Abstract
While worldwide efforts for improving COVID-19 vaccines are currently considered a top priority, the role of the genetic variants responsible for virus receptor protein stability is less studied. Angiotensin-converting enzyme-2 is the primary target of the SARS-CoV-1/SARS-CoV-2 spike (S) glycoprotein, enabling entry into the human body. Here, we applied computational saturation mutagenesis approaches to determine the folding energy caused by all possible mutations in ACE2 proteins within ACE2 - SARS-CoV-1-S/ACE2 - SARS-CoV-2-S complexes. We observed ACE2 mutations at residue D350 causing the most stabilizing effects on the protein. In addition, we identified ACE2 genetic variations in African Americans (rs73635825, rs766996587, and rs780574871), Latino Americans (rs924799658), and both groups (rs4646116 and rs138390800) affecting stability in the ACE2 - SARS-CoV-2-S complex. The findings in this study may aid in targeting the design of stable neutralizing peptides for treating minority patients.
Introduction
Following the discovery of ACE2 as the SARS-CoV-2 Spike (S) receptor, a substantial number of articles have appeared attempting to characterize the pathology of the infection and help with the creation of therapies (1). As a cellular receptor for SARS viruses, ACE2 protein acts as a port of its entry, allowing the coronavirus to invade and begin replicating. Notably, the S protein of SARS-CoV-2 binds to ACE2 more strongly than SARS-CoV-1 (1).
SARS-CoV-2 replication can be widespread; it is not confined to the lungs, but it can also affect the heart, intestines, blood vessels, muscles, and brain, leading to a myriad of post-COVID sequelae and comorbidities (2–5). Accordingly, many medical conditions aggravating the course of SARS-CoV-2 are characterized by changes in mRNA levels or the membrane abundance of ACE2. Key risk factors for COVID-19 include obesity, diabetes, cardiovascular diseases, and smoking (6–9). In particular, upregulation of ACE2 is a pathophysiological feature of diabetes and hyperglycemic states (10), obesity (11), smoking and air pollution (12, 13). On the other hand, asthma patients show lower expression of ACE2, and it has been reported that asthma exerts a protective effect against SARS-CoV-2 infection as well as severe COVID-19 outcomes (14, 15).
In addition, through the renin-angiotensin system (RAS) (16), ACE2 plays a crucial role in the development of hypertension, atherosclerotic plaque development, and chronic kidney disease (17). Under physiological conditions, the ACE enzyme alters angiotensin I and converts it into angiotensin II, which causes blood vessels to constrict. The tightening of the blood vessels leads to an increase in blood pressure. Recently, ACE2 was identified in pericytes of the heart (17). There are more ACE2 receptors on the surface of cells in the heart muscle in people with established cardiovascular disease than in those without disease (17, 18).
The ACE2 receptor has two functional domains: the N-terminal peptidase M2 domain and the C-terminal collectrin domain. The homodimer side chain domain (residues 19-768) includes the following regions: peptidase domain (PD): 19-65, C-terminal collectrin-like domain (CLD): 616-768, and N-terminal amino acid residues (17 to 537) (19). In both ACE2-SARS-CoV-1/2 complexes, the peptidase and N-terminal domains interact with the spike glycoprotein of SARS-CoV-1 and SARS-CoV-2; however, the N-terminal residues (303-537) are the binding site associated with the spike glycoprotein (20).
For a majority of human proteins, the levels of ACE2 depend on underlying variation in human genomes. Moreover, ACE2 protein species may differ in their amino acid sequences, encoded by genetic alleles unevenly distributed in patients of various ethnic backgrounds. Now that the 1,000 Genomes (16) and the gnomAD (21) projects are completed, common ACE2 variants have already been identified. However, detailed analyses of ACE2 sequences in various African/Latino American populations are still warranted. Several coding variants of ACE2 in humans have been associated with health conditions such as cardiovascular disorders, hypertension, and diabetes. GWASs have described the changes in the coronavirus spike protein and host ACE2 receptor by associating risk variants common contributing to comorbid states (22). A majority of uncovered genetic variation, however, is not directly responsible for a diseased state but serves as biomarkers instead, by pinpointing a set of disease-related loci on the human genome map, by their genetic associations. Occasionally, a variant may be used to seek out and isolate the disease-causing gene directly (23).
Due to widespread inconsistencies in mapping and quantifying underlying genetic variation in world populations, the role of ACE2 gene variation in differential susceptibility to COVID-19 infections cannot be ruled out. Underprivileged populations, particularly African and Latino Americans, are nearly three times more likely to be hospitalized with COVID-19 than white Americans (24). On the one hand, in these populations, both comorbidities and socioeconomic factors contribute to the higher COVID-19-attributed mortality (25). On the other hand, the African American population shows increased molecular expression of ACE2 (26). Moreover, in 2020, the infection rate for African Americans was 62 per 10,000 compared with 23 per 10,000 for Caucasian Americans (27). The ethnic differences in ACE2 expression may underline the higher infectiveness of SARS-CoV-2 among African Americans (28). Similar to African Americans, Hispanics/Latinos are 1.6 times more likely to develop COVID-19 than their non-Hispanic white counterparts, 3.3 times more likely to be hospitalized from COVID-19, and 2.2 times more likely to die from COVID-19 (28). The Latino population also has a higher infection rate (73 per 10,000) than African Americans (27).
