- 1Clinical Sciences Department, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates
- 2Department of Pathology, H.H. Sheikh Khalifa Specialty Hospital, Ras Al Khaimah, United Arab Emirates
A tumor is a result of stepwise accumulation of genetic and epigenetic alterations. This notion has deepened the understanding of cancer biology and has introduced the era of targeted therapies. On the other hand, there have been a series of attempts of using the immune system to treat tumors, dating back to ancient history, to sporadic reports of inflamed tumors undergoing spontaneous regression. This was succeeded by modern immunotherapies and immune checkpoint inhibitors. The recent breakthrough has broadened the sight to other players within tumor tissue. Tumor microenvironment is a niche or a system orchestrating reciprocal and dynamic interaction of various types of cells including tumor cells and non-cellular components. The output of this complex communication dictates the functions of the constituent elements present within it. More complicated factors are biochemical and biophysical settings unique to TME. This mini review provides a brief guide on a range of factors to consider in the TME research.
Introduction
The earliest form of cancer immunotherapy using infection started around 1550 BCE (1). In the modern era, an incidental observation of tumor regression after surgical wound infection was advanced into a more controlled approach using bacterial vaccines to treat sarcoma (2). This journey was then succeeded by application of Bacillus Calmette-Guerin (BCG), various types of oncolytic viruses and Immune Checkpoint Inhibitors (ICIs) (3). Substantial efficacy and superior safety profiles with tumor-agnostic features have immediately positioned ICIs in the main treatment arm in most advanced cancers. This has turned the focus from genetic and epigenetic alterations of tumor cells to immune cells. However, ICIs are no exception in primary or secondary resistance of drugs. This has led the investigators to place a heavier emphasis on other players and the surroundings of tumor cells. Long before the era of ICIs, histologic description of tumor tissues had already provided some insights in tumor surroundings. For instance, melanomas are characterized by fibrosis, melanophages (a type of macrophage), new blood vessels and infiltration of lymphocytes in and around the nests of dying tumor cells (4). Exuberant lymphoid reaction was the hallmark of colorectal cancer (CRC) with high microsatellite instability (MSI-high) (5). The study of CRC with MSI-high, either in Lynch syndrome or sporadic cases has indicated the hypermutator phenotype and MSI is still the most relevant predictive biomarker of ICIs currently (6). It is quite logical to speculate that the tumor mutational burden (TMB) follows MSI. However, the TMB is not a one-marker-fit-for-all (7). An example that displays this fact to the furthest extent was from an animal study where fibroblasts having inactivated TGF-β type II receptor induced precancerous lesions and carcinomas from an otherwise normal epithelium (8). With all these factors to consider, the center of attention always has been revolving around tumor cells. Environment is defined as the circumstances, objects, or conditions by which one is surrounded (9). The circumstances surrounding tumor cells theoretically ranges from ions, humoral factors and matrikines to various types of cells and tissues and even to host itself. Like the stem cell niche, tumor cells reside in their own niche or TME, and also have a reciprocal non-static spatiotemporal coordination with each other to regulate functions and differentiation of tumor cells and non-tumor cells, under the influence of specific physicochemical conditions (10–16). The current mini-review aims to cover as many attributes in this complex system, ranging from ions to cell and extracellular matrix (ECM), to physico-chemical properties of TME in an attempt to assist future studies.
Definition of tumor microenvironment
