
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med. , 04 January 2023
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.1001879
This article is part of the Research Topic Gluten: Yes, No, Maybe View all 6 articles
 Shakira Yoosuf1,2,3*†
Shakira Yoosuf1,2,3*† Caitlin G. Barrett1†
Caitlin G. Barrett1† Konstantinos Papamichael1
Konstantinos Papamichael1 Sarah E. Madoff1,4
Sarah E. Madoff1,4 Satya Kurada1,5
Satya Kurada1,5 Joshua Hansen1,2,6
Joshua Hansen1,2,6 Jocelyn A. Silvester1,2,3
Jocelyn A. Silvester1,2,3 Amelie Therrien1,2
Amelie Therrien1,2 Prashant Singh1,2,7
Prashant Singh1,2,7 Melinda Dennis1,2
Melinda Dennis1,2 Daniel A. Leffler1,2,8
Daniel A. Leffler1,2,8 Ciaran P. Kelly1,2
Ciaran P. Kelly1,2Background: Pancreatic Exocrine Insufficiency (PEI) is a possible cause of recurrent/persistent symptoms in celiac disease. Although pancreatic enzyme supplementation may be used to treat non-responsive celiac disease (NRCD) in clinical practice, clinical outcomes are variable and there is limited and low quality evidence to support this practice. The aim of this study was to assess the efficacy of pancreatic enzyme supplements (PES) for improvement of gastrointestinal symptoms in NRCD.
Methods: Prospective, randomized, placebo-controlled, double-blind, cross-over trial in adults with NRCD examining Celiac Disease-Gastrointestinal Symptom Rating Scale (CeD-GSRS) scores on PES (pancrelipase co-administered with omeprazole) versus placebo (omeprazole only) during a 10-day treatment period. The study was registered under the clinical trials registry (https://clinicaltrials.gov/ number, NCT02475369) on 18 Jun 2015.
Results: Twelve participants (nine female) were included in the per-protocol analysis; one participant had low fecal elastase-1. Pancrelipase was not associated with significant change in CeD-GSRS compared to placebo (−0.03 versus −0.26; P = 0.366). There was a significant decrease in mean values of total CeD-GSRS scores (3.58 versus 2.90, P = 0.004), abdominal pain (2.92 versus 2.42, P = 0.009), and diarrhea sub-scores (3.44 versus 2.92, P = 0.037) during the run-in period with omeprazole.
Conclusion: In this prospective, cross-over randomized, placebo-controlled study, PES did not improve symptoms in patients with NRCD. It is unclear whether this is a trial effect or related to administration of omeprazole.
Non-responsive celiac disease (NRCD) is characterized by persistent/recurrent symptoms of celiac disease (CeD) despite 6–12 months of adherence to a gluten-free diet (GFD) (1). Pancreatic Exocrine Insufficiency (PEI) is one of the suspected contributory factors for these symptoms, occurring in an estimated 12% of NRCD patients (2). Multiple mechanisms have been proposed to account for PEI in CeD, including enterokinase deficiency secondary to villous atrophy which may impair activation of pancreatic enzymes (3, 4). Although pancreatic enzyme supplementation may be used to treat NRCD in clinical practice, results are variable and there is limited and low quality evidence to support this practice.
Based on symptomatic response to a trial of pancreatic enzyme supplements (PES), the prevalence of PEI has been found to be 10–18% in NRCD patients in retrospective chart reviews (5, 6). Further, one previous clinical trial showed PES to be useful in alleviating persistent symptoms in NRCD, but exclusively included patients with low fecal elastase-1 levels, and studied their diarrheal symptoms only (7). While earlier reports suggested that loss of >90% pancreatic function (severe PEI) is required to cause clinically significant diarrhea, more recent studies point to symptoms occurring in mild-moderate PEI (8), which may not necessarily be accompanied by low fecal elastase-1 levels (9). Therefore, we examined the efficacy of PES in patients with a broader range of NRCD symptoms, such as abdominal pain and bloating, irrespective of the presence of clinically demonstrable pancreatic dysfunction as assessed by fecal elastase-1 levels.