Our study utilizes computational approaches to investigate the effects of every possible ACE2 missense mutation on protein stability. First, we applied structure-based energy calculations to measure the total effects of ACE2 mutations on the protein stability of ACE2-S complexes. Second, we identified key genetic mutations altering ACE2 stability in African/Latino American populations. Finally, we discussed the target residues that need future experimental validation to design neutralizing peptides against SARS-CoV-2.
Materials and methods
Structure preparation
The structures ACE2 - SARS-CoV-1-S (PDB ID: 2AJF) and ACE2 - SARS-CoV-2-S (PDB ID: 6LZG) were retrieved from the Protein Data Bank (29). PyMol was employed to visualize structural models and determine structural similarity in both complexes (30). Genetic variations were filtered specifically for the ACE2 gene in African/Latino Americans from gnomAD v4.3 (21). TM-align was used to maximize residue alignment based on structural similarity (30).
Free energy calculations
FoldX was utilized for all energy calculations, computing the change in the free folding free energy ΔΔG (the difference between the free energy of the mutant and wild-type protein). Each residue position underwent mutagenesis by using the FoldX algorithm (31). The initial step used the “RepairPdb” command to repair the wild-type protein structure, which functions by mutating certain residues to itself to lessen the total free energy of the protein structure. The “Build Model” command (31) is used to calculate the folding energy changes upon mutation. We performed computational saturation mutagenesis on the ACE2 protein and measured the effects of mutations on the protein stability of the S-ACE2 complex. 23,860 mutations were analyzed in the ACE2 chains in both ACE2-Spike protein complexes (PDB: 6LZG and 2AJF, respectively). In addition, we also analyzed the effects of these mutations in ACE2 chains alone by removing the spike chains from the protein complexes.
A value of ΔΔG > 0 shows that the mutation is destabilizing, while a ΔΔG < 0 is a stabilizing mutation. This algorithm has been used in many studies involving protein stabilization experiments (32). Prior to performing all energy analyses, the “RepairPDB” function in FoldX was used to correct bad angles, van der Waals energy clashes, and rotation assignments. For free folding energy calculation, the unfolded protein is considered as two parts, which include a three-residue segment with the mutation in the center and then all other residues. The values of protein stability between the mutant (MUT) and the wild-type structures were computed based on the folding energy change (ΔΔG) by using the following equations:
The computational predictions of protein stability changes upon mutations from mCSM (Cutoff Scanning Matrix) (33) and SAAFEC-SEQ (Sequence-based Single Amino Acid Folding Free Energy Changes) (34) were used to compare with the results from FoldX. The R graphical packages were used to generate heatmaps, line graphs, and boxplots (35).
Sequence-based analysis
Screening for non-acceptable polymorphisms (SNAP), a neural network-based tool used for the evaluation of functional effects of single amino acid substitutions in proteins, was used to predict whether a mutation is likely to alter protein function (23). The ACE2 protein sequences of PDB structures were used as the inputs of SNAP. R-programming was used to represent data by creating suggestable relationships by boxplots to compare the SNAP values.
Results
Structural alignment of ACE2 structures in complexes
We performed structural alignment of ACE2 chains in ACE2 - SARS-CoV-1-S/ACE2 - SARS-CoV-2-S complexes (Figure 1A). The root-mean-square deviation (RMSD) was 0.53, which indicates a great similarity of ACE2 conformations between these two complexes. The ΔΔG values of ACE2 mutations in both complexes were compared (Figure 1B), and the stability effects of mutations on both complexes were correlated (R2 = 0.8246). Structural alignment and correlation analysis revealed great similarity in ACE2 mutation effects between ACE2 - SARS-CoV-2-S and ACE2 - SARS-CoV-1-S complexes. In addition, we removed the Spike chains in complexes and calculated the ΔΔG of mutations on ACE2 chains alone. As shown in Supplementary Figure 1, the stability effects of mutations on ACE2 monomers are highly correlated with those in the complexes (R2 = 0.9631 for ACE2 - SARS-CoV-2-S and R2 = 0.9633 for ACE2 - SARS-CoV-1-S). The top six residue positions that strongly stabilize or destabilize ACE2 protein are listed in Table 1 based on the mean ΔΔG values in complexes. These residues also have common top values mean ΔΔG in ACE2 alone.
