
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 10 October 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1008450
This article is part of the Research Topic From Portal Hypertension to Acute-on-Chronic Liver Failure: New insights to Pathophysiology and Management of Liver Cirrhosis View all 11 articles
 Johannes Vogg1,2†
Johannes Vogg1,2† Constantin Maier-Stocker1†
Constantin Maier-Stocker1† Stefan Munker1,3
Stefan Munker1,3 Alexander Mehrl1
Alexander Mehrl1 Sophie Schlosser1
Sophie Schlosser1 Hauke Christian Tews1
Hauke Christian Tews1 Karsten Gülow1
Karsten Gülow1 Martina Müller1
Martina Müller1 Stephan Schmid1*
Stephan Schmid1*Background and aims: Liver diseases are frequent causes of morbidity and mortality worldwide. Liver diseases can lead to cirrhosis, with the risk of acute-on-chronic liver failure (ACLF). For the detection of changes in hepatic hemodynamics, Doppler ultrasonography is a well-established method. We investigated hepatic hemodynamics via serial Doppler ultrasonography to determine the predictive value of changes in hepatic perfusion for the outcome in patients with severe liver diseases compared to established prognostic models such as the MELD (Model for End-Stage Liver Disease) or CLIF-C (Chronic Liver Failure-Consortium) ACLF score.
Methods: In this prospective cohort study, hepatic perfusion was quantified at baseline before the initiation of treatment and every third day by means of serial measurements of the hepatic artery resistance index (HARI) and the maximum portal vein velocity (PVv) using Doppler ultrasonography in 50 consecutive patients with severe liver diseases admitted to a medical intensive care unit (MICU). The recorded hemodynamic parameters were compared to the MELD score, and the CLIF-C ACLF score to analyze their utility for the prediction of the outcome of patients with severe liver diseases, liver cirrhosis, and ACLF.
Results: The changes (delta) obtained by serial measurements of the MELD score, HARI, and PVv were analyzed through scatter plots. Bivariate correlation analysis yielded a new positive linear correlation between the delta-HARI and the delta-MELD score (r = 0.469; p < 0.001). In addition, our data revealed a new negative linear correlation between delta-PVv and the delta-MELD score (r = −0.279, p = 0.001). The leading cause of MICU mortality was acute-on-chronic liver failure (ACLF). A subgroup analysis of patients with liver cirrhosis revealed a positive linear correlation between the delta-HARI and the delta-CLIF-C-ACLF score (r = 0.252, p = 0.005). Of clinical relevance, non-survivors of ACLF exhibited a significantly higher mean value for the delta-HARI (0.010 vs. −0.005; p = 0.015) and a lower mean value for the delta-PVv (−0.7 vs. 1.9 cm/s; p = 0.037) in comparison to survivors of ACLF.
Conclusion: This study shows the prognostic value of the assessment of hepatic perfusion in critical care patients with severe liver diseases by bedside Doppler ultrasound examination and its utility as an accurate predictor of the outcome in patients with ACLF. Increasing HARI and a decreasing PVv are predictors of an adverse outcome. Delta-HARI and delta-PVv are new biomarkers of prognosis and ACLF-related mortality in patients with liver diseases. Delta-HARI and delta-PVv may be helpful in guiding clinical decision-making, especially in catecholamine and fluid management.
Liver diseases are significant causes of morbidity and mortality worldwide (1). The progression of liver diseases to cirrhosis and decompensation associated with critical illness is a significant cause of mortality in these patients (2, 3). Acute-on-chronic liver failure (ACLF) can occur in patients with liver cirrhosis and is a recently described entity diagnosed in patients with chronic liver diseases and a combination of hepatic and extrahepatic organ failures (kidney, respiratory, coagulation, circulatory, brain). Early diagnosis and treatment of ACLF are essential for the outcome of these critically ill patients (4, 5).
Organ perfusion plays an essential role in liver diseases, while the mechanisms regulating hepatic perfusion in patients with liver diseases, fibrosis, cirrhosis, and ACLF are only partially known (6). Portal venous flow depends mainly on the influx of splanchnic perfusion (7).
In contrast to portal venous flow, arterial flow is subject to pressure-dependent autoregulation. A second mechanism termed hepatic artery buffer response (HABR) is a central mechanism in the intrinsic regulation of hepatic blood flow (8). HABR describes the ability of the hepatic artery to compensate for changes in the flow of the portal vein by counter-directed hemodynamic adjustment of its perfusion. HABR causes arterial vasodilation through reduced portal flow and, vice versa, arterial vasoconstriction through increased portal flow (9). HABR is also preserved in patients with inflammatory liver diseases, even in advanced liver fibrosis and cirrhosis (10, 11).
Abdominal and Doppler ultrasonography are well-established methods for evaluating liver diseases and detecting changes in hepatic hemodynamics. International guidelines recommend using ultrasound scans to evaluate liver diseases (2, 3). Ultrasound scans are an easily accessible, inexpensive, and non-invasive procedure, which can be repeated as often as needed, even at the bedside of critical care patients. Such scans have high specificity in diagnosing liver fibrosis, liver cirrhosis, and portal hypertension. The diagnosis of liver cirrhosis by conventional ultrasound is based on changes in liver morphology and signs of portal hypertension. In addition, Doppler ultrasonography is a valuable tool for evaluating hemodynamic changes in cirrhotic liver tissue (12).
Little is known about the predictive value of serial measurements of hepatic hemodynamics in patients with severe liver diseases, particularly in patients with acutely decompensated cirrhosis at risk of developing acute-on-chronic liver failure.
The aim of this study was 1. to determine the predictive value of changes in hepatic perfusion for the outcome in patients with severe liver diseases compared to well-established prognostic models such as the MELD or CLIF-C ACLF score in the context of critical care treatment (13, 14), 2. to analyze the role of liver perfusion as an early predictor of mortality due to ACLF, and 3. to identify potential new hemodynamic targets in critical care for early therapeutic intervention in ACLF.
This prospective cohort study enrolled 50 patients with severe acute and chronic liver injury (ALI and CLI) and acute-on-chronic liver failure (ACLF) (Table 1). The diagnosis of liver cirrhosis was based on non-invasive tests following the current European Association for the Study of the Liver (EASL) Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis (15). Accordingly, we diagnosed liver cirrhosis by detecting specific morphological changes of the liver by ultrasound and computed tomography (CT) in combination with examination of clinical and laboratory chemistry parameters (16, 17). In our cohort, liver cirrhosis was diagnosed in 36 of the 50 patients studied. The patients were treated in a medical intensive care unit (MICU) of a German University Hospital that specializes in the treatment of liver diseases.
The aim of the study was to assess the potential of Doppler ultrasound as a predictor of the outcome of patients with severe liver diseases and as a novel prognostic biomarker for ACLF. The study was approved by the Ethics Committee of the University of Regensburg, Regensburg, Germany (registration number 18-920-101). All patients provided written, informed consent before the study, in accordance with the principles of the Declaration of Helsinki [revision of (18)].
A flow chart of eligible patients with liver disease, data collection, and analysis is given in Figure 1.
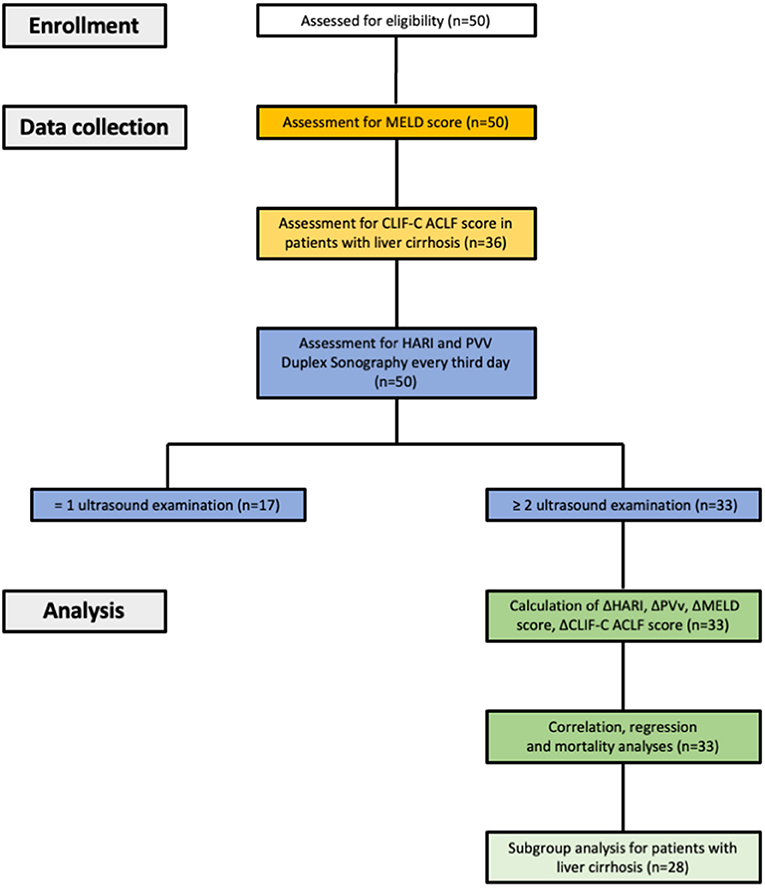
Figure 1. Study design, data collection and data analysis. HARI, hepatic artery resistance index; PVv, portal vein velocity; CLIF-C ACLF score, chronic liver failure-consortium acute-on-chronic liver failure score.