The National Cancer Institute defines the TME as “The normal cells, molecules, and blood vessels that surround and feed a tumor cell. A tumor can change its microenvironment, and the microenvironment can affect how a tumor grows and spreads.” (17). This definition may appear simple at first, but encompasses the idea of reciprocal interaction and regulation of a tumor cell behavior. The most common ones are based on a structural view (18). Regularly emphasized is the dynamic nature of the cell population, such as the resident players and non-resident cellular components (19, 20). However, these definitions do not specifically identify other elements, such as tumor interstitial fluid, and physicochemical properties. To better depict a dynamic symbiotic system, “Seed and Soil,” an analogy of the stem cell niche, was introduced (14). “The TME comprises of a diverse cellular and acellular milieu, in which cancer stem cells (CSCs) develop and thrive, and various stromal and immune cells are recruited to form and maintain this self-sustained environment” (21). In that regard, the definition of “seed and soil” is comprehensive enough to cover all components in TME.
Cellular component
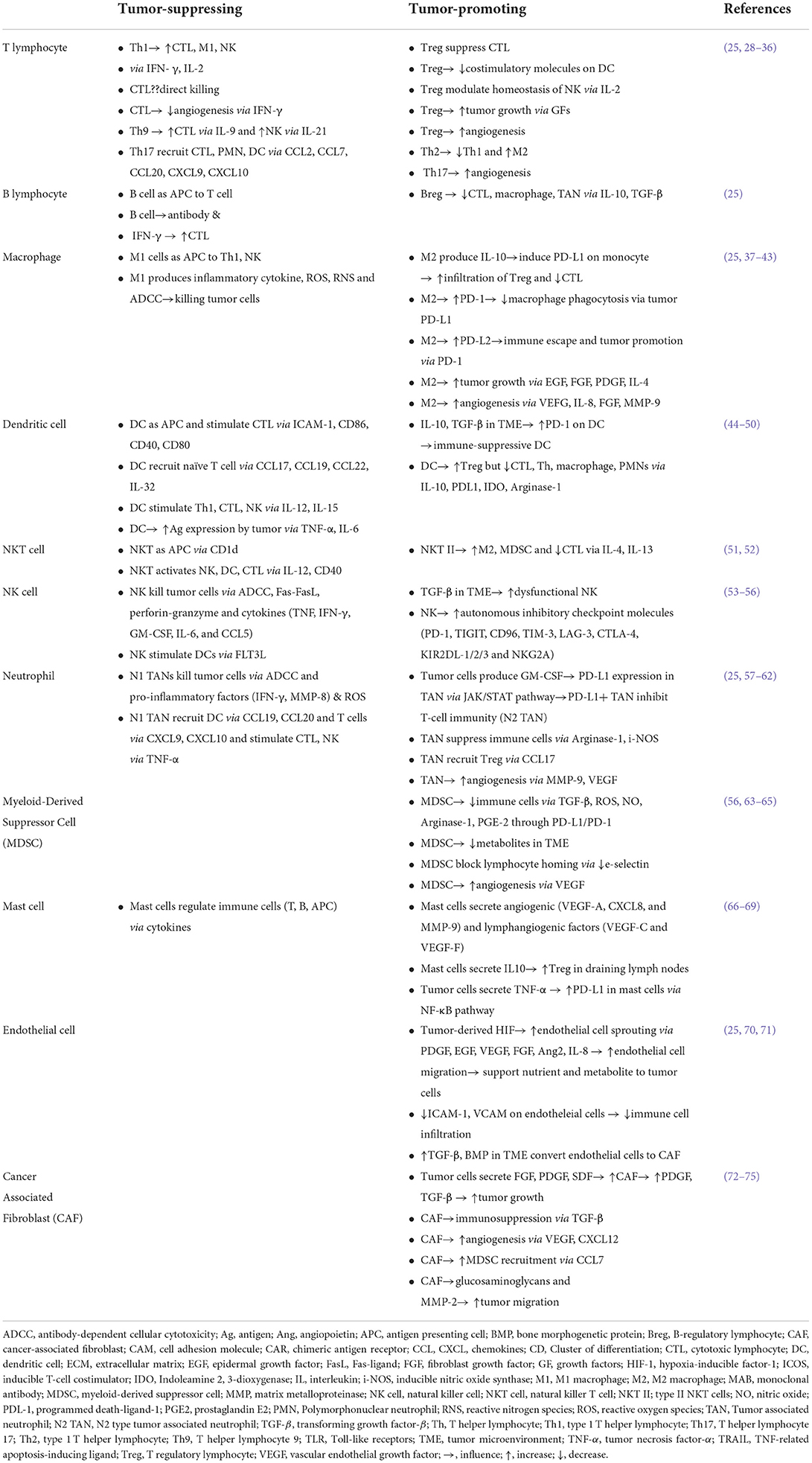
Histologic observation of tumors shows cancer cells intricately mixed with various inflammatory cells, fibroblasts, fibrotic stroma and blood vessels. One of the most studied examples is colorectal cancer (CRC) with high microsatellite instability (MSI). The tumor cells exhibit morphologic alterations such as mucinous change, signet ring cell feature and medullary histology (22). The presence of other cellular players is observed such as high number of tumor infiltrating lymphocytes (TILs) and peritumoral lymphoid follicles reminiscent of the inflammatory pattern of Crohn's disease (5). There are many cases providing morphologic evidence of multiple players in tumor tissues (6). On the other hand, data-driven approach was able to characterize complex alterations from genes to transcription, and has brought in molecular classifications agnostic about morphology (23). However, immune cells are still the major focus in the era of ICIs, and the classification systems based on proportion of these cells have been proposed (24–26). Two tier system such as a hot tumor vs. a cold tumor is widely accepted one. A three tier system, such as immune infiltrated/inflamed, immune excluded, and immune silent/desert is also a commonly used method of classification (25).
Back to the role of each population in TME, cells are generally classified as tumor-promoting vs. tumor-suppressing (27) (Table 1). In this scheme, players are not simply dysfunctional in TME, but also actively suppress other immune cells and promote tumor cells, ranging from growth, invasion, metastasis to immune evasion (27). Members found to promote tumors are regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), M2 tumor-associated macrophages (TAMs), resident or derived from bone marrow/spleen, N2 tumor-associated neutrophils (TANs), cancer-associated fibroblasts (CAFs), tolerogenic dendritic cells (DCs) and more details are summarized in Table 1 (76–78). Once cells migrate into the TME, they are polarized or differentiated under the local condition, and in return, these cells accelerate the immune-suppressive and tumor-promoting environment (37). Hence, the state is not static but can be dynamic depending on the context or milieu of cytokines and signaling molecules. For example, M1 macrophage can turn into the M2 type and vice versa, while an intermediate form between M1 and M2 has been discovered (37). Proportion-wise, cancer-associated fibroblasts (CAFs) are the most abundant component in the tumor tissue (13). CAFs have a critical position in all steps, from tumor initiation to metastasis, and even being related to therapeutic resistance (8, 79). CAFs are derived from resident fibroblasts and other cells such as smooth muscle cells, vascular pericytes and bone marrow-derived mesenchymal cells, adipocytes and this process is caused by various factors [stromal cell-derived factor 1 (SDF1), platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), fibroblast growth factor 2 (FGF2)] produced by tumor cells and immune cells (18, 80–83). CAFs then reciprocally promote tumor progression by production of growth factors (PDGF, TGF-β, epidermal growth factor (EGF), bone morphogenetic proteins (BMP) and C-X-C motif chemokine 12 (CXCL12), CXCL13) and these cells also stimulate angiogenesis by secreting vascular endothelial growth factor (VEGF), CXCL12 and FGF2 (72–75). Recently, focus was turned to rare cell populations in TME such as mast cells, basophils, eosinophils (84–86). The next-generation pathology, together with the single-cell analysis and systems pathology, will provide new insightful hints for developing effective therapeutic protocols targeting the TME (87, 88).
