proven to be a safe and effective therapy in the short, medium and long term (35). Treatment-emergent adverse events of sacral neuromodulation were pain at the stimulator and lead sites, lead migration, infection, and the requirement for surgical revision (36). Sam Tilborghs et al. reported that surgical reintervention rates were high, with a median of 33.2% (range: 8–34%) in studies with at least 24 months of follow-up (35). Some studies also reported the long-term efficacy and safety of peripheral tibial nerve stimulation (37–39). Although some patients had discomfort and mild pain at the site of stimulation, as well as tingling and acid swelling of the leg, peripheral tibial nerve stimulation was safe.
In terms of cost, all third-line treatments are more expensive than drug treatments, and sacral neuromodulation is considered to be the most expensive treatment compared with other interventions in the short term (40–42), which may be an important factor affecting its application. However, sacral neuromodulation seems to be either cost saving or acceptably cost effective compared with ongoing medical therapy, peripheral tibial nerve stimulation, or OnabotulinumtoxinA in the long term (43).
There were several limitations to the current network meta-analysis study. The current study primarily compared short-term efficacy at the 12-week follow-up, and we also extracted outcome data closest to the 12-week follow-up in this study, resulting in the lack of comparison of long-term efficacy. Further research is needed to evaluate the long-term efficacy of these interventions. In addition, placebo differed in their mode of administration. In the OnabotulinumtoxinA trials, placebo was administered as a single injection, and in the mirabegron and anticholinergics trials, the placebos were administered as daily oral tablets, while in the sacral neuromodulation and peripheral tibial nerve stimulation experiment, sham stimulation was used as a placebo. To link these interventions of interest into a network, we hypothesized that different placebo treatments would be equally effective in the same population. However, one study (44) found that different administrations affected the relative efficacy of placebo for osteoarthritis of the knee, which is a limitation of this network meta-analysis.
This study further justifies the current guidelines, namely, the gradual adoption of the three-line treatments. Moreover, we should pay attention to the advantages of sacral neuromodulation in the treatment of overactive bladder, especially considering the better efficacy and fewer complications. Although behavioral therapy and drug therapy are the first- and second-line treatments for overactive bladder, the overall treatment effect is usually small. Because of limited efficacy and severe adverse effects, some patients were refractory to behavioral therapy and medical treatment. Therefore, third-line treatments should be implemented as early as possible for patients who have not benefit from medicine treatment. Besides, sacral neuromodulation should be the first choice among the third-line treatments (OnabotulinumtoxinA, sacral neuromodulation and peripheral tibial nerve stimulation) according to this study.
This network meta-analysis revealed that sacral neuromodulation and OnabotulinumtoxinA achieved the best results in most outcomes at the 12-week follow-up. As there is a lack of head-to-head comparison studies among anticholinergics, mirabegron, OnabotulinumtoxinA, sacral neuromodulation, and peripheral tibial nerve stimulation for the treatment of adult overactive bladder symptoms, the present network meta-analysis provides the best available evidence for comparing these five interventions. Additionally, well-designed and head-to-head RCTs are needed to assess the efficacy and treatment-emergent adverse events of interventions in managing OAB.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
PL, YL, BS, QZ, and HG: conception and design and administrative support. PL and YL: provision of study materials or patients and data analysis and interpretation. PL: collection and assembly of data. All authors: manuscript writing and final approval of manuscript.