To quantify hepatic perfusion, the hepatic artery resistance index (HARI) and the maximum portal vein velocity (PVv) were determined at admission to the MICU and then every third day using Doppler ultrasonography. A total of 187 ultrasound and Doppler examinations were performed in 50 patients (mean 3.74; range 1–12). Seventeen patients were examined once, 8 patients twice, 4 patients three times, and 21 patients four or more times. A standardized protocol was used for the positioning and breathing/ventilation of the patients during the ultrasound examination and Doppler sonography according to the literature (19, 20). We determined the hepatic artery resistance index (HARI) and maximum portal vein velocity (PVv).
Ultrasound scans were performed by experienced examiners. Imaging and processing of the recordings were carried out with the mobile ultrasound system Noblus® (Hitachi Aloka Medical, Ltd., Japan). The hemodynamic parameters were recorded using a convex transducer with a 1–5 MHz frequency range. Each examination consisted of three independent measurements of PVv and the HARI. The mean values were calculated and recorded. An example of a Doppler ultrasound examination in a patient with ACLF is shown in Figure 2.
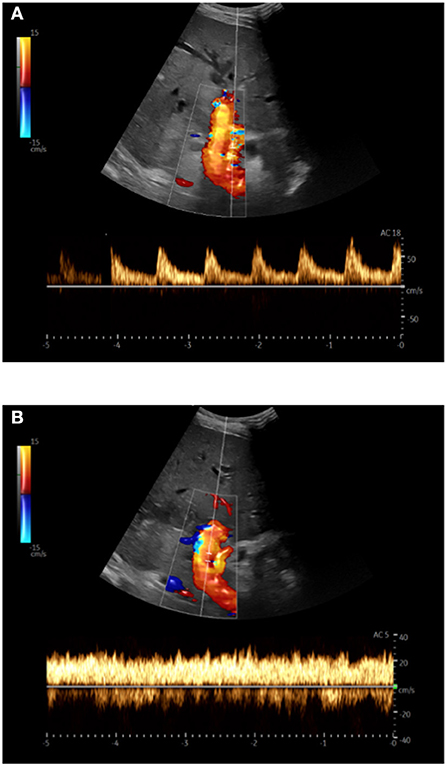
Figure 2. Doppler sonography in triplex technique (B-image + color Doppler + spectral Doppler) in a patient with acute-on-chronic liver failure; (A) Hepatic artery: Derivation of the arterial flow signal; (B) Portal vein: The maximum flow velocity was measured at the level of the hepatic artery after angle correction.
The PVv was measured using Pulsed-Wave-(PW)-Doppler in the hepatoduodenal ligament at the crossing level of the proper hepatic artery and the portal vein. The HARI was recorded at the crossing level of the hepatic artery and the portal vein using PW-Doppler (Figure 2). The resistance index was automatically calculated using the following equation (21):
The MELD score, a well-established indicator of the mortality of patients with end-stage liver disease, was calculated for each patient—simultaneously with the ultrasound examination—using the following equation (22, 23):
In patients with decompensated liver cirrhosis—in addition to the MELD score—the Chronic Liver Failure Consortium (CLIF)-C ACLF score, a score derived and validated by the CLIF consortium, was determined to predict the mortality of patients with ACLF (24). The following formula was used for the calculation, wherein CLIF-C OF score was raised according to (24):
To analyze the impact of life support in the MICU on liver perfusion, we recorded whether invasive ventilation (Servo-i®, Getinge, Sweden), renal replacement therapy (multiFiltrate Ci-Ca®, Fresenius, United States of America) or catecholamine therapy was required during intensive care treatment. Ventilation was pressure-controlled or pressure-supported; positive end-expiratory pressure (PEEP) and peak pressure (Ppeak) at the time of the ultrasound examination were collected. None of the patients received non-invasive ventilation during the ultrasound examination. The continuously administered catecholamines during the ultrasound examination were recorded in their respective dosage (Norepinephrine in mg/h, epinephrine in mg/h, dobutamine in mg/h, terlipressin in mcg/h, and vasopressin in IU/h).
Data were analyzed using SPSS Statistics, version 25 (IBM, USA). Correlation analyses of perfusion parameters, the MELD score, and the CLIF-C ACLF score were performed according to Pearson. The strength and direction of the correlations were described by the determined correlation coefficient (r). In addition, linear regression analyses of the perfusion parameters and the MELD score were carried out and described using the R2-value. As part of the study, differences in parameters were determined for specific groups. Mann-Whitney-U-tests were used for non-normally distributed variables and t-tests for normally distributed variables with equal variance. A p-value of 0.05 was set as the level of significance. Multiple regression analyses were performed to examine the extent to which liver perfusion was affected by life support at the MICU.
A total of 50 patients were enrolled in the study. The demographic and clinical characteristics of these 50 study patients are summarized in Table 1. Thirty-four patients were male, and 16 were female. The age of the cohort ranged from 39 to 90 years (mean 59.7; SD ± 10.3 years). The study included patients with different stages of acute and chronic liver diseases. Thirty-six patients were diagnosed with liver cirrhosis. The leading etiology of liver cirrhosis was alcohol-related (n = 25), which was diagnosed in 3 patients with hepatocellular carcinoma (HCC). Other causes of liver cirrhosis were autoimmune liver diseases (n = 4), viral hepatitis (n = 4), and cirrhosis of idiopathic origin (n = 3). The patients with liver cirrhosis were categorized according to the Child-Pugh classification, 1 patient was classified with liver cirrhosis Child-Pugh A, 12 patients with liver cirrhosis Child-Pugh B, and 23 patients with liver cirrhosis Child-Pugh C. In summary, the majority of our patient cohort had advanced stages of liver cirrhosis and were at high risk of developing acute-on-chronic liver failure (Table 1).
The patients without underlying liver cirrhosis (n = 14) had been admitted to the MICU due to drug-induced acute liver failure (ALF) (n = 6), cholangiosepsis (n = 5), and liver diseases of different etiologies (n = 3).
On average, the patients were treated at the MICU for 15.3 (SD ± 13.8) days. The length of the MICU stay ranged from a minimum of 2 days to a maximum of 72 days. Twenty-three of the 50 patients studied (=46%) underwent renal replacement therapy, and 13 patients (=26%) required mechanical ventilation therapy during intensive care treatment. Twenty-nine patients (=58%) required vasoactive medication for circulatory support during the MICU stay. Sixteen of the 50 examined patients (32%) died during the MICU stay. The leading cause of death was acute-on-chronic liver failure (93% of deceased patients, n = 15). In the non-survivors, ACLF resulted, despite maximum intensive care therapy, in multiorgan failure, coagulation failure, and circulation failure (15/16 patients). One patient (1/16 patients) newly diagnosed with congestive hepatopathy died of septic shock due to severe pneumonia.
Precipitating events for ACLF were infections and gastrointestinal bleedings. In 9 patients, infections were the precipitating events for ACLF (6/9 patients with pneumonia and 3/9 patients with urosepsis). In 6 patients, gastrointestinal bleeding was the precipitating events for ACLF (4/6 patients with varicose bleeding and 2/6 patients with non-varicose upper gastrointestinal bleeding).
HARI, PVv, and MELD score were collected and analyzed at the time of admission to the ICU. Overall n = 50 patients were studied. On average, the HARI was 0.74, the maximum PVv was 19.1 cm/s, and the MELD score was 25.2 at admission (Table 1). The mean values of HARI, PVv, and MELD score were compared for deceased and survived patients by t-test and Mann-Whitney U-test, respectively (Table 4A). On admission to the ICU, non-survivors had, on average, a higher MELD score (29.7 vs. 23.2, p = 0.010). Liver perfusion at admission did not differ significantly between non-survivors and survivors [HARI (0.75 vs. 0.73, p = 0.342), PVv (14.4 cm/s vs. 21.3 cm/s, p = 0.129)].