FIGURE 1
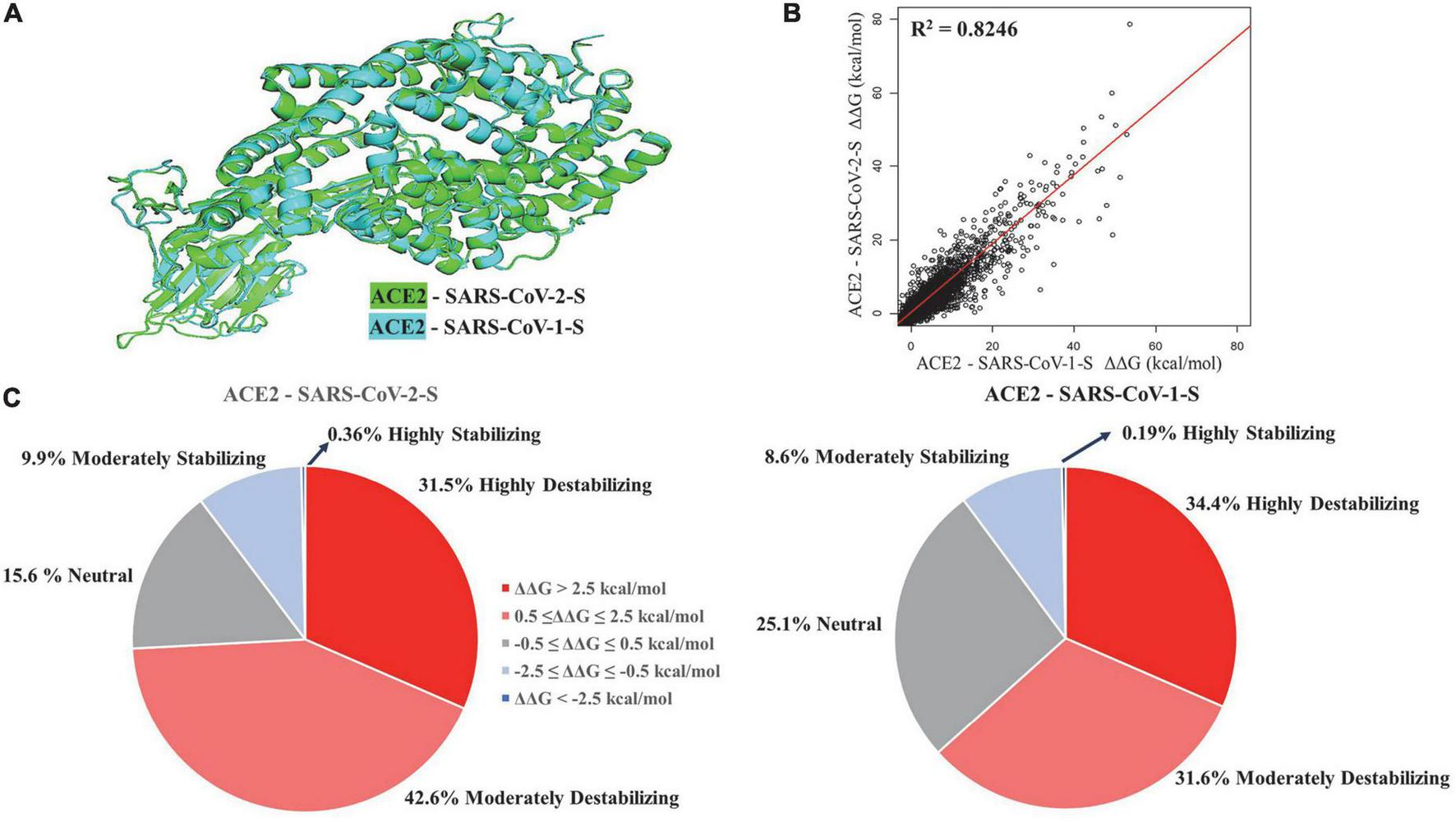
Structural alignment (A) and regression analysis (B) of ACE2 - SARS-CoV-2-S and ACE2 - SARS-CoV-1-S complexes. Pie charts (C) displaying the distribution of effects of mutations on protein stability effects of missense mutations.
TABLE 1
| Residue position | FoldX | mCSM | SAAFEC-SEQ | ||||
| Score | Effect | Score | Effect | Score | Effect | ||
| ACE2 - SARS-CoV-2-S | ACE2 Mutation | ||||||
| G561 | 24.80 | 25.46 | D | 1.23 | D | 1.57 | D |
| G486 | 23.94 | 24.35 | D | 0.94 | D | 1.49 | D |
| G268 | 17.15 | 17.60 | D | 1.08 | D | 1.15 | D |
| G405 | 17.00 | 11.48 | D | 1.22 | D | 1.42 | D |
| G399 | 14.80 | 14.56 | D | 0.93 | D | 1.36 | D |
| G173 | 14.62 | 14.63 | D | 1.33 | D | 1.19 | D |
| D350 | –1.52 | –1.28 | S | 0.001 | D | –0.51 | S |
| D382 | –1.08 | –0.12 | S | –1.02 | S | –0.5 | S |
| G66 | –0.92 | –0.87 | S | –0.98 | S | –0.48 | S |
| S105 | –0.84 | –0.86 | S | –0.52 | S | –0.58 | S |
| S47 | –0.84 | –0.76 | S | –0.77 | S | –0.75 | S |
| S167 | –0.79 | –0.60 | S | –0.63 | S | –0.46 | S |
| ACE2 - SARS-CoV-1-S | ACE2 Mutation | ||||||
| G561 | 29.23 | 29.38 | D | 1.23 | D | 1.57 | D |
| G486 | 20.73 | 21.08 | D | 0.94 | D | 1.49 | D |
| G173 | 17.61 | 17.20 | D | 1.33 | D | 1.48 | D |
| G405 | 16.03 | 15.74 | D | 1.22 | D | 1.42 | D |
| G399 | 13.82 | 13.33 | D | 0.93 | D | 1.36 | D |
| G268 | 14.20 | 14.65 | D | 1.08 | D | 1.15 | D |
| D350 | –1.58 | –1.38 | S | 0.19 | D | –0.60 | S |
| E375 | –1.46 | –1.39 | S | –0.87 | S | –0.70 | S |
| D543 | –1.43 | –1.33 | S | –0.15 | S | –0.10 | S |
| T324 | –1.29 | –1.29 | S | –0.73 | S | –0.49 | S |
| E430 | –1.29 | –1.03 | S | –0.24 | S | –0.17 | S |
| S43 | –1.10 | –1.06 | S | –0.89 | S | –0.52 | S |
Comparison of target mutations among different computational tools on ACE2 – SARS-CoV-1-S/ACE2 – SARS-CoV-2-S ΔΔGMean (kcal/mol).