Extracellular matrix
Tumor stroma shows fibrosis or even desmoplasia in certain types of tumors, such as biliary cancer and gray-colored myxoid change, likely due to the ECM alteration (89, 90). ECM undergoes a remodeling process in physiologic and pathologic conditions, and it is an intricate phenomenon involving more than 700 proteins (91, 92). The characteristics of the remodeled ECM eventually affect the fate of cells (91, 92). The major alterations of tumor ECM are degradation, stiffening and physical remodeling (18, 93). In TME, acidic condition, excessive amount of proteases [i.e., matrix metalloproteases (MMPs), disintegrin and metalloproteinases (ADAMs), disintegrin and metalloproteinases with thrombospondin motifs (ADAMTS)] and production of reactive oxygen species (ROS) from tumor cells, CAF, TAN and TAM cause degradation of ECM (18). During this process, Extracellular Matrix-Derived Fragments are produced. These undertake active biological functions as matrikines leading to various effects such as acceleration of matrix production, promoting or suppressing tumor progression and angiogenesis (93, 94). Neoplastic tumors are stiffer than adjacent normal tissues and this is due to an excessive laydown of ECM and altered post-translational modification (PTM) (18). At first, CAFs secrete ECM in excess, including collagens, glycoproteins, proteoglycans, and polysaccharides (18). Then, the hypoxic condition enhances the cross-linking via production of lysyl oxidase (LOX) and transglutaminase from CAGs (95, 96). These modified rigid collagen fibrils are known to facilitate tumor cell migration and progression (97–100). In addition to the structural changes, PTM of ECM directly controls the tumor cell behavior by modulating the function of various growth factors embedded in the matrix (46, 101–103). For example, heparan sulfate proteoglycans (HSPGs) have different binding and releasing capacity of growth factors, depending on the sulphation pattern. This pattern is modified by the enzyme called endosulphatase (Sulf). In tumor tissue, the isotypes of Sulf are differentially expressed that the sulphation pattern made by Sulf1 inhibits the signaling pathways promoting tumors, while contrastingly, the other formed by Sulf2 enhances them (101, 102). Altered glycosylation patterns are reported in tumor tissues, and are currently under research (22, 104, 105). Lastly, mechanical force causes physical remodeling of the ECM, and makes fibers aligned to make routes for tumor cell migration (93). In TME, the ECM is continuously remodeled in terms of the amount, structure and chemical properties and this process shapes the interplay of the components modulating the fate of tumor cells in their progression (93). High-throughput proteomics approach is expected to acquire more insight from this process (91, 106).
Biochemical component
One of the approaches to understand the biochemical property of TME is to look into the fluid of tumor or tumor interstitial fluid (TIF) (107, 108). TIF is characterized by high PCO2, low PO2 and low pH, and these parameters are linked with each other (11, 12). Hypoxia in tumor tissues is the major contributor to acidic environment. Rapid proliferation of tumor cells and insufficient oxygen supply cause hypoxia. This condition reprograms tumor cells favoring aerobic glycolysis with production of lactate (109). Major regulators in this process are hypoxia-inducible factor (HIF)-1α, c-Myc, and p53 (110–114). Hypoxia induces inhibition of prolyl-hydroxylases and this stabilizes the HIFs. HIF-1α switches metabolisms in tumor by upregulating the transcription of enzymes of glycolysis, such as hexokinase 1/2 (HK I/II) and pyruvate kinase isoenzyme M2 (PKM2), glucose transporters (Glut) such as Glut-1 and 3, alongside other genes inhibiting oxidative phosphorylation (115–118). As the dimer form of PKM2 prevails in the tumor, glucose metabolism is shifted to lactate production (118, 119). Abnormal vessels are unable to clear hydrogen ions effectively and hydration of CO2 by carbonic anhydrase IX in hypoxic areas further increase acidity (120). This altered biochemical environment reconditions the cells under its influence forming a selective pressure which favors cancer cells over normal cells (120–128). This situation promotes tumorigenesis, tumor progression and immune evasion and is related with a poor clinical prognosis and resistance to therapy. Recently reported findings suggest that the lactic acid not only intensifies acidity but also directly impacts cellular signaling pathways preferentially polarizing TAM to M2 type (129).
What about the ions in TME? Previous studies have shown that the concentration of ions in TIF is similar to that in plasma (130). Recently, this notion has been revisited. More sophisticated analysis revealed that the potassium concentration is higher in TIF, while other ions such as sodium, chloride and magnesium remain within normal range (131). Higher potassium level was found to suppress activation and effector function of T cells (131). A starvation response is induced by local hyperkalemia, and this in turn reduces nutrient uptake, resulting in the imbalance of Acetyl Co-A (AcCoA) level in subcellular compartments (132). In this setting, mitochondrial AcCoA is relatively higher than nucleocytosolic AcCoA, and this disproportionate state causes reduction of histone acetylation promoting stemness of T cells, eventually impeding the activation of effector genes (132).
ROS are known as the byproduct of hypoxic environment produced by tumor cells in TME, and the up-to-date interpretation is that ROS are not only radicals having damaging effect, but also have diverse biologic effects such as stabilization of HIFs to promote angiogenesis, activation of cell proliferation, as well as survival pathways, metabolic reprogramming, differentiation of CAFs and deregulation of immune cells (133). Reactive Nitrogen Species (RNS) are also rich in TME, due to an increase in arginine metabolism within tumor cells and tumor-infiltrating myeloid cells (134). RNS causes nitration of chemokine (C-C motif) ligand 2 (CCL2), and this modification suppresses infiltration and effector function of lymphocytes (134, 135).
Altered metabolic condition is a common survival strategy by tumor cells (136–139). Clinically, cachexia represents increased catabolic status to feed cancer cells (140, 141). Abnormally increased anabolism is also seen in cancer patients. Non-Islet Cell Tumor Hypoglycemia (NICTH) is a paraneoplastic syndrome where non-endocrine tumors cause hypoglycemia, while promoting anabolism of tumor cells by aberrantly producing insulin-like growth factor II (IGF-II), insulin receptor antibodies and various cytokines (tumor necrosis factor-α, interleukin-1 and−6) (142–145). Metabolic condition comes into play at microscopic level as well. As immune cells enter into tumor tissue, those cells face hypoglycemia and a scant amount of essential amino acids including glutamine and lipids. This condition hinders all steps of immune cell functions such as infiltration, proliferation and effector because these tasks have great demand for energy, nutrition and metabolic reprogramming (136–139). This competitive condition places the immune system in an anergy and exhaustion state (146, 147).
Extracellular vesicles (EVs) are rich in TIF (148). EVs such as exosomes, microvesicles, and apoptotic bodies carry active signaling and regulatory molecules like mRMA, miRNA, signaling proteins, microRNAs (miRNAs), long non-coding RNAs (LncRNAs), and circular RNAs (circRNAs) (149–151). All types of cells including cancer stem cells are known to secrete them (152, 153). Isolated EVs enriched in TME have the capability of promoting angiogenesis, modulating immune cells, enhancing tumor migration and epithelial-mesenchymal transition (EMT), metastasis and increasing drug resistance (148, 154, 155). However, EVs in TIF are not always tumor-promoting. Some EVs were found to exhibit anti-tumor effects (156, 157). This concept can be applied to patient treatment via an EV engineering. EVs derived from proven fighters such as active TILs and chimeric antigen receptor (CAR)-T cells may potentially recondition dysfunctional or anergic immune cells in tumor tissue (158–162). There are other humoral factors not mentioned here. Proteomic approach is expected to find unique signatures of TIF and further develop our understanding of the complex nature of TME.