The primary goal of our study was to analyze the changes over time of the perfusion parameters HARI and PVv and to investigate their utility as prognostic biomarkers. In patients (n = 33) who were examined more than once during their MICU stay, the course over time parameters (n = 137) was calculated from the varying absolute values of each examination and are further referred to as delta-values (Table 1). The mean of the MELD score of the patients during their MICU stay was 25.8 points and decreased by 0.3 points with each examination. The mean HARI was 0.74 and decreased by 0.003 during the MICU stay. In contrast, the mean maximum PVv was 19.2 cm/s and increased by 0.4 cm/s during the MICU stay.
The prognostic value of routine Doppler evaluation of hepatic perfusion on the MICU was determined by means of a correlation analysis of the delta-HARI and delta-PVv with the delta-MELD score. Correlation analysis was performed by bivariate correlation analyses according to Pearson (Table 2). There was a significant positive linear correlation between the delta-MELD score and the delta-HARI (r = 0.469; p < 0.001) and a negative linear correlation between the delta-MELD score and delta-PVv (r = −0.279, p = 0.001). The correlations between delta-MELD score, delta-HARI, and delta-PVv are shown in scatter plots in Figure 3. In summary, patients with increasing HARI or decreasing PVv showed an increase in their MELD Score, which reflects the worsening of their liver disease.
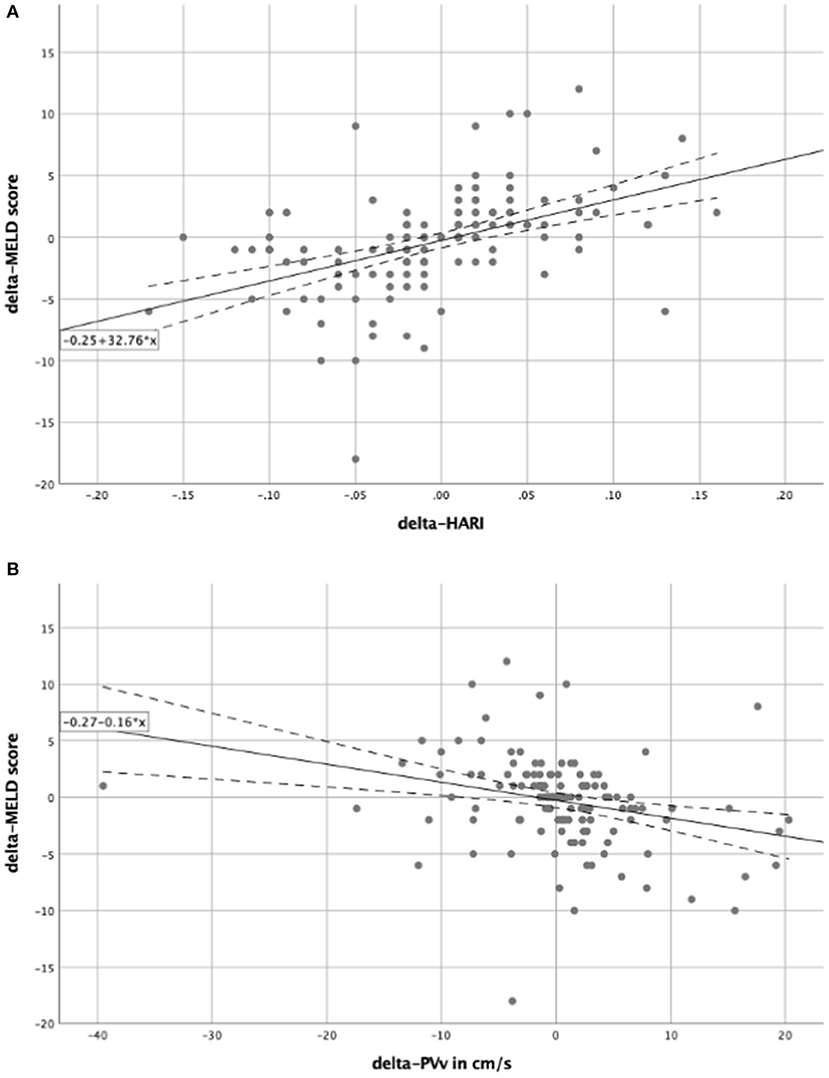
Figure 3. Scatter plots for the correlation between delta-MELD score and the perfusion parameters. In both scatter plots, the solid line represents the regression equation, which is also shown in the box. The dashed lines equate to the 95% confidence interval. (A) Showing a positive linear correlation between the delta-MELD score and the delta-HARI; (B) showing a negative linear correlation between the delta-MELD score and delta-PVv. HARI, hepatic artery resistance index; PVv, portal vein velocity.
To further investigate the influence of the delta-HARI and delta-PVv on the delta-MELD score, regression analyses (Table 2) showed an R2-value of 0.220 and 0.078. Both regression models presented p-values of < 0.05. The regression of the delta-HARI and the delta-MELD score resulted in a regression coefficient of 32.76 with a p-value of < 0.001 and a 95% confidence interval ranging from 22.27 to 43.25. The regression coefficient for delta-PVv and the delta-MELD score was −0.16 with a p-value of 0.001 and a 95% confidence interval ranging from −0.25 to −0.07. The determined coefficients were used to set up the following regression equations to be able to predict the course over time of the MELD score as a function of the delta-HARI and delta-PVv:
delta-MELD score = −0.25 + 32.76 × delta-HARI (Figure 3A).
delta-MELD score = −0.27−0.16 × delta-PVv (Figure 3B).
Furthermore, the relation between delta-HARI and delta-PVv was analyzed by bivariate correlation analyses according to Pearson, r = −0.159, p = 0.063. This suggests that the hepatic artery buffer response (HABR) is impaired in our patient cohort. If the HABR were functional, we would expect a positive correlation (10).
The mean values of delta-HARI, delta-PVv, and delta-MELD score were calculated for the 33 patients who had been examined more than once during their MICU stay. The mean values of these parameters were then compared to evaluate their utility as prognostic markers in patients with severe liver disease (Table 3). Patients who did not survive ACLF were characterized by an increase of their MELD score on average by 1.3 points and of the hepatic artery resistance index by 0.01, whereas maximum portal vein velocity decreased on average by 0.7 cm/s per examination. In survivors, the MELD score decreased on average by 1.9 points and the hepatic artery resistance index by 0.005, whereas maximum portal vein velocity increased on average by 1.9 cm/s per examination. The distribution of delta-HARI, delta-PVv, and delta-MELD score for non-survivors and patients who recovered is shown in boxplots in Figure 4. The comparison of non-survivors and survivors showed statistically significant differences in the mean values of delta-HARI (p = 0.015), delta-PVv (p = 0.037), and delta-MELD score (p = 0.002). Of clinical relevance, each of the three parameters was useful as a prognostic biomarker for patients with ACLF. Cohen's d was calculated and interpreted to quantify the size of each effect. Figure 4 shows the newly described statistically significant differences in the mean values of delta-HARI (Figure 4A) and delta-PVv (Figure 4B) between deceased and surviving patients.
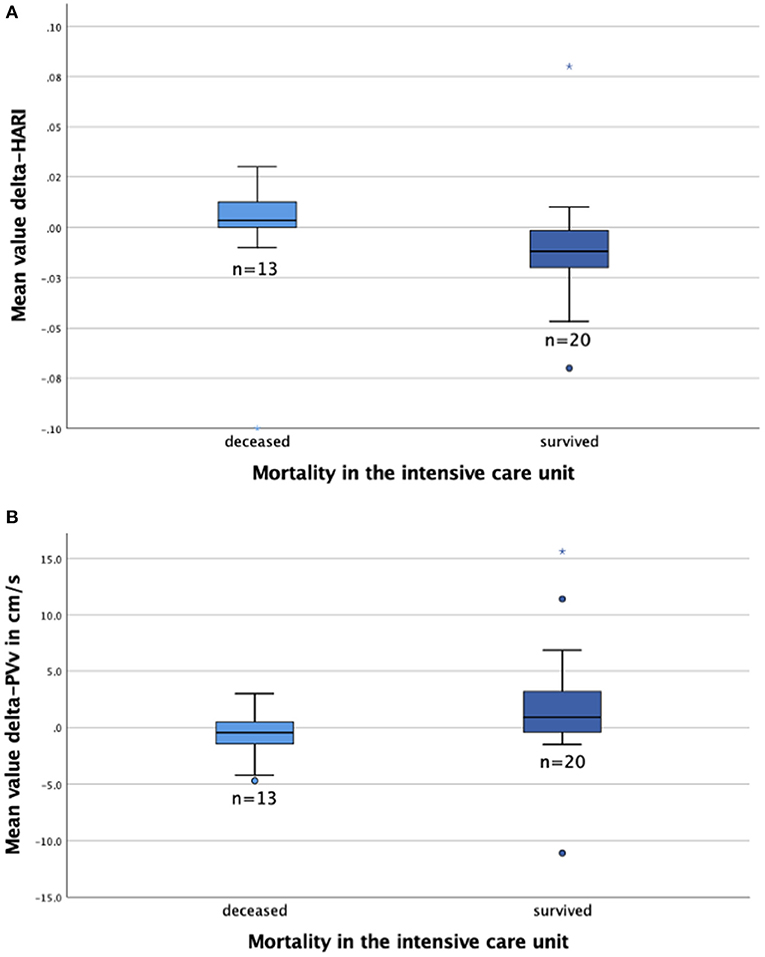
Figure 4. Boxplots to compare the MELD score and perfusion parameters for deceased (n = 13) and survived (n = 20) patients. The figures show the median (line in the middle of the box), 1st/3rd quartile (lower/upper edge of the box), minimum (lower whisker), maximum (upper whisker) and outliers*°. (A) Delta-HARI is higher in deceased patients than in survived patients; (B) Delta-PVv is lower in deceased patients than survived patients. HARI, hepatic artery resistance index; PVv, portal vein velocity.