D, destabilization; S, stabilization.
Effects of ACE2 mutations on protein stability
The percentage of mutations in both complexes was grouped based on highly destabilizing, moderately destabilizing, neutral, moderately stabilizing, and highly stabilizing (Figure 1C). In the ACE2-SARS-CoV-2-S complex, 31.5% of the mutations displayed high destabilization (ΔΔG > 2.5 kcal/mol), while 42.6% of the mutations showed moderate destabilization (0.5 ≤ ΔΔG ≤ 2.5 kcal/mol). 15.6% of the mutations were neutral (−0.5 ≤ ΔΔG ≤ 0.5 kcal/mol), 9.9% of mutations can moderately stabilize ACE2 (−2.5 ≤ ΔΔG ≤ −0.5 kcal/mol), and 0.36% of the mutations were highly stabilizing (ΔΔG < −2.5 kcal/mol). In the ACE2-SARS-CoV-1-S complex, 34.4% of the mutations displayed high destabilization (ΔΔG > 2.5 kcal/mol), while 31.6% of the mutations showed moderate destabilization (0.5 ≤ ΔΔG ≤ 2.5 kcal/mol). 25.1% of the mutations were neutral (−0.5 ≤ ΔΔG ≤ 0.5 kcal/mol), 8.6% of the mutations had a moderate stabilizing effect (−2.5 ≤ ΔΔG ≤ −0.5 kcal/mol), and 0.19% of the mutations were highly stabilizing (ΔΔG < −2.5 kcal/mol).
The mean values of ΔΔG at each ACE2 residue position and ΔΔG value of substitutions to alanine are shown in the line charts of Figure 2. In the ACE2-SARS-CoV-2-S complex, the mutations in G561 cause the maximum destabilizing effects (mean ΔΔG = 24.80 kcal/mol), while the mutations in D350 have the highest stabilizing effects (mean ΔΔG = −1.52 kcal/mol). A similar pattern of mean ΔΔG was observed in the ACE2 - SARS-CoV-1-S complex, with a range from 29.23 kcal/mol in G561 to -1.58 kcal/mol in D350. The heatmaps of top residues with the maximum destabilizing/stabilization effects of mutations on ACE2 stability and the structural representations of these key residues are shown in Figure 2. Here, we probed for the most destabilizing residues that were common to both complexes. As shown in Figure 2 and Table 1, the mutations at Glycine (G) residue positions G561, G486, G268, G405, and G399 have the maximum destabilizing effects on ACE2 in both complexes. Glycine is the simplest and non-essential amino acid because it has only a hydrogen atom as its side chain. Mutation of G to other larger amino acids will result in adverse conformation variations and destabilize the protein complex, which makes it responsible for over 15% of genetic diseases (36). Its size is often critical in allowing polypeptide chains to make tight turns or to approach one another closely. Residues that replace glycine disturb the helix fold, subjecting chains to additional hydroxylation and glycosylation. Hence, since glycine is crucial in helix formation, substitution at numerous nucleotide sites will result in a clinically evident phenotype (37). Consequently, glycine to glutamic acid substitution can reduce the activity of ACE2 in the central nervous system, thereby affecting the signal transduction between ACE2 in pleural cavities. Interestingly, when G is mutated to tryptophan (W), it causes the highest destabilization energy. A G to W substitution can interact with other aromatic or positively charged residues. W is the largest amino acid and is found to have the highest probability of causing disease [Klein (38)]. In both complexes, the greatest energy instability was seen when G was mutated to W at positions 561, 405, and 399. The greatest unstable energy value was also seen in G561W in both ACE2 - SARS-CoV-1-S/ACE2 - SARS-CoV-2-S (ΔΔG = 78.58 and 53.58 kcal/mol, respectively).
FIGURE 2
Visualization of key residues affecting protein in panel (A) ACE2 - SARS-CoV-2 and (B) ACE2 - SARS-CoV-1-S complexes. The lines (mean ΔΔG, kcal/mol) and bubbles (ΔΔG of alanine mutations, kcal/mol) shown the destabilization (red) and stabilization (blue) effects (The scales of y-axis for two effects are different for clear visualization). ACE2 is shown in green or cyan, and the SARS-CoV-2/1 (S) protein is shown in gray. In the heatmaps of key residues, maximum values (orange), minimum (magenta), and the genetic variants (green) are labeled for the substitutions.