Biophysical component
Highly cellular tumors like lymphoma, seminoma, and Ewing sarcoma frequently present characteristic bulging cut surfaces. These features are related to an increased pressure inside tumor tissue (163). High tissue pressure is due to an increase in the proliferation and migration of tumor cells, alteration of ECM and increased interstitial fluid pressure (IFP) (163). The increased IFP is caused by the abnormal vessels having higher permeability, lack of pericytes, vascular compression by tumor growth and abundant ECM (164–167). IFP is elevated by 10–40 mmHg in tumor tissues (168, 169). Increased IFP generates an outward tissue flow and cell velocity flow, which hinders an inward penetration of cells, antibodies and drugs (164, 165, 170, 171). Interestingly, high pressure itself has been shown to enhance tumor proliferation and is often related to a poor clinical outcome (172–174). Vascular endothelial growth factor inhibitors, pegylated human recombinant hyaluronidase-α, collagenase and angiotensin inhibitors are suggested for potential drugs which can reduce IFP and promote the delivery of various molecules into tumor tissues (165). Migration and homing of immune cells is an entrenched process involving various chemokines, gradients and APC interaction (175–179). However, movement of immune cells under high IFP and altered ECM are not well studied, requiring further research.
Conclusion
The main stream in cancer research has been about decoding genetic and epigenetic alterations in tumor cells. This scheme has been powerful to understand the nature of cancer diseases, and has led to the discovery of means to restore it. Meanwhile, a distinct course of ideas appeared long ago from the ancient time to the modern concept of immunotherapies and ICIs. This different perspective has widened sight to other attributes within tumor tissue. TME is a system consisting of a reciprocal communication network among components under unique physicochemical conditions. This process influences all components and the output influences TME in an iterative way. Various attempts such as data-driven approaches will rapidly improve understanding of surroundings of tumor cells and lead to several discoveries of predictive biomarkers and an eventual control of resistance. Another aspect not discussed in this mini review is about the host factors such as host genetic makeup. Certain single nucleotide polymorphisms (SNPs) in genes of the immune system were found to affect cancer susceptibility of an individual and these may also influence response to ICIs (180–182). There are case reports on renal cell carcinomas undergoing regression after transfusion of plasma from another patient of the same family (183, 184). This may indicate the presence of an inherited resistance to cancer. Even though these are still speculative and can be explained by other mechanisms, this macro-environment also needs to be considered in the dimension of future studies.
Author contributions
BK drafted the initial version of the manuscript. IT reviewed it and added comments. Both authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Seohyun Kim, a medical student at Universita Cattolica del Sacro Cuore, for English editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dawson WR, Ebbell B. The papyrus ebers; the greatest egyptian medical document. J Egypt Archaeol. (1938) 24:250–51. doi: 10.1177/030751333802400149
2. Coley WB. Treatment of inoperable malignant tumors with toxins of erysipelas and the bacillus prodigiosus. Trans Am Surg Assn. (1894) 12:183–212.
3. Dobosz P, Dzieciatkowski T. The intriguing history of cancer immunotherapy. Front Immunol. (2019) 10:2965. doi: 10.3389/fimmu.2019.02965
4. Kang S, Barnhill RL, Mihm MC Jr, Sober AJ. Histologic regression in malignant melanoma: an interobserver concordance study. J Cutan Pathol. (1993) 20:126–29. doi: 10.1111/j.1600-0560.1993.tb00228.x
5. Alexander J, Watanabe T, Wu T, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. (2001) 158:527–35. doi: 10.1016/S0002-9440(10)63994-6
6. Chmielik E. Pathology and tumor microenvironment: past, present, and future. Pathobiol. (2020) 87:55–7. doi: 10.1159/000507222
7. McGrail DJ, Pilie PG, Rashid NU, Chang JT, Moulder SL, Lin SY. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. (2021) 32:661–72. doi: 10.1016/j.annonc.2021.02.006
8. Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. (2004) 303:848–51. doi: 10.1126/science.1090922
9. Merriam-Webster.com Dictionary. Environment. (2022). Available online at: https://www.merriam-webster.com/dictionary/environment (accessed June 5, 2022).
10. Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol. (2005) 167:475–88. doi: 10.1016/S0002-9440(10)62991-4
11. Baronzio G, Parmar G, Baronzio M, Kiselevsky M. Tumor interstitial fluid: proteomic determination as a possible source of biomarkers. Cancer Genomics Proteomics. (2014) 11:225–37.
12. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. (2012) 92:1005–60. doi: 10.1152/physrev.00037.2011
13. Han C, Liu T, Yin R. Biomarkers for cancer-associated fibroblasts. Biomark Res. (2020) 8:64. doi: 10.1186/s40364-020-00245-w
14. Paget S. The distribution of secondary growths in cancer of the breast. Lancet. (1889) 133:571–3. doi: 10.1016/S0140-6736(00)49915-0
15. Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. (2016) 1370:82–96. doi: 10.1111/nyas.13016
16. Jhala D. A review on extracellular matrix mimicking strategies for an artificial stem cell niche. Polymer Reviews. (2015) 55:561–95. doi: 10.1080/15583724.2015.1040552
17. National cancer Institute. NCI Dictionaries. Tumor Microenvironment. Available online at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/tumor-microenvironment (accessed June 22, 2022).