Thus, delta-HARI and delta-PVv can predict the mortality of critical care patients with severe liver diseases to a similar extent as the delta-MELD score. Differences in the mean values of the delta-MELD score indicate a slightly stronger effect on mortality and higher prognostic predictive value of the delta-MELD in comparison to the delta-HARI. In our dataset, the area under the curve (AUC) for the prediction of ICU mortality for delta-HARI was 0.76 (95% Confidence Interval: 0.58–0.94, p = 0.012) and thus only slightly lower than that of the delta-MELD with an AUC of 0.84 (95% Confidence Interval: 0.70–0.97, p = 0.01). Increasing HARI and decreasing PVv are early predictors of an adverse outcome of patients with severe liver diseases. A summary of the data is given in Figure 5.
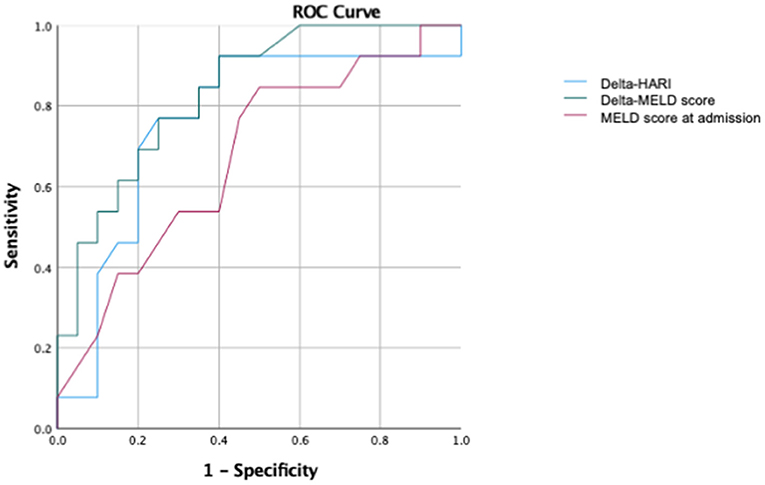
Figure 5. ROC analysis for the prediction of ICU mortality. AUC of delta-HARI was 0.76 (95% Confidence Interval: 0.58–0.94, p = 0.012), AUC of delta-MELD was 0.84 (95% Confidence Interval: 0.70–0.97, p = 0.01), and AUC of MELD-Score at admission was 0.71 (95% Confidence Interval: 0.53–0.89, p = 0,049).
Multiple regression analyses were performed to examine the extent to which delta-HARI and delta-PVv were affected by factors other than the delta-MELD score. Here, we primarily focused on the effects of intensive care therapeutic procedures.
Forty-six percentage of the patients underwent renal replacement therapy (Table 1). In our study, no ultrasound examinations were performed while the patients were dialyzed. Liver perfusion did not significantly differ in patients with dialysis or without dialysis (delta-HARI 0.006 vs. −0.008, p = 0.074, delta-PVv 0.8 cm/s vs. 0.9 cm/s, p = 0.868, Mann-Whitney U-test). Twenty-six percentage of the patients required mechanical ventilation during intensive care treatment. There was no correlation between ventilation pressures (PEEP and Ppeak) and liver perfusion over time. Fifty-eight percentage of the patients required vasoactive medication for circulatory support during the ICU stay, with norepinephrine being the most frequently used catecholamine. A combination therapy of vasoactive agents was required in 11 of the 29 patients receiving vasoactive medication. Delta-norepinephrine (r = 0.247, p = 0.004) and delta-epinephrine (r = 0.244, p = 0.004) showed a positive correlation with the delta-HARI. Delta-dobutamine (r = −0.18, p = 0.036) correlated statistically significantly with delta-PVv. Details concerning the effect of life support in the MICU on liver perfusion are shown in Table 4A.
In multiple regression analyses, factors that potentially influence liver perfusion such as delta-norepinephrine, delta-epinephrine, delta-dobutamine, delta-terlipressin, delta-vasopressin, delta-PEEP, and delta-Ppeak were compared with the delta-MELD score concerning their effect on the respective liver perfusion parameters. The regression for delta-HARI yielded a corrected R2 of 0.290 with a model significance of p < 0.001. Delta-MELD score (p < 0.001), delta-norepinephrine (p = 0.033), and delta-dobutamine (p = 0.027) showed a significant effect on the course of HARI, with delta-MELD score clearly exerting the most significant influence (standardized coefficient beta: 0.439 vs. 0.189 and 0.168, respectively) (Table 4B). The regression for delta-PVv yielded a corrected R2 of 0.116 with a model significance of p = 0.003. For delta-MELD score (p = 0.001) and delta-dobutamine (p = 0.010) a significant effect on delta-PVv could be determined. Again, delta-MELD score affected the course of PVv more pronounced than delta-dobutamine (standardized coefficient beta: −0.281 vs. −0.219) (Table 4B).
In summary, delta-HARI and delta-PVv are significantly influenced by the delta-MELD score and not by dialysis or mechanical ventilation. Norepinephrine and dobutamine have a mild to moderate impact on liver perfusion, while the delta-MELD score exerts the most significant effect on the course of the perfusion parameters HARI and PVv. Our analyses also reveal that optimized catecholamine therapy and fluid management are potential therapeutic targets to improve liver perfusion.
Of clinical relevance, the leading cause of death in our patient cohort was an acute-on-chronic liver failure (93% of deceased patients, n = 15). Therefore, we performed a correlation analysis between delta-HARI and delta-PVv with the delta-MELD and delta-CLIF-C ACLF score in a subgroup analysis of the 36 patients with liver cirrhosis.
The CLIF-C ACLF score is a score that was derived and validated by the chronic liver failure (CLIF) consortium to predict the mortality of patients with ACLF (24). The CLIF-C ACLF score combines the age of the patient and the white blood cell count with the chronic liver failure (CLIF) organ failure score (CLIF OF score), which is a modified version of the Sequential Organ Failure Assessment (SOFA) score (24–26). The CLIF OF score system comprises the organs/systems liver, kidney, brain, coagulation, circulatory, and respiratory with the respective subscores 1–3 (24).
For the subgroup analysis of patients with liver cirrhosis (n = 33), HARI, PVv, and MELD score were collected at admission to the MICU and compared for deceased and survived patients by t-test and Mann-Whitney U-test, respectively (Table 6A). Analogous to the complete patient collective, the liver perfusion parameters at admission showed no significant differences regarding the mortality of the patients. MELD score was a predictor of mortality on admission.
Changes over time in the liver perfusion parameters were analyzed in the subgroup of patients with liver cirrhosis. Our analyses showed a significant positive linear correlation between the delta-HARI and the delta-MELD score (r = 0.517; p < 0.001) and the delta-CLIF-C ACLF score (r = 0.252; p = 0.005). In addition, we could demonstrate a concomitant negative linear correlation between delta-PVv and the delta-MELD score (r = −0.316; p < 0.001).
There was no significant correlation between delta-PVv and delta-CLIF-C ACLF score (r = −0.106, p = 0.246).
For further investigation of the correlation of delta-HARI or delta-PVv with the delta-MELD score and the delta-CLIF-C ACLF score, regression analyses were performed. These showed an R2-value of 0.261 for the influence of delta-HARI on the delta-MELD score and an R2-value of 0.063 for the influence of delta-HARI on delta-CLIF-C ACLF score, respectively. Both regression models showed p-values of < 0.05. For the effect of the delta-PVv on the delta-MELD score an R2-value of 0.1 was calculated (p < 0.05).