We compared the computational predictions from mCSM and SAAFEC-SEQ to the FoldX results (Table 1). For the six residues with destabilizing effects, all three tools give consistent predictions for the mutation stability effects. FoldX and SAAFEC-SEQ predict that mutations in D350 can stabilize ACE2 protein stability, while mCSM prediction shows that mutations in this residue have neutral effects. For the other five top residues with stabilizing effects, all three tools give negative mean ΔΔG values. The results indicate that the FoldX predication is reliable for identifying the key residues altering protein stability.
Mutation pathogenic analysis of ACE2
The results from SNAP combine exome and genome data from a variety of algorithms to summarize possible pathogenicity. For this study, amino acid changes were compared to calculated SNAP scores (23). The boxplot functions were used since the generated graphs take up less space and are therefore particularly useful for comparing distributions between several groups or data sets. The SNAP score utilizes various physicochemical features of nsSNPs (non-synonymous single nucleotide polymorphisms) substitutions, as well as evolutionary data.
The boxplots of SNAP scores of mutation groups with different folding energy change (ΔΔG) intervals for ACE2 - SARS-CoV-2-S/ACE2 - SARS-CoV-1-S stability are shown in Figure 3. The SNAP scores were compared versus five categories of stability energy intervals in both complexes, and their means were significantly different (ANOVA test, p-value = 0.0036973). The SNAP scores of mutations with strong destabilizing/stabilizing effects (ΔΔG > 2.5 or ΔΔG < -2.5 kcal/mol) were higher than those with moderate effects (0.5 < ΔΔG < 2.5 or no effects (-0.5 ≤ ΔΔG ≤ 0.5 kcal/mol) in both complexes. The results indicate that mutations altering protein stability have damaging effects on ACE2 protein functions.
FIGURE 3
Boxplots for the SNAP scores of ACE2 mutation groups with different folding energy change intervals in panel (A) ACE2 - SARS-CoV-2 and (B) ACE2 - SARS-CoV-1 complexes.
Genetic variants affecting protein stability
dbNSFP (39) contains genetic variants from numerous databases (gnomAD, dbSNP, 1000 Genomes, Ensembl, etc.). We collected 231 genetic variants present in gnomAD from dbNSFP v4.3 (Supplementary Table 1). Among these variants, 40 unique missense mutations were observed in African Americans, while 42 unique missense mutations were present in Latino Americans. The selected target genetic variants affecting ACE2 stability in African/Latino Americans are listed in Table 2. rs4646116 (K26R) (more cases are reported in Latino Americans) and rs138390800 (K341R) are found in both ethnic groups, with effects being neutral in both complexes. The variants rs73635825 (S19P), rs146676783 (E37K), and rs766996587 (M82I) identified in African Americans but not Latino Americans can destabilize the ACE2 protein.
TABLE 2
| Genetic variation | gnomAD allele count* | ΔΔ G (kcal/mol) | ||||
| dbSNP ID | Mutation | All | AFR | AMR | ACE2 - SARS-CoV-2-S | ACE2 - SARS-CoV-1-S |
| rs73635825 | S19P | 46 | 45 | 0 | 0.973 | 0.350 |
| rs4646116 | K26R | 728 | 13 | 90 | 0.461 | 0.418 |
| rs146676783 | E37K | 6 | 1 | 0 | 0.943 | 2.857 |
| rs766996587 | M82I | 2 | 2 | 0 | 1.570 | 1.136 |
| rs759579097 | G326E | 1 | 1 | 0 | –0.195 | –0.732 |
| rs924799658 | F40 L | 3 | 0 | 3 | 0.104 | –0.354 |
| rs780574871 | E312K | 2 | 2 | 0 | 0.276 | 0.299 |
| rs138390800 | K341R | 59 | 52 | 5 | –0.079 | 0.305 |
Key genetic variants altering ACE2 stability in African/Latino Americans.
*All, all populations; AFR, African American; AMR, Latino/Admixed American.
Discussion
There are several reasons why African American and Latino communities are being disproportionately affected by the coronavirus. In particular, these populations are also at higher risk of chronic health problems aggravating the clinical course of COVID-19, including diabetes, heart disease and obesity (6, 40). Here, we concentrated on genetic variants that affect the African/Latino American ethnicities, also facing the greatest socioeconomic and racial disparities (41). Structural studies have reported numerous genetic variations affecting the stability of the ACE2 - SARS-CoV-2-S complex (42). In African Americans, rs766996587 (M82I) and rs73635825 (S19P) appear with an allelic frequency of 1.0 × 10–5. rs766996587 represents a methionine to isoleucine change that destabilizes the protein structure and affects the pathogenicity of ACE2-driven viral infections (43). In our study, predicted mutation values show a correlation between ACE2 - SARS-CoV-1-S/ACE2 - SARS-CoV-2-S on respective destabilization (44). rs73635825 (S19P) is a change of a serine to a proline close to the N-end of ACE2 protein. By in silico evaluation using PolyPhen-2, rs73635825 has been predicted to have damaging effects, and multiple sources have concluded that the change from serine to proline decreases immune resistance against SARS-CoV-2-S due to a decrease in stability (28). rs73635825 is common in European populations (Allele Frequency = 2.13 × 10–4). There have been some published structural and homology modeling studies showing that this variant destabilizes the ACE2 receptor in European populations but not in African Americans (Allele Frequency = 2.1 × 10–3) (45). rs146676783 (E37K), a change from glutamic acid to lysine, is detected in African Americans, with an allelic frequency of 3.0 × 10–5 appearing in this race (46). E37K contributes to decreased stability in ACE2 - SARS-CoV-2-S (ΔΔG = 0.943 kcal/mol) and in ACE2 - SARS-CoV-1-S (ΔΔG = 2.857 kcal/mol). rs4646116 (K26R), seen predominantly in Latino Americans, destabilizes the ACE2 receptor domain. rs759579097 (G326E), albeit showing a neutral effect on protein stability, increases the binding affinity between ACE2 and SARS-CoV-2-S (47).