We conducted a prospective, randomized, double-blind, placebo-controlled, cross-over trial to determine the efficacy of PES for treatment of gastrointestinal (GI) symptoms in NRCD patients. Subjects with NRCD on a strictly GFD recruited at Beth Israel Deaconess Medical Center (BIDMC) between July 2015 and April 2017 were randomized 1:1 to initial treatment with either pancrelipase (Viokace®, Confab Laboratories, St-Hubert, QC, Canada) coadministered with omeprazole, or placebo with omeprazole (Figure 1). Coadministration of omeprazole with Viokace® is recommended in order to prevent gastric inactivation of these non-enteric coated PES. Therefore, we had a 1-week run-in period to ensure omeprazole was therapeutic prior to initiation of pancreatic enzyme supplementation. Inclusion criteria were: biopsy-confirmed CeD; age 18–80 years; self-reported strict adherence to GFD as assessed by an expert dietitian and the Celiac Dietary Adherence Test (CDAT) (10); and persistent GI symptoms (Celiac Disease-Gastrointestinal Symptom Rating Scale or CeD-GSRS score ≥3 in the highest domain at baseline visit) (11). Those with pork allergy, limited English proficiency, lactose intolerance, pregnancy, history of chronic active GI disease (other than CeD), major GI surgeries or other conditions that would interfere with subjects’ participation or confound study results were excluded.
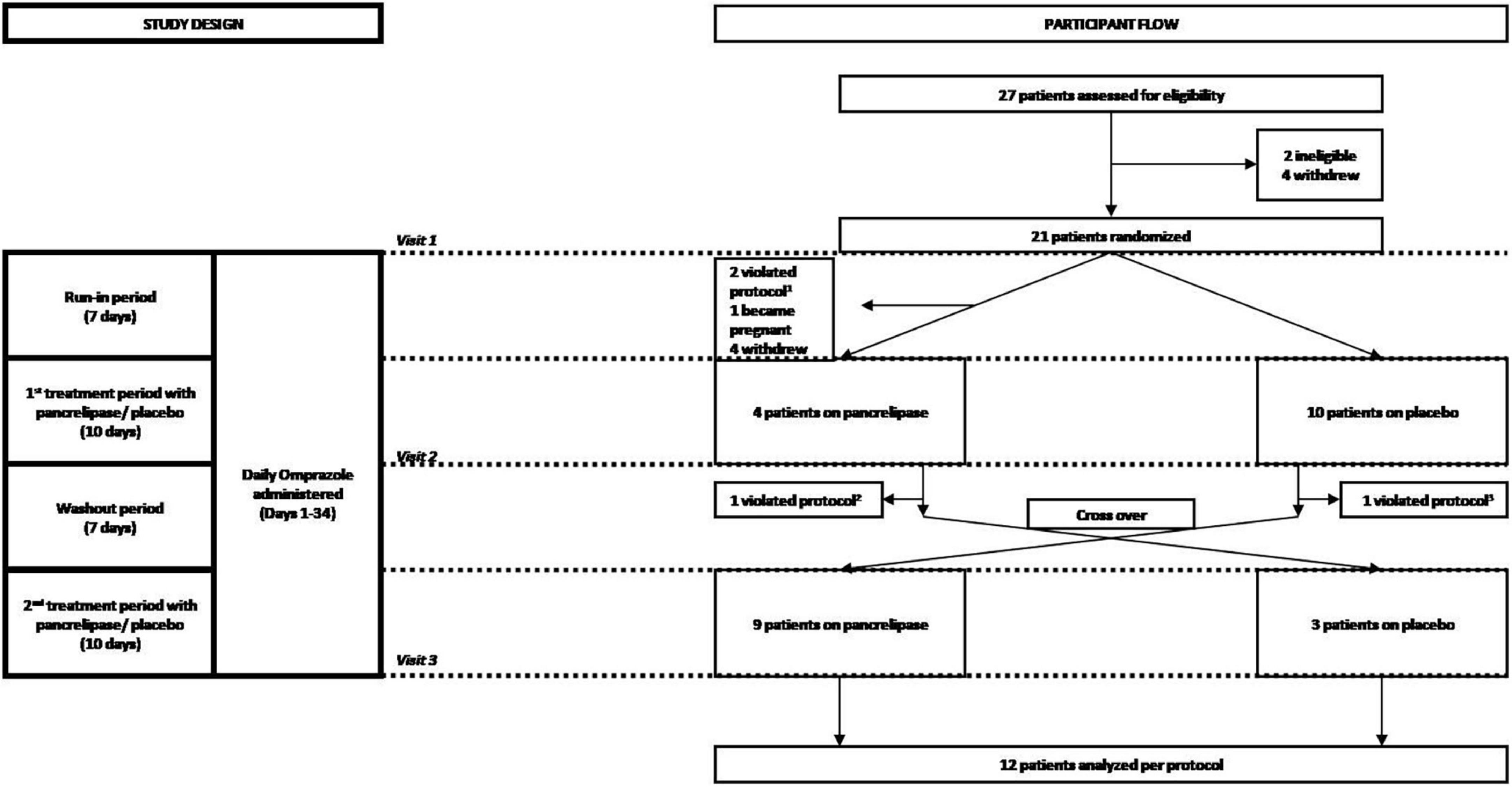
Figure 1. Consort diagram of the randomization and follow-up of study participants. Participants were screened for eligibility, assessed for severity of celiac disease symptoms and randomized at visit 1, which corresponded to the beginning of a 7 day run-in period during which participants received 20 mg omeprazole orally. Omeprazole was also administered during the first 10-day period of treatment with either pancreatic enzyme supplements (pancrelipase) or placebo, a 7 day wash-out (starting with visit 2) and the second 10-day treatment with pancreatic enzyme supplements or placebo (starting with visit 3). Participants who completed the overall 34-day course were then analyzed per-protocol. 1Two patients failed run-in; 2one patient did not complete data collection; 3one patient started placebo before washout.
Subjects were screened and randomized at visit 1, which corresponded to the beginning of a 7-day run-in period during which participants received omeprazole 20 mg orally. Omeprazole administration was continued through to the end of the study, including the first 10-day period of treatment with either PES (pancrelipase) or placebo, followed by a 7-day wash-out and the second 10-day treatment with pancrelipase or placebo (Figure 1). Participants who completed the 34-day study were analyzed per-protocol. Fecal