Regression analyses for the effect of delta-PVv on the delta-CLIF-C ACLF score showed R2-values of 0.011 and were not significant, p = 0.245.
Data are shown in Tables 5, 6 and Figure 6.
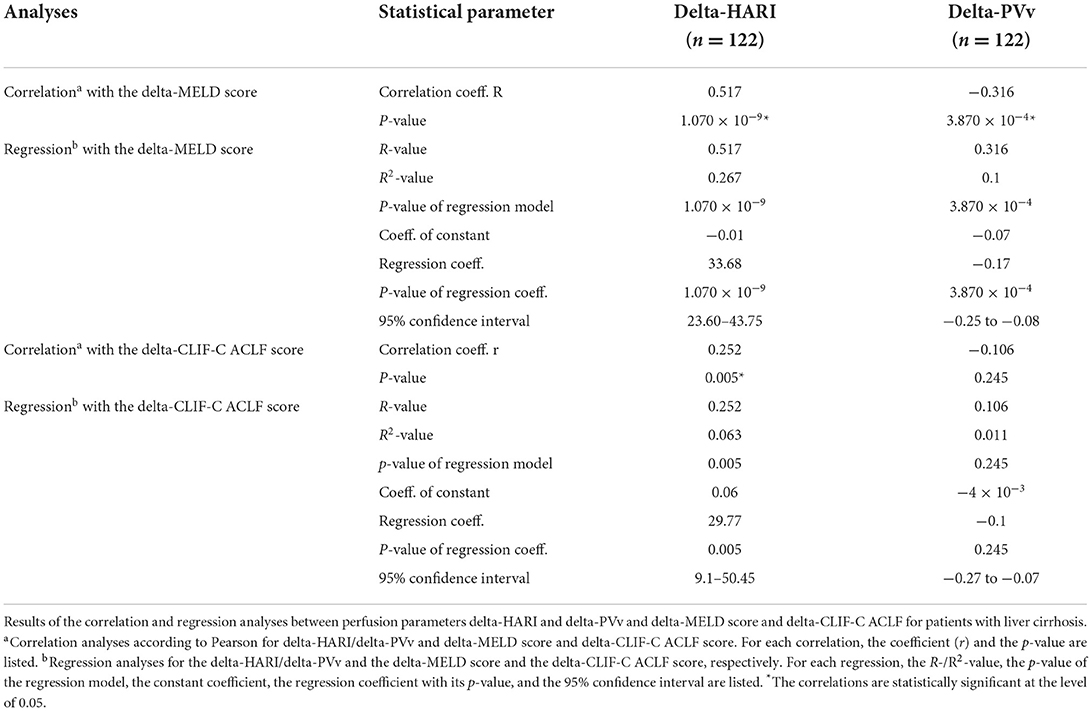
Table 5. Subgroup analyses—Correlation and regression analyses between perfusion parameters and delta-MELD score and delta-CLIF-C ACLF score for patients with liver cirrhosis.
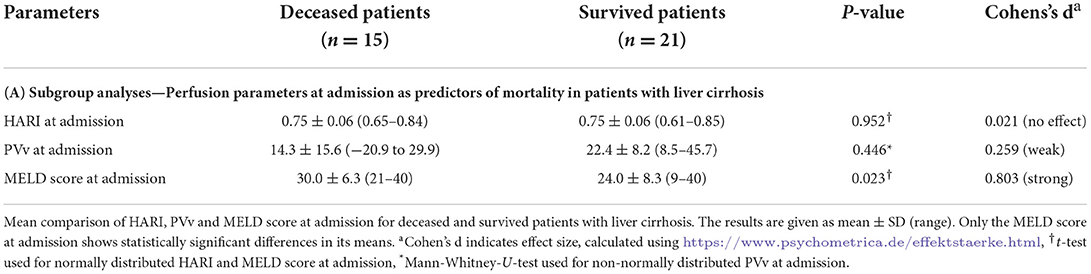
Table 6A. Subgroup analyses—Liver perfusion parameters as predictors of mortality in patients with liver cirrhosis.

Table 6B. Subgroup analyses—Differences in perfusion parameters over time predicting mortality in patients with liver cirrhosis.
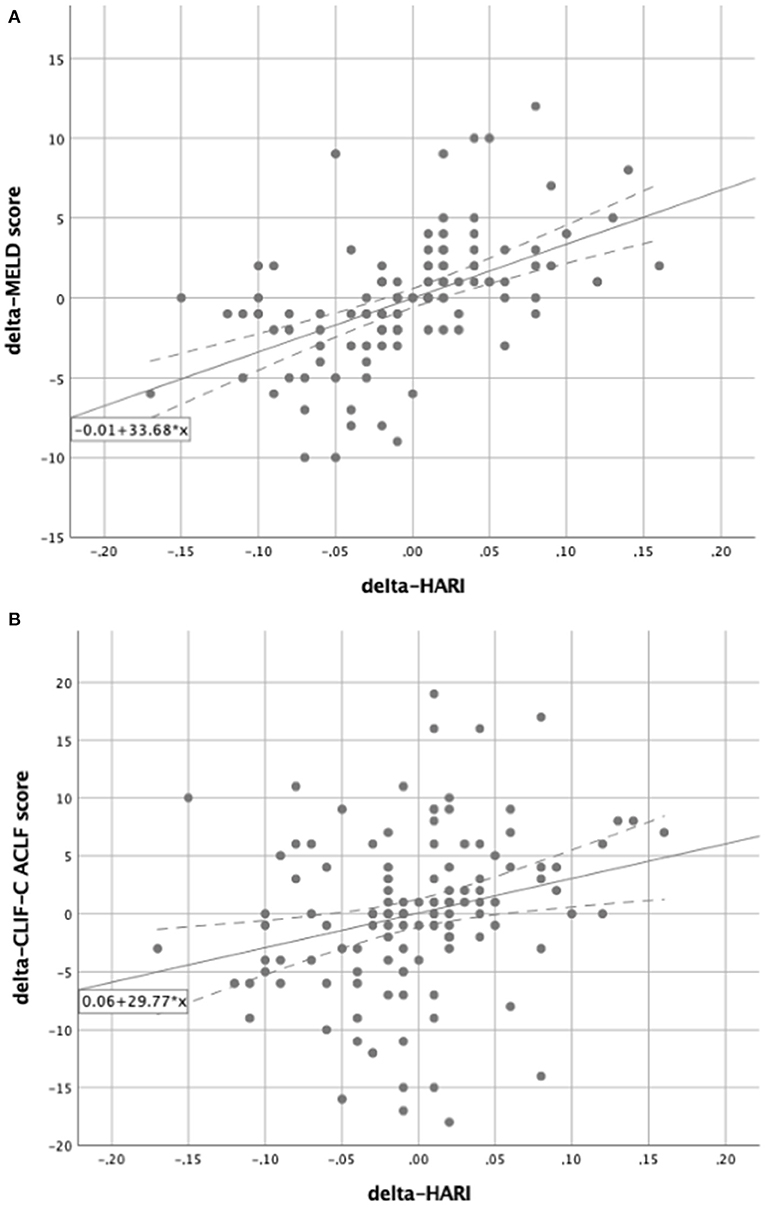
Figure 6. Subgroup analysis for patients with liver cirrhosis. Scatter plots for the correlation between delta-HARI and delta-MELD or delta-CLIF-C ACLF score for patients with liver cirrhosis. (A) Showing a positive linear correlation between delta-HARI and delta-MELD score. (B) showing a positive linear correlation between delta-HARI and delta-CLIF-C ACLF score.
Using the determined coefficients, the following regression equitation can predict the course over time of the delta-MELD score and the delta-CLIF-C-ACLF score as a function of delta-delta-HARI and delta-PVv.
Delta-MELD score = −0.01 + 33.68 x delta-HARI (Figure 6A).
Delta-CLIF-C ACLF score = 0.06 + 29.77 x delta-HARI (Figure 6B).
Delta-MELD score = −0.07 to 0.17 × delta-PVv
Delta-CLIF-C ACLF score was not calculated as a function of delta-PVv as the regression analyses were not significant.
In summary, in our subgroup analysis for patients with liver cirrhosis and ACLF, we could confirm the correlation of the delta-HARI and delta-PVv with the delta-MELD score, which were evident in the entire cohort. The correlations in the subgroup of patients with liver cirrhosis and ACLF were even higher than in the entire cohort, highlighting the relevance of our findings for patients with cirrhosis and ACLF.