We hypothesize that by analyzing the mutant protein stability of both ACE2 - SARS-CoV-1-S/ACE2 - SARS-CoV-2-S complexes, our contribution can aid in predicting future mutations. Increased protein stability can be a significant factor in the study of protein evolution (48). Stabilized proteins can adapt to a broad range of mutations and determine the gradual change of the proteins. Stabilizing mutations offer an advantage to the virus by improving the ratio of correctly folded proteins to decreased protein deficiency inside the cell. Changes in significant amino acids of the ACE2 receptor were projected to decrease or increase SARS-CoV-2-S recognition (49). For example, the hemagglutinin protein of influenza virus has demonstrated that at higher stability, the virulence and infectivity rate are higher (38). Our results show that mutations in residue D350 can increase the stability of the ACE2 receptor in both ACE2 - SARS-CoV-2-S (mean ΔΔG = −1.52 kcal/mol) and ACE2 - SARS-CoV-1-S (mean ΔΔG = −1.58 kcal/mol) complexes. Most COVID-19 vaccines are designed based on the SARS-CoV-2 S protein, and their effectiveness could be influenced by the emergence of new SARS-CoV-2 variants with multiple S mutations. The designed ACE2 could be a potential new therapeutic against COVID-19 (50). Increasing protein stability is an important goal for protein engineering. Target residues can be analyzed and verified by using bioinformatic tools and molecular biology experiments. The replacement of more functional amino acids in these key sites can generate more stable therapeutic peptides. Given the transmission and rapid spread of SARS-CoV-2, it is important to use new computational approaches to design new therapies. Theory and computational systems along with advanced laboratory techniques will increase our understanding of the functional mechanism of SARS-CoV-2 and its human receptor ACE2.
Conclusion
The excess of publications associated with the SARS-CoV-2-S virus-related disease outbreak reveals the intense effort by researchers to tackle both molecular mechanisms and therapeutic methods useful for treating current and future variants of the coronavirus outbreak. In our research, we showed that mutations in some residues, such as D350, stabilize the ACE2 receptor. Our data indicate that genetic variants, such as rs73635825, rs4646116, rs146676783, and rs766996587, in African/Latino American populations may cause significant decreases in the stability of ACE2. Further research in the areas of antiviral discovery will enhance our understanding of the effects of these mutations in hopes of designing therapeutic peptides capable of disrupting the complexes between the virus and ACE2.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
VM and ST: conceptualization, software, investigation, data curation, and writing—original draft preparation. VM: methodology and formal analysis. ST, QT, FZ, AB, VM, RR, AO, and MJ: writing—review and editing. QT and ST: supervision. ST: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Howard University start-up funds (U100193) and the National Science Foundation (DBI 2000296 and IIS 1924092). This project was supported (in part) by the National Institute on Minority Health and Health Disparities of the National Institutes of Health (2U54MD007597).
Acknowledgments
We thank the Howard University Research Centers for Minority Institutions (RCMI) program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1002187/full#supplementary-material
Supplementary Figure 1The regression analysis of ΔΔG (kcal/mol) of mutations in ACE2 chain alone and in complex for ACE2 - SARS-CoV-2-S (left) and ACE2 - SARS-CoV-1-S (right) complexes.
Supplementary Table 1Summary of ACE2 genetic variants with their gnomAD allele counts and ΔΔG values (kcal/mol).
References
1.
GuoYRCaoQDHongZSTanYYChenSDJinHJet alThe origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status.Military Med Res. (2020) 7:11. 10.1186/s40779-020-00240-0
2.
Zamorano CuervoNGrandvauxN. ACE2: evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities.eLife. (2020) 9:e61390. 10.7554/eLife.61390
3.
PatersonCDavisDRocheMBissettBRobertsCTurnerMet alWhat are the long-term holistic health consequences of COVID-19 among survivors? An umbrella systematic review.J Med Virol. (2022) 94:5653–68. 10.1002/jmv.28086
4.
BaranovaACaoHChenJZhangF. Causal association and shared genetics between asthma and COVID-19.Front Immunol. (2022) 13:705379. 10.3389/fimmu.2022.705379
5.
BaranovaACaoHZhangF. Causal effect of COVID-19 on Alzheimer’s disease: A Mendelian randomization study.J Med Virol. (2022). 10.1002/jmv.28107
6.
DochertyABHarrisonEMGreenCAHardwickHEPiusRNormanLet alFeatures of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study.BMJ. (2020) 369:m1985. 10.1136/bmj.m1985
7.