elastase-1 and celiac serology levels were determined at the baseline visit. There were two additional visits at the end of each treatment period. At all three visits, medication logs were checked and symptoms were assessed with the Celiac Symptom Index (CSI; modified to have a 1-week recall period, from the original 4-week recall) and the CeD-GSRS (total score as well as scores in three domains – abdominal pain, indigestion, and diarrhea) (12).
Participants were randomized in a 1:1 ratio using a computer-generated sequence in blocks of four. The hospital pharmacy group performed the randomization and dispensed the study drug with no further involvement in the study. To maintain allocation concealment, identical, sequentially numbered containers were used for treatment and placebo. During the treatment phase, participants received Viokace, a porcine derived pancrelipase that contains lipase, amylase, and protease administered with meals and snacks. Allocation concealment was maintained using equal numbers of visually indistinguishable capsules for placebo and active treatment. Each capsule of Viokace contained 20,880 USP units of lipase, 78,300 USP units of protease and 78,300 USP units of amylase and the number of capsules was based upon weight: >59 kg: 6/meal, 4/snack; 53–59 kg: 5/meal, 4/snack; 47–52 kg: 5/meal, 3/snack; and 40–46 kg: 4/meal, 3/snack.
The primary outcome was change in mean CeD-GSRS on PES versus placebo. Secondary outcomes included change in mean CSI, and CeD-GSRS individual domain scores (abdominal pain, indigestion, and diarrhea) on PES versus placebo and correlation of these outcomes with baseline fecal elastase-1. For sample size calculation, we hypothesized a mean difference in CeD-GSRS of 0.4 between treatment and placebo (11). Assuming SD = 0.55, we needed 32 patients to achieve 80% power at α = 0.05. The primary outcome was evaluated using Wilcoxon signed rank test and linear mixed effects model using the nlme package (13). In the model, treatment (PES or placebo), time (before or after treatment), treatment × time interaction, sequence (PES first or placebo first), period (before or after the crossover), age and sex were considered fixed effects and intercepts for subjects were random effects. Additionally, proportions of patients with >30 and >50% reductions in CeD-GSRS total and abdominal pain scores were compared between the two interventions using McNemar’s test. All statistical tests were performed with R (R Core Team, 3.6.1). A two-sided P-value < 0.05 was considered statistically significant. The study was approved by the BIDMC Institutional Review Board; ClinicalTrials.gov number, NCT02475369. Informed consent was obtained from all individual participants included in the study.