Furthermore, we identified a new significant correlation of the delta-HARI with the delta-CLIF-C ACLF score in this cohort, which is of high clinical relevance because the CLIF-C ACLF score is -up to date- the prognostic score, which is the best predictor of mortality in ACLF (26). The correlations between delta-HARI and delta-CLIF-C ACLF are lower than the correlations between the delta-HARI and the delta-MELD score. We attribute this to the fact that all organ systems are included in the prediction of mortality of the CLIF-C ACLF score, whereas in our study, the ultrasound examinations were focused on the liver. In this context, the MELD score is more specific for the system “liver” in terms of the parameters included, which explains the better correlation of the delta-HARI with the delta-MELD score.
In addition, in the subgroup of patients with liver cirrhosis, ROC (Receiver operating characteristic) analyses were performed to predict ICU mortality (Figure 7). In this subgroup of patients with liver cirrhosis, the AUC for the prediction of ICU mortality for delta-HARI was 0.78 (95% Confidence Interval: 0.60–0.97, p = 0.011) and thus only slightly lower than that of the delta-MELD 0.81 (95% Confidence Interval: 0.65–0.97, p = 0.005). The AUC of delta-CLIF-C ACLF in this subgroup was highest with 0.815 (95% Confidence Interval: 0.66–0.97, p = 0.005), which is in accordance with the literature regarding the CLIF-C ACLF score as the best prognostic score of ACLF.
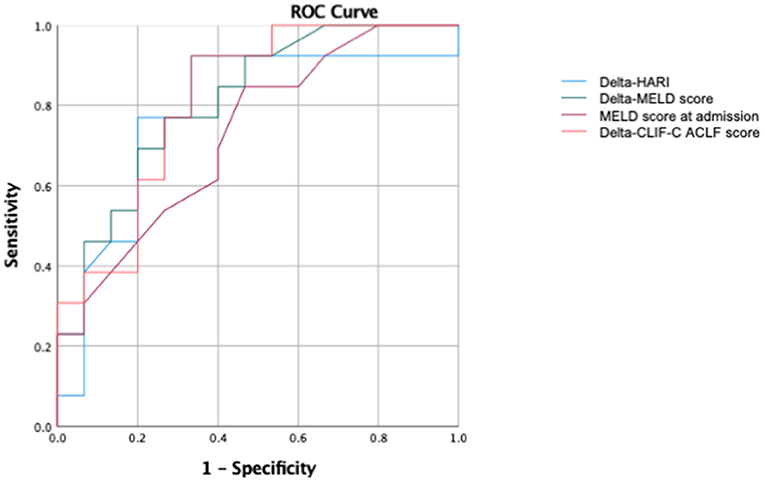
Figure 7. Subgroup ROC analysis for patients with liver cirrhosis of the prediction of ICU mortality. AUC of delta-HARI was 0.78 (95% Confidence Interval: 0.60–0.97, p = 0.011), AUC of delta-MELD was 0.81 (95% Confidence Interval: 0.65–0.97, p = 0.005), AUC of MELD-Score at admission was 0.73 (95% Confidence Interval: 0.54–0.914, p = 0.040), and AUC of delta-CLIF-C-ACLF was 0.815 (95% Confidence Interval: 0.66–0.97, p = 0.005).
In summary, we show a positive correlation of the delta-HARI with the delta-MELD score (r = 0.469, p < 0.001) and a negative correlation of the delta-PVv with the delta-MELD score (r = −0.279, p = 0.001). Compared with the mean values of the delta-HARI (−0.003) and the delta-PVv (0.4 cm/s), the MELD score decreased throughout the MICU stay with simultaneously decreasing resistance indices of the hepatic artery and increasing maximum portal flow velocity. As a decreasing MELD score is a positive prognostic factor, both decreasing HARI and increasing PVv can be considered as novel positive prognostic factors for patients with severe liver diseases. In contrast, increasing HARI and decreasing PVv constitute negative prognostic biomarkers for patients with severe liver diseases. These findings were confirmed in a subgroup analysis of patients with liver cirrhosis. Here we established a new correlation between the delta-HARI and the delta-CLIF-C-ACLF score (r = 0.252; p = 0.005) and confirmed the delta-HARI as an accurate predictor of the outcome of patients with ACLF.
This prospective cohort study aims 1. to determine the predictive value of changes in hepatic perfusion for the outcome in patients with severe liver diseases, 2. to analyze the role of liver perfusion as a new predictor for mortality due to ACLF, and 3. to establish perfusion-based biomarkers as early readouts for therapy guidance in patients with severe liver diseases and ACLF.
We have analyzed changes in hepatic perfusion of critical care patients with acute and chronic liver diseases over the course of their MICU stay to establish new biomarkers of prognosis and therapy guidance. This is the first report of a prospective time series measurement using Doppler sonography in patients with liver disease in the MICU. An increase in the hepatic artery resistance index (HARI) and a decrease in portal vein velocity (PVv) during the MICU stay predicted an adverse outcome and increased mortality.
Previous studies have focused on hepatic hemodynamics in general and on the importance of ultrasound examination in particular (27–30), and data were collected retrospectively or as part of a cross-sectional study (31–34). Only a few studies have examined the correlation between Doppler sonographic measurements of hepatic perfusion and the MELD score so far (31–33, 35, 36).
The patients included in our study showed a mean hepatic artery resistance index of 0.74 ± 0.08 (range 0.55–0.95) and a maximum portal vein velocity of 19.2 ± 15.7 cm/s (range −43.8 to 49.2 cm/s). These results are comparable to those of other studies in patients with liver cirrhosis of alcoholic vs. viral etiology (14, 21, 37, 38). In our study, the absolute mean values of the HARI and PVv reflect the momentary/current status of hepatic perfusion, whereas the time series measurement of hepatic perfusion—reflected by the parameter delta-HARI and delta-PVv—accurately describe the development of hepatic perfusion over time during hospitalization at the MICU. Our study recorded a mean of −0.003 ± 0.057 (range −0.170 to 0.160) for delta-HARI and a mean of 0.4 ± 7.0 cm/s (range −39.5 to 20.3) for delta-PVv. In patients with a good prognosis arterial resistance in the liver decreased, and the maximum portal flow velocity increased over time and with recovery. On the contrary, increasing HARI and decreasing PVv were predictors of an adverse outcome in critically ill patients with different stages of acute and chronic liver diseases. Non-survivors showed a higher delta-HARI (0.010 vs. −0.005; p = 0.015) and lower delta-PVv (−0.7 vs. 1.9 cm/s; p = 0.037) in comparison to patients who survived. Of note, it is the change over time of these perfusion parameters, which most accurately predicts outcome and mortality. Thus, we identified delta-HARI and delta-PVv as early predictors of mortality in acute and chronic liver diseases.
Mortality in our patient cohort was predominantly due to acute-on-chronic liver failure (ACLF). Worldwide, ACLF is emerging as a major cause of mortality in patients with cirrhosis and chronic liver diseases (5). A systematic review of the global burden of ACLF recently reported a prevalence among patients admitted with decompensated cirrhosis of 35% and 90-day mortality of 55% (1). Of note, the exact definition of ACLF varies worldwide (3, 39–42). In Europe, ACLF is generally defined according to the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium as an acute deterioration of pre-existing chronic liver disease associated with organ failure and high short-term mortality (i.e., death < 28 days after hospital admission) (25). In collaboration with the ESAL-CLIF Consortium, Jalan et al. (24) established a prognostic score to predict mortality in patients with acute-on-chronic liver failure, the CLIF-Consortium ACLF (CLIF-C ACLF) score. This score combines the CLIF-C Organ Failure Score [a modification of the Sequential Organ Failure Assessment (SOFA) score] with two other independent predictors of mortality (age and white cell count). Compared to other prognostic scores, such as the MELD score and Child-Pugh score, the CLIF-C ACLF score is the best available score for the prediction of 28-day mortality among patients with ACLF (43, 44). Of note, none of these scores includes liver perfusion parameters.
By demonstrating an association between delta-HARI and delta-PVv with ACLF-related mortality, our study shows for the first time that the course over time of hepatic perfusion plays a crucial role in the prognosis of patients with ACLF. We were able to show a clear utility for liver hemodynamic parameters as prognostic biomarkers by comparing serial measurements of HARI with the established prognostic scores delta-CLIF-C-ACLF in the early prediction of ACLF-related mortality.