CaoHBaranovaAWeiXWangCZhangF. Bidirectional causal associations between type 2 diabetes and COVID-19.J Med Virol. (2022). 10.1002/jmv.28100
8.
ZhangFBaranovaA. Smoking quantitatively increases risk for COVID-19.Eur Respir J. (2021) 2101273. 10.1183/13993003.01273-2021
9.
XiaoFZhouYCZhangMBChenDPengSLTangHNet alHyperglycemia and blood glucose deterioration are risk factors for severe COVID-19 with diabetes: a two-center cohort study.J Med Virol. (2022) 94:1967–75. 10.1002/jmv.27556
10.
SrivastavaARockman-GreenbergCSareenNLionettiVDhingraS. An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning β-cell survival and cardiovascular conditions in diabetic patients.Mol Cell Biochem. (2022) 477:1681–95. 10.1007/s11010-022-04396-2
11.
Al-BennaS. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients.Obes Med. (2020) 19:100283. 10.1016/j.obmed.2020.100283
12.
HeijinkIHHackettTLPouwelsSD. Effects of cigarette smoking on SARS-CoV-2 receptor ACE2 expression in the respiratory epithelium(dagger).J Pathol. (2021) 253:351–4. 10.1002/path.5607
13.
SmithJCSausvilleELGirishVYuanMLVasudevanAJohnKMet alCigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract.Dev Cell. (2020) 53:514–29.e3. 10.1016/j.devcel.2020.05.012
14.
CarliELardaniLFitzgibbonRFambriniEBagattoniS. Periodontology part 3: hereditary gingival fibromatosis (HGF): from diagnosis to treatment in the paediatric age.Eur J Paediatr Dent. (2022) 23:249–50. 10.23804/ejpd.2022.23.03.13
15.
BaranovaACaoHZhangF. Severe COVID-19 increases the risk of schizophrenia.Psychiatry Res. (2022) 317:114809. 10.1016/j.psychres.2022.114809
16.
ClarkeLZheng-BradleyXSmithRKuleshaEXiaoCTonevaIet alThe 1000 Genomes Project: data management and community access.Nat Methods. (2012) 9:459–62. 10.1038/nmeth.1974
17.
ThumTSARS-CoV-2 receptor ACE2 expression in the human heart: cause of a post-pandemic wave of heart failure?Eur Heart J. (2020) 41:1807–9. 10.1093/eurheartj/ehaa410
18.
Herman-EdelsteinMGuettaTBarneaAWaldmanMBen-DorNBaracYDet alExpression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the renin angiotensin system.Cardiovasc Diabetol. (2021) 20:90.
19.
WrappDWangNCorbettKSGoldsmithJAHsiehC-LAbionaOet alCryo-EM structure of the 2019-nCoV spike in the prefusion conformation.Science. (2020) 367:1260–3.
20.
PrabakaranPXiaoXDimitrovDS. A model of the ACE2 structure and function as a SARS-CoV receptor.Biochem Biophys Res Commun. (2004) 314:235–41. 10.1016/j.bbrc.2003.12.081
21.
KarczewskiKJFrancioliLCTiaoGCummingsBBAlföldiJWangQet alThe mutational constraint spectrum quantified from variation in 141,456 humans.Nature. (2020) 581:434–43.
22.
RaoSBaranovaACaoHChenJZhangXZhangF. Genetic mechanisms of COVID-19 and its association with smoking and alcohol consumption.Brief Bioinform. (2021) 22:bbab284.
23.
BrombergYRostB. SNAP: predict effect of non-synonymous polymorphisms on function.Nucleic Acids Res. (2007) 35:3823–35.
24.
CarboneMLednickyJXiaoSYVendittiMBucciE. Coronavirus 2019 infectious disease epidemic: where we are, what can be done and hope for.J Thorac Oncol. (2021) 16:546–71.
25.
YehiaBRWinegarAFogelRFakihMOttenbacherAJesserCet alAssociation of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals.JAMA Network Open. (2020) 3:e2018039.
26.
BusehAG.Empowering Resilience: Improving Health Care Delivery in War-Impacted African Countries : A Case Study of Liberia.Lanham, MD: University Press of America (2008). 279 p.
27.
WilliamsLBFernanderAFAzamTGomezMLKangJMoodyCLet alCOVID-19 and the impact on rural and black church Congregants: results of the C-M-C project.Res Nurs Health. (2021) 44:767–75. 10.1002/nur.22167
28.
HussainMJabeenNRazaFShabbirSBaigAAAmanullahAet alStructural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein.J Med Virol. (2020) 92:1580–6.
29.
BermanHM. The protein data bank.Nucleic Acids Res. (2000) 28:235–42. 10.1016/j.apsb.2020.02.006
30.
ZhangYSkolnickJ. Scoring function for automated assessment of protein structure template quality.Proteins. (2004) 57:702–10.
31.
DelgadoJRaduskyLGCianferoniDSerranoL. FoldX 5.0: working with RNA, small molecules and a new graphical interface.Bioinformatics. (2019) 35:4168–9. 10.1093/bioinformatics/btz184
32.
BussORudatJOchsenreitherK. FoldX as protein engineering tool: better than random based approaches?Comput Struct Biotechnol J. (2018) 16:25–33. 10.1016/j.csbj.2018.01.002
33.