18. Brassart-Pasco S, Brezillon1 S, Brassart B, Ramont L, Oudart J, Monboisse JC. Tumor microenvironment: extracellular matrix alterations influence tumor progression. Front Oncol. (2020) 10:397. doi: 10.3389/fonc.2020.00397
19. Schiavoni G, Gabriele L, Mattei F. The tumor microenvironment: a pitch for multiple players. Front Oncol. (2013) 3:90. doi: 10.3389/fonc.2013.00090
20. Dvorak HF, Weaver VM, Tlsty TD, Bergers G. Tumor microenvironment and progression. J Surg Oncol. (2011) 103:468–74. doi: 10.1002/jso.21709
21. Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol. (2021) 12:656364. doi: 10.3389/fimmu.2021.656364
22. Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. (2012) 107:1315–30. doi: 10.1038/ajg.2012.161
23. Zhao L, Lee CHF, Ng MK, Yan H, Bijlsma MF. Molecular subtyping of cancer: current status and moving toward clinical applications. Brief Bioinform. (2019) 20:572–84. doi: 10.1093/bib/bby026
24. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. (2017) 541:321–30. doi: 10.1038/nature21349
25. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–5. doi: 10.1016/j.cub.2020.06.081
26. Ren X, Guo S, Guan X, Kang Y, Liu J, Yang X. Immunological classification of tumor types and advances in precision combination immunotherapy. Front Immunol. (2022) 13:790113. doi: 10.3389/fimmu.2022.790113
27. Farc D, Cristea V. An overview of the tumor microenvironment, from cells to complex networks. Exp Ther Med. (2021) 21:96. doi: 10.3892/etm.2020.9528
28. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. (2004) 10:942–9. doi: 10.1038/nm1093
29. Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, Mempel TR. Dynamic treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. (2014) 124:2425–40. doi: 10.1172/JCI66375
30. Ziai J, Gilbert HN, Foreman O, Eastham-Anderson J, Chu F, Huseni M, et al. CD8+ T cell infiltration in breast and colon cancer: a histologic and statistical analysis. PLoS ONE. (2018) 13:e0190158. doi: 10.1371/journal.pone.0190158
31. Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol. (2018) 9:14. doi: 10.3389/fimmu.2018.00014
32. Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, et al. Distinct role of antigen-specific t helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. (1999) 190:617–27. doi: 10.1084/jem.190.5.617
33. Lorvik KB, Hammarstrom C, Fauskanger M, Haabeth OA, Zangani M, Haraldsen G, et al. Adoptive transfer of tumor-specific Th2 cells eradicates tumors by triggering an in situ inflammatory immune response. Cancer Res. (2016) 76:6864–76. doi: 10.1158/0008-5472.CAN-16-1219
34. Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, Mele V, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. (2017) 66:692–704. doi: 10.1136/gutjnl-2015-310016
35. Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. (2009) 30:92–107. doi: 10.1016/j.immuni.2008.11.005
36. Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. (2009) 114:1141–9. doi: 10.1182/blood-2009-03-208249
37. Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. (2014) 40:274–88. doi: 10.1016/j.immuni.2014.01.006
38. Wynn T, Chawla A, Pollard J. Macrophage biology in development, homeostasis and disease. Nature. (2013) 496:445–55. doi: 10.1038/nature12034
39. Romano E, Kusio-Kobialka M, Foukas P, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. (2015) 112:6140–5. doi: 10.1073/pnas.1417320112
40. Gordon S, Maute R, Dulken B, Hutter G, George B, McCracken M, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. (2017) 545:495–9. doi: 10.1038/nature22396
41. Wen Z, Liu H, Gao R, Zhou M, Ma J, Zhang Y, et al. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. J Immunother Cancer. (2018) 6:151. doi: 10.1186/s40425-018-0452-5
42. Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. (2010) 116:3311–20. doi: 10.1182/blood-2010-02-271981
43. Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. (2008) 113:1387–95. doi: 10.1002/cncr.23712
44. Sathe A, Grimes S, Lau B, Chen J, Suarez C, Huang R, et al. Single-cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin Cancer Res. (2020) 26:2640–53. doi: 10.1158/1078-0432.CCR-19-3231
45. Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. (2018) 24:1178–91. doi: 10.1038/s41591-018-0085-8
46. Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, et al. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J Biol Chem. (2003) 278:23107–17. doi: 10.1074/jbc.M302203200
47. Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. (1998) 393:480–3. doi: 10.1038/31002
48. Gardner A, de Mingo Pulido A, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. (2020) 11:924. doi: 10.3389/fimmu.2020.00924
49. Eisenring M, vom Berg J, Kristiansen G, Saller E, Becker B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. (2010) 11:1030–8. doi: 10.1038/ni.1947
50. Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. (2013) 1:145. doi: 10.1158/2326-6066.CIR-13-0102
51. Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. (2016) 139:869–81. doi: 10.1002/ijc.30134
52. Dhodapkar MV, Kumar V. Type II NKT cells and their emerging role in health and disease. J Immunol. (2017) 198:1015–21. doi: 10.4049/jimmunol.1601399
53. Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. (2006) 6:940–52. doi: 10.1038/nri1983
54. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. (2016) 17:1025–36. doi: 10.1038/ni.3518
55. Habif G, Crinier A, Andre P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. (2019) 16:415–22. doi: 10.1038/s41423-019-0224-2
56. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. (2018) 19:108–19. doi: 10.1038/s41590-017-0022-x
57. Mihaila AC, Ciortan L, Macarie RD, Vadana M, Cecoltan S, Preda MB, et al. Transcriptional profiling and functional analysis of N1/N2 neutrophils reveal an immunomodulatory effect of S100A9-blockade on the pro-inflammatory N1 subpopulation. Front Immunol. (2021) 12:708770. doi: 10.3389/fimmu.2021.708770
58. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
59. Kargl J, Zhu X, Zhang H, Yang G, Friesen T, Shipley M, et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight. (2019) 4:e130850. doi: 10.1172/jci.insight.130850
60. Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. (2017) 66:1900–11. doi: 10.1136/gutjnl-2016-313075