We enrolled and randomized 21 patients (17 female, median age: 41 years, range: 23–70 years). Initial treatment randomizations were 11 PES and 10 placebo (Figure 1). The trial was terminated prematurely by the sponsor for administrative reasons. Due to dropouts, a per-protocol analysis was performed on the 12 participants that completed the study (9 female; 3 initial treatment placebo) (Table 1). None of the eight dropouts in the initial PES arm were related to adverse events (AE). Also, dropouts had similar baseline characteristics. Treatment compliance, as assessed by pill count, was >90% on PES and >70% on placebo, with no severe AE reported.
Celiac Disease-Gastrointestinal Symptom Rating Scale total (3.58 versus 2.90, P = 0.004), and abdominal pain scores (2.92 versus 2.42, P = 0.009) were significantly reduced following the 7-day run-in period. Comparing pre- and post-intervention time-points, no significant changes were found in CeD-GSRS or CSI on PES compared with placebo by the Wilcoxon method and mixed effects model (Table 2). However, there was a statistically significant period effect associated with improvement in the CeD-GSRS total (P = 0.04) and indigestion domain (P = 0.008) scores.
No subject on PES and three (25%) on placebo had a >30% reduction in both total CeD-GSRS and the abdominal pain domain score compared to the end of run-in period (P = 0.008), with none having >50% reduction. Only one subject had low fecal elastase-1 at baseline (8%, 95% CI: 1.5–35%). Five subjects (42%) had fecal elastase-1 between 200 and 500 μg/g stool and six subjects (50%) had values >500 μg/g stool. Baseline fecal elastase-1 did not correlate significantly with differences in total CeD-GSRS score on PES versus placebo (R = 0.34, P = 0.280).
This is the first prospective, controlled trial to examine the efficacy of PES in NRCD. PES was not associated with significant symptom improvement as assessed by CeD-GSRS or CSI. However, CeD-GSRS decreased significantly during the run-in period during which omeprazole was administered. Thus, it is unclear whether improvement is attributable to the well-documented trial effect in CeD whereby enrollment in a trial leads to symptomatic improvement, presumably due to stricter GFD adherence (11, 14) or if there is a beneficial effect of omeprazole. The same factors could also explain the significant period effect associated with improvement of CeD-GSRS total and indigestion domain scores after the run-in period, even though overall, the improvement in these scores was not significant in the mixed effects model. Literature on the role of proton pump inhibitors (PPIs) in NRCD is scant, but a significant proportion of our cohort had PPI-responsive symptoms such as epigastric pain, which is known to occur frequently in NRCD (1).
Our cohort had one subject (8%, 95% CI: 1.5–35%) with low fecal elastase-1 levels, which is consistent with prior studies of NRCD (5, 15). Thus, it did not include exclusively individuals with known PEI, unlike a previous uncontrolled open-label trial that demonstrated significant reduction of diarrhea in 18 out of 20 NRCD subjects, all having low fecal elastase-1 levels (7). Further, our cohort was more representative of the general NRCD population given that we recruited patients with a wide variety of symptoms including abdominal pain and bloating, and not specifically diarrhea. Similarly, the baseline CeD-GSRS and CSI scores are similar to those from other general NRCD cohorts recruited in previous studies (11, 16). Compliance to PES was adequate with weight-based dosing with meals and/or snacks and co-administration of omeprazole, as is recommended for non-enteric coated pancreatic enzymes (17). The normal fecal elastase-1 levels and lack of response to PES in our subjects indicate that most symptoms were unlikely to be attributable to PEI. This is consistent with a previous retrospective study at our center, showing an absence of response to PES in the NRCD population (18). Furthermore, the only patient with low fecal elastase-1 in our study failed to show resolution of diarrhea with PES, but fecal elastase-1 may have been artificially low in the liquid stool sample (19).