PiresDEVAscherDBBlundellTL. mCSM: predicting the effects of mutations in proteins using graph-based signatures.Bioinformatics. (2014) 30:335–42.
34.
LiGPandaySKAlexovE. SAAFEC-SEQ: a sequence-based method for predicting the effect of single point mutations on protein thermodynamic stability.Int J Mol Sci. (2021) 22:606. 10.3390/ijms22020606
35.
JatainAA review study on big data analysis using R studio. In: Ranjan A editor. Proceedings of the International Journal of Computer Science and Mobile Computing. (Vol. 6), IJCSMC: Stockholm (2017). p. 8–13.
36.
VitkupDSanderCChurchGM. The amino-acid mutational spectrum of human genetic disease.Genome Biol. (2003) 4:R72.
37.
MariniJCForlinoACabralWABarnesAMSan AntonioJDMilgromSet alConsortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans.Hum Mutat. (2007) 28:209. 10.1002/humu.20429
38.
KleinEYBlumenkrantzDSerohijosAShakhnovichEChoiJMRodriguesJVet alStability of the influenza virus hemagglutinin protein correlates with evolutionary dynamics.mSphere. (2018) 3:e554–517. 10.1128/mSphereDirect.00554-17
39.
LiuXLiCMouCDongYTuY. dbNSFP v4: a comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs.Genome Med. (2020) 12:103. 10.1186/s13073-020-00803-9
40.
IslamMSKamalA-HMKabirASouthernDLKhanSHHasanSMMet alCOVID-19 vaccine rumors and conspiracy theories: the need for cognitive inoculation against misinformation to improve vaccine adherence.PLoS One. (2021) 16:e0251605. 10.1371/journal.pone.0251605
41.
HarveyHDunifonRPilkauskasN. Under whose roof? understanding the living arrangements of children in doubled-up households.Demography. (2021) 58:821–46. 10.1215/00703370-9101102
42.
CruzJDOConceiçãoIMCASousaSMBLuizonMR. Functional prediction and frequency of coding variants in human at binding sites with SARS-CoV-2 spike protein on different populations.J Med Virol. (2021) 93:71–3.
43.
BhattacharjeeMJLinJJChangCYChiouYTLiTNTaiCWet alIdentifying Primate ACE2 variants that confer resistance to SARS-CoV-2.Mol Biol Evol. (2021) 38:2715–31.
44.
ShangJYeGShiKWanYLuoCAiharaHet alStructural basis of receptor recognition by SARS-CoV-2.Nature. (2020) 581:221–4. 10.1038/s41586-020-2179-y
45.
ChoudharySSreenivasuluKMitraPMisraSSharmaP. Role of genetic variants and gene expression in the susceptibility and severity of COVID-19.Ann Lab Med. (2021) 41:129–38. 10.3343/alm.2021.41.2.129
46.
DarbaniB. The expression and polymorphism of entry machinery for COVID-19 in human: juxtaposing population groups, gender, and different tissues.Int J Environ Res Public Health. (2020) 17:3433. 10.3390/ijerph17103433
47.
BakhshandehBSorboniSGJavanmardARMottaghiSSMehrabiMRSorouriFet alVariants in ACE2; potential influences on virus infection and COVID-19 severity.Infect Genet Evol. (2021) 90:104773. 10.1016/j.meegid.2021.104773
48.
BrownEEFRezaeiRJamiesonTRDaveJMartinNTSingaraveluRet alCharacterization of critical determinants of ACE2–SARS CoV-2 RBD interaction.Int J Mol Sci. (2021) 22:2268. 10.3390/ijms22052268
49.
RotemASerohijosAWRChangCBWolfeJTFischerAEMehokeTSet alEvolution on the biophysical fitness landscape of an RNA virus.Mol Biol Evol. (2018) 35:2390–400. 10.1093/molbev/msy131
50.
PayandehZRahbarMRJahangiriAHashemiZSZakeriAJafarisaniMet alDesign of an engineered ACE2 as a novel therapeutics against COVID-19.J Theor Biol. (2020) 505:110425. 10.1016/j.jtbi.2020.110425
Summary
Keywords
ACE2, SARS-CoV-1-S, SARS-CoV-2-S, genetic variations, protein stability
Citation
Mahase V, Sobitan A, Rhoades R, Zhang F, Baranova A, Johnson M, Otolorin A, Tang Q and Teng S (2022) Genetic variations affecting ACE2 protein stability in minority populations. Front. Med. 9:1002187. doi: 10.3389/fmed.2022.1002187
Received
24 July 2022
Accepted
10 October 2022
Published
31 October 2022
Volume
9 - 2022
Edited by
Deepak Y. Patil, National Institute of Virology (ICMR), India
Reviewed by
Wenhan Guo, The University of Texas at El Paso, United States; Christian Gruber, Innophore GmbH, Austria; Yu-Shi Tian, Osaka University, Japan
Updates
Copyright
© 2022 Mahase, Sobitan, Rhoades, Zhang, Baranova, Johnson, Otolorin, Tang and Teng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaolei Teng, shaolei.teng@howard.eduQiyi Tang, qiyi.tang@howard.edu
This article was submitted to Infectious Diseases – Surveillance, Prevention and Treatment, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.