This work was supported by the Shandong Medical and Health Science and Technology Development Plan Project (2018WS333), the National Natural Science Foundation of China (Grant Nos. 81970661 and 81900637), and the Tai Shan Scholar Foundation to BS (ts201511092).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1014291/full#supplementary-material
1. Peyronnet B, Mironska E, Chapple C, Cardozo L, Oelke M, Dmochowski R, et al. A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur Urol. (2019) 75:988–1000. doi: 10.1016/j.eururo.2019.02.038
2. Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. (2006) 50:1306–14. doi: 10.1016/j.eururo.2006.09.019
3. Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and treatment of overactive bladder (Non-Neurogenic) in adults: AUA/SUFU guideline amendment 2019. J Urol. (2019) 202:558–63. doi: 10.1097/JU.0000000000000309
4. Reeves P, Irwin D, Kelleher C, Milsom I, Kopp Z, Calvert N, et al. The current and future burden and cost of overactive bladder in five European countries. Eur Urol. (2006) 50:1050–7. doi: 10.1016/j.eururo.2006.04.018
5. Tezer T, Yıldız N, Sarsan A, Alkan H. Short-term effect of magnetic stimulation added to bladder training in women with idiopathic overactive bladder: a prospective randomized controlled trial. Neurourol Urodyn. (2022) 41:1380–9. doi: 10.1002/nau.24957
6. Celenay ST, Karaaslan Y, Ozdemir E. Effects of pelvic floor muscle training on sexual dysfunction, sexual satisfaction of partners, urinary symptoms, and pelvic floor muscle strength in women with overactive bladder: a randomized controlled study. J Sex Med. (2022) 19:1421–30. doi: 10.1016/j.jsxm.2022.07.003
7. Kelleher C, Hakimi Z, Zur R, Siddiqui E, Maman K, Aballéa S, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol. (2018) 74:324–33. doi: 10.1016/j.eururo.2018.03.020
8. Welk B. The differential risk of mortality among users of overactive bladder anticholinergic medications and β3 agonists. Eur Urol Focus. (2022). [Epub ahead of print]. doi: 10.1016/j.euf.2022.08.002
9. De Nunzio C, Nacchia A, Gravina C, Turchi B, Gallo G, Trucchi A, et al. Adverse events related to antimuscarinics and beta-3-agonist: “real-life” data from the Eudra-Vigilance database. Minerva Urol Nephrol. (2022). [Epub ahead of print]. doi: 10.23736/S2724-6051.22.04849-2
10. Staskin D, Frankel J, Varano S, Kennelly M, Newman DK, Rosenberg MT, et al. Vibegron for the treatment of patients with dry and wet overactive bladder: a subgroup analysis from the EMPOWUR trial. Int J Clin Pract. (2022) 2022:6475014. doi: 10.1155/2022/6475014
11. Gratzke C, Chapple C, Mueller ER, Robinson D, Rolland C, Staskin D, et al. Efficacy and safety of combination pharmacotherapy for patients with overactive bladder: a rapid evidence assessment. Eur Urol. (2019) 76:767–79. doi: 10.1016/j.eururo.2019.07.010
12. Sakalis V, Sfiggas V, Vouros I, Salpiggidis G, Papathanasiou A, Apostolidis A. Detrusor overactivity may be a prognostic factor for better response to combination therapy over monotherapy in male patients with benign prostatic enlargement and storage lower urinary tract symptoms. Int Neurourol J. (2021) 25:69–76. doi: 10.5213/inj.2040188.094
13. Gratzke C, van Maanen R, Chapple C, Abrams P, Herschorn S, Robinson D, et al. Long-term safety and efficacy of mirabegron and solifenacin in combination compared with monotherapy in patients with overactive bladder: a randomised, multicentre phase 3 study (SYNERGY II). Eur Urol. (2018) 74:501–9. doi: 10.1016/j.eururo.2018.05.005