This study attempted to address the knowledge gap concerning the use of PES for NRCD irrespective of the presence of demonstrable PEI, which is a clinically relevant question. Limited data from previous retrospective studies have documented symptomatic improvement with trials of PES in 10–18% of NRCD patients (5, 6). However, with a rigorous study design, validated measures of GI symptoms and GFD adherence and standardized weight-based dosing of pancreatic enzymes, our trial showed contrary results. Study limitations include lack of a run-in period prior to omeprazole and trial termination before reaching an adequate sample size, with potential for a type 2 error. Also, despite randomization, protocol violations resulted in an unbalanced distribution in the two initial treatment groups (nine subjects assigned to placebo and three to pancrelipase); however, that did not significantly alter the relative distribution of baseline characteristics between the groups. Despite the small sample size, most patients showed no improvement on PES, which indicates that PEI may not be a common contributor to persistent symptoms in an unselected NRCD population.
Although empiric trials of PES have been suggested, this should not be routine until larger trials with robust study designs similar to ours demonstrate efficacy. As well, future studies may benefit from incorporation of recently available fecal and urine tests for gluten immunogenic peptides to select or stratify the population based upon ongoing gluten exposure, and provide objective information to control for a potential trial effect related to improved GFD adherence/reduced gluten exposure. Although representative of the NRCD population, the degree of symptoms of patients in our study was mild-moderate, which reduced the ability to detect a small improvement. To further characterize the effectiveness of PES, future prospective studies should examine larger NRCD cohorts with varying fecal elastase levels and with a wider range of symptom severity.
In this prospective cross-over randomized controlled trial of PES versus placebo, patients showed no significant improvement on PES, which indicates that PEI may not be a common contributor to persistent symptoms in NRCD population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation. The patients/participants provided their written informed consent to participate in this study.
CK and DL: study concept, design, and supervision. CB, CK, DL, JS, JH, MD, PS, SK, SM, and SY: acquisition of patient data. CK, DL, JS, KP, PS, and SY: statistical analysis. AT, CB, CK, DL, JS, KP, MD, PS, and SY: drafting of the manuscript, data interpretation, and critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
The trial sponsor, Allergan plc. had no role in trial design or data collection or analysis, but did review the manuscript. JS and PS received salary support from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers: K23DK119584 and T32DK07760. AT received funding from the Association des Gastroentérologues du Québec, Fond de Recherche du Québec – Santé Phase 2 award, ACG Junior Faculty Career Development Award and Research support from IMGX003-NIAID-1821.
CK: scientific advisor/consultant: Cour Pharma, Glutenostics, ImmunogenX, Innovate, Janssen, Kanyos/ Anokion, Merck, Milky Way Life Sciences, Takeda and Teravance; research support: Allergan; clinical study investigator: Allergan, Innovate, ImmunogenX, Merck, Milky Way Life Sciences, and Takeda Pharmaceuticals. DL: medical director: Takeda Pharmaceuticals. JS: scientific advisor/consultant: Mozart Therapeutics, Takeda Pharmaceuticals, and Teva Pharmaceuticals; research support: Biomedal S.L., Glutenostics LLC, and Milky Way Life Sciences; clinical study investigator: Cour Pharma and Takeda Pharmaceuticals. KP: received lecture fees from Mitsubishi Tanabe Pharma, Physicians Education Resource LLC, and Grifols and scientific advisory board fees from ProciseDx Inc., and Scipher Medicine Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AE, adverse events; BIDMC, Beth Israel Deaconess Medical Center; CDAT, Celiac Dietary Adherence Test; CeD, celiac disease; CeD-GSRS, Celiac Disease-Gastrointestinal Symptom Rating Scale; CSI, Celiac Symptom Index; GFD, gluten-free diet; GI, gastrointestinal; NRCD, non-responsive celiac disease; PEI, Pancreatic Exocrine Insufficiency; PES, pancreatic enzyme supplements; PPI, proton pump inhibitor.