Our results are in accordance with Mehta et al. who showed that the development of ACLF and its associated inflammatory response markedly changes intrahepatic hemodynamics with a subsequent decrease in hepatic blood flow and an increase in intrahepatic resistance, which predicted mortality (45). Solís-Muñoz et al. reported that the portal vein velocity was significantly lower in acutely decompensated patients with cirrhosis who developed ACLF than in those who did not develop ACLF (46). Furthermore, our data are in line with the results of Kuroda et al. (34) who analyzed hepatic perfusion using contrast-enhanced ultrasound (CEUS) in patients with acute liver failure (ALF) and investigated its utility as a prognostic tool (47). The authors recorded the time interval (TI) between the time to peak of the hepatic artery (HA) and liver parenchyma (LP) by performing CEUS at baseline and after 7 days. TI (HA, LP) was significantly shorter in non-survivors than in survivors and emerged as the only independent factor for predicting adverse prognosis in patients with ALF, with a 94.4% sensitivity and 90.6% specificity. This underlines the importance of preventing increasing HARI and decreasing PVv and implementing serial Doppler sonographic or CEUS-bases liver perfusion measurements in managing patients with acute and chronic liver disease. The transferability to daily clinical practice and the cost-effectiveness in the guidance of treatment is undoubtedly easier using routine Doppler sonography in comparison with CEUS.
Thus, the newly established correlation between hepatic perfusion and mortality, delta-HARI and delta-PVv, may present new valuable targets in the guidance of critical care therapy for patients with severe liver diseases by optimizing hepatic perfusion, for example, through calculated volume therapy or modulation of vasoactive medication. Thus, initial fluid resuscitation in ACLF following the recommendations of the International Guidelines for the Management of Sepsis (48) could be guided by repeated Doppler sonographic measurements to restore hemodynamics and to optimize liver perfusion. The choice of resuscitation fluid in patients with cirrhosis and ACLF is unclear. This issue was addressed by Maiwall et al. who compared the efficacy and safety of 20% Albumin to plasmalyte in the treatment of sepsis-induced hypotension (49). In critically ill patients with cirrhosis and sepsis-induced hypotension 20% albumin restores arterial pressure more quickly but causes more pulmonary complications than plasmalyte. Plasmalyte is safer and can be considered for volume resuscitation in these patients. The optimal management of the critically ill patient with sepsis and cirrhosis has not been well-defined and follows guidelines made for management of patients without cirrhosis with sepsis. Despite the lack of strong evidence, we usually follow an analogous (to patients without cirrhosis) approach to sepsis management in patients with cirrhosis, including the choice of fluids, vasopressors, and antibiotics. According to our data, we suggest monitoring fluids and vasopressors using routine Doppler sonography of the liver in patients with liver cirrhosis and ACLF. Monitoring of vasopressors is central because vasoactive medication can affect liver perfusion. We performed multiple regression analyses to identify potential effectors on liver perfusion. Renal replacement therapy and mechanical ventilation did not affect HARI and PVv. The latter has been described by Saner et al. who reported no significant differences in liver transplanted patients for maximal PVv and HARI when ventilated with different PEEP settings (50). The identified correlations between liver perfusion parameters and vasoactive medication are in accordance with previous publications (51–53). Consequently, monitoring vasopressors by Doppler sonography may help prevent adverse effects on (delta) HARI and PVv.
This study has several limitations. First, the sample size is small. Larger-scale prospective clinical studies are needed to confirm these findings. Second, Doppler sonography is an operator-dependent method. Third, this study is a single-center observational study which yielded clinically relevant results with respect to the use of liver perfusion parameters to guide volume and catecholamine therapy in patients with severe liver disease. The limitation lies in the observational nature of the study. A follow up interventional study should be designed including multiple participating sites to validate the efficacy of Doppler sonographic measurements of liver perfusion to guide volume resuscitation and vasopressor therapy of patients with ACLF.
Here, we show that delta-HARI and delta-PVv are new predictors of outcome in patients with ACLF. Furthermore, we could show that the course over time of the HARI correlates with the CLIF-C ACLF score during ICU treatment, underlining that serial measurement of liver perfusion parameter is an early predictor of mortality due to ACLF.
Our study establishes a clear utility of routine Doppler sonography evaluating hepatic perfusion in critical care patients with severe liver diseases. In addition, the correlation between hepatic perfusion and mortality, described here for the first time, may be seen as an opportunity to improve and guide the treatment of critical care patients with severe liver diseases by optimizing hepatic perfusion, for example, through balanced volume therapy or additional vasoactive medication.
We recommend introducing a regular routine Doppler sonographic evaluation of liver perfusion in critical care patients with severe liver diseases and liver cirrhosis in everyday clinical practice to assess prognosis and to guide therapeutic management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the University of Regensburg, Regensburg, Germany. The patients/participants provided their written informed consent to participate in this study.
SSchm, JV, CM-S, SM, KG, and MM: study concept, design, drafting of the manuscript, analyses, and interpretation of data. JV, CM-S, and SSchm: acquisition of data. JV, CM-S, SM, AM, SSchl, HT, KG, MM, and SSchm: writing and critical revision of the manuscript for important intellectual content. MM: supervision. All authors contributed to the article and approved the submitted version.
We thank our patients for participating in the study. We thank the interprofessional team of the Medical Intensive Care Unit of the Department of Internal Medicine I at the University Hospital Regensburg, Regensburg, Germany, for continuous support. We thank Monika Schoell and Jillena Zinsser-Krys, MD, University Hospital Regensburg, Regensburg, Germany for language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACLF, acute-on-chronic liver failure; ALF, acute liver failure; ALI, acute liver injury; CCC, cholangiocellular carcinoma; CEUS, contrast-enhanced ultrasound; CLI, chronic liver injury; CLIF-C, chronic liver failure-consortium; CT, computed tomography; EASL, European association for the study of the liver; EDV, end-diastolic velocity; HA, hepatic artery; HARI, hepatic artery resistance index; HABR, hepatic artery buffer response; HCC, hepatocellular carcinoma; LP, liver parenchyma; MELD, model for end-stage liver disease; MICU, medical intensive care unit; PEEP, positive end-expiratory pressure; Ppeak, peak pressure; PSV, peak systolic velocity; PVv, portal vein velocity; PW, doppler, pulsed-wave-doppler; ROC, receiver operating characteristic; SD, standard deviation; TI, time interval.
1. Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, et al. Global burden of disease: Acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. (2022) 71:148–55. doi: 10.1136/gutjnl-2020-322161
2. Angeli P, Bernardi M, Villanueva C, Francoz C, Mookerjee RP, Trebicka J, et al. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
3. Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian pacific association for the study of the liver (APASL): an update. Hepatol Int. (2019) 13:353–90. doi: 10.1007/s12072-019-09946-3
4. Nanchal R, Subramanian R, Karvellas CJ, Hollenberg SM, Peppard WJ, Singbartl K, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary and renal considerations: executive summary. Crit Care Med. (2020). doi: 10.1097/CCM.0000000000004193
5. Bajaj J, O'Leary J, Lai J, Wong F, Long M, Wong R, et al. Acute-on-chronic liver failure clinical guidelines. Am J Gastroenterol. (2022) 117:225–52. doi: 10.14309/ajg.0000000000001595
6. Khanam A, Kottilil S. Acute-on-chronic liver failure: pathophysiological mechanisms and management. Front Med. (2021) 8:1–18. doi: 10.3389/fmed.2021.752875
7. Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. (2010) 2:208–20. doi: 10.4254/wjh.v2.i6.208
8. Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J Gastroenterol. (2010) 16:6046–57. doi: 10.3748/wjg.v16.i48.6046
9. Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology. (1990) 98:1024–8. doi: 10.1016/0016-5085(90)90029-Z
10. Gülberg V, Haag K, Rössle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatology. (2002) 35:630–4. doi: 10.1053/jhep.2002.31722