14. De Nunzio C, Brucker B, Bschleipfer T, Cornu JN, Drake MJ, Fusco F, et al. Beyond antimuscarinics: a review of pharmacological and interventional options for overactive bladder management in men. Eur Urol. (2021) 79:492–504. doi: 10.1016/j.eururo.2020.12.032
15. Raju R, Linder BJ. Evaluation and treatment of overactive bladder in women. Mayo Clin Proc. (2020) 95:370–7. doi: 10.1016/j.mayocp.2019.11.024
16. Al-Danakh A, Safi M, Alradhi M, Almoiliqy M, Chen Q, Al-Nusaif M, et al. Posterior tibial nerve stimulation for overactive bladder: mechanism, classification, and management outlines. Parkinsons Dis. (2022) 2022:2700227. doi: 10.1155/2022/2700227
17. Chartier-Kastler E, Le Normand L, Ruffion A, Dargent F, Braguet R, Saussine C, et al. Sacral neuromodulation with the InterStim™ system for intractable lower urinary tract dysfunctions (SOUNDS): results of clinical effectiveness, quality of life, patient-reported outcomes and safety in a french multicenter observational study. Eur Urol Focus. (2021) 7:1430–7. doi: 10.1016/S0302-2838(21)00419-X
18. Liao L, Zhou Z, Chen G, Xu Z, Huang B, Chong T, et al. Sacral neuromodulation using a novel device with a six-contact-point electrode for the treatment of patients with refractory overactive bladder: a multicenter, randomized, single-blind, parallel-control clinical trial. Eur Urol Focus. (2022). [Epub ahead of print]. doi: 10.1016/j.euf.2022.04.006
19. Sayner AM, Rogers F, Tran J, Jovanovic E, Henningham L, Nahon I. Transcutaneous tibial nerve stimulation in the management of overactive bladder: a scoping review. Neuromodulation. (2022). [Epub ahead of print]. doi: 10.1016/j.neurom.2022.04.034
20. Chapple CR, Cruz F, Cardozo L, Staskin D, Herschorn S, Choudhury N, et al. Safety and efficacy of mirabegron: analysis of a large integrated clinical trial database of patients with overactive bladder receiving mirabegron, antimuscarinics, or placebo. Eur Urol. (2020) 77:119–28. doi: 10.1016/j.eururo.2019.09.024
21. Drake MJ, Nitti VW, Ginsberg DA, Brucker BM, Hepp Z, McCool R, et al. Comparative assessment of the efficacy of onabotulinumtoxinA and oral therapies (anticholinergics and mirabegron) for overactive bladder: a systematic review and network meta-analysis. BJU Int. (2017) 120:611–22. doi: 10.1111/bju.13945
22. Lo CW, Wu MY, Yang SS, Jaw FS, Chang SJ. Comparing the efficacy of onabotulinumtoxinA, sacral neuromodulation, and peripheral tibial nerve stimulation as third line treatment for the management of overactive bladder symptoms in adults: systematic review and network meta-analysis. Toxins. (2020) 12:128. doi: 10.3390/toxins12020128
23. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
24. Bagos PG. Meta-analysis in Stata using gllamm. Res Synth Methods. (2015) 6:310–32. doi: 10.1002/jrsm.1157
25. Hu D, O’Connor AM, Wang C, Sargeant JM, Winder CB. How to conduct a bayesian network meta-analysis. Front Vet Sci. (2020) 7:271. doi: 10.3389/fvets.2020.00271
26. Sheng L, Gao J, Xu Q, Zhang X, Huang M, Dai X, et al. Selection of optimal first-line immuno-related therapy based on specific pathological characteristics for patients with advanced driver-gene wild-type non-small cell lung cancer: a systematic review and network meta-analysis. Ther Adv Med Oncol. (2021) 13:17588359211018537. doi: 10.1177/17588359211018537
27. Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. (2013) 33:618–40. doi: 10.1177/0272989X13485157
28. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London: National Institute for Health and Care Excellence (2014).
29. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
30. Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. (2004) 3:46–53. doi: 10.1016/S1474-4422(03)00622-7
31. Wagg A, Cardozo L, Nitti VW, Castro-Diaz D, Auerbach S, Blauwet MB, et al. The efficacy and tolerability of the β3-adrenoceptor agonist mirabegron for the treatment of symptoms of overactive bladder in older patients. Age Ageing. (2014) 43:666–75. doi: 10.1093/ageing/afu017
32. Eldred-Evans D, Sahai A. Medium- to long-term outcomes of botulinum toxin A for idiopathic overactive bladder. Ther Adv Urol. (2017) 9:3–10. doi: 10.1177/1756287216672180
33. Clyne M. Incontinence: onabotulinumtoxinA safer than abobotulinumtoxinA for OAB. Nat Rev Urol. (2013) 10:253. doi: 10.1038/nrurol.2013.64
34. Kaaki B, Gupta D. Medium-term outcomes of sacral neuromodulation in patients with refractory overactive bladder: a retrospective single-institution study. PLoS One. (2020) 15:e0235961. doi: 10.1371/journal.pone.0235961
35. Tilborghs S, De Wachter S. Sacral neuromodulation for the treatment of overactive bladder: systematic review and future prospects. Expert Rev Med Devices. (2022) 19:161–87. doi: 10.1080/17434440.2022.2032655
36. De Wachter S, Vaganee D, Kessler TM. Sacral neuromodulation: mechanism of action. Eur Urol Focus. (2020) 6:823–5. doi: 10.1016/j.euf.2019.11.018
37. Bianchi D, Iacovelli V, Parisi I, Petta F, Gaziev G, Topazio L, et al. Real-life data on long-term follow-up of patients successfully treated with percutaneous tibial nerve stimulation. Minerva Urol Nephrol. (2021) 73:260–4. doi: 10.23736/S2724-6051.19.03492-1
38. Te Dorsthorst MJ, Heesakkers J, van Balken MR. Long-term real-life adherence of percutaneous tibial nerve stimulation in over 400 patients. Neurourol Urodyn. (2020) 39:702–6. doi: 10.1002/nau.24254
39. Peters KM, Carrico DJ, MacDiarmid SA, Wooldridge LS, Khan AU, McCoy CE, et al. Sustained therapeutic effects of percutaneous tibial nerve stimulation: 24-month results of the STEP study. Neurourol Urodyn. (2013) 32:24–9. doi: 10.1002/nau.22266
40. Arlandis S, Castro D, Errando C, Fernández E, Jiménez M, González P, et al. Cost-effectiveness of sacral neuromodulation compared to botulinum neurotoxin a or continued medical management in refractory overactive bladder. Value Health. (2011) 14:219–28. doi: 10.1016/j.jval.2010.08.006
41. Barnett G, Ockrim J. Re: cost of neuromodulation therapies for overactive bladder: percutaneous tibial nerve stimulation versus sacral nerve stimulation: M. Martinson, S. MacDiarmid and E. Black J Urol 2013; 189: 210-216. J Urol. (2013) 190:1444–5. doi: 10.1016/j.juro.2013.04.131
42. Siddiqui NY, Amundsen CL, Visco AG, Myers ER, Wu JM. Cost-effectiveness of sacral neuromodulation versus intravesical botulinum A toxin for treatment of refractory urge incontinence. J Urol. (2009) 182:2799–804.
43. Marcelissen T, Cornu JN, Antunes-Lopes T, Geavlete B, Delongchamps NB, Rashid T, et al. Management of idiopathic overactive bladder syndrome: what is the optimal strategy after failure of conservative treatment? Eur Urol Focus. (2018) 4:760–7. doi: 10.1016/j.euf.2018.05.004
Keywords: network meta-analysis, overactive bladder, sacral neuromodulation (SNM), peripheral tibial nerve stimulation, antimuscarinics, mirabegron
Citation: Liu P, Li Y, Shi B, Zhang Q and Guo H (2022) Comparison of different types of therapy for overactive bladder: A systematic review and network meta-analysis. Front. Med. 9:1014291. doi: 10.3389/fmed.2022.1014291
Received: 08 August 2022; Accepted: 04 October 2022;
Published: 20 October 2022.
Edited by:
Nithesh Naik, Manipal Academy of Higher Education, IndiaReviewed by:
Harrina Erlianti Rahardjo, Universitas Indonesia – Cipto Mangunkusumo Hospital, IndonesiaCopyright © 2022 Liu, Li, Shi, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Guo, Z3VvaHU5N0BzZHUuZWR1LmNu; Qiujie Zhang, MTg1NjAwODI1NTJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.