61. Konerding MA, Malkusch W, Klapthor B, van Ackern C, Fait E, Hill SA, et al. Evidence for characteristic vascular patterns in solid tumours: quantitative studies using corrosion casts. Br J Cancer. (1990) 80:724–32. doi: 10.1038/sj.bjc.6690416
62. Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. (2006) 103:12493–98. doi: 10.1073/pnas.0601807103
63. Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. (2019) 120:16–25. doi: 10.1038/s41416-018-0333-1
64. Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene. (2014) 33:2956–67. doi: 10.1038/onc.2013.257
65. Lupu M, Caruntu A, Caruntu C, Papagheorghe LML, Ilie MA, Voiculescu V, et al. Neuroendocrine factors: the missing link in non-melanoma skin cancer (Review). Oncol Rep. (2017) 38:1327–40. doi: 10.3892/or.2017.5817
66. Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. (2013) 13:362–75. doi: 10.1038/nri3427
67. Sammarco G, Varricchi G, Ferraro V, Ammendola M, De Fazio M, Altomare D, et al. Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. Int J Mol Sci. (2019) 20:2106. doi: 10.3390/ijms20092106
68. Lv Y, Zhao Y, Wang X, Chen N, Mao F, Teng Y, et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway. J Immunother Cancer. (2019) 7:54. doi: 10.1186/s40425-019-0530-3
69. Gan PY, Summers SA, Ooi JD, O'Sullivan KM, Tan DS, Muljadi RC, et al. Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J Am Soc Nephrol. (2012) 23:1955–66. doi: 10.1681/ASN.2012060572
70. Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. (2016) 30:1002–19. doi: 10.1101/gad.279737.116
71. Michiels C. Endothelial cell functions. J Cell Physiol. (2003) 196:430–43. doi: 10.1002/jcp.10333
72. Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A. (2014) 111:14776–81. doi: 10.1073/pnas.1416498111
73. Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Nat Acad Sci U S A. (2002) 99:12877–82. doi: 10.1073/pnas.162488599
74. Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. (2005) 65:3772–80. doi: 10.1158/0008-5472.CAN-04-4510
75. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. (2005) 121:335–48. doi: 10.1016/j.cell.2005.02.034
76. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. (2010) 116:935–44. doi: 10.1182/blood-2009-07-234872
77. Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. (2013) 501:346–54. doi: 10.1038/nature12626
78. Li X, Yang Y, Huang Q, Deng Y, Guo F, Wang G, et al. Crosstalk between the tumor microenvironment and cancer cells: a promising predictive biomarker for immune checkpoint inhibitors. Front Cell Dev Biol. (2021) 9:738373. doi: 10.3389/fcell.2021.738373
79. Louault K, Li R, DeClerck YA. Cancer-associated fibroblasts: understanding their heterogeneity. Cancers. (2020) 12:3108. doi: 10.3390/cancers12113108
80. Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE. (2012) 7:e30563. doi: 10.1371/journal.pone.0030563
81. Martin M, Wei H, Lu T. Targeting microenvironment in cancer therapeutics. Oncotarget. (2016) 7:52575–83. doi: 10.18632/oncotarget.9824
82. Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. (2005) 7:513–20. doi: 10.1016/j.ccr.2005.05.024
83. Santi A, Kugeratski FG, Zanivan S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics. (2018) 18:e1700167. doi: 10.1002/pmic.201700167
84. Komi DEA, Redegeld FA. Role of mast cells in shaping the tumor microenvironment. Clin Rev Allergy Immunol. (2020) 58:313–25. doi: 10.1007/s12016-019-08753-w
85. Marone G, Gambardella AR, Mattei F, Mancini F, Schiavoni G, Varricchi G. Basophils in tumor microenvironment and surroundings. Adv Exp Med Biol. (2020) 1224:21–34. doi: 10.1007/978-3-030-35723-8_2
86. Mattei F, Andreone S, Marone G, Gambardella AR, Loffredo S, Varricchi G, et al. Eosinophils in the tumor microenvironment. Adv Exp Med Biol. (2020) 1273:1–28. doi: 10.1007/978-3-030-49270-0_1
87. Caie PD, Harrison DJ. Next-generation pathology. Methods Mol Biol. (2016) 1386:61–72. doi: 10.1007/978-1-4939-3283-2_4
88. Lee J, Hyeon DY, Hwang D. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med. (2020) 52:1428–42. doi: 10.1038/s12276-020-0420-2
89. Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. (1996) 76:69–125. doi: 10.1152/physrev.1996.76.1.69
90. Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatol. (2014) 59:2397–402. doi: 10.1002/hep.26762
91. Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. (2016) 49:10–24. doi: 10.1016/j.matbio.2015.06.003
92. Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. (2011) 4:165–78. doi: 10.1242/dmm.004077
93. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. (2020) 11:5120. doi: 10.1038/s41467-020-18794-x
94. Kai FB, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell. (2019) 49:332–46. doi: 10.1016/j.devcel.2019.03.026
95. Emon B, Bauer J, Jain Y, Jung B, Saif T. Biophysics of tumor microenvironment and cancer metastasis - a mini review. Comput Struct Biotechnol J. (2018) 16:279–87. doi: 10.1016/j.csbj.2018.07.003
96. Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. (2019) 36:171–98. doi: 10.1007/s10585-019-09966-1
97. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. (2012) 125:5591–6. doi: 10.1242/jcs.116392
98. Levental KR Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. (2009) 139:891–906. doi: 10.1016/j.cell.2009.10.027
99. Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. (1998) 186:262–8. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3
100. Zhou Z, Ji CD, Xiao HL, Zhao HB, Cui YH, Bian XW. Reorganized collagen in the tumor microenvironment of gastric cancer and its association with prognosis. J Cancer. (2017) 8:1466–76. doi: 10.7150/jca.18466
101. Lanzi C, Zaffaroni N, Cassinelli G. Targeting heparan sulfate proteoglycans and their modifying enzymes to enhance anticancer chemotherapy efficacy and overcome drug resistance. Curr Med Chem. (2017) 24:2860–86. doi: 10.2174/0929867324666170216114248
102. Rosen SD, Lemjabbar-Alaoui H. Sulf-2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. (2010) 14:935–49. doi: 10.1517/14728222.2010.504718
103. Nawroth R, van Zante A, Cervantes S, McManus M, Hebrok M, Rosen SD. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS ONE. (2007) 2:e392. doi: 10.1371/journal.pone.0000392
104. Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. (2014) 511:319–25. doi: 10.1038/nature13535
105. Shurer CR, Kuo JCH, Roberts LM, Gandhi JG, Colville MJ, Enoki TA, et al. Physical principles of membrane shape regulation by the glycocalyx. Cell. (2019) 177:1757–70. doi: 10.1016/j.cell.2019.04.017
106. Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. (2018) 174:1373–87. doi: 10.1016/j.cell.2018.08.039
107. Wagner M, Wiig H. Tumor interstitial fluid formation, characterization, and clinical implications. Front Oncol. (2015) 5:115. doi: 10.3389/fonc.2015.00115
108. Wiig H, Tenstad O, Iversen PO, Kalluri R, Bjerkvig R. Interstitial fluid: the overlooked component of the tumor microenvironment? Fibrogenesis Tissue Repair. (2010) 3:12. doi: 10.1186/1755-1536-3-12
109. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. (1927) 8:519–30. doi: 10.1085/jgp.8.6.519
110. Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. (2013) 155:397–409. doi: 10.1016/j.cell.2013.09.025
111. Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. (2007) 4:99–124. doi: 10.1007/2789_2008_091
112. Arora A, Singh S, Bhatt AN, Pandey S, Sandhir R, Dwarakanath BS. Interplay between metabolism and oncogenic process: role of microRNAs. Transl Oncogenomics. (2015) 7:11–27. doi: 10.4137/TOG.S29652
113. Bost F, Decoux-Poullot AG, Tanti JF, Clavel S. Energy disruptors: rising stars in anticancer therapy? Oncogenesis. (2016) 5:e188. doi: 10.1038/oncsis.2015.46
114. Slaninova V, Krafcikova M, Perez-Gomez R, Steffal P, Trantirek L, Bray SJ, et al. Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open Biol. (2016) 6:150155. doi: 10.1098/rsob.150155
115. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. (2006) 70:1469–80. doi: 10.1124/mol.106.027029
116. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. (2006) 3:187–97. doi: 10.1016/j.cmet.2006.01.012
117. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. (2006) 3:177–85. doi: 10.1016/j.cmet.2006.02.002
118. Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. (2008) 452:230–3. doi: 10.1038/nature06734
119. Zahra K, Dey T, Ashish, Mishra SP, Pandey U. Pyruvate Kinase M2 and Cancer: the Role of PKM2 in promoting tumorigenesis. Front Oncol. (2020) 10:159. doi: 10.3389/fonc.2020.00159
120. Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci. (2014) 369:20130099. doi: 10.1098/rstb.2013.0099
121. Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. (2018) 7:10. doi: 10.1038/s41389-017-0011-9
122. Corbet C, Feron O. Tumour acidosis: From the passenger to the driver's seat. Nat Rev Cancer. (2017) 17:577–93. doi: 10.1038/nrc.2017.77
123. Obata S, Goi T, Nakazawa T, Kimura Y, Katayama K, Yamaguchi A. Changes in CO2 concentration increase the invasive ability of colon cancer cells. Anticancer Res. (2013) 33:1881–5.
124. Nevler A, Brown SZ, Nauheim D, Portocarrero C, Rodeck U, Bassig J, et al. Effect of hypercapnia, an element of obstructive respiratory disorder, on pancreatic cancer chemoresistance and progression. J Am Coll Surg. (2020) 230:659–67. doi: 10.1016/j.jamcollsurg.2019.12.033
125. Kikuchi R, Tsuji T, Iwai Y, Nakamura Y, Aoshiba K. High CO2 tumor microenvironment confers chemoresistance in lung cancer cells. Eur Respir J. (2017) 50:OA4865. doi: 10.1183/1393003.congress-2017.OA4865
126. Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sanchez-Garcia FJ. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol. (2016) 7:52. doi: 10.3389/fimmu.2016.00052
127. Rofstad EK, Rasmussen H, Galappathi K, Mathiesen B, Nilsen K, Graff BA. Hypoxia promotes lymph node metastasis in human melanoma xenografts by up-regulating the urokinase-type plasminogen activator receptor. Cancer Res. (2002) 62:1847–53.
128. Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. (2013) 123:3664–71. doi: 10.1172/JCI67230
129. Zhou HC, Yan XY, Yu WW, Liang XQ, Du XY, Liu ZV, et al. Lactic acid in macrophage polarization: the significant role in inflammation and cancer. Review Int Rev Immunol. (2022) 41:4–18. doi: 10.1080/08830185.2021.1955876
130. Gullino PM, Clark SH, Grantham FH. The interstitial fluid of solid tumors. Cancer Res. (1964) 24:780–98.
131. Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. (2016) 537:539–43. doi: 10.1038/nature19364
132. Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. (2019) 363:eaau0135. doi: 10.1126/science.aau0135
133. Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers. (2019) 11:1191. doi: 10.3390/cancers11081191
134. De Sanctis F, Sandri S, Ferrarini G, Pagliarello I, Sartoris S, Ugel S, et al. The emerging immunological role of post-translational modifications by reactive nitrogen species in cancer microenvironment. Front Immunol. (2014) 5:69. doi: 10.3389/fimmu.2014.00069
135. Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. (2011) 208:1949–62. doi: 10.1084/jem.20101956
136. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. (2017) 7:68. doi: 10.3389/fonc.2017.00068
137. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. (2012) 12:253–68. doi: 10.1038/nri3175
138. Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. (2004) 64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465
139. Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. (2006) 176:6752–61. doi: 10.4049/jimmunol.176.11.6752
140. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. (2009) 89:381–410. doi: 10.1152/physrev.00016.2008
141. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. (2015) 7:17–29. doi: 10.4251/wjgo.v7.i4.17
142. de Groot JWB, Rikhof B, van Doorn J, Bilo HJ, Alleman MA, Honkoop AH, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat cancer. (2007) 14:979–93. doi: 10.1677/ERC-07-0161
143. Schovanek J, Cibickova L, Ctvrtlik F, Tudos Z, Karasek D, Iacobone M, et al. Hypoglycemia as a symptom of neoplastic disease, with a focus on insulin-like growth factors producing tumors. J Cancer. (2019) 10:6475–80. doi: 10.7150/jca.30472
144. Bergman D, Halje M, Nordin M, Engstrom W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontol. (2013) 59:240–9. doi: 10.1159/000343995
145. Cerrato F, Sparago A, Verde G, De Crescenzo A, Citro V, Cubellis MV, et al. Different mechanisms cause imprinting defects at the IGF2/H19 locus in Beckwith-Wiedemann syndrome and Wilms' tumour. Hum Mol Genet. (2008) 17:1427–35. doi: 10.1093/hmg/ddn031
146. Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. (2015) 162:1229–41. doi: 10.1016/j.cell.2015.08.016
147. Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. (2013) 25:214–21. doi: 10.1016/j.coi.2012.12.003
148. Andaloussi SE, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. (2013) 12:347–57. doi: 10.1038/nrd3978
149. Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. (2017) 114:E9066–75. doi: 10.1073/pnas.1704862114
150. Zhou Y, Xia L, Lin J, Wang H, Oyang L, Tan S, et al. Exosomes in Nasopharyngeal Carcinoma. J Cancer. (2018) 9:767–77. doi: 10.7150/jca.22505
151. Tao SC, Guo SC. Role of extracellular vesicles in tumour microenvironment. Cell Cell Commun Signal. (2020) 18:163. doi: 10.1186/s12964-020-00643-5
152. Brown TJ, James V. The role of extracellular vesicles in the development of a cancer stem cell microenvironment niche and potential therapeutic targets: a systematic review. Cancers. (2021) 13:2435. doi: 10.3390/cancers13102435
153. Zhang X, Liu D, Gao Y, Lin C, An Q, Feng Y, et al. The biology and function of extracellular vesicles in cancer development. Front Cell Dev Biol. (2021) 9:777441. doi: 10.3389/fcell.2021.777441
154. Hu C, Chen M, Jiang R, Guo Y, Wu M, Zhang X. Exosome-related tumor microenvironment. J Cancer. (2018) 9:3084–92. doi: 10.7150/jca.26422
155. Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. (2018) 6:18. doi: 10.3389/fcell.2018.00018
156. Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. (2012) 30:1985–98. doi: 10.1002/stem.1161
157. Reza AMT, Choi YJ, Yasuda H, Kim JH. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. (2016) 6:38498. doi: 10.1038/srep38498
158. Cavallari C, Camussi G, Brizzi MF. Extracellular vesicles in the tumour microenvironment: eclectic supervisors. Int J Mol Sci. (2020) 21:6768. doi: 10.3390/ijms21186768
159. Beuzelin D, Kaeffer B. Exosomes and miRNA-loaded biomimetic nanovehicles, a focus on their potentials preventing type-2 diabetes linked to metabolic syndrome. Front Immunol. (2018) 9:2711. doi: 10.3389/fimmu.2018.02711
160. Schwarzenbach H, Gahan PB. MicroRNA shuttle from cell-to-cell by exosomes and its impact in cancer. Noncoding RNA. (2019) 5:28. doi: 10.3390/ncrna5010028
161. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. (2008) 16:782–90. doi: 10.1038/mt.2008.1
162. Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. (2017) 7:2732–45. doi: 10.7150/thno.18752
163. Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors i role of intersititial pressure and convection. Microvascr Res. (1989) 37:77–104. doi: 10.1016/0026-2862(89)90074-5
164. Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. (2004) 64:3731–6. doi: 10.1158/0008-5472.CAN-04-0074
165. Kim HG, Yu AR, Lee JJ, Lee YJ, Lim SM, Kim JS. Measurement of tumor pressure and strategies of imaging tumor pressure for radioimmunotherapy. Nucl Med Mol Imaging. (2019) 53:235–41. doi: 10.1007/s13139-019-00598-7
166. Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. (2003) 163:1801–15. doi: 10.1016/S0002-9440(10)63540-7
167. Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. (2004) 427:695. doi: 10.1038/427695a
168. Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. (2007) 101:937–49. doi: 10.1002/jcb.21187
169. Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. (2004) 4:806–13. doi: 10.1038/nrc1456
170. Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. (1987) 47:3039–51.
171. Wu M, Frieboes HB, McDougall SR, Chaplain MAJ, Cristini V, Lowengruba J. The effect of interstitial pressure on tumor growth: coupling with the blood and lymphatic vascular systems. J Theor Biol. (2013) 320:131–51. doi: 10.1016/j.jtbi.2012.11.031
172. Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. (2006) 8:89–95. doi: 10.1593/neo.05469
173. Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. (1992) 52:6371–4.
174. Nathanson SD, Nelson L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann Surg Oncol. (1994) 1:333–8. doi: 10.1007/BF03187139
175. Hampton HR, Chtanova T. Lymphatic migration of immune cells. Front Immunol. (2019) 10:1168. doi: 10.3389/fimmu.2019.01168
176. Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. (2006) 25:989–1001. doi: 10.1016/j.immuni.2006.10.011
177. Hugues S, Scholer A, Boissonnas A, Nussbaum A, Combadiere C, Amigorena S, et al. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol. (2007) 8:921–30. doi: 10.1038/ni1495
178. Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A. (1997) 94:3909–13. doi: 10.1073/pnas.94.8.3909
179. Dupre L, Houmadi R, Tang C, Rey-Barroso J. T lymphocyte migration: an action movie starring the actin and associated actors. Front Immunol. (2015) 6:586. doi: 10.3389/fimmu.2015.00586
180. Wagner M, Jasek M, Karabon L. Immune checkpoint molecules - inherited variations as markers for cancer risk. Front Immunol. (2020) 11:606721. doi: 10.3389/fimmu.2020.606721
181. Dong W, Gong M, Shi Z, Xiao J, Zhang J, Peng J. Programmed cell death-1 polymorphisms decrease the cancer risk: a meta-analysis involving twelve case-control studies. PLoS ONE. (2016) 11:e0152448. doi: 10.1371/journal.pone.0152448
182. Parakh A, Musafer A, Paessler S, Witkowski T, Suen CSNLW, Tutuka CSA, et al. PDCD1 polymorphisms may predict response to Anti-PD-1 blockade in patients with metastatic melanoma. Front Immunol. (2021) 12:672521. doi: 10.3389/fimmu.2021.672521
183. Horn L, Horn HL. An immunological approach to the therapy of cancer? Lancet. (1971) 2:466–9. doi: 10.1016/S0140-6736(71)92632-8
Keywords: tumor microenvironment, Immune Checkpoint Inhibitors, immune system, network, immunotherapy
Citation: Talaat IM and Kim B (2022) A brief glimpse of a tangled web in a small world: Tumor microenvironment. Front. Med. 9:1002715. doi: 10.3389/fmed.2022.1002715
Received: 25 July 2022; Accepted: 28 July 2022;
Published: 15 August 2022.
Edited by:
Luca Mastracci, University of Genoa, ItalyReviewed by:
Alessandro Gambella, University of Turin, ItalyCopyright © 2022 Talaat and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byoungkwon Kim, YmtraW0zMzNAZ21haWwuY29t
 Iman M. Talaat
Iman M. Talaat Byoungkwon Kim
Byoungkwon Kim