11. Iwao T, Toyonaga A, Shigemori H, Oho K, Sakai T, Tayama C, et al. Hepatic artery hemodynamic responsiveness to altered portal blood flow in normal and cirrhotic livers. Radiology. (1996) 200:793–8. doi: 10.1148/radiology.200.3.8756933
12. Afif AM, Chang JP-E, Wang YY, Lau SD, Deng F, Goh SY, et al. A sonographic doppler study of the hepatic vein, portal vein and hepatic artery in liver cirrhosis: correlation of hepatic hemodynamics with clinical Child Pugh score in Singapore. Ultrasound. (2017) 25:213–21. doi: 10.1177/1742271X17721265
13. Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. (2003) 52:134–9. doi: 10.1136/gut.52.1.134
14. Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. (2015) 62:232–42. doi: 10.1002/hep.27795
15. Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, Petta S, Thiele M. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. (2021) 75:659–89. doi: 10.1016/j.jhep.2021.05.025
16. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. (2014) 383:1749–61. doi: 10.1016/S0140-6736(14)60121-5
17. Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
18. World Medical Association. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
19. Sacerdoti D, Gaiani S, Buonamico P, Merkel C, Zoli M, Bolondi L, et al. Interobserver and interequipment variability of hepatic, splenic, and renal arterial Doppler resistance indices in normal subjects and patients with cirrhosis. J Hepatol. (1997) 27:986–92. doi: 10.1016/S0168-8278(97)80141-9
20. Sabbà C, Merkel C, Zoli M, Ferraioli G, Gaiani S, Sacerdoti D, et al. Interobserver and interequipment variability of echo-doppler examination of the portal vein: effect of a cooperative training program. Hepatology. (1995) 21:428–33. doi: 10.1016/0270-9139(95)90103-5
21. Sacerdoti D, Merkel C, Bolognesi M, Amodio P, Angeli P, Gatta A. Hepatic arterial resistance in cirrhosis with and without portal vein thrombosis: Relationships with portal hemodynamics. Gastroenterology. (1995) 108:1152–8. doi: 10.1016/0016-5085(95)90214-7
22. Kamath P. A model to predict survival in patients with end-stage liver disease. Hepatology. (2001) 33:464–70. doi: 10.1053/jhep.2001.22172
23. Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. (2007) 45:797–805. doi: 10.1002/hep.21563
24. Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. (2014) 61:1038–47. doi: 10.1016/j.jhep.2014.06.012
25. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. (2013) 144:1426–37. doi: 10.1053/j.gastro.2013.02.042
26. Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. (2018) 22:1–8. doi: 10.1186/s13054-018-2156-0
27. Baik SK. Haemodynamic evaluation by doppler ultrasonography in patients with portal hypertension: a review. Liver Int. (2010) 30:1403-13. doi: 10.1111/j.1478-3231.2010.02326.x
28. Lafortune M, Burns PN, Patriquin H, Dauzat M. Splanchnic vessels. Ultrasound Med Biol. (2000) 26:73–5. doi: 10.1016/S0301-5629(00)00170-8
29. Maruyama H, Yokosuka O. Ultrasonography for noninvasive assessment of portal hypertension. Gut Liver. (2017) 11:464–73. doi: 10.5009/gnl16078
30. Sudhamshu KC, Matsutani S, Maruyama H, Akiike T, Saisho H. Doppler study of hepatic vein in cirrhotic patients: correlation with liver dysfunction and hepatic hemodynamics. World J Gastroenterol. (2006) 12:5853–8. doi: 10.3748/wjg.v12.i36.5853
31. Park HS, Desser TS, Jeffrey RB, Kamaya A. Doppler ultrasound in liver cirrhosis: correlation of hepatic artery and portal vein measurements with model for end-stage liver disease score. J Ultrasound Med. (2017) 36:725–30. doi: 10.7863/ultra.16.03107
32. Shateri K, Mohammadi A, Moloudi F, Nosair E. Ghasemi-rad M. Correlation between sonographic portal vein diameter and flow velocity with the clinical scoring systems MELD and CTP in cirrhotic patients: is there a relationship? Gastroenterol Res. (2012) 5:112–9. doi: 10.4021/gr369w
33. Topal NB, Sarkut P, Dündar HZ, Ayyildiz T, Kiyici M, Kaya E, et al. The correlation between doppler US measurement of hepatic arterial flow and the MELD score in patients with chronic liver disease. Eur Rev Med Pharmacol. (2016) 20:291–6.
34. Salman A, Sholkamy A, Salman M, Omar M, Saadawy A, Abdulsamad A, et al. Study of early postoperative doppler changes post living donor liver transplantation and their impact on early mortality and small-for-size syndrome: a retrospective study. Int J Gen Med. (2021) 14:309–17. doi: 10.2147/IJGM.S280456
35. Glišić TM, Perišić MD, Dimitrijevic S, Jurišić V. Doppler assessment of splanchnic arterial flow in patients with liver cirrhosis: correlation with ammonia plasma levels and MELD score. J Clin Ultrasound. (2014) 42:264–9. doi: 10.1002/jcu.22135
36. Abdel-Razik A, Mousa N, Elhelaly R, Tawfik A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the model for end-stage liver disease scoring system. Eur J Gastroenterol Hepatol. (2015) 27:585–92. doi: 10.1097/MEG.0000000000000325
37. Colli A, Cocciolo M, Mumoli N, Cattalini N, Fraquelli M, Conte D. Hepatic artery resistance in alcoholic liver disease. Hepatology. (1998) 28:1182–6. doi: 10.1002/hep.510280503
38. Stulic M, Culafic D, Obrenovic R, Jankovic G, Alempijevic T, Lalosevic MS, et al. The clinical importance of cystatin C and hepatic artery resistive index in liver cirrhosis. Medicina. (2018) 54:37. doi: 10.3390/medicina54030037
39. Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, et al. Survival in infection-related acute-on-chronic liver failure is defined by extra-hepatic organ failures. Hepatology. (2014) 60:250–6. doi: 10.1002/hep.27077
40. Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, et al. Acute-on chronic liver failure. J Hepatology. (2012) 57:1336–48. doi: 10.1016/j.jhep.2012.06.026
41. Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Prim. (2016) 2:16041. doi: 10.1038/nrdp.2016.41
42. Kumar R, Mehta G, Jalan R. Acute-on-chronic liver failure. Clin Med. (2020) 20:501–4. doi: 10.7861/clinmed.2020-0631
43. Engelmann C, Tacke F. Decompensated liver cirrhosis and acute-on-chronic liver failure. Der Gastroenterologe. (2020) 15:22–33. doi: 10.1007/s11377-019-00407-9
44. Picon RV, Bertol FS, Tovo CV, de Mattos ÂZ. Chronic liver failure-consortium acute-on-chronic liver failure and acute decompensation scores predict mortality in Brazilian cirrhotic patients. World J Gastroenterol. (2017) 23:5237–45. doi: 10.3748/wjg.v23.i28.5237
45. Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver International. (2015) 35:724–34. doi: 10.1111/liv.12559
46. Solís-Muñoz P, Willars C, Wendon J, Auzinger G, Heneghan M. De la Flor-Robledo M, et al. Interlobar artery resistive index predicts acute-on-chronic liver failure syndrome in cirrhotic patients with acute decompensation. Ultraschall Med. (2018) 39:39–47. doi: 10.1055/s-0042-120258
47. Kuroda H, Abe T, Fujiwara Y, Nagasawa T, Suzuki Y, Kakisaka K, et al. Contrast-enhanced ultrasonography–based hepatic perfusion for early prediction of prognosis in acute liver failure. Hepatology. (2021) 73:2455–67. doi: 10.1002/hep.31615
48. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
49. Maiwall R, Kumar A, Pasupuleti SSR, Hidam AK, Tevethia H, Kumar G, et al. A randomized-controlled trial comparing 20% albumin to plasmalyte in patients with cirrhosis and sepsis-induced hypotension [ALPS trial]. J Hepatol. (2022) 77:670–82. doi: 10.1016/j.jhep.2022.03.043
50. Saner FH, Pavlaković G, Gu Y, Fruhauf NR, Paul A, Radtke A, et al. Does PEEP impair the hepatic outflow in patients following liver transplantation? Intensive Care Med. (2006) 32:1584–90. doi: 10.1007/s00134-006-0357-5
51. Richardson PD, Withrington PG. Liver blood flow. II Effects of drugs and hormones on liver blood flow. Gastroenterology. (1981) 81:356–75. doi: 10.1016/S0016-5085(81)80071-6
52. Kawasaki T, Moriyasu F, Kimura T, Someda H, Hamato N, Okuma M. Effects of dobutamine on hepatosplanchnic hemodynamics in patients with chronic liver disease. Scand J Gastroenterol. (1994) 29:1044–54. doi: 10.3109/00365529409094884
53. Kalambokis G, Economou M, Paraskevi K, Konstantinos P, Pappas C, Katsaraki A, et al. Effects of somatostatin, terlipressin and somatostatin plus terlipressin on portal and systemic hemodynamics and renal sodium excretion in patients with cirrhosis. J Gastroenterol Hepatol. (2005) 20:1075–81. doi: 10.1111/j.1440-1746.2005.03902.x
Keywords: liver disease, liver cirrhosis, acute-on-chronic liver failure, liver perfusion, doppler ultrasound, critical care medicine
Citation: Vogg J, Maier-Stocker C, Munker S, Mehrl A, Schlosser S, Tews HC, Gülow K, Müller M and Schmid S (2022) Hepatic perfusion as a new predictor of prognosis and mortality in critical care patients with acute-on-chronic liver failure. Front. Med. 9:1008450. doi: 10.3389/fmed.2022.1008450
Received: 31 July 2022; Accepted: 20 September 2022;
Published: 10 October 2022.
Edited by:
Dominik Bettinger, University of Freiburg Medical Center, GermanyReviewed by:
Yasuyuki Tamai, Mie University Hospital, JapanCopyright © 2022 Vogg, Maier-Stocker, Munker, Mehrl, Schlosser, Tews, Gülow, Müller and Schmid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan Schmid, c3RlcGhhbi5zY2htaWRAdWtyLmRl
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.