1. Rubio-Tapia A, Hill I, Kelly C, Calderwood A, Murray J. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. (2013) 108:656–76. doi: 10.1038/ajg.2013.79
2. Singh V, Haupt M, Geller D, Hall J, Quintana Diez P. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. (2017) 23:7059–76. doi: 10.3748/wjg.v23.i39.7059
3. Lebenthal E, Antonowicz I, Shwachman H. The interrelationship of enterokinase and trypsin activities in intractable diarrhea of infancy, celiac disease, and intravenous alimentation. Pediatrics. (1975) 56:585–91. doi: 10.1542/peds.56.4.585
4. Regan P, DiMagno E. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology. (1980) 78:484–7. doi: 10.1016/0016-5085(80)90860-4
5. Abdulkarim A, Burgart L, See J, Murray J. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. (2002) 97:2016–21. doi: 10.1111/j.1572-0241.2002.05917.x
6. Fine K, Meyer R, Lee E. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. (1997) 112:1830–8. doi: 10.1053/gast.1997.v112.pm9178673
7. Leeds J, Hopper A, Hurlstone D, Edwards S, McAlindon M, Lobo A, et al. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. (2007) 25:265–71. doi: 10.1111/j.1365-2036.2006.03206.x
8. Lindkvist B, Phillips M, Domínguez-Muñoz J. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: prevalence and diagnostic use. Pancreatology. (2015) 15:589–97. doi: 10.1016/j.pan.2015.07.001
9. Carroccio A, Verghi F, Santini B, Lucidi V, Iacono G, Cavataio F, et al. Diagnostic accuracy of fecal elastase 1 assay in patients with pancreatic maldigestion or intestinal malabsorption: a collaborative study of the Italian society of pediatric gastroenterology and hepatology. Dig Dis Sci. (2001) 46:1335–42. doi: 10.1023/A:1010687918252
10. Leffler D, Dennis M, Edwards George J, Jamma S, Magge S, Cook E, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. (2009) 7:530–6. doi: 10.1016/j.cgh.2008.12.032
11. Leffler D, Kelly C, Green P, Fedorak R, DiMarino A, Perrow W, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. (2015) 148:1311.e–9.e. doi: 10.1053/j.gastro.2015.02.008
12. Kelly C, Green P, Murray J, Dimarino A, Colatrella A, Leffler D, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. (2013) 37:252–62. doi: 10.1111/apt.12147
13. Pinheiro J, Bates D, R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-158. (2022). Available online at: https://CRAN.R-project.org/package=nlme (accessed July 18, 2022).
14. Murray J, Kelly C, Green P, Marcantonio A, Wu T, Mäki M, et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. (2017) 152:787.e–98.e. doi: 10.1053/j.gastro.2016.11.004
15. O’Mahony S, Howdle P, Losowsky M. Review article: management of patients with non-responsive coeliac disease. Aliment Pharmacol Ther. (1996) 10:671–80. doi: 10.1046/j.1365-2036.1996.66237000.x
16. Lähdeaho M, Scheinin M, Vuotikka P, Taavela J, Popp A, Laukkarinen J, et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol. (2019) 4:948–59. doi: 10.1016/S2468-1253(19)30264-X
17. Trang T, Chan J, Graham D. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21(st) century. World J Gastroenterol. (2014) 20:11467–85. doi: 10.3748/wjg.v20.i33.11467
18. Leffler D, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly C. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. (2007) 5:445–50. doi: 10.1016/j.cgh.2006.12.006
Keywords: diarrhea, sprue, malabsorption, exocrine pancreatic insufficiency, proton pump inhibitors, pancreas, dyspepsia
Citation: Yoosuf S, Barrett CG, Papamichael K, Madoff SE, Kurada S, Hansen J, Silvester JA, Therrien A, Singh P, Dennis M, Leffler DA and Kelly CP (2023) Pancreatic enzyme supplementation versus placebo for improvement of gastrointestinal symptoms in non-responsive celiac disease: A cross-over randomized controlled trial. Front. Med. 9:1001879. doi: 10.3389/fmed.2022.1001879
Received: 24 July 2022; Accepted: 29 November 2022;
Published: 04 January 2023.
Edited by:
Stefano Guandalini, The University of Chicago, United StatesReviewed by:
Gary James Connett, University Hospital Southampton NHS Foundation Trust, United KingdomCopyright © 2023 Yoosuf, Barrett, Papamichael, Madoff, Kurada, Hansen, Silvester, Therrien, Singh, Dennis, Leffler and Kelly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shakira Yoosuf, ✉ c2hha2lyYTg5MUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.